Abstract
Neuronal migration is essential for the development of the cerebral cortex. Mutations leading to defective migration are associated with numerous brain pathologies. An important challenge in the field is to understand the intrinsic and extrinsic mechanisms that regulate neuronal migration during normal development and in disease. Many small GTPases are expressed in the central nervous system during embryonic development. Recent findings have shown that Rap1 and its downstream partners Ral, Rac and Cdc42 are involved in the maintenance of N-Cadherin at the plasma membrane which is necessary for the correct polarization of migrating neurons. The activation of Rap1 is triggered by Reelin, an extracellular protein known for its role in the organization of the cortex into layers of neurons. In the absence of Reelin, neurons exhibit a broader and irregular pattern of positioning. The prevailing model suggests that Reelin signals to neurons during the last step of their migration, a notion that is inconsistent with new data describing an effect of Reelin on early steps of migration. In regard to these recent findings I suggest a revised model, which I call the “polarity model,” that further refines our understanding of the developmental function played by Reelin and its downstream small GTPases.
Keywords: brain, cadherin, cortex, mouse, neuronal migration, polarity, Rap, reeler, Reelin
Introduction
Small GTPases are guanine nucleotide binding proteins that function as molecular switches by cycling between active GTP-bound and inactive GDP-bound states. They are activated by Guanine nucleotide Exchange Factors (GEFs) that induce GTP loading and inhibited by GTPase Activating Proteins (GAPs) that return them into their GDP loaded inactive form.1 The large superfamily of small GTPases (also named the Ras superfamily) is comprised of the Ras/Rap/Ral, Rho, Rab, Arf and Ran families.2 They regulate a wide variety of essential cellular processes such as cell division, adhesion, polarity, migration and differentiation.3 It is thus not surprising that many of their members are involved directly or indirectly in numerous pathological conditions including cancer and brain developmental diseases.
This extra view will focus on the Rap subfamily of small GTPases. It is composed of five related proteins, Rap1 (A and B) and Rap2 (A, B and C) which have overlapping functions and patterns of expression. Rap1 in particular has been intensively studied for its role in the regulation of integrin-mediated cell adhesion and the control of endothelial and epithelial cadherin-based cell-cell junction integrity.4 We have recently demonstrated a new function of Rap1 in regulating the polarity of migrating neurons in the developing mouse brain cortex through the control of N-Cadherin (NCad).5 We found that Reelin, an extracellular matrix protein which has an important function in the organization of the cortex, triggers the activation of Rap1 in cortical neurons when they are midway through their migration path, at a stage where re-polarization occurs. These new findings do not fit with the current model in which Reelin affects neurons at the end of their migration. Here, I suggest a revised model of action for the Reelin signaling pathway with a central function for small GTPases.
I will start this extra view with a description of the development of the cerebral cortex and the recently discovered role played by Rap1. Then I will discuss the downstream proteins involved in this function, followed by the upstream Reelin signal.
Cortical Development and Rap1 Small GTPases
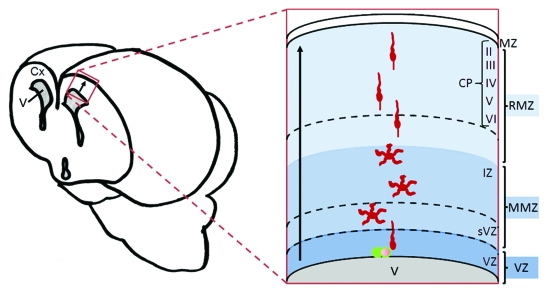
The development of the brain requires that neurons migrate away from their birth place in order to perform their functions properly. In addition, neurons have to extend neurites and ultimately differentiate and communicate with each other. Characterization of the molecular signaling pathways involved in cerebral cortex development is important for the understanding of brain pathologies such as lissencephaly, microcephaly, periventricular heterotopia, epilepsy, dyslexia, mental retardation, schizophrenia, bipolar disorder, and many others resulting from defective cortical architecture, connectivity and function. The majority of neurons in the cortex are the excitatory glutamatergic neurons that are generated from progenitor cells located at the ventricular zone (VZ) which lines the ventricle (Fig. 1). These neurons undergo different phases of migration starting with a polarized migration within the VZ. This is followed by a more complex movement within the multipolar morphology zone (MMZ), which is made up of the sub-ventricular zone (sVZ) and the lower part of the intermediate zone (IZ), where they undergo more divisions.6,7 Importantly, at this stage, the neurons lose their bipolar morphology and elongate several neurites which is why they have been referred as having a multipolar morphology. This occurs along with a slower migration and a few switches in their direction of movement. The neurons then migrate within the radial morphology zone (RMZ), comprising the upper part of the IZ and the cortical plate (CP), to reach the top of the CP. During the transition from MMZ to RMZ, they change morphology to become bipolar once again. Within the CP, new neurons migrate past the older ones already installed resulting in “inside-out layering,” that is, a gradient of cells with younger neurons in the outermost field of the CP and older neurons more inside.
