Abstract
Proteins of the phosphatidylinositol 3-kinase-related protein kinase (PIKK) family are activated by various cellular stresses, including DNA damage, premature termination codon and nutritional status, and induce appropriate cellular responses. The importance of PIKK functions in the maintenance of genome integrity, accurate gene expression and the proper control of cell growth/proliferation is established. Recently, ATPase associated diverse cellular activities (AAA+) proteins RUVBL1 and RUVBL2 (RUVBL1/2) have been shown to be common regulators of PIKKs. The RUVBL1/2 complex regulates PIKK-mediated stress responses through physical interactions with PIKKs and by controlling PIKK mRNA levels. In this review, the functions of PIKKs in stress responses are outlined and the physiological significance of the integrated regulation of PIKKs by the RUVBL1/2 complex is presented. We also discuss a putative “PIKK regulatory chaperone complex” including other PIKK regulators, Hsp90 and the Tel2 complex.
Keywords: AAA+, ATM, ATR, DNA damage response, DNA-PKcs, mTOR, PIKK, RUVBL, SMG-1, TRRAP
Introduction
Genome maintenance and precise gene expression are critically important issues for all organisms. Cells have evolved defense systems from gene mutations, errors in gene expression and various environmental stresses. Phosphatidylinositol 3-kinase-related protein kinase (PIKK) family proteins engage with these defense systems at each level of gene expression. Six PIKKs, ATM (ataxia telangiectasia mutated), ATR (ATM- and Rad3-related), DNA-PKcs (DNA-dependent protein kinase catalytic subunit), SMG-1 (suppressor with morphogenetic effect on genitalia-1), TOR (target of rapamycin) and TRRAP (transformation/transcription domain associated protein), have been identified in vertebrates. All PIKKs, except for TRRAP, function as protein kinases and transduce cellular stresses as phosphorylation signals to downstream effectors and induce proper stress responses. In addition to the importance of each PIKK function, recent studies have suggested an interplay among PIKKs. In this review, we provide an overview of the functions of PIKKs and present recent findings of common regulators of PIKKs. We also discuss a possible role of common regulators of PIKKs in the coordination of PIKKs in cellular stress responses.
PIKK-Mediated Defense Systems Against Various Cellular Stresses
PIKK family
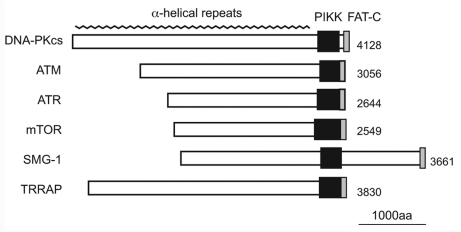
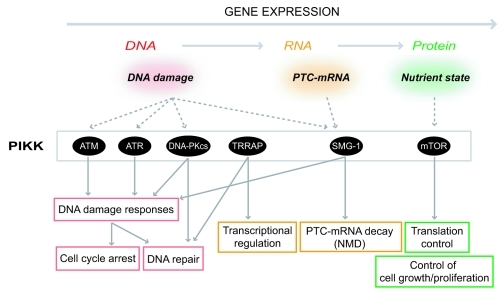
The phosphatidylinositol 3-kinase-related protein kinase (PIKK) family is known as an atypical Ser/Thr protein kinase family that has sequence homology to the catalytic domain of lipid PI3-kinases.1 These kinases are characterized as large proteins (270−470 kDa) with shared domain structures: a highly conserved catalytic domain, FAT-C (FRAP, ATM and TRRAP C-terminal) and successive α-helical repeats in the N-terminal region that provides protein-protein interaction surfaces (Fig. 1). Among the six PIKKs reported, ATM, ATR, TRRAP and TOR are evolutionally conserved from Saccharomyces cerevisiae to Homo sapiens, whereas DNA-PKcs and SMG-1 appeared during metazoan evolution. ATM, ATR, DNA-PKcs and SMG-1 preferentially phosphorylate Ser or Thr followed by Glu; therefore, these proteins are called S/TQ directed kinases.2 Every PIKK forms a protein complex with specific binding partners that can regulate the recruitment of PIKK to the activation site, substrate binding and kinase activity.3 The PIKK complexes play central roles in cellular responses to various stresses, including DNA damage, aberrant mRNAs and nutrient availability (Fig. 2).
Figure 1. The domain structures of human PIKK family members. PIKKs share the highly conserved catalytic PIKK domain and the FAT-C (FRAP, ATM, and TRRAP C-terminal) domain. Although the PIKK domain has sequence homology to the catalytic domain of PI3-kinases, PIKKs act as Ser/Thr protein kinases except for TRRAP. The FAT-C domain located near the PIKK domain is thought to modulate the kinase activity. The N-terminal region of PIKK is composed of α-helical repeats, which contribute to protein-protein interactions.
Figure 2. Summary of PIKK-mediated stress responses. PIKKs are activated various cellular stresses and induce proper cellular responses at various steps of gene expression. ATM and ATR are activated by DNA damages including DSBs to arrest cell cycle and activate DNA repair pathways. DNA-PKcs engages in a DSB repair process called NHEJ. TRRAP regulates transcription as a HAT complex component. SMG-1 recognizes PTC-mRNAs and leads to PTC-mRNA degradation. mTOR controls cellular translation activity and cell growth in response to nutrient status. Except for TRRAP, each PIKK induces proper stress responses through phosphorylations of downstream effector proteins.
ATM (reviewed in refs. 4 and 5)
ATM functions in responses to DNA double-strand breaks (DSBs), which are formed by ionizing radiation (IR) and DNA damaging agents. When DSBs appear, ATM is recruited to the adjacent region of the DSBs and is partially activated by autophosphorylation that transforms an inactive dimer to active monomers. In this early stage, ATM phosphorylates substrates including histone H2AX and p53. Phosphorylated histone H2AX becomes an initial signal for DNA damage and recruits DNA damage recognition/repair factors. Phosphorylated p53 induces the G1 checkpoint. Monomer ATM is recruited to DSBs by the Mre11-Rad50-Nbs1 (MRN) complex and is fully activated. Active ATM phosphorylates diverse downstream effectors and DNA break associated proteins, including Chk2, Nbs1, MDC1, BRCA1 and induces cell-cycle checkpoint, DNA repair and stress-induced transcription.
Besides DNA damage responses, ATM is involved in vesicle transport in the cytoplasm. For example, ATM associates with β-adaptin, one of the components of the clathrin-mediated endocytosis adaptor complex.6 Cytoplasmic vesicular localization of ATM, including peroxisome, is also observed and ATM deficient cells show increased lysosomal accumulation and reduced oxidative metabolism.7,8 The cytoplasmic localization of ATM is especially appreciable in neural cells and ATM forms a complex with VAMP2 and Synapsin-I, two synaptic vesicle proteins, and modulates synaptic function through the regulation of the synaptic vesicular release cooperatively with ATR.9 ATM also participates in insulin signaling by phosphorylating 4EBP1, a cap dependent negative translation regulator, collaborating with mTOR.10
Mutations of the ATM gene are responsible for ataxia telangiectasia (A-T), an autosomal recessive disorder characterized by cerebellar ataxia telangiectasia, immunodeficiency, radiosensitivity and cancer susceptibility.11,12 ATM deficient mice show growth retardation, immunedefects, infertility, neurological defects and the majority of the mice develop thymic lymphomas.7,13 ATM depletion also impairs stem cell maintenance and causes aged phenotypes.14,15
ATR (reviewed in ref. 16)
ATR was originally discovered as a gene with sequence homology to ATM and is biochemically similar to ATM. In contrast to ATM, ATR is activated by a stalled replication fork during S phase and many types of DNA damage that give rise to single strand DNA (ssDNA) structures, including DSBs, base-crosslinks and agents, which cause DNA replication stress and DNA damage. ATR is recruited to the ssDNA coated with replication protein A (RPA) through the interaction with ATRIP. RPA also localizes the RAD9-RAD1-Hus1 (9–1-1) complex to the RPA-ssDNA sites. The 9–1-1 complex recruits TopBP1, an ATR activator, to ATR and induces ATR activation. Although ATR phosphorylates numerous substrates and regulates DNA replication, the cell cycle checkpoint and DNA repair, the best studied ATR substrate is Chk1. Activated Chk1 phospho-inactivates Ccd25 proteins, Cdk activators, thereby preventing the cell cycle transition. ATR-mediated Chk1 signaling is also critical for regulating DNA replication. ATR also phosphorylates replication related proteins, including PCNA, Polε, RPA, MCM proteins and DNA repair related proteins, including BRCA1, WRN and BLM. However, the physiological significance of these phosphorylation events is poorly understood. The kinase activity of ATR is also involved in replication-dependent histone mRNA degradation together with Upf1, a NMD transacting factor.17
As expected in the critical regulation of replication stress, ATR is essential for viability across many organisms ranging from yeast to mammals.18,19 Moreover, deletion of the ATR gene in adult mice causes aged phenotypes and stem cell loss, in a similar manner to the ATM gene deletion.20 Mutations of the ATR gene have been found in a few patients of the Seckel syndrome, an autosomal recessive disorder characterized by intrauterine growth retardation, microcephaly and mental retardation.21
DNA-PKcs (reviewed in refs. 22 and 23)
DNA-PKcs is the catalytic subunit of the DNA-PK holoenzyme, which is composed of DNA-PKcs and the Ku70/80 heterodimer. DNA-PK (the DNA-PKcs and Ku70/80 complex) and its kinase activity are essential for non-homologous end-joining (NHEJ), a major DSB repair pathway in mammals. In NHEJ, Ku70/80 recognizes DNA ends and recruits DNA-PKcs to DSBs; thereby two DNA-PK molecules interact to connect the DNA ends. This interaction leads to the autophosphorylation of DNA-PK in trans, which induces conformational changes of DNA-PKcs and the release of the DNA-PKcs from the DNA ends, allowing the NHEJ- and repair factors to access the DSB and subsequent end-processing.23 Besides autophoshorylation, a number of DNA-PK substrates including NHEJ factors have been identified in vitro. However, the physiological roles of these phosphorylation events in vivo have not been well defined. DNA-PK-mediated DNA end-processing is also required for the rejoining of DSBs generated by V(D)J recombination during T- and B-cell development, and a DNA-PKcs inactive mutation causes severe combined immunodeficiency (SCID).24,25 In addition to the role in NHEJ, recent studies have uncovered the involvement of DNA-PKcs in DNA damage signaling. For example, in response to IR, DNA-PKcs promotes cell survival through phosphorylation of Thr308 and Ser473 (this site is also phosphorylated by mTORC2, see below) residues of Akt (also called PKB) together with PDK1 and the subsequent transcriptional regulation of the p53-p21 pathway.26 IR-dependent phospho-activation of nuclear caspase-2 by DNA-PKcs also contributes to NHEJ and the maintenance of the ATM-mediated G2/M checkpoint.27 Parts of DNA-PK are localized to lipid rafts, microenvironments on cell membranes where signaling molecules accumulate, and such localization has been suggested to mediate DNA damage signals through phosphorylations in response to IR.28 In addition, DNA-PKcs mediates exchange of UV-induced translation profiles, including the promotion of the synthesis of DNA-repair related proteins and the inhibition of global translation.29 DNA-PKcs is also involved in replication stress induced histone mRNA destabilization together with Upf1, similarly to that observed for ATR.30 Furthermore, DNA-PK associates with telomeres and DNA-PK defects induce telomere fusion and telomere aneuploidy without telomere shortening, suggesting DNA-PK’s critical role in telomere capping.31
SMG-1 (reviewed in refs. 32 and 33)
SMG-1 forms an SMG-1 complex (SMG1C) with SMG-8 and SMG-934 and functions in an mRNA quality control mechanism called nonsense-mediated mRNA decay (NMD). NMD selectively degrades premature termination codon (PTC)-containing mRNAs, which can be generated by gene mutation, splicing and transcription errors. PTC-mRNAs also arise in a physiological process, the V(D)J recombination during T- and B-cell maturation.35 Therefore NMD suppresses the production of potentially harmful or nonfunctional polypeptides and ensures the accuracy of gene expression. SMG-1 plays an essential role in NMD by phosphorylating Upf1, a central regulator of NMD. When a ribosome recognizes a PTC, SMG-1, Upf1 and eukaryotic releasing factors (eRF1 and eRF3) assemble to form the SMG-1-Upf1-eRF1-eRF3 (SURF) complex on the PTC-recognizing ribosome.36 If an exon junction complex (EJC), a multiprotein complex deposited on an exon-junction in a splicing dependent manner, exists downstream of the PTC, the SURF associates with the EJC. The association between SURF and EJC establishes PTC recognition and induces SMG-1-mediated Upf1 phosphorylation.36 Phosphorylated Upf1 recruits mRNA decay factors and phopho-Upf1 recognizing NMD factors,37-39 and advances subsequent decay processes. Therefore SMG-1-mediated Upf1 phosphorylation is an essential step in NMD. Although Upf1 is also identified as a substrate of other PIKKs (ATM, ATR, DNA-PKcs, see below), the function of SMG-1 in NMD cannot be compensated with other PIKKs.
