Abstract
In flowering plants, seed development and seed filling are intricate genetically programmed processes that correlate with changes in metabolite levels and that are spatially and temporally regulated by a complex signaling network mediated mainly by sugars and hormones. ASIL1, a member of the plant-specific trihelix family of DNA-binding transcription factors, was isolated based on its interaction with the GT-element of the Arabidopsis thaliana 2S albumin At2S3 promoter. Mutation of ASIL1 derepressed expression of a subset of embryonic genes resulting in accumulation of 2S albumin and embryo-specific lipids in leaves.1 It was recently reported that mutation of ASIL1 led to early embryo development in Arabidopsis.2 In this study, we demonstrated that ASIL1 acts as a temporal regulator of seed filling. In developing siliques, mutation of ASIL1 led to earlier expression of master regulatory genes LEC2, FUS3 and ABI3 as well as genes for seed storage reserves. Moreover, the 12S globulin accumulated to a much higher level in the developing seeds of asil1–1 compared with wild type. To our knowledge, this is the first evidence that ASIL1 not only functions as a negative regulator of embryonic traits in seedlings but also contributes to the maintenance of precise temporal control of seed filling. Thus, ASIL1 represents a novel component of the regulatory framework controlling embryonic gene expression in Arabidopsis.
Keywords: Arabidopsis, ASIL1, regulation, seed development, seed filling
Seeds offer plants a unique opportunity to suspend their life cycles in a desiccated state. This enables them to endure adverse environmental conditions and then resume growth using endogenous storage reserves when more favorable conditions develop. Seed formation is an intricate genetically programmed process that is correlated with changes in metabolite levels and is regulated by a complex signaling network mediated mainly by hormone and sugar levels.3,4 Seed development can be divided into two stages, embryo morphogenesis and maturation. The hallmarks of maturation include storage compound accumulation, acquisition of desiccation tolerance, growth arrest and entry into a dormancy period that is broken upon germination.5 In Arabidopsis, three members of the B3 family of transcription factors, LEAFY COTYLEDON (LEC) 2, ABSCISIC ACID-INSENTITIVE 3 (ABI3) and FUSCA 3 (FUS3) as well as a fourth regulator, a HAP3 subunit of the CCAAT-box binding transcription factor (CBF) LEC1, are master regulators of seed maturation processes. A redundant gene regulatory network linking these regulators was elucidated by examining the expression of ABI3, FUS3 and LEC2 in abi3, fus3, lec1 and lec2 single, double and triple mutants.6 In combination with ABA, GA, auxin and sugar signaling, this regulatory network governs most seed-specific maturation traits in a partially redundant manner.6
The regulatory networks controlling the seed maturation program in flowering plants are repressed prior to germination so that seed storage reserves do not accumulate during vegetative development. A number of regulatory proteins have been implicated in these regulatory networks.7 For example, ASIL1, a protein characteristic of the plant-specific trihelix family of transcription factors, plays a critical role in preventing expression of some of the foremost embryonic and seed maturation genes in seedlings. The seedlings of asil1 mutants exhibit a global shift in gene expression to resemble late embryogenesis. Consistent with this, asil1 seedlings accumulate 2S albumin and oil comprised of fatty acids similar to that of seed-derived lipid.1
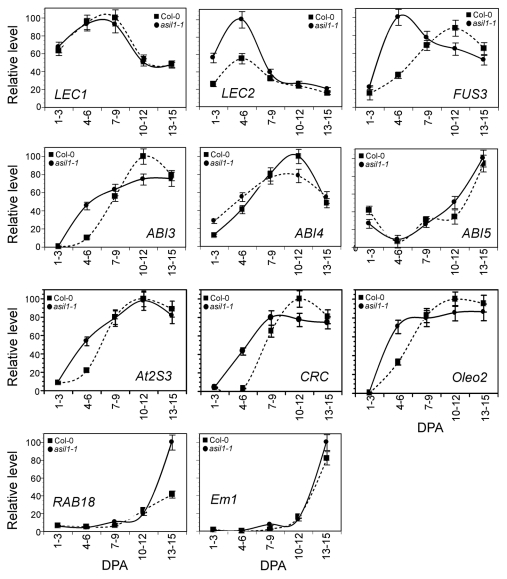