Figure 1. Migration of glutamatergic neurons in the mammalian cerebral cortex. Neurons are born in the ventricular zone (VZ) from progenitor cells (green cells). They start migration as bipolar cells then migrate as multipolar cells when they move into the Multipolar Morphology Zone (MMZ), which contains the sub-ventricular zone (sVZ) and the lower part of the intermediate zone (IZ). Neurons resume a bipolar migration when they enter the Radial Morphology Zone (RMZ) comprising the upper part of the IZ and the cortical plate (CP). Within the CP, neurons migrate past the other cells already installed and settle just beneath the marginal zone (MZ) resulting in formation of an inside-out layered structure: The CP is divided into layers II to VI, with layer II consisting of younger later-born cells and layer VI consisting of the oldest cells. Cx, cortex; V, ventricle; arrows show direction of migration.
During the multipolar stage, and in spite of the multiple changes of direction of migration, the net movement is still directed toward the CP. Rap1 has emerged as a critical regulator of this polarization.5 Similar to all small GTPases, Rap proteins are regulated by specific GAPs or GEFs. Important clues for their involvement in brain development came from the phenotype of a hypomorphic mutant mouse for C3G, a Rap-specific GEF, which exhibits an accumulation of neurons within the MMZ.8 This arrest in cell migration is mainly due to the highly disorganized radial glia fibers (the main migration substrate for this type of cell) and disintegration of the basement membrane. Moreover, these mice die around E14.5 making it difficult to study the migration of later born neurons. C3G is not the only GEF regulating Rap during the development of the cortex. In fact, a dorsal telencephalon-specific knockout of PDZ-GEF-1, another activating protein for Rap1 and Rap2 enzymes, results in the accumulation of neurons underneath a normally developed cortex.9 The involvement of Rap activators in cortical development suggested that Rap enzymes might also play an important role. Indeed, the neuron-specific inhibition of Rap (i.e., without affecting radial glial cells) in vivo induces an ectopic accumulation of neurons within the MMZ.5 Time lapse video-microscopy revealed that this phenotype is not the result of defective neuronal motility of the affected multipolar cells, but is rather due to a defect in their polarization toward the RMZ. This is because the movement of the Rap-inhibited neurons is randomized with a decreased net movement toward the RMZ. This phenotype is not due to a complete arrest of invasion of the RMZ because many cells migrate out of the MMZ, albeit with a significant delay when compared with control cells. Surprisingly, the subsequent radial bipolar migration along glial fibers (also called glia-guided locomotion) is not affected by the absence of Rap activity. This absence of effect on locomotion has been confirmed in an in vitro lattice culture system where dissociated neurons move along glial fibers. Together, these observations suggest that Rap is important for the initial polarization of neurons but not migration per se.
Rap1 Polarizes Neurons Through Its Regulation of N-Cadherin, with a Potential Involvement of Ral, Rac and Cdc42
Signaling through the small GTPase Rap1 has been implicated in both integrin-mediated and cadherin-mediated adhesion events. To date, studies examining neuron-specific deletion of β1 Integrins10 or the Integrin downstream effector FAK (focal adhesion kinase)11 did not observe any defect in glia-guided migration. On the other hand, both Rap1 and cadherins and their interaction are emerging as important regulators during the brain development. Classical cadherins are single-pass transmembrane adhesion receptors involved in cell-cell contact and epithelial polarity through calcium dependent homophilic binding. Intracellularly, cadherins interact with catenin family members. p120-catenin binds to the juxtamembrane region of cadherin to stabilize it at the plasma membrane, while α- and β-catenin serve a dynamic role in linking cadherin to the actin cytoskeleton. Many members of the cadherin family are expressed in the central nervous system and one of them, NCad, has recently attracted the interest of neuroscientists.
