Abstract
Previous studies showed that LOW QUANTUM YIELD OF PHOTOSYSTEM II 1 (LQY1), a small thylakoid zinc finger protein was involved in maintenance and repair of Photosystem II (PSII). Here the author provide additional evidence for the role of LQY1 in PSII maintenance and repair and further commentary on the occurrence of LQY1 protein among land plants. After exposure to high light, Arabidopsis thaliana mutants lacking functional LQY1 gene (At1g75690) are more photoinhibited than wild-type control plants; display higher total non-photochemical quenching and photoinhibitory quenching. These results are consistent with the initial observation that lqy1 mutants have lower PSII efficiency than wild-type plants after high-light treatment. The low-PSII-efficiency phenotype can be suppressed upon complementation of lqy1 mutants with the LQY1 gene from wild-type plants. This further demonstrates that LQY1 is important in maintaining the activity of photosystem II in Arabidopsis. LQY1 homologs are present in land plants but are absent from sequenced genomes of green algae and cyanobacteria, which may reflect plant adaptation to excess light stress during the transition to land.
Keywords: adaptation, complementation, non-photochemical quenching, photoinhibitory quenching, photosystem II, PSII repair and reassembly, zinc finger
Sunlight is essential for plant’s growth and development. However, too much exposure can cause damage to the plants. Under high-light conditions, photosystem II (PSII) undergoes rapid damage and repair cycle to degrade and replace photodamaged reaction center proteins.1 This is a dynamic process that involves dozens of proteins and hundreds of cofactors.2 Recently, it was found that a thylakoid membrane zinc finger protein, LOW QUANTUM YIELD OF PSII 1 (LQY1), has protein disulfide isomerase activity and it interacts with PSII core monomer and CP43-less monomer (a marker for ongoing PSII repair).3 LQY1 is primarily localized in thylakoid grana margin and stroma lamellae, where critical steps of PSII repair and reassembly occur.3 Under high-light conditions, the lqy1-deficient mutants have faster degradation and synthesis of reaction center protein D1, less PSII-light harvesting complex II (LHCII), lower PSII efficiency, than wild type.3 These results suggest that LQY1 may act in maintenance and repair of photodamaged PSII complexes.3
In this paper, the author reports the non-photochemical quenching (NPQ) kinetics of lqy1 mutants, the characterization of lqy1 complementation lines, and phylogenetic analysis of LQY1 and homologs in other land plants. The results further demonstrate that lqy1 mutants are more prone to photoinhibition, and that the low-PSII-efficiency phenotype in lqy1 mutants is indeed caused by mutations in the LQY1 gene. Phylogenetic analysis of LQY1 and homologs suggests that LQY1 might be important to plant adaptation to excess light during the transition from water to land.
The lqy1 Mutants Are More Prone to Photoinhibition
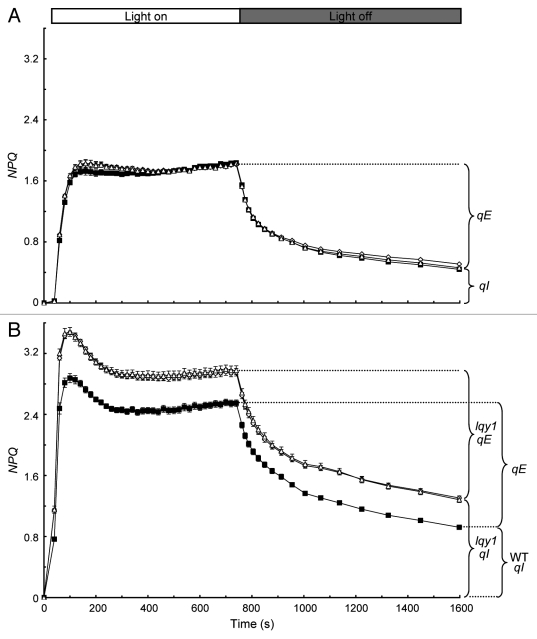
The initial characterization of lqy1 mutants showed that lqy1 mutants have much higher minimum fluorescence (F0) and much lower maximum PSII efficiency (Fv/Fm) than wild-type plants, after exposure to high light.3 This suggests that lqy1 mutants are more photoinhibited than wild-type plants under high light. To further test this hypothesis, the author monitored the induction and relaxation of NPQ before and after 3 h high-light (1,500 – 1,700 μmol photons m−2 s−1) treatment. Before high-light treatment, lqy1 mutants had similar, but not identical, induction and relaxation kinetics to wild-type plants (Fig. 1A). Induction of NPQ occurred during the 12 min exposure to actinic light (531 μmol photons m−2 s−1) and relaxation of NPQ occurred after actinic light was switched off. It is interesting that both lqy1 mutants had slightly higher NPQ values than wild-type plants during the first 6 min of actinic exposure (Student’s t-test, p < 0.05). However, this trend was not observed in the last 6 min of actinic exposure.