In addition to NMD, SMG-1 is implicated in other stress responses, including DNA damage,40 oxidative stress, hypoxia41,42 and cytokine signaling.43 In a similar fashion to ATM and ATR, SMG-1 activates by IR or UV and phosphorylates p53.40 Moreover, SMG-1 depletion causes spontaneous DNA damage and sensitizes cells to IR.40 SMG-1 also associates with the telomere and protects the telomere by inhibiting the association of telomeric repeat-containing RNA (TERRA) with telomeric DNA.44
SMG-1 is essential for mouse embryogenesis.45 SMG-1 null mutants in C. elegans and D. melanogaster are viable,46,47 and inactivation of SMG-1 shows oxidative stress resistance and longevity in analogy to TOR in C. elegans.48 Since NMD suppresses the dominant phenotype of the heterozygote caused by a nonsense mutation and because NMD is not essential for viability in C. elegans, a temperature sensitive mutant of SMG-1 can be used for genetic screening to identify gene mutations in heterozygotes of C. elegans. Temperature sensitive mutants of SMG-1 have also been used for inducible expression of transgenes with long 3′UTRs, which are a NMD target.49
mTOR (reviewed in ref. 50)
TOR was originally identified as the target protein of rapamycin, a macrolide produced by bacterium Streptomyces hygroscopicus.51,52 TOR regulates various cellular activities, including cell size control, cell proliferation, translation activity and cell metabolism in response to external stresses and nutritional status. From yeast to mammals, two distinct functional TOR complexes have been identified: TORC1 and TORC2. Mammalian TORC1 (mTORC1), which is directly inhibited by rapamycin, is composed of mTOR, Raptor and mLST8 (also called as GβL), whereas rapamycin insensitive mTORC2 is composed of mTOR, Rictor, mLST8, SIN1 and Protor.
mTORC1 functions as a sensor of external signals, such as growth factors, nutrients, redox stress and controls cellular translation activity.53 The mTORC1 phosphorylates the p70 ribosomal S6 kinase (S6K) and eIF4E binding protein (4EBP), two key regulators of cap-dependent translation, thereby facilitating translation together with the regulation of ribosome biogenesis via transcriptional regulation.54 mTORC1 also enhances the translation efficiency of newly synthesized spliced mRNAs through activation of S6K recruited to the spliced mRNAs by SKAR, an EJC component.55 mTORC1-mediated S6K phosphorylation and translation enhancement are linked to cell size control.56 mTORC1 also acts as a conserved negative regulator of autophagy in response to nutrient availability.57
In contrast, mTORC2 regulates actin cytoskeletal organization by phosphorylating PKCα58,59; however, the upstream signals remain unclear. mTORC2 also phosphorylates Ser473 of Akt and controls cell growth, proliferation and cell migration.60 Recently, another (m)TORC2 target, serum glucocorticoid–induced protein kinase 1 (SGK1) has been identified.61 Through the phosphorylation of SGK1, TORC2 regulates fat metabolism, body size and development in C. elegans.62,63
Based on the critical role of mTOR in regulating cell proliferation and growth, mTOR knockout mice are embryonic lethal and mTOR-deficient embryonic stem (ES) cells fail to be maintained because of proliferation arrest.64,65 Moreover, (m)TORC1 signaling is linked to aging and the selective inhibition of TORC1 signaling commonly extends the life span of yeast, worms, flies and mice.66
TRRAP (reviewed in ref. 67)
TRRAP, the only catalytically inactive PIKK member, was identified as an essential co-factor of c-Myc and E2F for the transcription/transformation activities of these oncogenic proteins and named “transformation/transcription domain–associated protein (TRRAP)”68. Beside c-Myc and E2F, TRRAP associates with various transcription factors, including p53, E1A, ERα/β, β-catenin and regulates transcription. Importantly, TRRAP is a shared and essential component of distinct histone acetyltransferase (HAT) complexes, including PCAF (SAGA in S. cerevisiae), TIP60 (NuA4 in S. cerevisiae), TFTC and SILK HAT complexes from yeast to mammals. A HAT complex, which is composed of a catalytic subunit and its associated proteins, acetylates Lys residues of core histone tails to promote transcription. TRRAP appears to play a role in the recruitment of HAT complexes to the chromatin as a mediator between a HAT complex and various transcription factors. A genome-wide analysis revealed that TRRAP regulates various gene expressions involved in cell cycle progression, the cytoskeleton, cell adhesion, protein turnover, metabolism and signal transduction.69 Consistent with this, TRRAP is essential for viability in S. cerevisiae and Mus musculus,70,71 and TRRAP-deficient cells show mitotic checkpoint failures and the severe suppression of cell proliferation.70 TRRAP is also involved in DNA repair processes through the TIP60 HAT complex72 and the NHEJ pathway.73
RUVBL1 and RUVBL2 are Multifunctional ATPases (reviewed in ref. 74)
RUVBL1 and RUVBL2 are conserved AAA+ family proteins
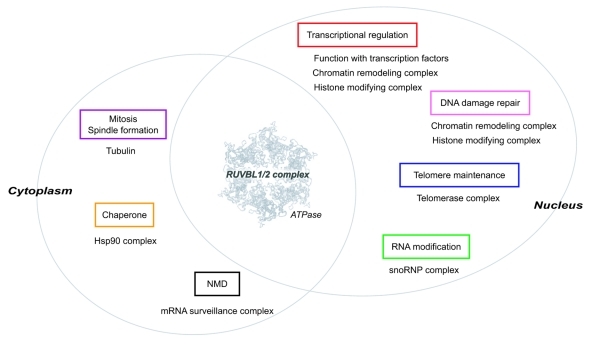
RuvB-like (RUVBL) 1 and RUVBL2, also known as Pontin and Reptin (or TIP48 and TIP49), are evolutionally conserved ATPases that belong to the ATPase associated diverse cellular activities (AAA+) family. They have sequence homology to bacterial RuvB, a DNA helicase involved in homologs recombination and DNA repair.75,76 Both have been shown to have ATPase and DNA helicase activity in vitro.77 RUVBL1 and RUVBL2 interact with each other and can form a double hexamer, probably consisting of two homo hexamers, which is a characteristic of AAA+ family proteins.78,79 In some circumstances, RUVBL1 and RUVBL2 act independently and have antagonistic effects.80,81 However, in most cases, they appear to form a complex and function together. The complex formation of RUVBL1 and RUVBL2 appears to be important in vivo, because the depletion of either protein causes co-depletion of the other protein.82,83 Based on their diverse functions (Fig. 3), both proteins are essential for viability/development of S. cerevisiae,77,85 D. melanogaster,86 and C. elegans [wormbase (http://www.wormbase.org/)].
Figure 3. The RUVBL1/2 complex participates in diverse cellular processes. The RUVBL1/2 complex is composed of RUVBL1 and RUVBL2, and both proteins possess ATPase activity. The RUVBL1/2 complex is localized to nucleus and cytoplasm, and participates in diverse cellular processes together with specific interactors (shown below each box). The ATPase activity of the RUVBL1/2 complex is thought to essential for their functions in each process. The atomic structure of RUVBL1 is derived from reference 84.
The RUVBL1/2 complex is involved in diverse cellular functions
While RUVBL1 and RUVBL2 participate in diverse nuclear processes, the best studied function of RUVBL1 and RUVBL2 is transcriptional control.87 RUVBL1 and RUVBL2 are shared components of the Ino80 and SRCAP (Swr1 in S. cerevisiae) chromatin remodeling complex,88,89 and the TIP60 HAT complex.90 The RUVBL1/2 complex is essential for the chromatin remodeling activity of the Ino80 complex91 and the HAT activity of the TIP60 complex.92 Both the Ino80 and TIP60 chromatin remodeling/modifying complexes are also implicated in DNA repair.93,94 Moreover, RUVBL1 and RUVBL2 interact with RNA polymerases and various transcription factors, including c-Myc, β-catenin, TATA binding protein and E2F, and regulate transcription of their target genes, thereby affecting cellular transformation, development and tumor metastasis.87 Conditional depletion of Rvb1 or Rvb2 in S. cerevisiae was reported to influence transcription of > 5% of the genes of yeast.95
The RUVBL1/2 complex is also involved in the maturation of small nucleolar RNPs (snoRNPs), which catalyze post-transcriptional modification of non-coding RNAs, such as rRNA (rRNA), tRNA (tRNA) and small nuclear RNA (snRNA).96 The RUVBL1/2 complex associates with different snoRNPs and controls accumulation of snoRNAs, proper localization of the components to the nucleolus and snoRNP assembly.97,98
The RUVBL1/2 complex also participates in telomere maintenance. The RUVBL1/2 complex associates with telomerase reverse transcriptase (TERT) and regulates the accumulation of the telomerase RNA component (TERC) and the assembly of the functional telomerase complex with its ATPase activity. Therefore, depletion of RUVBL1 or RUVBL2 severely reduces telomerase activity.83 The association of the RUVBL1/2 complex with TERT is observed to peak in the S phase,83 suggesting that the RUVBL1/2 complex regulates telomerase activity in a cell cycle dependent manner.
In addition, the RUVBL1/2 complex was identified as one of interacting proteins of Hsp90, an essential molecular chaperone for cellular homeostasis, together with RPAP3 and NOP17 (Tah1 and Pih1 in S. cerevisiae, respectively), two conserved Hsp90 co-factors99,100 (described later, see Section 4). The RUVBL1/2 complex was also found in another chaperone-like URI/prefoldin complex, which is involved in (m)Tor-dependent nutrient signaling101 (described later, see Section 4).
RUVBL1 and RUVBL2 are also involved in mitosis. RUVBL1 interacts with α- and γ-tubulin, and regulates spindle assembly,102 whereas RUVBL2 is localized to the midbody and suggested to function in cytokinesis.103,104
As mentioned above, the RUVBL1/2 complex participates in various cellular processes. In most cases, the RUVBL1/2 complex functions as a component of multiprotein complexes containing nucleic acids (DNA/RNA), and it commonly regulates the assembly of these functional complexes.82,83,91 Inhibition of the RUVBL1/2 complex causes immature complex assembly and functional defects, suggesting a similar mode of action of the RUVBL1/2 complex on macromolecular complex formation/remodeling. Although the detailed mechanisms are unknown, their ATPase activity and nucleic binding properties may be important for these processes.
Integrated Regulation of the PIKK Family by the RUVBL1/2 Complex
The RUVBL1/2 complex regulates PIKK function through physical interaction and controls the levels of these kinases
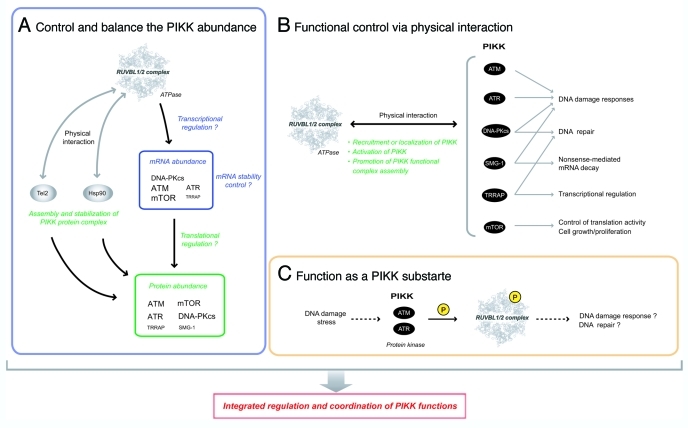
Recently, we found an unexpected link between the RUVBL1/2 complex and the PIKK family. We had originally identified RUVBL1 and RUVBL2 as SMG-1 interacting proteins. Subsequent analyses revealed that the RUVBL1/2 complex associates not only with SMG-1 but also with any PIKK.82 In addition to the physical interactions, the RUVBL1/2 complex regulates the levels of all PIKKs (Fig. 4A). Either knockdown of RUVBL1 or RUVBL2 clearly decreased all PIKK proteins and suppressed PIKK signaling.82 Thus, the RUVBL1/2 complex can modulate PIKK functions as a common interactor and regulator of their protein abundance.