The prevailing view is that cadherin functions to mediate adhesion between stationary cells, thereby maintaining tissue integrity and segregation of different cell populations. Indeed, throughout development, NCad is highly expressed in the vertebrate central nervous system and its conditional deletion in the dorsal telencephalon results in disruption of the adherens junctions localized at the apical end of neuroepithelial cells, where NCad is most highly concentrated. This results in a general disruption of neuroepithelial integrity and aberrant radial glia fibers that do not expand toward the pial surface.12 However, evidence is emerging that cadherins also regulate cellular motility. In the rat caudal hindbrain, classic cadherins regulate tangential migration of precerebellar neurons.13 In the zebrafish, NCad concentrates transiently at the front of cerebellar granule cells during the initiation of their chain-migration and is required for them to polarize prior to migrate.14 In the early chick embryo PDGF signaling controls NCad expression in mesoderm cells, which is required for efficient migration.15 Interestingly, C3G and PDZ-GEF1, two of the Rap-specific GEFs known to be important for mammalian brain development (see above), have also been linked to the cadherins. In epithelial cells, C3G directly interacts with E-cadherin and is important for the initial steps of adherens junction formation,16,17 while PDZ-GEF1 is recruited by MAGI-1 at VE-cadherin-mediated endothelial cell-cell adhesions.18
Our recent findings demonstrated that in the mammalian cerebral cortex NCad has an important function in polarizing cortical neurons before they are able to start migrating into the RMZ.5 The inhibition of cadherins in post-mitotic neurons without affecting progenitor cells and their radial glia fibers, recapitulates the phenotype induced by inhibition of Rap1 i.e., loss of polarity during the multipolar migration with no effect on the speed of migration as multipolar or bipolar neurons. Several experiments confirmed that NCad functions downstream from Rap1. First, inhibition of Rap1 in vivo and in vitro reduced the presence of NCad at the plasma membrane with a concomitant increase in intracellular NCad. Second, a functional assay demonstrated that inhibition of Rap1 reduced the binding of neurons to the NCad extracellular domain. And finally, overexpression of NCad in the cortex is able to partially rescue the cell positioning defect due to inhibition of Rap1. These data suggest that Rap1 activity is important in migrating neurons in order to maintain the high level of NCad at the plasma membrane necessary to allow cells to polarize correctly. Yet we do not know whether other cadherins, also expressed at the MMZ, might have some redundant function with NCad. In addition, how NCad allows the polarization of cortical neurons is still under investigation. Nevertheless, hypotheses might be suggested. NCad may be activated locally in order to increase the binding to radially-oriented processes on other neurons or glial fibers. This adhesion could stabilize the position of the centrosome. A similar model has been suggested for the directional chain migration of cerebellar granule neurons in the zebrafish with NCad transiently accumulating at the front of the cells.14 However, in other cell types, it is the cadherin-free cell edge that shows the polarity of migration. Indeed, cadherin-mediated cell-cell interactions induce the centrosome and Golgi apparatus to move toward the free cell edges in cultured astrocytes19 and stimulate protrusions at the free edge in Xenopus neural crest cells.20 Alternatively, NCad may be a regulator for other cell surface receptors that respond to directional signals from the CP. For example, NCad modulates FGF-2 signaling in MCF-7 breast cancer cells21 and VE-cadherin regulates TGFβ signaling in endothelial cells.22 This model has parallels with the migration of Drosophila border cells, where Drosophila ECad (DECad) is required in the migrating cells as well as in the cells they migrate between.23 The border cells extend a long leading process, whose direction is specified by a growth factor gradient but whose formation requires DECad.24 The induction of the long extensions on border cells may be analogous to the induction of a radial leading process on cortical neurons, and in both cases surface cadherin expression may be key to developing the polarity needed for migration.
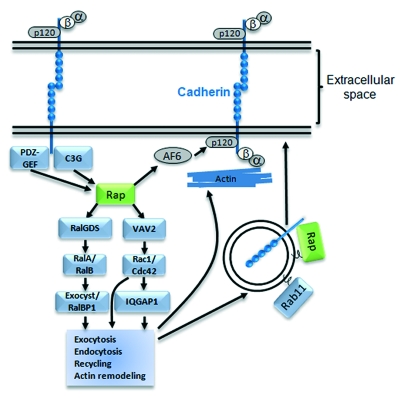
According to studies in epithelial and endothelial cells, Rap uses several mechanisms to regulate cadherin traffic (Fig. 2). The Rap effector RalGDS activates RalA, which docks secretory vesicles to the exocyst complex and recycles E-Cadherin to epithelial cell-cell junctions.25 It has been shown that Rap1, Rac1 and Cdc42, activated by nectins, are able to trigger the formation of adherens junctions in epithelial cells and fibroblasts.26,27 This positions them upstream of cadherin function. However, they also can be activated downstream of cadherins,17,28 suggesting a potential positive feedback loop. In epithelial cells, Rap1 is important for the recruitment of E-cadherin into nascent cell-cell contact sites16,29 and in this process it functions upstream of Rac1 and Cdc42 by recruiting their GEF Vav2.16,17 Cdc42 has been reported to regulate the trafficking of basolateral membrane proteins30 as well as to modulate the association of cell-cell contacts with the actin cytoskeleton.31 Finally, activation of Rac1 and Cdc42 by Rap1 could also stabilize cadherins at the membrane through their target IQGAP1. Studies have suggested two possibilities as to how they work. First, it has been proposed that IQGAP1 destabilizes cadherins by inducing their dissociation from α-catenin and IQGAP1 is negatively regulated by Rac1 and Cdc42.31,32 Second, another study suggested that IQGAP stabilizes cadherins through the reorganization of the actin cytoskeleton and is positively regulated by Rac1 and Cdc42.33 Regardless of which of these pathways downstream of Rap1 are predominant, perhaps depending on cell types or experimental conditions, all those results indicate that, in epithelial cells, stimulation of Rap1 may induce the activation of Ral, Rac and/or Cdc42, which in turn might facilitate the directional vesicle transport of E-cadherin and/or organize the actin cytoskeleton, enabling neighboring cells to contact one another. Other regulatory pathways have also been proposed such as the interaction of Rap1 with AF6, increasing AF6 association with p120 catenin which in turn strengthens the interaction of p120 catenin with E-cadherin, reducing its internalization and/or degradation.34