Figure 1.
Time courses for induction and relaxation of NPQ before (A) and after (B) 3 h high-light treatment. Measurements of chlorophyll fluorescence parameters were done on 4-week-old plants after 20 min of dark adaptation. Forty seconds after initial determination of F0 and Fm, actinic light (531 μmol photons m−2 s−1) was switched on for 715 sec. After termination of actinic light, relaxation of NPQ (the qE component of NPQ actually) was monitored for 840 sec. The remainder of NPQ primarily represents the qI component of NPQ. NPQ values in (B) were calculated with Fm determined before the high-light (1,500 – 1,700 μmol photons m−2 s−1) treatment. Data for Col wild-type (closed squares), lqy1–1 (open diamonds) and lqy1–2 (open triangles) plants are presented as mean ± SE (n = 8).
The difference in NPQ kinetics between lqy1 mutants and wild-type plants are much more pronounced after treating the plants under high light for 3 h (Fig. 1B). Throughout the 12 min actinic exposure, the lqy1 mutants had much higher NPQ values than the wild-type control plants (Student’s t-test, p < 0.05). After the actinic light was turned off, NPQ in lqy1 mutants and wild-type plants relaxed at similar rate. This is consistent with the similar energy-dependent quenching (qE) in lqy1 mutants and wild-type plants, as reported previously.3 The remainder of NPQ mostly represents photoinhibitory quenching (qI).4,5 The lqy1 mutants demonstrated a much larger portion of NPQ that was not relaxed during the 14 min dark period (Fig. 1B), consistent with reportedly higher qI in lqy1 mutants.3
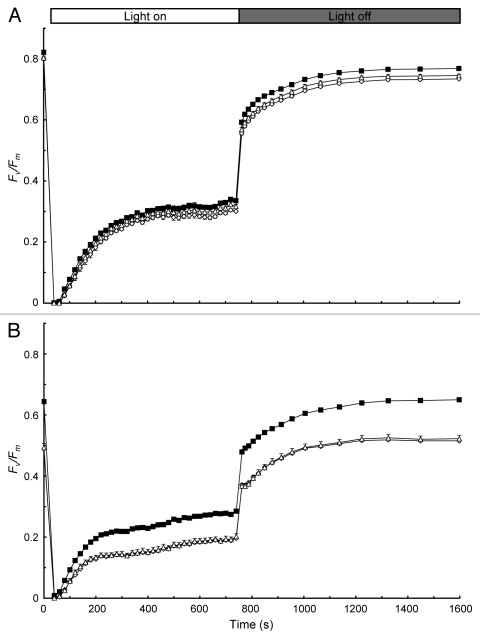
The author also monitored the changes in Fv/Fm during NPQ induction and relaxation kinetics before and after 3 h high-light treatment (Fig. 2). Consistent with the initial mutant characterization,3 the lqy1 mutants have slightly lower Fv/Fm than wild-type plants before the 3 h high-light exposure, and they have much lower Fv/Fm than wild-type plants after the 3 h high-light exposure (Fig. 2). The data from the time course of NPQ kinetics further demonstrated that the lqy1 mutants are more prone to high-light-induced photoinhibition, and that PSII in lqy1 mutants is less efficient that wild-type plants under high light.
Figure 2.
Fv/Fm during the induction and relaxation of NPQ before (A) and after (B) 3 h high-light treatment. Measurements of chlorophyll fluorescence parameters were done as described in Figure 1 legend. Data for Col wild-type (closed squares), lqy1–1 (open diamonds) and lqy1–2 (open triangles) plants are presented as mean ± SE (n = 8).
