Abstract
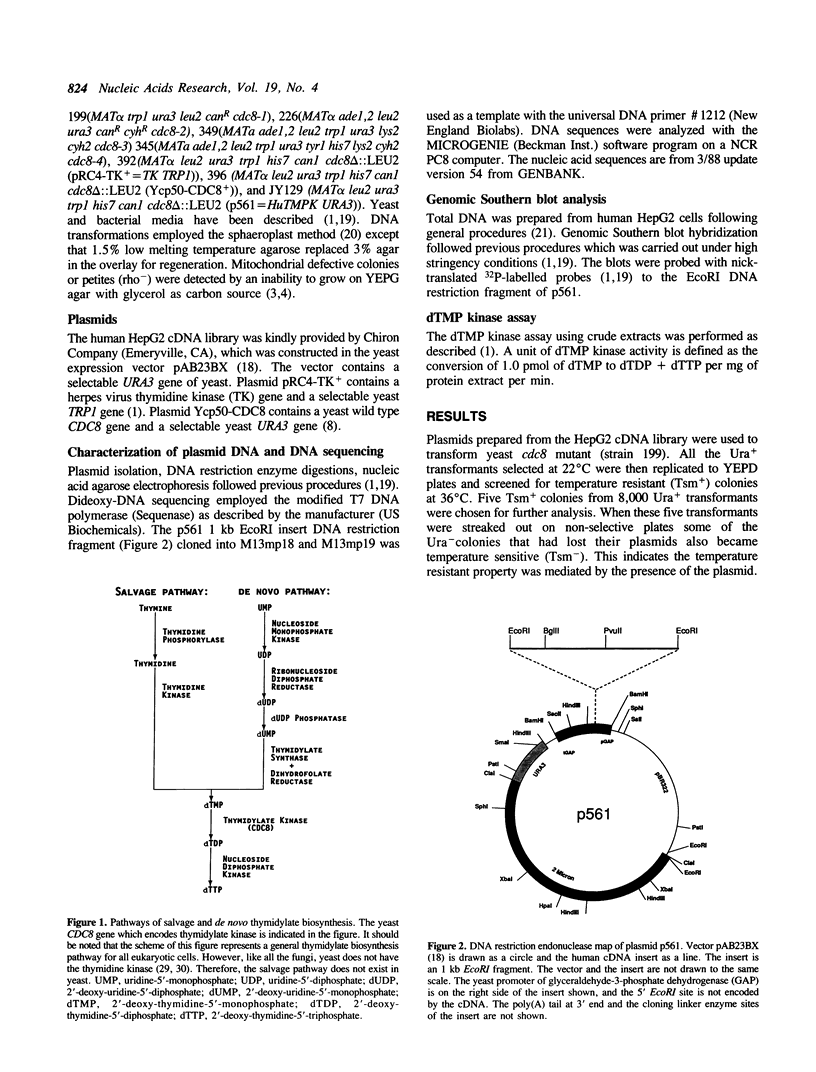
(Deoxy)thymidylate (dTMP) kinase is an enzyme which phosphorylates dTMP to dTDP in the presence of ATP and magnesium. This enzyme is important in cellular DNA synthesis because the synthesis of dTTP, either via the de novo pathway or through the exogenous supply of thymidine, requires the activity of this enzyme. It has been suggested that the activities of the enzymes involved in DNA precursor biosynthesis, such as thymidine kinase, thymidylate synthase, thymidylate kinase, and dihydrofolate reductase, are subjected to cell cycle regulation. Here we describe the cloning of a human dTMP kinase cDNA by functional complementation of a yeast dTMP kinase temperature-sensitive mutant at the non-permissive temperature. The nucleotide sequence of the cloned human cDNA is predicted to encode a 24 KD protein that shows considerable homology with the yeast and vaccinia virus dTMP kinase enzymes. The human enzyme activity has been investigated by expressing it in yeast. In this work, we demonstrate that the cloned human cDNA, when expressed in yeast, produces dTMP kinase activity.