Figure 2. Regulation of cadherins by Rap1 and other downstream small GTPases. Rap1 induces adherens junction formation by stabilizing cadherins at the plasma membrane. Maintenance of cadherins at junctions can take the form of increased exocytosis, decreased endocytosis, increased recycling at the plasma membrane or remodeling of the actin cytoskeleton underneath the junctions. Those functions can be accomplished by the activation of different pathways downstream of Rap1 such as signals involving the small GTPases Ral, Rac, Cdc42 or Rab11. Rap1 also inhibits the targeting of cadherins for degradation through an AF6/p120-catenin pathway. Activation of Rap1 depends on specific GEFs such as PDZ-GEF or C3G that are recruited by cadherin clustering or by other signals.
Although migrating neurons are different from static epithelial cells making contacts, Rap1, Ral, Rac and Cdc42 may function similarly to regulate cell to cell contact of cortical neurons through the regulation of NCad.5 Our inhibition and rescue experiments in the animal and in vitro suggested that Ral, Rac and Cdc42 may also play a role influencing Rap1’s effect on the presence of NCad at the plasma membrane and the resulting function on polarity of cortical neurons.5 This process is likely to be very dynamic and control of the amount of cadherins at the cell surface must be tightly regulated by a balance between endocytosis and recycling to the sites of new contact formation. Of note, a recent study demonstrated the involvement of Rab5-dependent endocytic and Rab11-dependent recycling pathways in the regulation of NCad in cortical neurons,35 while, in epithelial cells, Rap1 has been shown to co-localize with E-cadherin at the Rab11-positive recycling endosome compartment.36 It would be of interest to determine whether Rap1 and the Rab pathways work in parallel or cooperatively in the regulation of NCad and neuronal migration. Another important next step would also be to investigate whether the exocyst, downstream of Ral, or IQGAP and other proteins downstream of Rac and Cdc42, play roles in the polarization of cortical neurons through the Rap1/NCad pathway.
Reelin Polarizes Multipolar Neurons Toward the RMZ Through Rap1 and NCad: A New Model of Action
An important question is what stimulates Rap1 and its downstream effectors in the polarization of migrating neurons.
Reelin is an extracellular protein secreted by Cajal-Retzius cells present in the marginal zone above the RMZ and is required for the correct organization of the CP.37 The prevailing model suggests that Reelin acts on migrating neurons when they arrive at the top of the RMZ during their final somal translocation (named “detach and go” because cells would detach from the radial glia then proceed through the final somal translocation).38 This model explains why, in the absence of Reelin, the inside-out layering of the cortex is inverted. However, it is important to point out that the reeler cortical phenotype is not simply an inversion of the neuronal layering. Even though the earliest neurons shift from a deep laminar position to form a superficially located superplate in the reeler brains, the later born neurons exhibit a broader and irregular distribution which is far more than just an inversion of laminar fate.39 This suggests that the current model may not fully explain the phenotype. Earlier studies already indicated that Reelin may signal the neurons before they start their radial migration within the RMZ. First, the active cleavage fragment of Reelin has been shown to diffuse from the marginal zone into the deep tissue.40 Second, cells in the MMZ express the highest level of functional Reelin receptors.41 Recently, we showed that inhibition of Reelin by in utero electroporation delays the cells at the MMZ and affects the localization of NCad at their plasma membrane, mimicking the phenotype caused by Rap inhibition. Rescue experiments in vivo confirmed that Rap1 is involved in this phenotype downstream of Reelin. These data suggest that Reelin is, at least in part, responsible for the Rap1/N-cadherin-mediated polarization function in neurons migrating within the MMZ. In agreement with this, NCad protein levels are decreased in the embryonic reeler mutant cerebral cortex exclusively at the MMZ.5 A function of Reelin through Rap1 and NCad on polarity of neurons before they enter the RMZ may better explain the disorganized positioning of late-born neurons. Indeed, in the absence of a polarizing signal, neurons would exit the MMZ in a disorderly manner disregarding their date of birth. It is surprising that Reelin affects the polarization of neurons because previous experiments showed that the localization of the source of Reelin is not important for its function in the CP.42,43 The polarization mediated by Reelin may thus be indirect. Reelin would rather act as a permissive signal allowing neurons to respond to another cue which still remains to be discovered.