It is worth mentioning that the NPQ induction curves in high-light treated samples exhibit a complex shape with a quick increase followed by a partial relaxation to steady-state during actinic light illumination (Fig. 1B). Similar behavior has been observed in high-light-acclimated Physcomitrella patens.6 This transient relaxation of quenching during the induction of NPQ may be attributed to the activation of the Calvin-Benson cycle, which consumes ATP and reduces ΔpH.6
The Low-PSII-Efficiency Phenotype in lqy1 Mutants can be Suppressed by Complementation
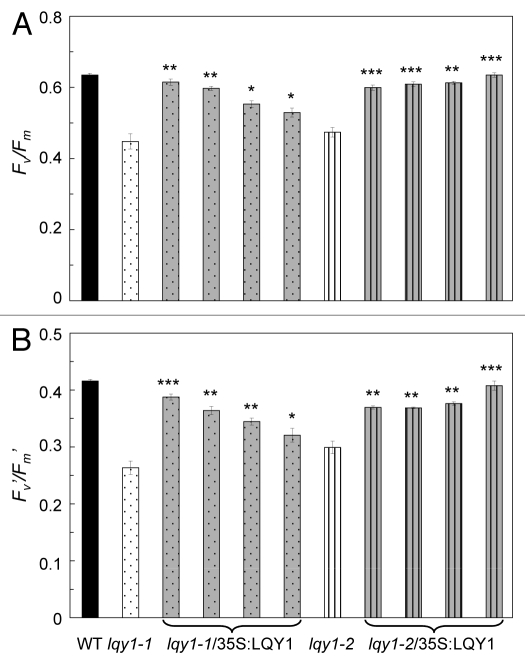
As mentioned above, PSII in lqy1 mutants is not as efficient as wild-type plants under high light irradiance. To test whether the low-PSII-efficiency phenotype in lqy1 mutants is caused by mutations in the LQY1 gene, wild-type LQY1 gene or empty vector was introduced into lqy1–1 and lqy1–2 mutants. The T2 generation transformants were treated under high light (1,500 – 1,700 μmol photons m−2 s−1) for 3 h and their PSII efficiency analyzed by chlorophyll fluorescence (Fig. 3). Complementing lqy1 mutants with wild-type LQY1 gene significantly increased Fv/Fm, which was measured in dark-adapted leaves. Similar results were observed in maximum PSII efficiency during actinic exposure, Fv’/Fm’. The four representative complementation lines in either lqy1–1 or lqy1–2 background show a wide range of efficiency, from partial complementation to complete complementation. This experiment confirms that mutations in the LQY1 gene are the cause of the low-PSII-efficiency phenotype in lqy1 mutants and that LQY1 plays an important role in regulating PSII activity, especially under high-light stress conditions.
Figure 3.
PSII efficiency of lqy1 complementation lines. Fv/Fm (A) was measured after 20 min of dark adaptation. Fv’/Fm’ (B) was measured after 5 min of actinic exposure at 531 μmol photons m−2 s−1. Data from T2 generation are presented as mean ± SE (n = 4). Each bar represents a single insertion event at T1 generation. Black bars, wild-type empty vector control; white dotted bars, lqy1–1 empty vector control; gray dotted bars, representative complementation lines in lqy1–1 background; white striped bars, lqy1–2 empty vector control; gray striped bars, representative complementation lines in lqy1–2 background. The asterisks indicate significant difference between lqy1 complementation lines and the corresponding lqy1 mutant (Student’s t-test, *, p < 0.05; **, p < 0.01; ***, p < 0.001).