Full text
PDF

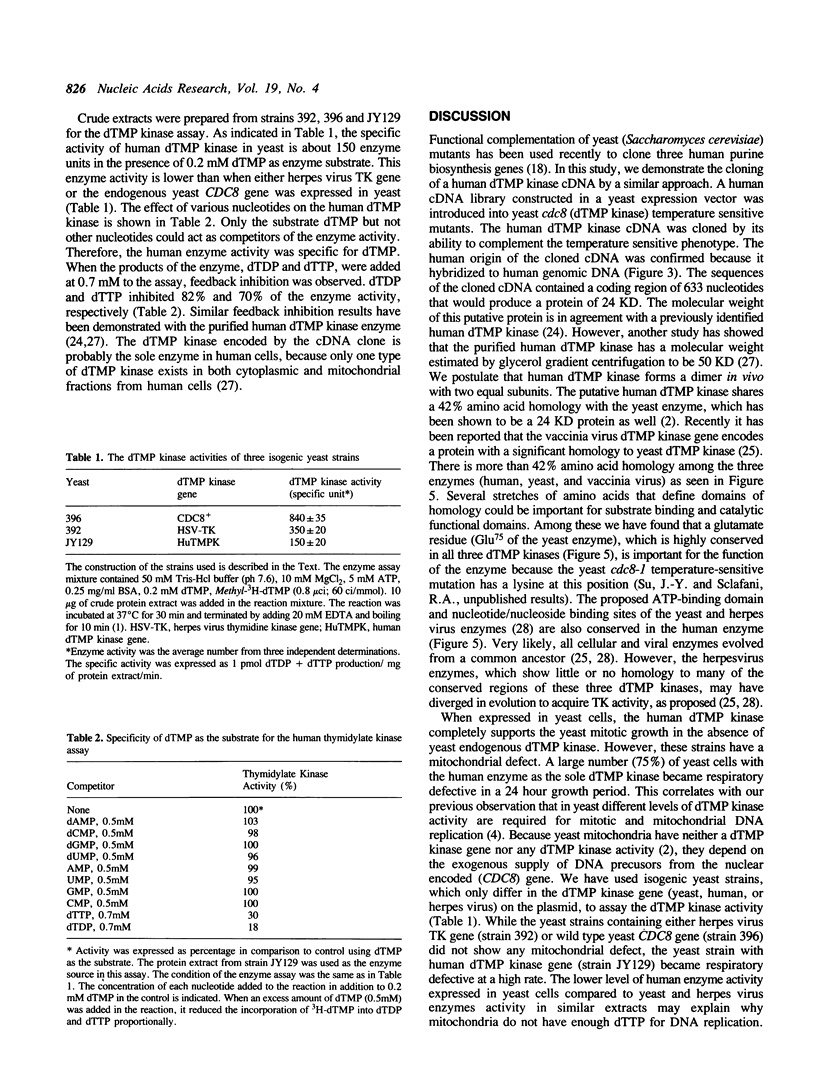

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bello L. J. Regulation of thymidine kinase synthesis in human cells. Exp Cell Res. 1974 Dec;89(2):263–274. doi: 10.1016/0014-4827(74)90790-3. [DOI] [PubMed] [Google Scholar]
- Bisson L., Thorner J. Thymidine 5'-monophosphate-requiring mutants of Saccharomyces cerevisiae are deficient in thymidylate synthetase. J Bacteriol. 1977 Oct;132(1):44–50. doi: 10.1128/jb.132.1.44-50.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celniker S. E., Campbell J. L. Yeast DNA replication in vitro: initiation and elongation events mimic in vivo processes. Cell. 1982 Nov;31(1):201–213. doi: 10.1016/0092-8674(82)90420-2. [DOI] [PubMed] [Google Scholar]
- Chen M. S., Prusoff W. H. Association of thymidylate kinase activity with pyrimidine deoxyribonucleoside kinase induced by herpes simplex virus. J Biol Chem. 1978 Mar 10;253(5):1325–1327. [PubMed] [Google Scholar]
- Farnham P. J., Schimke R. T. Transcriptional regulation of mouse dihydrofolate reductase in the cell cycle. J Biol Chem. 1985 Jun 25;260(12):7675–7680. [PubMed] [Google Scholar]
- Grivell A. R., Jackson J. F. Thymidine kinase: evidence for its absence from Neurospora crassa and some other micro-organisms, and the relevance of this to the specific labelling of deoxyribonucleic acid. J Gen Microbiol. 1968 Dec;54(2):307–317. doi: 10.1099/00221287-54-2-307. [DOI] [PubMed] [Google Scholar]
- Hereford L. M., Hartwell L. H. Defective DNA synthesis in permeabilized yeast mutants. Nat New Biol. 1971 Dec 8;234(49):171–172. doi: 10.1038/newbio234171a0. [DOI] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenh C. H., Rao L. G., Johnson L. F. Regulation of thymidylate synthase enzyme synthesis in 5-fluorodeoxyuridine-resistant mouse fibroblasts during the transition from the resting to growing state. J Cell Physiol. 1985 Jan;122(1):149–154. doi: 10.1002/jcp.1041220122. [DOI] [PubMed] [Google Scholar]
- Jong A. Y., Kuo C. L., Campbell J. L. The CDC8 gene of yeast encodes thymidylate kinase. J Biol Chem. 1984 Sep 10;259(17):11052–11059. [PubMed] [Google Scholar]
- Kucera R., Brown C. L., Paulus H. Cell cycle regulation of ribonucleoside diphosphate reductase activity in permeable mouse L cells and in extracts. J Cell Physiol. 1983 Nov;117(2):158–168. doi: 10.1002/jcp.1041170205. [DOI] [PubMed] [Google Scholar]
- Kuo C. L., Campbell J. L. Cloning of Saccharomyces cerevisiae DNA replication genes: isolation of the CDC8 gene and two genes that compensate for the cdc8-1 mutation. Mol Cell Biol. 1983 Oct;3(10):1730–1737. doi: 10.1128/mcb.3.10.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. L., Campbell J. L. Purification of the cdc8 protein of Saccharomyces cerevisiae by complementation in an aphidicolin-sensitive in vitro DNA replication system. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4243–4247. doi: 10.1073/pnas.79.14.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. S., Cheng Y. Human thymidylate kinase. Purification, characterization, and kinetic behavior of the thymidylate kinase derived from chronic myelocytic leukemia. J Biol Chem. 1977 Aug 25;252(16):5686–5691. [PubMed] [Google Scholar]
- Mariani B. D., Slate D. L., Schimke R. T. S phase-specific synthesis of dihydrofolate reductase in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4985–4989. doi: 10.1073/pnas.78.8.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews C. K., Slabaugh M. B. Eukaryotic DNA metabolism. Are deoxyribonucleotides channeled to replication sites? Exp Cell Res. 1986 Feb;162(2):285–295. doi: 10.1016/0014-4827(86)90335-6. [DOI] [PubMed] [Google Scholar]