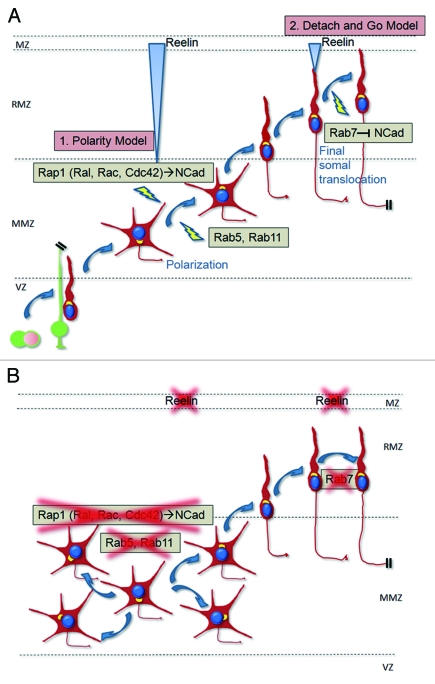
After neurons have received the polarizing cue triggered by Reelin, the signal is downregulated. Previous works have shown that neurons downregulate the signal and/or become less responsive to Reelin once they commence migration within the RMZ. For example, Reelin induces the downregulation of its functional receptors at the time neurons migrate within the RMZ.41,44 Also, Reelin induces the phosphorylation of the intracellular adaptor Dab1 which is consequently degraded.45,46 It is therefore likely that membrane-associated NCad levels are downregulated because the Reelin signal is not any longer there to maintain it at the membrane. Indeed, NCad also exhibits a downregulation of its expression in the wild-type RMZ, which is much less pronounced in the reeler brain.5 Interestingly, the downregulation of the Reelin signal seems to be an important event for a correct neuronal positioning.45,46 A testable prediction would be that the downregulation of NCad depends on the decreased Reelin signal. Indeed, a recent study showed that Rab7-dependent degradation of NCad is also important for the final phase of migration when neurons reach the top of the RMZ.35 Therefore, I propose a model (Fig. 3) in which Reelin first triggers the polarization of neurons when they are at the MMZ by activating Rap1 and stabilizing NCad at the plasma membrane but, and at the same time as phosphorylated Dab1 and the Reelin signal are downregulated, NCad is also degraded through a Rab7 pathway when neurons migrate as bipolar cells. This downregulation at the end of their migration might be as important as the upregulation at the multipolar stage. In this view, the final somal translocation is a consequence of the downregulation of the Reelin signal that was initiated when the cells were in the MMZ.
Figure 3. Regulation of neuronal migration within the mammalian cerebral cortex by the Reelin/Rap1/N-cadherin pathway and other small GTPases. (A) Reelin signals through Rap1 (and its downstream enzymes Ral, Rac and Cdc42) and N-cadherin on neurons at the MMZ to polarize them toward the RMZ. Rab5 and Rab11 might also be involved. The subsequent glia-guided migration within the RMZ is independent of Reelin, Rap1 or N-cadherin. During this migration, the Reelin signal is downregulated (degradation of phosphorylated Dab1, downregulation of functional receptors) and N-cadherin is downregulated by the Rab7 pathway which may be a consequence of the downregulation of the Reelin pathway. When neurons reach the top of the RMZ, they undergo a final somal translocation. This final somal translocation could depend on the reduction of the Reelin signal or, alternatively, Reelin may induce a second set of intracellular signals in cells performing the final somal translocation as they are more mature than when they encountered Reelin for the first time in the RMZ and are in a different biological context. (B) In the absence of Reelin, Rap1 or N-cadherin, neurons are disoriented within the MMZ. Rab5 or Rab11 inhibition induces a similar phenotype. In the absence of Reelin or Rab7, the final somal translocation is also affected and could be, at least in part, a consequence of defective N-cadherin downregulation.