Phylogenetic Analysis of LQY1 Protein and its Homologs in Other Land Plants
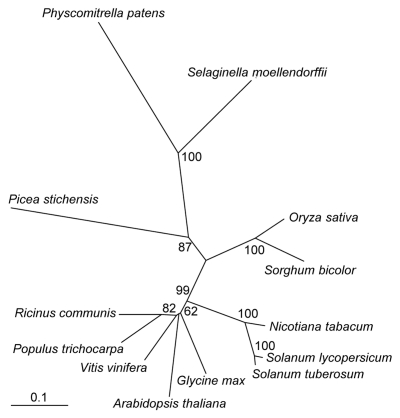
LQY1 homologs are found in the sequenced genomes of other land plants, for example, bryophyte moss Physcomitrella patens, lycopod Selaginella moellendorffii, gymnosperm Picea stichensis, monocots Oryza sativa and Sorghum bicolor, and eudicots (Fig. 4). However, LQY1 homologs are not found in either cyanobacteria or algae.3 This may due to the differences in habitat conditions between land plants and their ancestor green algae. For example, both land plants and algae can suffer from photoinhibition when they are exposed to extreme conditions, such as heat, low temperature and excess light.7 Because the ocean has higher heat capacity than the atmosphere, algae are not exposed to as much heat stress as land plants. The immobile life form of land plants also prevent escape from rapid fluctuation of light intensity by swimming away or deeper, a typical algae behavior.8 The combination of these conditions requires land plants to use a more efficient system to mitigate the damage of photoinhibition.
Figure 4.
Phylogenetic analysis of LQY1 protein and its homologs in other land plants. Multiple alignments and phylogenetic reconstruments were performed by the ClustalX and PHYLIP 3.68 programs. Bootstrap values (1,000 replicates) above 50% are given on branches. Bar = 0.1 amino acid substitutions.
The present work provided addition support for the role of LQY1 in maintaining the activity of PSII, especially under high-light stress conditions. NPQ kinetics show that lqy1 mutants have much higher total NPQ and qI, and much lower Fv/Fm than wild-type plants (Figs. 1–2). Complementation experiments demonstrate that the low-PSII-efficiency phenotype of lqy1 mutants can be rescued by introducing wild-type LQY1 gene into the mutant backgrounds (Fig. 3). Phylogenetic analysis of LQY1 and homologs suggests that LQY1 might be important during the transition of plants from water to land (Fig. 4). Further investigation may lead to the identification of the direct interacting partner(s) of LQY1 and a more detailed understanding of the process and regulation of PSII repair and reassembly.
Materials and Methods
Complementation of lqy1 Mutants
A full-length LQY1 gene containing a 3′-untranslated region was amplified using Col-0 wild type Arabidopsis leaf DNA, Pfu DNA polymerase (Promega) with a forward primer 5′- ACACATCTAGAATGCCAGTTTCAGCTCCATC-3′ (XbaI site underlined) and a reverse primer 5′-ACACAGGATCCAAGTACAAGAAGAAACATTT-3′ (BamHI site underlined). The resulting PCR product was AT cloned into a pGEM-T vector and sequenced to check for errors. An XbaI/BamHI-digested LQY1 fragment was subcloned into a binary vector as previously described.9,10 The modified binary vector containing the LQY1 gene was transformed into lqy1–1 and lqy1–2 mutants by Argobacterium tumefaciens-mediated floral-dip method.11 Gentamycin-resistant plants were selected at the T1 generation and genotyped to verify transformation.
Chlorophyll fluorescence analysis
NPQ kinetics of wild-type and lqy1 mutant plants were determined as described previously.3NPQ was calculated using the following equation: NPQ = (Fm - Fm’)/Fm’. For Fv/Fm and Fv’/Fm’ measurements of wild-type and lqy1 complementation lines, initial determination of Fo and Fm was done by the application of a saturation pulse (2,800 μmol photons m−2 s−1) after 20 min of dark adaptation, as described previously.12,13 After a 40 sec delay, the actinic light (531 μmol photons m−2 s−1) was switched on for 360 sec. At the end of actinic exposure, another saturation pulse was applied and Fo’ and Fm’ determined. Actinic illumination, saturation pulse and measuring light were provided by an array of 44 high-power royal blue (450 nm) LED-lamps equipped with collimating optics. The intensity of actinic illumination was carefully chosen according to the quality of the light source and the performance of Arabidopsis plants at that light intensity.14Fv/Fm and Fv’/Fm’ were calculated using the following equations: Fv/Fm = (Fm - Fo)/Fm; Fv’/Fm’ = (Fm’ - Fo’)/Fm’.