Figure 4. The RUVBL1/2 complex can regulate PIKK functions through several ways. Three possible mechanisms for the RUVBL1/2 complex to regulate PIKK functions. (A) Control and balance the abundance of PIKK. The RUVBL1/2 complex and its ATPase activity is required for the maintenance of PIKK protein abundance. The RUVBL1/2 complex affects the mRNA level of some PIKKs. The character size of each PIKK shows the extent of the sensitivity. The RUVBL1/2 complex is also involved in the assembly and stabilization of newly synthesized PIKK protein complex probably together with Hsp90 and the Tel2 complex. (B) Functional control via physical interactions. The RUVBL1/2 complex physically interacts with PIKK and facilitates proper PIKK-mediated stress responses. Three mechanisms to control PIKK function; recruitment/localization of PIKK, activation of PIKK through posttranslational modification, and promotion of the functional complex assembly of PIKK during stress responses. (C) Function as a PIKK substrate. RUVBL2 is phosphorylated by ATM/ATR in response to DNA damage stress.105 The RUVBL1/2 complex may have a role as a downstream effector protein of PIKKs. The atomic structure of RUVBL1in Figure 4 is derived from reference 84.
The detailed mechanism describing how the RUVBL1/2 complex controls the quantities of PIKKs is unknown; however, regulation appears to be at the mRNA level and the ATPase activities of both RUVBL1 and RUVBL2 are involved.82 As one possibility, the RUVBL1/2 complex may regulate transcriptional activity of PIKKs together with E2F1 and c-Myc, because E2F1, one of RUVBL1 interacting transcription factors and regulated by c-Myc, can promote transcriptional activity of ATM and DNA-PKcs.106,107 E2F1 and c-Myc also facilitate translation activity of target mRNAs by inducing cap methylation;108 therefore, the RUVBL1/2 complex may influence the translation activity of PIKK mRNAs. Actually, the effect of RUVBL1/2 knockdown on the PIKK protein levels is more severe than that on the PIKK mRNA levels,82 indicating that an undefined mechanism at the protein level participates in the process. Given the association of the RUVBL1/2 complex with Hsp90 and the Tel2 complex, the RUVBL1/2 complex probably acts through the Hsp90 chaperone pathway for maturation and stabilization of PIKK proteins (Fig. 4A, described later, see Putative “PIKK Regulatory Chaperone Complexes” Consisting of the RUVBL1/2 Complex, the Tel2 Complex and HSP90).
As described above, the RUVBL1/2 complex directly participates in the functions of at least two PIKKs, TRRAP and SMG-1. TRRAP and the RUVBL1/2 complex function together in transcriptional regulation and DNA repair processes as essential components of the TIP60 HAT complex.72,87,90 On the other hand, the RUVBL1/2 complex associates with SMG-1 and facilitates rearrangement of the SMG-1-containing complex during NMD.82 Since RUVBL1 and RUVBL2 interact with the N-terminal region of SMG-1,82 the RUVBL1/2 complex is expected to interact with α-helical repeats of other PIKKs (Fig. 1). The α-helical region of PIKKs provides protein-protein interaction surfaces important for their functions, such as ATM-Nbs1, ATR-ATRIP, mTOR-Raptor and SMG-1-SMG-8/SMG-9;109-112 therefore the association of the RUVBL1/2 complex possibly influences the formation of PIKKs complexes.
In a different manner from TRRAP and SMG-1, a direct relationship between the RUVBL1/2 complex and other PIKKs has not been reported. However, previous studies suggest the involvement of the RUVBL1/2 complex in PIKK-mediated DNA damage response and repair. For example, the RUVBL1/2 complex-containing the TIP60 HAT complex acetylates the FAT-C domain of ATM, thereby activating ATM in response to DNA damage.113 The requirement of the RUVBL1/2 complex for the TIP60 HAT activity92 indicates a critical role of the RUVBL1/2 complex in ATM activation and the DNA damage response. The FAT-C domain is conserved among PIKKs and critical for kinase activity (Fig. 1);114–117 therefore other PIKKs may be activated by similar acetylation events.118 The RUVBL1/2 complex may also be involved in ATR recruitment through physical interactions with RPA3,85 a subunit of RPA, an ATR recruiter. Moreover, RUVBL2 is a DNA damage-induced ATM/ATR substrate.105 These observations indicate that the RUVBL1/2 complex directly participates in the PIKK-mediated DNA damage response and repair process in addition to the quantity control of PIKKs (Fig. 4B and C).
Although ATM, ATR and DNA-PKcs have been established as nuclear kinases, the RUVBL1/2 complex associates with PIKKs both in the nucleus and cytoplasm (unpublished data), suggesting that the RUVBL1/2 complex may also influence the nuclear localization of PIKKs or their cytoplasmic functions (see Section 1). For instance, a part of ATM, ATR and DNA-PKcs localizes to the centrosome119 and ATM/ATR activates the cell cycle checkpoint by inhibiting spindle assembly in response to DNA damage during mitosis.120 As mentioned above, the RUVBL1/2 complex associates with α- and γ-tubulin103,121 and RUVBL1 regulates microtubule assembly during mitosis,102 implying a relationship to the ATM/ATR-mediated DNA damage response during mitosis.
Functional relationships between the RUVBL1/2 complex and TOR have also been suggested. The (m)TORC1 acts as a positive regulator of transcription of rRNAs and ribosomal proteins.54 In addition, TORC1 controls rRNA maturation through snoRNP localization/accumulation in the nucleolus like RUVBL1 in C. elegans,122 suggesting that TOR and RUVBL1 function in the same pathway. A further study indicated that the RUVBL1/2 complex participates in (m)TOR signaling as components of the unconventional prefoldin URI complex together with RPB5101 (described later, see Putative “PIKK Regulatory Chaperone Complexes” Consisting of the RUVBL1/2 Complex, the Tel2 Complex and HSP90).
Taken together, the RUVBL1/2 complex can regulate PIKK functions thorough several ways: (1) control of PIKKs levels (Fig. 4A); (2) activation of PIKKs via post translational modifications (Fig. 4B); (3) recruitment or localization of PIKKs; (4) promote assembly/rearrangement of PIKK complexes (Fig. 4B); and (5) function as a downstream effector of PIKK signaling (Fig. 4C).
Functional links among PIKKs and a possible role of the RUVBL1/2 complex in the coordination of PIKK-mediated stress responses
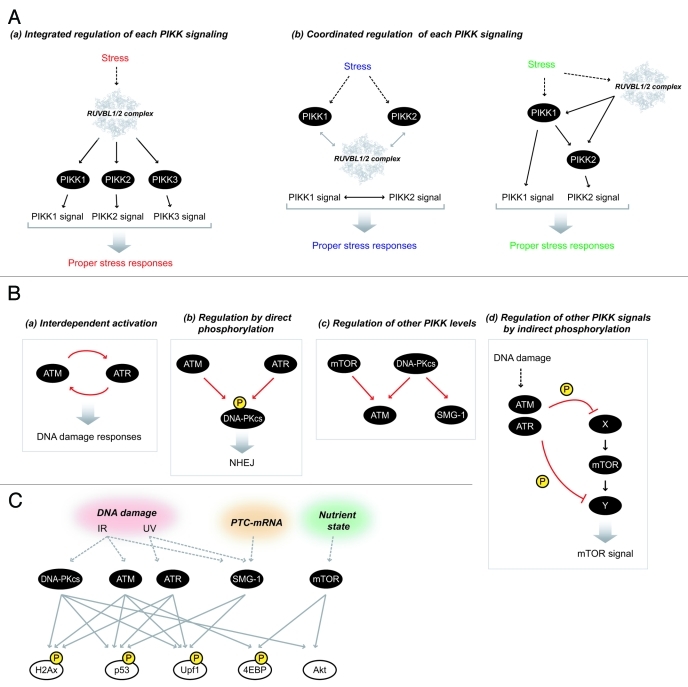
What is the significance of common PIKK regulators? Based on previous observations suggesting functional links among PIKKs, the RUVBL1/2 complex may participate in coordinating and regulating PIKK signaling for the appropriate stress responses (Possible models are illustrated in Figure 5A).
Figure 5. Crosstalk and regulation among PIKKs. (A) Possible models of the regulation of PIKK signaling by the RUVBL1/2 complex. (a) The RUVBL1/2 complex integrates each PIKK signaling as an upstream regulator and induces proper stress responses. (b) When multiple PIKKs cooperatively function in response to stress signals, the RUVBL1/2 complex assists this process and coordinates multiple PIKK signals (the left model). The RUVBL1/2 complex coordinates the cross-regulation among PIKKs [see also (B)] thereby induce proper stress responses (the right model). The atomic structure of RUVBL1 is derived from reference.84 (B) Cross-regulation among PIKKs. Several regulatory mechanisms among PIKKs have been observed. (a) Interdependent activation of ATM and ATR in response to DNA damage. (b) Regulation of other PIKK by direct phosphorylation: DNA-PKcs is phosphorylated by ATM and ATR in response to DNA damage stress to regulate cellular radio-resistance and NHEJ. (c) Regulation of other PIKK levels: DNA-PKcs and mTOR are required for the maintenance of ATM abundance. DNA-PKcs is also involved in the maintenance of SMG-1 abundance. (d) Regulation of other PIKK signals by indirect phosphorylations: Both upstream and downstream factors of mTORC1 signal are ATM/ATR substrates and mTORC1 signal is downregulated by DNA damage stresses. (C) Shared substrates among PIKKs. Histone H2Ax, p53, and Upf1 are shared substrates of DNA-PKcs, ATM, ATR and SMG-1. 4EBP and Akt, two well known mTOR substrates, are also phosphorylated by ATM and DNA-PKcs respectively.
Previous studies have often suggested functional relationships among PIKKs in DNA damage responses. For example, ATM and ATR are postulated to be activated by separate signals and act independently. However, interdependent activation between ATM and ATR is also observed [Fig. 5B-(a)].123-125 In addition, ATM or ATR phosphorylates DNA-PKcs in response to IR or UV/replication stress and the former is important for cellular radio-resistance and NHEJ [Fig. 5B-(b)].126,127 Conversely, DNA-PKcs regulates the abundance of ATM and SMG-1 [Fig. 5B-(c)].128,129 SMG-1 also activates in response to IR and UV, and phosphorylates p53 together with ATM/ATR.40 In DNA repair processes, TRRAP contributes to efficiency/fidelity of NHEJ in a HAT activity independent manner, in addition to DNA-PKcs.73 ATM/ATR-mediated DNA damage signals link to various signal pathways. Upstream and downstream factors of mTORC1, Akt, TSC1, 4EBP and S6K have been identified as possible ATM/ATR substrates induced by IR105 and downregulation of mTORC1 signaling by DNA damage stress has been reported [Fig. 5B-(d)].130 In contrast, mTOR regulates ATM levels [Fig. 5B-(c)].128 In addition, (m)TORC1 inhibition and tor1 (one of tor genes in S. pombe and forms TORC2) mutants show high sensitivity to DNA-damaging agents,131-133 suggesting a link between ATM/ATR-mediated DNA damage responses and (m)TOR signaling. We speculate that PIKKs function in DNA damage response and DNA repair in collaboration with each other at multiple levels, and this is important for proper DNA damage responses. In this context, the RUVBL1/2 complex can serve as a mediator among PIKKs and organize DNA damage responses.