Flowering plants require proper timing for control of their development. This requires the correct timing of cell division and a change in the balance of expression of numerous genes.8 How developmental timing is controlled during seed development and seed filling is still not well understood. ASIL1 expression is dynamically regulated during seed development and relatively high levels of ASIL1 transcript accumulated during early- and mid-embryogenesis.1 Recently, ASIL1 was found to be involved in the temporal regulation of embryo maturation in Arabidopsis in a partially redundant manner.2 To examine whether ASIL1 is involved in the regulation of seed filling, we compared changes in the expression of seed-specific genes in wild-type and asil1–1 mutant plants1 by real-time RT-PCR analysis with RNA isolated from siliques corresponding to five different stages of silique development: early-embryogenesis I (1–3 DPA), early-embryogenesis II (4–6 DPA), mid-embryogenesis (7–9 DPA), late-embryogenesis I (10–12 DPA) and late-embryogenesis II (13–15 DPA). Seeds were mature and completely dry after 15 DPA. As shown in Figure 1, of five classes of seed maturation-related genes examined, RAB18 (a Group II LEA or dehydrin) showed increased expression during the very late stages of embryogenesis in the asil1 mutant, whereas AtEm1 (Group I LEA genes) had little or no change in expression. In contrast, the three classes of storage reserve genes examined (the 2S albumin At2S3, the 12S globulin CRC, and the oleosin gene Oelo2) were expressed during early-embryogenesis either earlier (CRC) or at higher levels (At2S3 and Oelo2) in asil1 than in the developing seeds of wild-type plants. The transcripts of At2S3 reached a maximum at 10–12 DPA in both wild-type and asil1 plants, whereas CRC mRNAs were detectable at early-embryogenesis in asil1, 3 DPA earlier than in wild type. The At2S3 and Oelo2 transcripts in asil1–1 were at least 2-fold more abundant at 4–6 DPA than in wild-type plants. This result suggests that developing asil1 seeds accumulate more storage reserves than wild type. In addition, as shown in Figure 1, At2S3 mRNAs accumulated at 4–6 DPA in wild-type plants whereas no transcript was detected at the same stages of silique development, indicating that the synthesis of albumin is earlier than that of globulin in Arabidopsis seeds. This difference in the expression pattern between At2S3 and CRC is consistent with the study in developing rape seed embryos (Brassica napus) whereby the synthesis of napin started a few days earlier than that of cruciferin.9
Figure 1.
Temporal effect of ASIL1 mutation on expression of seed maturation genes in developing seeds. Total RNA was isolated from siliques of wild type (Col-0) and asil1–1 corresponding to five developmental stages as indicated (DPA) and subjected to real-time RT-PCR analysis with ACT2 mRNA as an internal reference and gene-specific primers as previously described.1 The mean and sd from qRT-PCR analysis were determined from three biological replicates of either Col-0 or asil1–1.
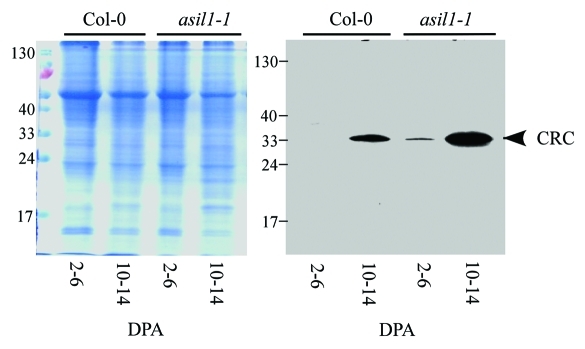
To further corroborate the finding that accumulation of seed storage proteins is influenced by disruption of the ASIL1 gene, we compared the 12S CRC accumulation profiles in asil1–1 and wild-type plants by immunoblot analysis. Proteins were extracted from siliques corresponding to stages 2–6 DPA and 10–14 DPA and subjected to immunoblot analysis with a monoclonal antibody raised against CRC. The globulin accumulated to a much higher level (approximately 5-fold at 10–14 DPA as analyzed by densitometry10) in the developing seeds of asil1–1 compared with wild type (Fig. 2). These results demonstrate that ASIL1 is important for regulating the timing of seed storage product synthesis in Arabidopsis.
Figure 2.