Phylogenetic analysis
Multiple alignments of protein sequences were performed by the ClustalX program (www.clustal.org). Phylogenetic reconstructions were performed using PHYLIP 3.68 (http://evolution.genetics.washington.edu/phylip.html). Protein distance matrixes were calculated with the Jones-Taylor-Thornton model using the PROTDIST program. An unrooted tree was generated with the neighbor-joining method using the NEIGHBOR program. 1000 trials of bootstrapping were performed and the consensus tree computed. The accession numbers for LQY1 and homologs were as described previously.3
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by U.S. National Science Foundation 2010 Project Grant MCB-0519740 and Western Michigan University startup fund. The author thanks Cheng Peng at Michigan State University for participation in chlorophyll fluorescence analysis on the rescue lines.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18022
References
- 1.Demmig-Adams B, Adams WW. Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:599–626. doi: 10.1146/annurev.pp.43.060192.003123. [DOI] [Google Scholar]
- 2.Mulo P, Sirpiö S, Suorsa M, Aro EM. Auxiliary proteins involved in the assembly and sustenance of photosystem II. Photosynth Res. 2008;98:489–501. doi: 10.1007/s11120-008-9320-3. [DOI] [PubMed] [Google Scholar]
- 3.Lu Y, Hall DA, Last RL. A small zinc finger thylakoid protein plays a role in maintenance of photosystem II in Arabidopsis thaliana. Plant Cell. 2011;23:1861–75. doi: 10.1105/tpc.111.085456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Müller P, Li XP, Niyogi KK. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001;125:1558–66. doi: 10.1104/pp.125.4.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krause GH, Jahns P. Pulse amplitude modulated chlorophyll fluorometry and its application in plant science. In Green BR, Parson WW, ed. Light-Harvesting Antennas in Photosynthesis. Dordrecht, The Netherlands: Kluwer Academic Publishers 2003:373-99. [Google Scholar]
- 6.Gerotto C, Alboresi A, Giacometti GM, Bassi R, Morosinotto T. Role of PSBS and LHCSR in Physcomitrella patens acclimation to high light and low temperature. Plant Cell Environ. 2011;34:922–32. doi: 10.1111/j.1365-3040.2011.02294.x. [DOI] [PubMed] [Google Scholar]
- 7.Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI. Photoinhibition of photosystem II under environmental stress. Biochim Biophys Acta. 20071767:414–21. doi: 10.1016/j.bbabio.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Alboresi A, Gerotto C, Giacometti GM, Bassi R, Morosinotto T. Physcomitrella patens mutants affected on heat dissipation clarify the evolution of photoprotection mechanisms upon land colonization. Proc Natl Acad Sci USA. 2010;107:11128–33. doi: 10.1073/pnas.1002873107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu Y, Steichen JM, Yao J, Sharkey TD. The role of cytosolic α-glucan phosphorylase in maltose metabolism and the comparison of amylomaltase in Arabidopsis and Escherichia coli. Plant Physiol. 2006;142:878–89. doi: 10.1104/pp.106.086850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang SC, Fernandez DE. Effect of regulated overexpression of the MADS domain factor AGL15 on flower senescence and fruit maturation. Plant Physiol. 2002;130:78–89. doi: 10.1104/pp.004721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–43. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 12.Lu Y, Savage LJ, Ajjawi I, Imre KM, Yoder DW, Benning C, et al. New connections across pathways and cellular processes: industrialized mutant screening reveals novel associations between diverse phenotypes in Arabidopsis. Plant Physiol. 2008;146:1482–500. doi: 10.1104/pp.107.115220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu Y, Savage LJ, Last RL. Chloroplast phenomics: systematic phenotypic screening of chloroplast protein mutants in Arabidopsis. In Jarvis RP, ed. Methods in Molecular Biology. Iotowa: Humana Press 2011; 775, Part 2:161-85. [DOI] [PubMed] [Google Scholar]
- 14.Johnson MP, Pérez-Bueno ML, Zia A, Horton P, Ruban AV. The zeaxanthin-independent and zeaxanthin-dependent qE components of nonphotochemical quenching Involve common conformational changes within the photosystem II antenna in Arabidopsis. Plant Physiol. 2009;149:1061–75. doi: 10.1104/pp.108.129957. [DOI] [PMC free article] [PubMed] [Google Scholar]