In the above model, Reelin signals only in multipolar cells. However, it is also conceivable that Reelin stimulates migrating neurons twice: first at the MMZ and a second hit later during the final somal translocation. Neurons might be refractory to Reelin signaling after the first stimulation but become sensitive again when they reach the top of the RMZ. It is then plausible that Reelin triggers two different intracellular signals in the immature multipolar and in the more mature bipolar neurons as they are predicted to express different sets of intracellular signaling molecules and are surrounded by different cues and/or extracellular matrix proteins. In this case, the “polarity model” is not mutually exclusive with, but complementary to the previous “detach and go” model because they affect migrating neurons at two different steps of their journey.
Future Directions
The next challenge in the field is to determine exactly how Rap1 and its client GTPases RalA/B, Rac and Cdc42 affect the polarity of cortical neurons. Other small GTPases such as members of the Rab family might also come into play. How all those small GTPases finely tune neuronal migration needs to be investigated in more details. How NCad, and maybe other cadherins, regulate the polarized movement of cortical neurons is also ground for future work. Reelin certainly does not work alone and it will be exciting to uncover the interconnection of the multiple signals that regulate the development of the cerebral cortex.
Acknowledgments
I gratefully thank J.A. Cooper, R.N. Eisenman, S.M. Parkhurst and V. Vasioukhin, for helpful comments on the manuscript. Y.J. is supported by the Fonds National de la Recherche Scientifique (Belgium) and the European Commission, 7th framework program, under the Marie Curie International Outgoing Fellowship Program.
Footnotes
Previously published online: www.landesbioscience.com/journals/smallgtpases/article/18283
References
- 1.Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–77. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 2.Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118:843–6. doi: 10.1242/jcs.01660. [DOI] [PubMed] [Google Scholar]
- 3.Frische EW, Zwartkruis FJ. Rap1, a mercenary among the Ras-like GTPases. Dev Biol. 2010;340:1–9. doi: 10.1016/j.ydbio.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 4.Boettner B, Van Aelst L. Control of cell adhesion dynamics by Rap1 signaling. Curr Opin Cell Biol. 2009;21:684–93. doi: 10.1016/j.ceb.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jossin Y, Cooper JA. Reelin, Rap1 and N-cadherin orient the migration of multipolar neurons in the developing neocortex. Nat Neurosci. 2011;14:697–703. doi: 10.1038/nn.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–44. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- 7.Shoukimas GM, Hinds JW. The development of the cerebral cortex in the embryonic mouse: an electron microscopic serial section analysis. J Comp Neurol. 1978;179:795–830. doi: 10.1002/cne.901790407. [DOI] [PubMed] [Google Scholar]
- 8.Voss AK, Britto JM, Dixon MP, Sheikh BN, Collin C, Tan SS, et al. C3G regulates cortical neuron migration, preplate splitting and radial glial cell attachment. Development. 2008;135:2139–49. doi: 10.1242/dev.016725. [DOI] [PubMed] [Google Scholar]
- 9.Bilasy SE, Satoh T, Ueda S, Wei P, Kanemura H, Aiba A, et al. Dorsal telencephalon-specific RA-GEF-1 knockout mice develop heterotopic cortical mass and commissural fiber defect. Eur J Neurosci. 2009;29:1994–2008. doi: 10.1111/j.1460-9568.2009.06754.x. [DOI] [PubMed] [Google Scholar]
- 10.Belvindrah R, Graus-Porta D, Goebbels S, Nave KA, Muller U. Beta1 integrins in radial glia but not in migrating neurons are essential for the formation of cell layers in the cerebral cortex. J Neurosci. 2007;27:13854–65. doi: 10.1523/JNEUROSCI.4494-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beggs HE, Schahin-Reed D, Zang K, Goebbels S, Nave KA, Gorski J, et al. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron. 2003;40:501–14. doi: 10.1016/S0896-6273(03)00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kadowaki M, Nakamura S, Machon O, Krauss S, Radice GL, Takeichi M. N-cadherin mediates cortical organization in the mouse brain. Dev Biol. 2007;304:22–33. doi: 10.1016/j.ydbio.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Taniguchi H, Kawauchi D, Nishida K, Murakami F. Classic cadherins regulate tangential migration of precerebellar neurons in the caudal hindbrain. Development. 2006;133:1923–31. doi: 10.1242/dev.02354. [DOI] [PubMed] [Google Scholar]