Another possible functional link among PIKKs is telomere maintenance. The telomere is a protective end structure of chromosomes in eukaryotes and is essential for genome stability.134 The telomere is maintained by telomerase, an RNP complex containing the telomerase reverse transcriptase catalytic subunit (TERT), and protected by multiple telomeric DNA binding proteins. Telomere maintenance closely links to DNA damage repair processes135 and at least four of the six PIKKs are involved in telomere maintenance. For example, Tel1 and Rad3 (ATM and ATM orthologs in S. pombe) promote the recruitment of telomere protective proteins and telomerase.136 ATM and ATR also cooperate with other repair machinery to form the proper telomeric structure on telomere replication.137 DNA-PKcs and Ku70/80 associate with telomeres and are suggested to function in telomere capping.31 SMG-1 also associates with telomeres and inhibits accumulation of TERRA around the telomere and SMG-1 depletion causes telomere loss and fusion.44 In most somatic cells, telomerase expression is low, while progenitor germ/stem cells and putative cancer stem cells possess high activity of telomerase. When a silent TERT gene reactivates, c-Myc, TRRAP and its associating HAT activities are required.138 TRRAP-containing SAGA HAT complex also regulates the turnover of critical telomere binding protein, TRF1.139 Several reports also suggest the involvement of mTOR in telomere regulation. For example, mTORC1 inhibition causes downregulation of TERT mRNA expression and reduced telomerase activity.140 On the other hand, Akt, a downstream effector of mTORC2, negatively regulates telomere length by phosphorylating TRF1,141 which is consistent with another study showing the elongation of the telomere in a tor1 mutant in S. pombe.131 As mentioned above, the RUVBL1/2 complex is critical for telomerase activity as this complex promotes the assembly of the telomerase complex.83 Although the direct relationship among PIKKs and the RUVBL1/2 complex in telomere maintenance has not been defined, their cooperative actions and the coordination of PIKKs by the RUVBL1/2 complex may be important for telomere maintenance.
In addition to the above mentioned cases, several PIKK substrates, including p53, histone H2AX, Upf1, 4EBP and Akt are shared by multiple PIKKs (Fig. 5C). Thus, the RUVBL1/2 complex may be involved in the selection of PIKKs through a cellular stress dependent mechanism.
Putative “PIKK Regulatory Chaperone Complexes” Consisting of the RUVBL1/2 Complex, the Tel2 Complex and Hsp90
Two other PIKK regulators, the Tel2 complex and Hsp90
In addition to the RUVBL1/2 complex, at least two common regulators of PIKK, the Tel2 complex and Hsp90, have been reported.
Tel2 (also called CLK2) is the mammalian homolog of S. cerevisiae telomere maintenance 2 (Tel2); however, the involvement of Tel2 in telomere maintenance has not been reported in mammals.142 Tel2 forms a complex with the Tel2-interacting protein 1 (Tti1) (also called SMG-10) and Tel2-interacting protein 2 (Tti2)128,143-145. Tel2, Tti1 and Tti2 are closely related and each protein level depends on one another.128,143,144 The Tel2 complex associates with newly synthesized PIKK proteins and is required for the formation of PIKK-functional complexes, such as mTORC1, mTORC2 and ATR-ATRIP complexes.144,146 Inhibition of the Tel2 complex causes destabilization of PIKK proteins without affecting their mRNA levels.142-144 Moreover, Tel2 is required for the recruitment of Tel1 (ATM ortholog in S. pombe) to DNA damage sites and the activation of the Rad3 (ATR ortholog in S. pombe) and Rad3 mediated replication checkpoint.147-150 Tti1 was also isolated as one of the genes required for IR-resistance and is involved in PIKK-mediated DNA damage responses.143
Hsp90 is an ATP-dependent molecular chaperone to specific proteins including various protein kinases. Hsp90 forms multiple complexes with its co-factors and facilitates structural maturation and complex assembly of its client proteins.151 Although physical interactions of Hsp90 with PIKKs have been observed only in DNA-PKcs and SMG-1,82,152 Hsp90 inhibition leads to a reduction in all PIKK proteins and their downstream phosphorylation signals.82,128,142 Moreover, Hsp90 inhibition impairs DSB repair and the cell cycle checkpoint to IR because of the attenuation of IR-induced ATM and DNA-PKcs activation without downregulation of ATM and DNA-PKcs.153 Hsp90 was also identified as a Raptor interacting protein and the reduction in phosphorylation of mTOR and its substrates, 4EBP and S6K, were observed under Hsp90 inhibition without affecting the levels of mTOR.154
The putative PIKK regulatory chaperone complex and its components
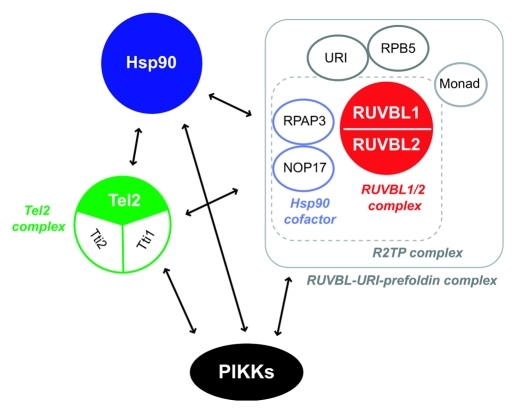
A recent report revealed that the Tel2 complex interacts with Hsp90 and that the associations between the Tel2 complex and PIKKs depends on the chaperone activity of Hsp90.144 In addition, the RUVBL1/2 complex associates with the Tel2 complex and Hsp90.82,128,144,166 Both the RUVBL1/2 complex and the Tel2 complex also interact with two evolutionarily conserved Hsp90 co-factors, RPAP3 and NOP17,82,128,144 which can affect ATPase activity of Hsp90.155,156 These observations strongly suggest that the RUVBL1/2 complex, the Tel2 complex and Hsp90 form a complex and function together to regulate PIKKs.
If it was the case, how do these molecules function together? One possibility is that the RUVBL1/2 complex and the Tel2 complex act as Hsp90 co-factors. Hsp90 cofactors play important roles in controlling Hsp90 chaperone activity through mediating client association and/or regulating the ATPase cycle of Hsp90.151,157 Since Tel2 directly interacts with ATM and mTOR,142 the Tel2 complex may promote the association of Hsp90 with PIKKs. Alternatively, the RUVBL1/2 complex and the Tel2 complex may affect the Hsp90 ATPase cycle, thereby regulating its chaperone activity. Considering that RUVBL1 and RUVBL2 are AAA+ family ATPases, which are widely involved in molecular remodeling events via ATP hydrolysis,158 it is also possible that the RUVBL1/2 complex may act as another molecular chaperone in PIKK regulation. Indeed, the ability of the RUVBL1/2 complex to maintain PIKK abundance requires its ATPase activity.82 In this context, the associations between the RUVBL1/2 complex and Hsp90 cofactors (RPAP3, NOP17) may link two molecular chaperones like Hop, which mediates and coordinates two chaperones, Hsp70 and Hsp90.159
Interestingly, the RUVBL1/2 complex interacts with another chaperone-related prefoldin complex containing URI, RPB5 and Monad.101,160 URI (also called RMP), an unconventional prefoldin, controls a part of nutrient sensitive gene expression and cell survival signaling downstream of (m)TOR,101,161 and its deficiency causes DNA breaks and cell cycle arrest in C. elegans.162 URI interacts with all PIKK proteins, the Tel2 complex, and Hsp90.128 RPB5, a shared subunit of RNA polymerases and a known URI interactor,163,164 associates with at least one PIKK, SMG-1, and is involved in NMD.82 Monad (also called WDR92) interacts with at least the RUVBL1/2 complex, Tti1, RPAP3, NOP17, URI and RPB5.82,128,160,165 Based on the above mentioned observations, multiple chaperone-containing complexes are expected to collaboratively function to regulate PIKKs (Fig. 6). Together with the previous analyses, the putative PIKK regulatory chaperone complex may not only assist the maturation of PIKK complexes when PIKK proteins are synthesized, but also facilitate the remodeling of PIKK complexes when PIKKs activate in response to stress signals. Interestingly, some molecules including RUVBL2 have putative phosphorylation sites by PIKK (see Table 1), suggesting that they can also function as PIKK downstream effectors and provide an additional intricate regulatory mechanism of PIKKs.
Figure 6. The putative “PIKK regulatory complex.” Three common PIKK regulators, the RUVBL1/2 complex, Hsp90 and the Tel2 complex interact with one another. Other factors (RPAP3, NOP17, RPB5, URI and Monad) are shared interactors of the RUVBL1/2 complex, Hsp90 and the Tel2 complex. They are possible PIKK regulators (see Table 1). The interaction between the RUVBL1/2-URI-prefoldin complex and the Tel2 complex is mediated by NOP17 in a Tel2 phosphorylation dependent manner.166
Table 1. List of common and possible PIKK regulators in mammals.
| Molecule | Domain/Motif | Character and related cellular process | Possible phosphorylation site by PIKKs** | |
|---|---|---|---|---|
| Common PIKK regulators |
RUVBL1 (RuvB-like 1) |
AAA+ domain, Walker A, WalkerB motif |
AAA+ family proteins, ATPase/DNA helicase activity, form a hexameric complex, transcriptional regulation, RNA modification/biogenesis, telomere maintenance, DNA repair, spindle formation, Hsp90 cofactor, NMD |
- |
| RUVBL2 (RuvB-like 2) |
Prediction & report: Ser220 (ref. 105) |
|||
| Hsp90 (Heatshock protein 90) |
Histidine kinase-like ATPases domain |
conserved molecular chaperone, ATPase, promotes protein folding/structural maturation/assembly/transport of specific client proteins |
Prediction & report: Thr297 (ref. 105) |
|
| Tel2 (telomere maintenance 2) |
- |
replication checkpoint, DNA damage response/checkpoint |
- |
|
| SMG-10/Tti1 (Tel2 interacting protein 1) |
HEAT repeat |
Tel2 complex component, DNA damage response/checkpoint |
prediction: Ser391 |
|
| Tti2 (Tel2 interacting protein 2) |
- |
Tel2 complex component, DNA damage response/checkpoint |
- |
|
| Possible PIKK regulators |
RPAP3 (RNAPII-Associated Protein 3) |
TPR motif |
RNA polymerase associated protein, Hsp90 cofactor, UV-induced DNA damage response and cell survival, TNF-α and cycloheximide-induced apoptosis |
prediction: Ser116 Ser481 |
| |
NOP17 (Nucleolar protein 17) |
PIH1 domain |
pre-rRNA processing/RNA modification, Hsp90 cofactor |
- |
| |
URI/RMP (Unconventional prefoldine RPB5 interactor/RPB5 mediating protein) |
Prefoldin α domain |
unconventional prefoldin, transcriptional regulation, regulation of survival signaling at mitocochondria |
phosphrylated at Ser371 by p70 S6K, downstream of mTOR (ref. 161) |
| RPB5 (RNA polymerase II subunit 5) |
- |
shared subunits of all three RNA polymerases, transcriptional regulation, NMD |
prediction: T29 |
|
| Monad/WDR92 (WD repeat domain 92) | WD40 domain | RNA polymerase associated protein, TNF-α and cycloheximide-induced apoptosis | - |
Notes: *All molecules are evolutionarily conserved in eukaryote; **possible phosphorylation sites were predicted by the scansite program (http://scansite.mit.edu/) with high stringency. Aabbreviations; AAA+, ATPase associated diverse cellular activities; TPR, tetratricopeptide repeat; HEAT, Huntingtin, elongation factor 3, A subunit of protein phosphatase 2A, and TOR1.
Given that the majority of the putative PIKK regulatory chaperone complex components also physically and functionally associate with transcriptional machinery167,168 and RNP biogenesis,169,170 similar complexes probably function in other cellular processes.
On the other hand, the inhibition of the RUVBL1/2 complex or the Tel2 complex has been observed to have a different effect on the PIKK mRNA levels.82,142,143 Concerning the regulation of the PIKK abundance, the mutual regulation among PIKKs is also exist [Fig. 5B-(c)]. The regulatory mechanisms of the PIKK family appear to be involved in multiple unknown mechanisms. Further studies are required to understand the detailed molecular mechanisms of PIKK regulation by the putative PIKK regulatory chaperone complex.