- McNeil J. B., Friesen J. D. Expression of the Herpes simplex virus thymidine kinase gene in Saccharomyces cerevisiae. Mol Gen Genet. 1981;184(3):386–393. doi: 10.1007/BF00352510. [DOI] [PubMed] [Google Scholar]
- Newlon C. S., Ludescher R. D., Walter S. K. Production of petites by cell cycle mutants of Saccharomyces cerevisiae defective in DNA synthesis. Mol Gen Genet. 1979 Jan 31;169(2):189–194. doi: 10.1007/BF00271670. [DOI] [PubMed] [Google Scholar]
- Patterson M., Sclafani R. A., Fangman W. L., Rosamond J. Molecular characterization of cell cycle gene CDC7 from Saccharomyces cerevisiae. Mol Cell Biol. 1986 May;6(5):1590–1598. doi: 10.1128/mcb.6.5.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson G. R., Whalley J. M. Evolution of the herpes thymidine kinase: identification and comparison of the equine herpesvirus 1 thymidine kinase gene reveals similarity to a cell-encoded thymidylate kinase. Nucleic Acids Res. 1988 Dec 9;16(23):11303–11317. doi: 10.1093/nar/16.23.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild D., Brake A. J., Kiefer M. C., Young D., Barr P. J. Cloning of three human multifunctional de novo purine biosynthetic genes by functional complementation of yeast mutations. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2916–2920. doi: 10.1073/pnas.87.8.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser C. A., Steglich C., deWet J. R., Scheffler I. E. Cell cycle-dependent regulation of thymidine kinase activity introduced into mouse LMTK- cells by DNA and chromatin-mediated gene transfer. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1119–1123. doi: 10.1073/pnas.78.2.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani R. A., Fangman W. L. Thymidine utilization by tut mutants and facile cloning of mutant alleles by plasmid conversion in S. cerevisiae. Genetics. 1986 Nov;114(3):753–767. doi: 10.1093/genetics/114.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani R. A., Fangman W. L. Yeast gene CDC8 encodes thymidylate kinase and is complemented by herpes thymidine kinase gene TK. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5821–5825. doi: 10.1073/pnas.81.18.5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherley J. L., Kelly T. J. Regulation of human thymidine kinase during the cell cycle. J Biol Chem. 1988 Jun 15;263(17):8350–8358. [PubMed] [Google Scholar]
- Smith G. L., de Carlos A., Chan Y. S. Vaccinia virus encodes a thymidylate kinase gene: sequence and transcriptional mapping. Nucleic Acids Res. 1989 Oct 11;17(19):7581–7590. doi: 10.1093/nar/17.19.7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J. Y., Belmont L., Sclafani R. A. Genetic and molecular analysis of the SOE1 gene: a tRNA(3Glu) missense suppressor of yeast cdc8 mutations. Genetics. 1990 Mar;124(3):523–531. doi: 10.1093/genetics/124.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamiya N., Yusa T., Yamaguchi Y., Tsukifuji R., Kuroiwa N., Moriyama Y., Fujimura S. Co-purification of thymidylate kinase and cytosolic thymidine kinase from human term placenta by affinity chromatography. Biochim Biophys Acta. 1989 Mar 16;995(1):28–35. doi: 10.1016/0167-4838(89)90229-x. [DOI] [PubMed] [Google Scholar]
- White J. H., Green S. R., Barker D. G., Dumas L. B., Johnston L. H. The CDC8 transcript is cell cycle regulated in yeast and is expressed coordinately with CDC9 and CDC21 at a point preceding histone transcription. Exp Cell Res. 1987 Jul;171(1):223–231. doi: 10.1016/0014-4827(87)90265-5. [DOI] [PubMed] [Google Scholar]