- 14.Rieger S, Senghaas N, Walch A, Koster RW. Cadherin-2 controls directional chain migration of cerebellar granule neurons. PLoS Biol. 2009;7:e1000240. doi: 10.1371/journal.pbio.1000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X, Chrisman H, Weijer CJ. PDGF signalling controls the migration of mesoderm cells during chick gastrulation by regulating N-cadherin expression. Development. 2008;135:3521–30. doi: 10.1242/dev.023416. [DOI] [PubMed] [Google Scholar]
- 16.Hogan C, Serpente N, Cogram P, Hosking CR, Bialucha CU, Feller SM, et al. Rap1 regulates the formation of E-cadherin-based cell-cell contacts. Mol Cell Biol. 2004;24:6690–700. doi: 10.1128/MCB.24.15.6690-6700.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuyama T, Ogita H, Kawakatsu T, Inagaki M, Takai Y. Activation of Rac by cadherin through the c-Src-Rap1-phosphatidylinositol 3-kinase-Vav2 pathway. Oncogene. 2006;25:8–19. doi: 10.1038/sj.onc.1209010. [DOI] [PubMed] [Google Scholar]
- 18.Sakurai A, Fukuhara S, Yamagishi A, Sako K, Kamioka Y, Masuda M, et al. MAGI-1 is required for Rap1 activation upon cell-cell contact and for enhancement of vascular endothelial cadherin-mediated cell adhesion. Mol Biol Cell. 2006;17:966–76. doi: 10.1091/mbc.E05-07-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dupin I, Camand E, Etienne-Manneville S. Classical cadherins control nucleus and centrosome position and cell polarity. J Cell Biol. 2009;185:779–86. doi: 10.1083/jcb.200812034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Theveneau E, Marchant L, Kuriyama S, Gull M, Moepps B, Parsons M, et al. Collective chemotaxis requires contact-dependent cell polarity. Dev Cell. 2010;19:39–53. doi: 10.1016/j.devcel.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suyama K, Shapiro I, Guttman M, Hazan RB. A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell. 2002;2:301–14. doi: 10.1016/S1535-6108(02)00150-2. [DOI] [PubMed] [Google Scholar]
- 22.Rudini N, Felici A, Giampietro C, Lampugnani M, Corada M, Swirsding K, et al. VE-cadherin is a critical endothelial regulator of TGF-beta signalling. EMBO J. 2008;27:993–1004. doi: 10.1038/emboj.2008.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niewiadomska P, Godt D, Tepass U. DE-Cadherin is required for intercellular motility during Drosophila oogenesis. J Cell Biol. 1999;144:533–47. doi: 10.1083/jcb.144.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fulga TA, Rorth P. Invasive cell migration is initiated by guided growth of long cellular extensions. Nat Cell Biol. 2002;4:715–9. doi: 10.1038/ncb848. [DOI] [PubMed] [Google Scholar]
- 25.Grindstaff KK, Yeaman C, Anandasabapathy N, Hsu SC, Rodriguez-Boulan E, Scheller RH, et al. Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell. 1998;93:731–40. doi: 10.1016/S0092-8674(00)81435-X. [DOI] [PubMed] [Google Scholar]
- 26.Ogita H, Takai Y. Activation of Rap1, Cdc42, and rac by nectin adhesion system. Methods Enzymol. 2006;406:415–24. doi: 10.1016/S0076-6879(06)06030-7. [DOI] [PubMed] [Google Scholar]
- 27.Fukuyama T, Ogita H, Kawakatsu T, Fukuhara T, Yamada T, Sato T, et al. Involvement of the c-Src-Crk-C3G-Rap1 signaling in the nectin-induced activation of Cdc42 and formation of adherens junctions. J Biol Chem. 2005;280:815–25. doi: 10.1074/jbc.M411099200. [DOI] [PubMed] [Google Scholar]
- 28.Kraemer A, Goodwin M, Verma S, Yap AS, Ali RG. Rac is a dominant regulator of cadherin-directed actin assembly that is activated by adhesive ligation independently of Tiam1. Am J Physiol Cell Physiol. 2007;292:C1061–9. doi: 10.1152/ajpcell.00073.2006. [DOI] [PubMed] [Google Scholar]
- 29.Price LS, Hajdo-Milasinovic A, Zhao J, Zwartkruis FJ, Collard JG, Bos JL. Rap1 regulates E-cadherin-mediated cell-cell adhesion. J Biol Chem. 2004;279:35127–32. doi: 10.1074/jbc.M404917200. [DOI] [PubMed] [Google Scholar]