Relationship of the RUVBL1/2 Complex to Cancer Biology
From a clinical point of view, PIKKs have been suggested to be potential therapeutic targets for cancer therapy. For example, the constitutive activation of mTOR signaling has been observed in multiple types of tumors, and rapamycin analogs, which inhibit mTORC1 and cause growth reduction of cancer cells, are under clinical trials as anti-cancer agents.171 Further, ATM, ATR or DNA-PKcs-mediated DNA damage responses and DNA repair pathways are potential targets for cancer therapy in combination with irradiation and DNA-damaging chemical agents.172 NMD inhibition is also attractive as a new therapeutic approach to cancer by inducing tumor immunity.173 In addition to the regulation of all PIKKs, the RUVBL1/2 complex is implicated in telomerase activity and the Hsp90 pathway,83,99 both of which are promising targets of cancer therapy and the inhibitors of which are under clinical trials.174,175 RUVBL1 and RUVBL2 are also involved in c-Myc-mediated cellular transformation and cancer metastasis through the transcriptional regulation with β-catenin and the TIP60 HAT complex.80,176 Thus, the RUVBL1/2 complex represents a molecular target for cancer therapy through the simultaneous suppression of the above mentioned multiple pathways. In support of this idea, suppression of the RUVBL1/2 complex induces growth arrest and increased apoptosis of tumor cells in vitro and in vivo.177
Conclusions and Perspectives
While much is known about the critical importance of PIKKs in cellular stress responses, their overall regulatory mechanisms and the interplay among PIKKs are not well defined. The finding that all PIKKs are regulated by common regulators provides important insights into these issues.
A common PIKK regulator, the RUVBL1/2 complex, can regulate each PIKK function by controlling PIKK levels and through physical interaction with each PIKK. This suggests that the RUVBL1/2 complex mediates PIKK signaling and coordinates each PIKK-mediated stress response as a common PIKK regulator. Based on its diverse cellular functions,74 the RUVBL1/2 complex possibly links the PIKK-mediated stress responses to other cellular processes, thereby facilitating correct stress responses. Although the molecular mechanisms of the RUVBL1/2 complex-mediated PIKK regulation remain to be fully elucidated, recent studies have indicated the existence of putative PIKK regulatory chaperone complexes, including the RUVBL1/2 complex, the Tel2 complex and Hsp90. Future studies will clarify the PIKK-regulatory roles of the RUVBL1/2 complex as an ATPase and the function of the Tel2 complex in the chaperone activity of Hsp90. The putative PIKK regulatory chaperone complex contains additional factors, and their function and PIKK preference should also be examined. In addition, global analyses to evaluate the interplay among PIKKs and the linkage of PIKK signals to other cellular processes are important. Further analyses will reveal the physiological significance of the common regulators of PIKKs and help our understanding of the basic mechanisms underlying proper stress responses in living organisms.
Acknowledgments
This work was supported, in part, by grants from the Japan Society for the Promotion of Science (to S.O. and N.I.), from the Ministry of Education, Culture, Sports, Science and Technology of Japan [a Grant-in-Aid for Scientific Research (A) (to S.O.), Young Scientists (A) (to A.Y.), Scientific Research on Innovative Areas “RNA regulation” (to A.Y.) and Scientific Research on Innovative Areas “Functional machinery for noncoding RNAs” (to A.Y.)], from the Japan Science and Technology Corporation (to A.Y. and S.O.), Takeda Science Foundation (to S.O.), Mitsubishi foundation (to S.O.), Uehara memorial foundation (to S.O.), and the Yokohama Foundation for Advancement of Medical Science (to A.Y.). N.I. is a Research Fellow of the Japan Society for the Promotion of Sciences.
Glossary
Abbreviations:
- PIKK
Phosphatidylinositol 3-kinase-related protein kinase
- ATM
ataxia telangiectasia mutated
- ATR
ATM- and Rad3-related
- DNA-PKcs
DNA-dependent protein kinase catalytic subunit
- SMG-1
suppressor with morphogenetic effect on genitalia-1
- mTOR
mammalian target of rapamycin
- TRRAP
transformation/ transcription domain associated protein
- AAA+
ATPase associated diverse cellular activities
- RUVBL1/2
RuvB-like 1 and RuvB-like 2
- FAT-C
FRAP, ATM, and TRRAP C-terminal
- DSBs
DNA double strand breaks
- IR
ionizing radiation
- UV
ultraviolet
- NHEJ
non-homologous end-joining
- NMD
nonsense-mediated mRNA decay
- EJC
exon junction complex
- PTC
premature termination codon
- SURF
SMG-1-Upf1-eRF1-eRF3
- TERT
telomerase reverse transcriptase
- TERRA
telomeric repeat-containing RNA
- HAT
histone acetyltransferase
- snoRNP
small nucleolar RNP
- MRN
Mre11-Rad50-Nbs1
Footnotes
Previously published online: www.landesbioscience.com/journals/nucleus/article/18926
References
- 1.Hunter T. When is a lipid kinase not a lipid kinase? When it is a protein kinase. Cell. 1995;83:1–4. doi: 10.1016/0092-8674(95)90225-2. [DOI] [PubMed] [Google Scholar]
- 2.Abraham RT. PI 3-kinase related kinases: 'big' players in stress-induced signaling pathways. DNA Repair (Amst) 2004;3:883–7. doi: 10.1016/j.dnarep.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Lovejoy CA, Cortez D. Common mechanisms of PIKK regulation. DNA Repair (Amst) 2009 doi: 10.1016/j.dnarep.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavin MF. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol. 2008;9:759–69. doi: 10.1038/nrm2514. [DOI] [PubMed] [Google Scholar]
- 5.Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–68. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 6.Lim DS, Kirsch DG, Canman CE, Ahn JH, Ziv Y, Newman LS, et al. ATM binds to beta-adaptin in cytoplasmic vesicles. Proc Natl Acad Sci USA. 1998;95:10146–51. doi: 10.1073/pnas.95.17.10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barlow C, Ribaut-Barassin C, Zwingman TA, Pope AJ, Brown KD, Owens JW, et al. ATM is a cytoplasmic protein in mouse brain required to prevent lysosomal accumulation. Proc Natl Acad Sci USA. 2000;97:871–6. doi: 10.1073/pnas.97.2.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watters D, Kedar P, Spring K, Bjorkman J, Chen P, Gatei M, et al. Localization of a portion of extranuclear ATM to peroxisomes. J Biol Chem. 1999;274:34277–82. doi: 10.1074/jbc.274.48.34277. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Han YR, Plummer MR, Herrup K. Cytoplasmic ATM in Neurons Modulates Synaptic Function. Curr Biol. 2009 doi: 10.1016/j.cub.2009.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang DQ, Kastan MB. Participation of ATM in insulin signalling through phosphorylation of eIF-4E-binding protein 1. Nat Cell Biol. 2000;2:893–8. doi: 10.1038/35046542. [DOI] [PubMed] [Google Scholar]
- 11.Savitsky K, Bar-Shira A, Gilad S, Rotman G, Ziv Y, Vanagaite L, et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995;268:1749–53. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- 12.Lavin MF, Shiloh Y. The genetic defect in ataxia-telangiectasia. Annu Rev Immunol. 1997;15:177–202. doi: 10.1146/annurev.immunol.15.1.177. [DOI] [PubMed] [Google Scholar]
- 13.Xu Y, Ashley T, Brainerd EE, Bronson RT, Meyn MS, Baltimore D. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev. 1996;10:2411–22. doi: 10.1101/gad.10.19.2411. [DOI] [PubMed] [Google Scholar]
- 14.Hishiya A, Ito M, Aburatani H, Motoyama N, Ikeda K, Watanabe K. Ataxia telangiectasia mutated (Atm) knockout mice as a model of osteopenia due to impaired bone formation. Bone. 2005;37:497–503. doi: 10.1016/j.bone.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Inomata K, Aoto T, Binh NT, Okamoto N, Tanimura S, Wakayama T, et al. Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell. 2009;137:1088–99. doi: 10.1016/j.cell.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 16.Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol. 2008;9:616–27. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaygun H, Marzluff WF. Regulated degradation of replication-dependent histone mRNAs requires both ATR and Upf1. Nat Struct Mol Biol. 2005;12:794–800. doi: 10.1038/nsmb972. [DOI] [PubMed] [Google Scholar]
- 18.de Klein A, Muijtjens M, van Os R, Verhoeven Y, Smit B, Carr AM, et al. Targeted disruption of the cell-cycle checkpoint gene ATR leads to early embryonic lethality in mice. Curr Biol. 2000;10:479–82. doi: 10.1016/S0960-9822(00)00447-4. [DOI] [PubMed] [Google Scholar]
- 19.Naumovski L, Friedberg EC. A DNA repair gene required for the incision of damaged DNA is essential for viability in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1983;80:4818–21. doi: 10.1073/pnas.80.15.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G, et al. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1:113–26. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Driscoll M, Ruiz-Perez VL, Woods CG, Jeggo PA, Goodship JA. A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat Genet. 2003;33:497–501. doi: 10.1038/ng1129. [DOI] [PubMed] [Google Scholar]
- 22.Collis SJ, DeWeese TL, Jeggo PA, Parker AR. The life and death of DNA-PK. Oncogene. 2005;24:949–61. doi: 10.1038/sj.onc.1208332. [DOI] [PubMed] [Google Scholar]
- 23.Mahaney BL, Meek K, Lees-Miller SP. Repair of ionizing radiation-induced DNA double-strand breaks by non-homologous end-joining. Biochem J. 2009;417:639–50. doi: 10.1042/BJ20080413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirchgessner CU, Patil CK, Evans JW, Cuomo CA, Fried LM, Carter T, et al. DNA-dependent kinase (p350) as a candidate gene for the murine SCID defect. Science. 1995;267:1178–83. doi: 10.1126/science.7855601. [DOI] [PubMed] [Google Scholar]
- 25.Blunt T, Finnie NJ, Taccioli GE, Smith GC, Demengeot J, Gottlieb TM, et al. Defective DNA-dependent protein kinase activity is linked to V(D)J recombination and DNA repair defects associated with the murine scid mutation. Cell. 1995;80:813–23. doi: 10.1016/0092-8674(95)90360-7. [DOI] [PubMed] [Google Scholar]
- 26.Bozulic L, Surucu B, Hynx D, Hemmings BA. PKBalpha/Akt1 acts downstream of DNA-PK in the DNA double-strand break response and promotes survival. Mol Cell. 2008;30:203–13. doi: 10.1016/j.molcel.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 27.Shi M, Vivian CJ, Lee KJ, Ge C, Morotomi-Yano K, Manzl C, et al. DNA-PKcs-PIDDosome: a nuclear caspase-2-activating complex with role in G2/M checkpoint maintenance. Cell. 2009;136:508–20. doi: 10.1016/j.cell.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Lucero H, Gae D, Taccioli GE. Novel localization of the DNA-PK complex in lipid rafts: a putative role in the signal transduction pathway of the ionizing radiation response. J Biol Chem. 2003;278:22136–43. doi: 10.1074/jbc.M301579200. [DOI] [PubMed] [Google Scholar]
- 29.Powley IR, Kondrashov A, Young LA, Dobbyn HC, Hill K, Cannell IG, et al. Translational reprogramming following UVB irradiation is mediated by DNA-PKcs and allows selective recruitment to the polysomes of mRNAs encoding DNA repair enzymes. Genes Dev. 2009;23:1207–20. doi: 10.1101/gad.516509. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Müller B, Blackburn J, Feijoo C, Zhao X, Smythe C. DNA-activated protein kinase functions in a newly observed S phase checkpoint that links histone mRNA abundance with DNA replication. J Cell Biol. 2007;179:1385–98. doi: 10.1083/jcb.200708106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilley D, Tanaka H, Hande MP, Kurimasa A, Li GC, Oshimura M, et al. DNA-PKcs is critical for telomere capping. Proc Natl Acad Sci USA. 2001;98:15084–8. doi: 10.1073/pnas.261574698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamashita A, Kashima I, Ohno S. The role of SMG-1 in nonsense-mediated mRNA decay. Biochim Biophys Acta. 2005;1754:305–15. doi: 10.1016/j.bbapap.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Abraham RT. The ATM-related kinase, hSMG-1, bridges genome and RNA surveillance pathways. DNA Repair (Amst) 2004;3:919–25. doi: 10.1016/j.dnarep.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Yamashita A, Izumi N, Kashima I, Ohnishi T, Saari B, Katsuhata Y, et al. SMG-8 and SMG-9, two novel subunits of the SMG-1 complex, regulate remodeling of the mRNA surveillance complex during nonsense-mediated mRNA decay. Genes Dev. 2009;23:1091–105. doi: 10.1101/gad.1767209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li S, Wilkinson MF. Nonsense surveillance in lymphocytes? Immunity. 1998;8:135–41. doi: 10.1016/S1074-7613(00)80466-5. [DOI] [PubMed] [Google Scholar]
- 36.Kashima I, Yamashita A, Izumi N, Kataoka N, Morishita R, Hoshino S, et al. Binding of a novel SMG-1-Upf1-eRF1-eRF3 complex (SURF) to the exon junction complex triggers Upf1 phosphorylation and nonsense-mediated mRNA decay. Genes Dev. 2006;20:355–67. doi: 10.1101/gad.1389006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohnishi T, Yamashita A, Kashima I, Schell T, Anders KR, Grimson A, et al. Phosphorylation of hUPF1 induces formation of mRNA surveillance complexes containing hSMG-5 and hSMG-7. Mol Cell. 2003;12:1187–200. doi: 10.1016/S1097-2765(03)00443-X. [DOI] [PubMed] [Google Scholar]
- 38.Isken O, Kim YK, Hosoda N, Mayeur GL, Hershey JW, Maquat LE. Upf1 phosphorylation triggers translational repression during nonsense-mediated mRNA decay. Cell. 2008;133:314–27. doi: 10.1016/j.cell.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okada-Katsuhata Y, Yamashita A, Kutsuzawa K, Izumi N, Hirahara F, Ohno S. N- and C-terminal Upf1 phosphorylations create binding platforms for SMG-6 and SMG-5:SMG-7 during NMD. Nucleic Acids Res. 2011 doi: 10.1093/nar/gkr791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brumbaugh KM, Otterness DM, Geisen C, Oliveira V, Brognard J, Li X, et al. The mRNA surveillance protein hSMG-1 functions in genotoxic stress response pathways in mammalian cells. Mol Cell. 2004;14:585–98. doi: 10.1016/j.molcel.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Chen RQ, Yang QK, Chen YL, Oliveira VA, Dalton WS, Fearns C, et al. Kinome siRNA screen identifies SMG-1 as a negative regulator of hypoxia-inducible factor-1alpha in hypoxia. J Biol Chem. 2009;284:16752–8. doi: 10.1074/jbc.M109.014316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown JA, Roberts TL, Richards R, Woods R, Birrell G, Lim YC, et al. A Novel Role for hSMG-1 in Stress Granule Formation. Mol Cell Biol. 2011;31:4417–29. doi: 10.1128/MCB.05987-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oliveira V, Romanow WJ, Geisen C, Otterness DM, Mercurio F, Wang HG, et al. A protective role for the human SMG-1 kinase against tumor necrosis factor-alpha-induced apoptosis. J Biol Chem. 2008;283:13174–84. doi: 10.1074/jbc.M708008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Azzalin CM, Reichenbach P, Khoriauli L, Giulotto E, Lingner J. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science. 2007;318:798–801. doi: 10.1126/science.1147182. [DOI] [PubMed] [Google Scholar]
- 45.McIlwain DR, Pan Q, Reilly PT, Elia AJ, McCracken S, Wakeham AC, et al. Smg1 is required for embryogenesis and regulates diverse genes via alternative splicing coupled to nonsense-mediated mRNA decay. Proc Natl Acad Sci USA. 2010;107:12186–91. doi: 10.1073/pnas.1007336107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hodgkin J, Papp A, Pulak R, Ambros V, Anderson P. A new kind of informational suppression in the nematode Caenorhabditis elegans. Genetics. 1989;123:301–13. doi: 10.1093/genetics/123.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Z, Smith KR, Batterham P, Robin C. Smg1 nonsense mutations do not abolish nonsense-mediated mRNA decay in Drosophila melanogaster. Genetics. 2005;171:403–6. doi: 10.1534/genetics.105.045674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Masse I, Molin L, Mouchiroud L, Vanhems P, Palladino F, Billaud M, et al. A novel role for the SMG-1 kinase in lifespan and oxidative stress resistance in Caenorhabditis elegans. PLoS ONE. 2008;3:e3354. doi: 10.1371/journal.pone.0003354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jorgensen EM, Mango SE. The art and design of genetic screens: caenorhabditis elegans. Nat Rev Genet. 2002;3:356–69. doi: 10.1038/nrg794. [DOI] [PubMed] [Google Scholar]
- 50.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 51.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–9. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 52.Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78:35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 53.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–18. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 54.Mayer C, Grummt I. Ribosome biogenesis and cell growth: mTOR coordinates transcription by all three classes of nuclear RNA polymerases. Oncogene. 2006;25:6384–91. doi: 10.1038/sj.onc.1209883. [DOI] [PubMed] [Google Scholar]
- 55.Ma XM, Yoon SO, Richardson CJ, Julich K, Blenis J. SKAR links pre-mRNA splicing to mTOR/S6K1-mediated enhanced translation efficiency of spliced mRNAs. Cell. 2008;133:303–13. doi: 10.1016/j.cell.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 56.Ruvinsky I, Sharon N, Lerer T, Cohen H, Stolovich-Rain M, Nir T, et al. Ribosomal protein S6 phosphorylation is a determinant of cell size and glucose homeostasis. Genes Dev. 2005;19:2199–211. doi: 10.1101/gad.351605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang YY, Juhasz G, Goraksha-Hicks P, Arsham AM, Mallin DR, Muller LK, et al. Nutrient-dependent regulation of autophagy through the target of rapamycin pathway. Biochem Soc Trans. 2009;37:232–6. doi: 10.1042/BST0370232. [DOI] [PubMed] [Google Scholar]
- 58.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 59.Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–8. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 60.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 61.García-Martínez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1) Biochem J. 2008;416:375–85. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- 62.Jones KT, Greer ER, Pearce D, Ashrafi K. Rictor/TORC2 regulates Caenorhabditis elegans fat storage, body size, and development through sgk-1. PLoS Biol. 2009;7:e60. doi: 10.1371/journal.pbio.1000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soukas AA, Kane EA, Carr CE, Melo JA, Ruvkun G. Rictor/TORC2 regulates fat metabolism, feeding, growth, and life span in Caenorhabditis elegans. Genes Dev. 2009;23:496–511. doi: 10.1101/gad.1775409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gangloff YG, Mueller M, Dann SG, Svoboda P, Sticker M, Spetz JF, et al. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol Cell Biol. 2004;24:9508–16. doi: 10.1128/MCB.24.21.9508-9516.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murakami M, Ichisaka T, Maeda M, Oshiro N, Hara K, Edenhofer F, et al. mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol Cell Biol. 2004;24:6710–8. doi: 10.1128/MCB.24.15.6710-6718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK. The TOR pathway comes of age. Biochim Biophys Acta. 2009;1790:1067–74. doi: 10.1016/j.bbagen.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murr R, Vaissiere T, Sawan C, Shukla V, Herceg Z. Orchestration of chromatin-based processes: mind the TRRAP. Oncogene. 2007;26:5358–72. doi: 10.1038/sj.onc.1210605. [DOI] [PubMed] [Google Scholar]
- 68.McMahon SB, Van Buskirk HA, Dugan KA, Copeland TD, Cole MD. The novel ATM-related protein TRRAP is an essential cofactor for the c-Myc and E2F oncoproteins. Cell. 1998;94:363–74. doi: 10.1016/S0092-8674(00)81479-8. [DOI] [PubMed] [Google Scholar]
- 69.Herceg Z, Li H, Cuenin C, Shukla V, Radolf M, Steinlein P, et al. Genome-wide analysis of gene expression regulated by the HAT cofactor Trrap in conditional knockout cells. Nucleic Acids Res. 2003;31:7011–23. doi: 10.1093/nar/gkg902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herceg Z, Hulla W, Gell D, Cuenin C, Lleonart M, Jackson S, et al. Disruption of Trrap causes early embryonic lethality and defects in cell cycle progression. Nat Genet. 2001;29:206–11. doi: 10.1038/ng725. [DOI] [PubMed] [Google Scholar]
- 71.Saleh A, Schieltz D, Ting N, McMahon SB, Litchfield DW, Yates JR, 3rd, et al. Tra1p is a component of the yeast Ada.Spt transcriptional regulatory complexes. J Biol Chem. 1998;273:26559–65. doi: 10.1074/jbc.273.41.26559. [DOI] [PubMed] [Google Scholar]
- 72.Murr R, Loizou JI, Yang YG, Cuenin C, Li H, Wang ZQ, et al. Histone acetylation by Trrap-Tip60 modulates loading of repair proteins and repair of DNA double-strand breaks. Nat Cell Biol. 2006;8:91–9. doi: 10.1038/ncb1343. [DOI] [PubMed] [Google Scholar]
- 73.Robert F, Hardy S, Nagy Z, Baldeyron C, Murr R, Dery U, et al. The transcriptional histone acetyltransferase cofactor TRRAP associates with the MRN repair complex and plays a role in DNA double-strand break repair. Mol Cell Biol. 2006;26:402–12. doi: 10.1128/MCB.26.2.402-412.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jha S, Dutta A. RVB1/RVB2: running rings around molecular biology. Mol Cell. 2009;34:521–33. doi: 10.1016/j.molcel.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.West SC. Processing of recombination intermediates by the RuvABC proteins. Annu Rev Genet. 1997;31:213–44. doi: 10.1146/annurev.genet.31.1.213. [DOI] [PubMed] [Google Scholar]
- 76.Yamada K, Ariyoshi M, Morikawa K. Three-dimensional structural views of branch migration and resolution in DNA homologous recombination. Curr Opin Struct Biol. 2004;14:130–7. doi: 10.1016/j.sbi.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 77.Kanemaki M, Kurokawa Y, Matsu-ura T, Makino Y, Masani A, Okazaki K, et al. TIP49b, a new RuvB-like DNA helicase, is included in a complex together with another RuvB-like DNA helicase, TIP49a. J Biol Chem. 1999;274:22437–44. doi: 10.1074/jbc.274.32.22437. [DOI] [PubMed] [Google Scholar]
- 78.Torreira E, Jha S, Lopez-Blanco JR, Arias-Palomo E, Chacon P, Canas C, et al. Architecture of the pontin/reptin complex, essential in the assembly of several macromolecular complexes. Structure. 2008;16:1511–20. doi: 10.1016/j.str.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Puri T, Wendler P, Sigala B, Saibil H, Tsaneva IR. Dodecameric structure and ATPase activity of the human TIP48/TIP49 complex. J Mol Biol. 2007;366:179–92. doi: 10.1016/j.jmb.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 80.Kim JH, Kim B, Cai L, Choi HJ, Ohgi KA, Tran C, et al. Transcriptional regulation of a metastasis suppressor gene by Tip60 and beta-catenin complexes. Nature. 2005;434:921–6. doi: 10.1038/nature03452. [DOI] [PubMed] [Google Scholar]