- 30.Kroschewski R, Hall A, Mellman I. Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells. Nat Cell Biol. 1999;1:8–13. doi: 10.1038/8977. [DOI] [PubMed] [Google Scholar]
- 31.Fukata M, Kuroda S, Nakagawa M, Kawajiri A, Itoh N, Shoji I, et al. Cdc42 and Rac1 regulate the interaction of IQGAP1 with beta-catenin. J Biol Chem. 1999;274:26044–50. doi: 10.1074/jbc.274.37.26044. [DOI] [PubMed] [Google Scholar]
- 32.Kuroda S, Fukata M, Nakagawa M, Fujii K, Nakamura T, Ookubo T, et al. Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin- mediated cell-cell adhesion. Science. 1998;281:832–5. doi: 10.1126/science.281.5378.832. [DOI] [PubMed] [Google Scholar]
- 33.Izumi G, Sakisaka T, Baba T, Tanaka S, Morimoto K, Takai Y. Endocytosis of E-cadherin regulated by Rac and Cdc42 small G proteins through IQGAP1 and actin filaments. J Cell Biol. 2004;166:237–48. doi: 10.1083/jcb.200401078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoshino T, Sakisaka T, Baba T, Yamada T, Kimura T, Takai Y. Regulation of E-cadherin endocytosis by nectin through afadin, Rap1, and p120ctn. J Biol Chem. 2005;280:24095–103. doi: 10.1074/jbc.M414447200. [DOI] [PubMed] [Google Scholar]
- 35.Kawauchi T, Sekine K, Shikanai M, Chihama K, Tomita K, Kubo K, et al. Rab GTPases-dependent endocytic pathways regulate neuronal migration and maturation through N-cadherin trafficking. Neuron. 2010;67:588–602. doi: 10.1016/j.neuron.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Balzac F, Avolio M, Degani S, Kaverina I, Torti M, Silengo L, et al. E-cadherin endocytosis regulates the activity of Rap1: a traffic light GTPase at the crossroads between cadherin and integrin function. J Cell Sci. 2005;118:4765–83. doi: 10.1242/jcs.02584. [DOI] [PubMed] [Google Scholar]
- 37.Jossin Y. Neuronal migration and the role of reelin during early development of the cerebral cortex. Mol Neurobiol. 2004;30:225–51. doi: 10.1385/MN:30:3:225. [DOI] [PubMed] [Google Scholar]
- 38.Cooper JA. A mechanism for inside-out lamination in the neocortex. Trends Neurosci. 2008;31:113–9. doi: 10.1016/j.tins.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Boyle MP, Bernard A, Thompson CL, Ng L, Boe A, Mortrud M, et al. Cell-type-specific consequences of reelin deficiency in the mouse neocortex, hippocampus, and amygdala. J Comp Neurol. 2011;519:2061–89. doi: 10.1002/cne.22655. [DOI] [PubMed] [Google Scholar]
- 40.Jossin Y, Gui L, Goffinet AM. Processing of Reelin by embryonic neurons is important for function in tissue but not in dissociated cultured neurons. J Neurosci. 2007;27:4243–52. doi: 10.1523/JNEUROSCI.0023-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uchida T, Baba A, Perez-Martinez FJ, Hibi T, Miyata T, Luque JM, et al. Downregulation of functional Reelin receptors in projection neurons implies that primary Reelin action occurs at early/premigratory stages. J Neurosci. 2009;29:10653–62. doi: 10.1523/JNEUROSCI.0345-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jossin Y, Ignatova N, Hiesberger T, Herz J, Lambert de Rouvroit C, Goffinet AM. The central fragment of Reelin, generated by proteolytic processing in vivo, is critical to its function during cortical plate development. J Neurosci. 2004;24:514–21. doi: 10.1523/JNEUROSCI.3408-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Magdaleno S, Keshvara L, Curran T. Rescue of ataxia and preplate splitting by ectopic expression of Reelin in reeler mice. Neuron. 2002;33:573–86. doi: 10.1016/S0896-6273(02)00582-2. [DOI] [PubMed] [Google Scholar]
- 44.Morimura T, Hattori M, Ogawa M, Mikoshiba K. Disabled1 regulates the intracellular trafficking of reelin receptors. J Biol Chem. 2005;280:16901–8. doi: 10.1074/jbc.M409048200. [DOI] [PubMed] [Google Scholar]
- 45.Bock HH, Jossin Y, May P, Bergner O, Herz J. Apolipoprotein E receptors are required for reelin-induced proteasomal degradation of the neuronal adaptor protein Disabled-1. J Biol Chem. 2004;279:33471–9. doi: 10.1074/jbc.M401770200. [DOI] [PubMed] [Google Scholar]
- 46.Simó S, Jossin Y, Cooper JA. Cullin 5 regulates cortical layering by modulating the speed and duration of Dab1-dependent neuronal migration. J Neurosci. 2010;30:5668–76. doi: 10.1523/JNEUROSCI.0035-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]