- 81.Diop SB, Bertaux K, Vasanthi D, Sarkeshik A, Goirand B, Aragnol D, et al. Reptin and Pontin function antagonistically with PcG and TrxG complexes to mediate Hox gene control. EMBO Rep. 2008;9:260–6. doi: 10.1038/embor.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Izumi N, Yamashita A, Iwamatsu A, Kurata R, Nakamura H, Saari B, et al. AAA+ proteins RUVBL1 and RUVBL2 coordinate PIKK activity and function in nonsense-mediated mRNA decay. Sci Signal. 2010;3:ra27. doi: 10.1126/scisignal.2000468. [DOI] [PubMed] [Google Scholar]
- 83.Venteicher AS, Meng Z, Mason PJ, Veenstra TD, Artandi SE. Identification of ATPases pontin and reptin as telomerase components essential for holoenzyme assembly. Cell. 2008;132:945–57. doi: 10.1016/j.cell.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matias PM, Gorynia S, Donner P, Carrondo MA. Crystal structure of the human AAA+ protein RuvBL1. J Biol Chem. 2006;281:38918–29. doi: 10.1074/jbc.M605625200. [DOI] [PubMed] [Google Scholar]
- 85.Qiu XB, Lin YL, Thome KC, Pian P, Schlegel BP, Weremowicz S, et al. An eukaryotic RuvB-like protein (RUVBL1) essential for growth. J Biol Chem. 1998;273:27786–93. doi: 10.1074/jbc.273.43.27786. [DOI] [PubMed] [Google Scholar]
- 86.Bauer A, Chauvet S, Huber O, Usseglio F, Rothbacher U, Aragnol D, et al. Pontin52 and reptin52 function as antagonistic regulators of beta-catenin signalling activity. EMBO J. 2000;19:6121–30. doi: 10.1093/emboj/19.22.6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gallant P. Control of transcription by Pontin and Reptin. Trends Cell Biol. 2007;17:187–92. doi: 10.1016/j.tcb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 88.Shen X, Mizuguchi G, Hamiche A, Wu C. A chromatin remodelling complex involved in transcription and DNA processing. Nature. 2000;406:541–4. doi: 10.1038/35020123. [DOI] [PubMed] [Google Scholar]
- 89.Mizuguchi G, Shen X, Landry J, Wu WH, Sen S, Wu C. ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science. 2004;303:343–8. doi: 10.1126/science.1090701. [DOI] [PubMed] [Google Scholar]
- 90.Ikura T, Ogryzko VV, Grigoriev M, Groisman R, Wang J, Horikoshi M, et al. Involvement of the TIP60 histone acetylase complex in DNA repair and apoptosis. Cell. 2000;102:463–73. doi: 10.1016/S0092-8674(00)00051-9. [DOI] [PubMed] [Google Scholar]
- 91.Jónsson ZO, Jha S, Wohlschlegel JA, Dutta A. Rvb1p/Rvb2p recruit Arp5p and assemble a functional Ino80 chromatin remodeling complex. Mol Cell. 2004;16:465–77. doi: 10.1016/j.molcel.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 92.Jha S, Shibata E, Dutta A. Human Rvb1/Tip49 is required for the histone acetyltransferase activity of Tip60/NuA4 and for the downregulation of phosphorylation on H2AX after DNA damage. Mol Cell Biol. 2008;28:2690–700. doi: 10.1128/MCB.01983-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morrison AJ, Shen X. DNA repair in the context of chromatin. Cell Cycle. 2005;4:568–71. doi: 10.4161/cc.4.4.1612. [DOI] [PubMed] [Google Scholar]
- 94.van Attikum H, Gasser SM. The histone code at DNA breaks: a guide to repair? Nat Rev Mol Cell Biol. 2005;6:757–65. doi: 10.1038/nrm1737. [DOI] [PubMed] [Google Scholar]
- 95.Jónsson ZO, Dhar SK, Narlikar GJ, Auty R, Wagle N, Pellman D, et al. Rvb1p and Rvb2p are essential components of a chromatin remodeling complex that regulates transcription of over 5% of yeast genes. J Biol Chem. 2001;276:16279–88. doi: 10.1074/jbc.M011523200. [DOI] [PubMed] [Google Scholar]
- 96.Matera AG, Terns RM, Terns MP. Non-coding RNAs: lessons from the small nuclear and small nucleolar RNAs. Nat Rev Mol Cell Biol. 2007;8:209–20. doi: 10.1038/nrm2124. [DOI] [PubMed] [Google Scholar]
- 97.King TH, Decatur WA, Bertrand E, Maxwell ES, Fournier MJ. A well-connected and conserved nucleoplasmic helicase is required for production of box C/D and H/ACA snoRNAs and localization of snoRNP proteins. Mol Cell Biol. 2001;21:7731–46. doi: 10.1128/MCB.21.22.7731-7746.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Watkins NJ, Lemm I, Ingelfinger D, Schneider C, Hossbach M, Urlaub H, et al. Assembly and maturation of the U3 snoRNP in the nucleoplasm in a large dynamic multiprotein complex. Mol Cell. 2004;16:789–98. doi: 10.1016/j.molcel.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 99.Zhao R, Davey M, Hsu YC, Kaplanek P, Tong A, Parsons AB, et al. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell. 2005;120:715–27. doi: 10.1016/j.cell.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 100.Te J, Jia L, Rogers J, Miller A, Hartson SD. Novel subunits of the mammalian Hsp90 signal transduction chaperone. J Proteome Res. 2007;6:1963–73. doi: 10.1021/pr060595i. [DOI] [PubMed] [Google Scholar]
- 101.Gstaiger M, Luke B, Hess D, Oakeley EJ, Wirbelauer C, Blondel M, et al. Control of nutrient-sensitive transcription programs by the unconventional prefoldin URI. Science. 2003;302:1208–12. doi: 10.1126/science.1088401. [DOI] [PubMed] [Google Scholar]
- 102.Ducat D, Kawaguchi S, Liu H, Yates JR, 3rd, Zheng Y. Regulation of microtubule assembly and organization in mitosis by the AAA+ ATPase Pontin. Mol Biol Cell. 2008;19:3097–110. doi: 10.1091/mbc.E07-11-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sigala B, Edwards M, Puri T, Tsaneva IR. Relocalization of human chromatin remodeling cofactor TIP48 in mitosis. Exp Cell Res. 2005;310:357–69. doi: 10.1016/j.yexcr.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 104.Skop AR, Liu H, Yates J, 3rd, Meyer BJ, Heald R. Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science. 2004;305:61–6. doi: 10.1126/science.1097931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, 3rd, Hurov KE, Luo J, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–6. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 106.Ren B, Cam H, Takahashi Y, Volkert T, Terragni J, Young RA, et al. E2F integrates cell cycle progression with DNA repair, replication, and G(2)/M checkpoints. Genes Dev. 2002;16:245–56. doi: 10.1101/gad.949802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Berkovich E, Ginsberg D. ATM is a target for positive regulation by E2F-1. Oncogene. 2003;22:161–7. doi: 10.1038/sj.onc.1206144. [DOI] [PubMed] [Google Scholar]
- 108.Cole MD, Cowling VH. Specific regulation of mRNA cap methylation by the c-Myc and E2F1 transcription factors. Oncogene. 2009;28:1169–75. doi: 10.1038/onc.2008.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Arias-Palomo E, Yamashita A, Fernandez IS, Nunez-Ramirez R, Bamba Y, Izumi N, et al. The nonsense-mediated mRNA decay SMG-1 kinase is regulated by large-scale conformational changes controlled by SMG-8. Genes Dev. 2011;25:153–64. doi: 10.1101/gad.606911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–75. doi: 10.1016/S0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 111.Ball HL, Myers JS, Cortez D. ATRIP binding to replication protein A-single-stranded DNA promotes ATR-ATRIP localization but is dispensable for Chk1 phosphorylation. Mol Biol Cell. 2005;16:2372–81. doi: 10.1091/mbc.E04-11-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.You Z, Chahwan C, Bailis J, Hunter T, Russell P. ATM activation and its recruitment to damaged DNA require binding to the C terminus of Nbs1. Mol Cell Biol. 2005;25:5363–79. doi: 10.1128/MCB.25.13.5363-5379.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sun Y, Jiang X, Chen S, Fernandes N, Price BD. A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM. Proc Natl Acad Sci USA. 2005;102:13182–7. doi: 10.1073/pnas.0504211102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Morita T, Yamashita A, Kashima I, Ogata K, Ishiura S, Ohno S. Distant N- and C-terminal domains are required for intrinsic kinase activity of SMG-1, a critical component of nonsense-mediated mRNA decay. J Biol Chem. 2007;282:7799–808. doi: 10.1074/jbc.M610159200. [DOI] [PubMed] [Google Scholar]
- 115.Priestley A, Beamish HJ, Gell D, Amatucci AG, Muhlmann-Diaz MC, Singleton BK, et al. Molecular and biochemical characterisation of DNA-dependent protein kinase-defective rodent mutant irs-20. Nucleic Acids Res. 1998;26:1965–73. doi: 10.1093/nar/26.8.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Beamish HJ, Jessberger R, Riballo E, Priestley A, Blunt T, Kysela B, et al. The C-terminal conserved domain of DNA-PKcs, missing in the SCID mouse, is required for kinase activity. Nucleic Acids Res. 2000;28:1506–13. doi: 10.1093/nar/28.7.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Takahashi T, Hara K, Inoue H, Kawa Y, Tokunaga C, Hidayat S, et al. Carboxyl-terminal region conserved among phosphoinositide-kinase-related kinases is indispensable for mTOR function in vivo and in vitro. Genes Cells. 2000;5:765–75. doi: 10.1046/j.1365-2443.2000.00365.x. [DOI] [PubMed] [Google Scholar]
- 118.Jiang X, Sun Y, Chen S, Roy K, Price BD. The FATC domains of PIKK proteins are functionally equivalent and participate in the Tip60-dependent activation of DNA-PKcs and ATM. J Biol Chem. 2006;281:15741–6. doi: 10.1074/jbc.M513172200. [DOI] [PubMed] [Google Scholar]
- 119.Zhang S, Hemmerich P, Grosse F. Centrosomal localization of DNA damage checkpoint proteins. J Cell Biochem. 2007;101:451–65. doi: 10.1002/jcb.21195. [DOI] [PubMed] [Google Scholar]
- 120.Smith E, Dejsuphong D, Balestrini A, Hampel M, Lenz C, Takeda S, et al. An ATM- and ATR-dependent checkpoint inactivates spindle assembly by targeting CEP63. Nat Cell Biol. 2009;11:278–85. doi: 10.1038/ncb1835. [DOI] [PubMed] [Google Scholar]
- 121.Gartner W, Rossbacher J, Zierhut B, Daneva T, Base W, Weissel M, et al. The ATP-dependent helicase RUVBL1/TIP49a associates with tubulin during mitosis. Cell Motil Cytoskeleton. 2003;56:79–93. doi: 10.1002/cm.10136. [DOI] [PubMed] [Google Scholar]
- 122.Sheaffer KL, Updike DL, Mango SE. The Target of Rapamycin pathway antagonizes pha-4/FoxA to control development and aging. Curr Biol. 2008;18:1355–64. doi: 10.1016/j.cub.2008.07.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jazayeri A, Falck J, Lukas C, Bartek J, Smith GC, Lukas J, et al. ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat Cell Biol. 2006;8:37–45. doi: 10.1038/ncb1337. [DOI] [PubMed] [Google Scholar]
- 124.Myers JS, Cortez D. Rapid activation of ATR by ionizing radiation requires ATM and Mre11. J Biol Chem. 2006;281:9346–50. doi: 10.1074/jbc.M513265200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stiff T, Walker SA, Cerosaletti K, Goodarzi AA, Petermann E, Concannon P, et al. ATR-dependent phosphorylation and activation of ATM in response to UV treatment or replication fork stalling. EMBO J. 2006;25:5775–82. doi: 10.1038/sj.emboj.7601446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen BP, Uematsu N, Kobayashi J, Lerenthal Y, Krempler A, Yajima H, et al. Ataxia telangiectasia mutated (ATM) is essential for DNA-PKcs phosphorylations at the Thr-2609 cluster upon DNA double strand break. J Biol Chem. 2007;282:6582–7. doi: 10.1074/jbc.M611605200. [DOI] [PubMed] [Google Scholar]
- 127.Yajima H, Lee KJ, Chen BP. ATR-dependent phosphorylation of DNA-dependent protein kinase catalytic subunit in response to UV-induced replication stress. Mol Cell Biol. 2006;26:7520–8. doi: 10.1128/MCB.00048-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Izumi N, Yamashita A, Hirano H, Ohno S. Hsp90 regulates PIKK family proteins together with the RUVBL1/2 and Tel2-containing co-factor complex. Cancer Sci. 2012;103:50–7. doi: 10.1111/j.1349-7006.2011.02112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Peng Y, Woods RG, Beamish H, Ye R, Lees-Miller SP, Lavin MF, et al. Deficiency in the catalytic subunit of DNA-dependent protein kinase causes down-regulation of ATM. Cancer Res. 2005;65:1670–7. doi: 10.1158/0008-5472.CAN-04-3451. [DOI] [PubMed] [Google Scholar]
- 130.Feng Z, Zhang H, Levine AJ, Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci USA. 2005;102:8204–9. doi: 10.1073/pnas.0502857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schonbrun M, Laor D, Lopez-Maury L, Bahler J, Kupiec M, Weisman R. TOR complex 2 controls gene silencing, telomere length maintenance, and survival under DNA-damaging conditions. Mol Cell Biol. 2009;29:4584–94. doi: 10.1128/MCB.01879-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Beuvink I, Boulay A, Fumagalli S, Zilbermann F, Ruetz S, O'Reilly T, et al. The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation. Cell. 2005;120:747–59. doi: 10.1016/j.cell.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 133.Shen C, Lancaster CS, Shi B, Guo H, Thimmaiah P, Bjornsti MA. TOR signaling is a determinant of cell survival in response to DNA damage. Mol Cell Biol. 2007;27:7007–17. doi: 10.1128/MCB.00290-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.O'Sullivan RJ, Karlseder J. Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol. 2010;11:171–81. doi: 10.1038/nrm2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.d'Adda di Fagagna F, Teo SH, Jackson SP. Functional links between telomeres and proteins of the DNA-damage response. Genes Dev. 2004;18:1781–99. doi: 10.1101/gad.1214504. [DOI] [PubMed] [Google Scholar]
- 136.Moser BA, Subramanian L, Khair L, Chang YT, Nakamura TM. Fission yeast Tel1(ATM) and Rad3(ATR) promote telomere protection and telomerase recruitment. PLoS Genet. 2009;5:e1000622. doi: 10.1371/journal.pgen.1000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Verdun RE, Karlseder J. The DNA damage machinery and homologous recombination pathway act consecutively to protect human telomeres. Cell. 2006;127:709–20. doi: 10.1016/j.cell.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 138.Nikiforov MA, Chandriani S, Park J, Kotenko I, Matheos D, Johnsson A, et al. TRRAP-dependent and TRRAP-independent transcriptional activation by Myc family oncoproteins. Mol Cell Biol. 2002;22:5054–63. doi: 10.1128/MCB.22.14.5054-5063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Atanassov BS, Evrard YA, Multani AS, Zhang Z, Tora L, Devys D, et al. Gcn5 and SAGA regulate shelterin protein turnover and telomere maintenance. Mol Cell. 2009;35:352–64. doi: 10.1016/j.molcel.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhou C, Gehrig PA, Whang YE, Boggess JF. Rapamycin inhibits telomerase activity by decreasing the hTERT mRNA level in endometrial cancer cells. Mol Cancer Ther. 2003;2:789–95. [PubMed] [Google Scholar]
- 141.Chen YC, Teng SC, Wu KJ. Phosphorylation of telomeric repeat binding factor 1 (TRF1) by Akt causes telomere shortening. Cancer Invest. 2009;27:24–8. doi: 10.1080/07357900802027081. [DOI] [PubMed] [Google Scholar]
- 142.Takai H, Wang RC, Takai KK, Yang H, de Lange T. Tel2 regulates the stability of PI3K-related protein kinases. Cell. 2007;131:1248–59. doi: 10.1016/j.cell.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 143.Hurov KE, Cotta-Ramusino C, Elledge SJ. A genetic screen identifies the Triple T complex required for DNA damage signaling and ATM and ATR stability. Genes Dev. 2010;24:1939–50. doi: 10.1101/gad.1934210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Takai H, Xie Y, de Lange T, Pavletich NP. Tel2 structure and function in the Hsp90-dependent maturation of mTOR and ATR complexes. Genes Dev. 2010 doi: 10.1101/gad.1956410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hayashi T, Hatanaka M, Nagao K, Nakaseko Y, Kanoh J, Kokubu A, et al. Rapamycin sensitivity of the Schizosaccharomyces pombe tor2 mutant and organization of two highly phosphorylated TOR complexes by specific and common subunits. Genes Cells. 2007;12:1357–70. doi: 10.1111/j.1365-2443.2007.01141.x. [DOI] [PubMed] [Google Scholar]
- 146.Kaizuka T, Hara T, Oshiro N, Kikkawa U, Yonezawa K, Takehana K, et al. Tti1 and Tel2 are critical factors in mammalian target of rapamycin complex assembly. J Biol Chem. 2010;285:20109–16. doi: 10.1074/jbc.M110.121699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Collis SJ, Barber LJ, Clark AJ, Martin JS, Ward JD, Boulton SJ. HCLK2 is essential for the mammalian S-phase checkpoint and impacts on Chk1 stability. Nat Cell Biol. 2007;9:391–401. doi: 10.1038/ncb1555. [DOI] [PubMed] [Google Scholar]
- 148.Anderson CM, Korkin D, Smith DL, Makovets S, Seidel JJ, Sali A, et al. Tel2 mediates activation and localization of ATM/Tel1 kinase to a double-strand break. Genes Dev. 2008;22:854–9. doi: 10.1101/gad.1646208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shikata M, Ishikawa F, Kanoh J. Tel2 is required for activation of the Mrc1-mediated replication checkpoint. J Biol Chem. 2007;282:5346–55. doi: 10.1074/jbc.M607432200. [DOI] [PubMed] [Google Scholar]
- 150.Rendtlew Danielsen JM, Larsen DH, Schou KB, Freire R, Falck J, Bartek J, et al. HCLK2 is required for activity of the DNA damage response kinase ATR. J Biol Chem. 2009;284:4140–7. doi: 10.1074/jbc.M808174200. [DOI] [PubMed] [Google Scholar]
- 151.Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11:515–28. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 152.Falsone SF, Gesslbauer B, Tirk F, Piccinini AM, Kungl AJ. A proteomic snapshot of the human heat shock protein 90 interactome. FEBS Lett. 2005;579:6350–4. doi: 10.1016/j.febslet.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 153.Dote H, Burgan WE, Camphausen K, Tofilon PJ. Inhibition of hsp90 compromises the DNA damage response to radiation. Cancer Res. 2006;66:9211–20. doi: 10.1158/0008-5472.CAN-06-2181. [DOI] [PubMed] [Google Scholar]
- 154.Ohji G, Hidayat S, Nakashima A, Tokunaga C, Oshiro N, Yoshino K, et al. Suppression of the mTOR-raptor signaling pathway by the inhibitor of heat shock protein 90 geldanamycin. J Biochem. 2006;139:129–35. doi: 10.1093/jb/mvj008. [DOI] [PubMed] [Google Scholar]
- 155.Eckert K, Saliou JM, Monlezun L, Vigouroux A, Atmane N, Caillat C, et al. The Pih1-Tah1 cochaperone complex inhibits Hsp90 molecular chaperone ATPase activity. J Biol Chem. 2010 doi: 10.1074/jbc.M110.138263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Millson SH, Vaughan CK, Zhai C, Ali MM, Panaretou B, Piper PW, et al. Chaperone ligand-discrimination by the TPR-domain protein Tah1. Biochem J. 2008;413:261–8. doi: 10.1042/BJ20080105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hahn JS. The Hsp90 chaperone machinery: from structure to drug development. BMB Rep. 2009;42:623–30. doi: 10.5483/BMBRep.2009.42.10.623. [DOI] [PubMed] [Google Scholar]
- 158.Hanson PI, Whiteheart SW. AAA+ proteins: have engine, will work. Nat Rev Mol Cell Biol. 2005;6:519–29. doi: 10.1038/nrm1684. [DOI] [PubMed] [Google Scholar]
- 159.Young JC, Agashe VR, Siegers K, Hartl FU. Pathways of chaperone-mediated protein folding in the cytosol. Nat Rev Mol Cell Biol. 2004;5:781–91. doi: 10.1038/nrm1492. [DOI] [PubMed] [Google Scholar]
- 160.Cloutier P, Al-Khoury R, Lavallee-Adam M, Faubert D, Jiang H, Poitras C, et al. High-resolution mapping of the protein interaction network for the human transcription machinery and affinity purification of RNA polymerase II-associated complexes. Methods. 2009;48:381–6. doi: 10.1016/j.ymeth.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Djouder N, Metzler SC, Schmidt A, Wirbelauer C, Gstaiger M, Aebersold R, et al. S6K1-mediated disassembly of mitochondrial URI/PP1gamma complexes activates a negative feedback program that counters S6K1 survival signaling. Mol Cell. 2007;28:28–40. doi: 10.1016/j.molcel.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 162.Parusel CT, Kritikou EA, Hengartner MO, Krek W, Gotta M. URI-1 is required for DNA stability in C. elegans. Development. 2006;133:621–9. doi: 10.1242/dev.02235. [DOI] [PubMed] [Google Scholar]
- 163.Woychik NA, Liao SM, Kolodziej PA, Young RA. Subunits shared by eukaryotic nuclear RNA polymerases. Genes Dev. 1990;4:313–23. doi: 10.1101/gad.4.3.313. [DOI] [PubMed] [Google Scholar]
- 164.Dorjsuren D, Lin Y, Wei W, Yamashita T, Nomura T, Hayashi N, et al. RMP, a novel RNA polymerase II subunit 5-interacting protein, counteracts transactivation by hepatitis B virus X protein. Mol Cell Biol. 1998;18:7546–55. doi: 10.1128/mcb.18.12.7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Itsuki Y, Saeki M, Nakahara H, Egusa H, Irie Y, Terao Y, et al. Molecular cloning of novel Monad binding protein containing tetratricopeptide repeat domains. FEBS Lett. 2008;582:2365–70. doi: 10.1016/j.febslet.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 166.Horejsí Z, Takai H, Adelman CA, Collis SJ, Flynn H, Maslen S, et al. CK2 phospho-dependent binding of R2TP complex to TEL2 is essential for mTOR and SMG1 stability. Mol Cell. 2010;39:839–50. doi: 10.1016/j.molcel.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 167.Jeronimo C, Forget D, Bouchard A, Li Q, Chua G, Poitras C, et al. Systematic analysis of the protein interaction network for the human transcription machinery reveals the identity of the 7SK capping enzyme. Mol Cell. 2007;27:262–74. doi: 10.1016/j.molcel.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Boulon S, Pradet-Balade B, Verheggen C, Molle D, Boireau S, Georgieva M, et al. HSP90 and its R2TP/Prefoldin-like cochaperone are involved in the cytoplasmic assembly of RNA polymerase II. Mol Cell. 2010;39:912–24. doi: 10.1016/j.molcel.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Boulon S, Marmier-Gourrier N, Pradet-Balade B, Wurth L, Verheggen C, Jady BE, et al. The Hsp90 chaperone controls the biogenesis of L7Ae RNPs through conserved machinery. J Cell Biol. 2008;180:579–95. doi: 10.1083/jcb.200708110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhao R, Kakihara Y, Gribun A, Huen J, Yang G, Khanna M, et al. Molecular chaperone Hsp90 stabilizes Pih1/Nop17 to maintain R2TP complex activity that regulates snoRNA accumulation. J Cell Biol. 2008;180:563–78. doi: 10.1083/jcb.200709061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Easton JB, Houghton PJ. mTOR and cancer therapy. Oncogene. 2006;25:6436–46. doi: 10.1038/sj.onc.1209886. [DOI] [PubMed] [Google Scholar]
- 172.Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8:193–204. doi: 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- 173.Pastor F, Kolonias D, Giangrande PH, Gilboa E. Induction of tumour immunity by targeted inhibition of nonsense-mediated mRNA decay. Nature. 2010;465:227–30. doi: 10.1038/nature08999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Harley CB. Telomerase and cancer therapeutics. Nat Rev Cancer. 2008;8:167–79. doi: 10.1038/nrc2275. [DOI] [PubMed] [Google Scholar]
- 175.Taldone T, Gozman A, Maharaj R, Chiosis G. Targeting Hsp90: small-molecule inhibitors and their clinical development. Curr Opin Pharmacol. 2008;8:370–4. doi: 10.1016/j.coph.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wood MA, McMahon SB, Cole MD. An ATPase/helicase complex is an essential cofactor for oncogenic transformation by c-Myc. Mol Cell. 2000;5:321–30. doi: 10.1016/S1097-2765(00)80427-X. [DOI] [PubMed] [Google Scholar]
- 177.Huber O, Menard L, Haurie V, Nicou A, Taras D, Rosenbaum J. Pontin and reptin, two related ATPases with multiple roles in cancer. Cancer Res. 2008;68:6873–6. doi: 10.1158/0008-5472.CAN-08-0547. [DOI] [PubMed] [Google Scholar]