Accumulation of the 12S globulin cruciferin in developing seeds of asil1 mutant. Total protein extracts were prepared from siliques of wild type (Col-0) and asil1–1 mutant plants corresponding to stages 2–6 DPA and 10–14 DPA. An equal amount of protein (10 µg) was loaded in each lane and subjected to SDS-PAGE followed by either Coomassie brilliant blue staining (left) or immunoblot analysis with monoclonal anti-12S cruciferin (CRC) antibody (right) as described previously.1
To elucidate the mechanism underlying the regulation of seed filling by ASIL1 during seed maturation, we examined the effects of asil1 mutation on the transcript levels of master regulatory genes affecting seed development programs. Total RNA was extracted from siliques at different developmental stages and subjected to real-time RT-PCR analysis. Similar to the previous gene expression analysis in wild-type plants,11-13 LEC1 and LEC2 transcripts were found to be abundant at early-embryogenesis stages, FUS3, ABI3 and ABI4 expression was at high levels from mid- to late-embryogenesis, whereas ABI5 was mostly expressed at late-embryogenesis stages (Fig. 1). In asil1–1 mutant plants, transcript levels of LEC1 and ABI5 were essentially identical to wild-type whereas ABI4 expression had increased at early-embryogenesis and decreased after mid-embryogenesis. In contrast, abundance of the LEC2 and ABI3 transcripts at early-embryogenesis was at least 2-fold higher in asil1–1 than in wild-type plants. Furthermore, FUS3 transcript peaked 4 d earlier in asil1 than in wild type (4–6 DPA vs. 10–12 DPA) (Fig. 1).
In conclusion, precise temporal regulation of gene expression is required for proper seed maturation. Understanding the timing mechanisms of seed development and seed filling has become an important goal for many plant biologists. We demonstrated that ASIL1 temporally regulates the expression of genes encoding the B3 family of master regulators (LEC2, FUS3 and ABI3) as well as seed storage protein (SSP) (2S albumin and 12S globulin) and oleosin genes. This indicates that ASIL1 contributes to the maintenance of the seed maturation program. We propose a model for the participation of ASIL1 in the negative regulation of SSP and oleosin genes during seed maturation through LEC2-FUS3/ABI3 signaling. ASIL1 negatively controls these genes either by interacting with DNA-binding elements to directly repress transcription of SSP and oleosin genes or by repressing the B3 regulators so that transcription of SSP and oleosin genes is inhibited indirectly.
Acknowledgments
We are grateful to the ABRC for distribution of Arabidopsis T-DNA insertion lines. This work was supported by a grant from the Agriculture and Agri-Food Canada -Canadian Crop Genomics Initiative.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18709
References
- 1.Gao M-J, Lydiate DJ, Li X, Lui H, Gjetvaj B, Hegedus DD, et al. Repression of seed maturation genes by a trihelix transcriptional repressor in Arabidopsis seedlings. Plant Cell. 2009;21:54–71. doi: 10.1105/tpc.108.061309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willmann MR, Mehalick AJ, Packer RL, Jenik PD. MicroRNAs regulate the timing of embryo maturation in Arabidopsis. Plant Physiol. 2011;155:1871–84. doi: 10.1104/pp.110.171355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber H, Borisjuk L, Wobus U. Molecular physiology of legume seed development. Annu Rev Plant Biol. 2005;56:253–79. doi: 10.1146/annurev.arplant.56.032604.144201. [DOI] [PubMed] [Google Scholar]
- 4.Holdsworth MJ, Bentsink L, Soppe WJ. Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytol. 2008;179:33–54. doi: 10.1111/j.1469-8137.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- 5.Harada JJ. Signaling in plant embryogenesis. Curr Opin Plant Biol. 1999;2:23–7. doi: 10.1016/S1369-5266(99)80005-3. [DOI] [PubMed] [Google Scholar]
- 6.To A, Valon C, Savino G, Guilleminot J, Devic M, Giraudat J, et al. A network of local and redundant gene regulation governs Arabidopsis seed maturation. Plant Cell. 2006;18:1642–51. doi: 10.1105/tpc.105.039925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao M-J, Gropp G, Wei S, Hegedus DD, Lydiate DL. Combinatorial networks regulating seed development and seed filling. In: KI Sato, ed, Embryogenesis. Rijeka, Croatia: InTech, 2012; ISBN 979-953-307-439-8. [Google Scholar]
- 8.Raz V, Bergervoet JH, Koornneef M. Sequential steps for developmental arrest in Arabidopsis seeds. Development. 2001;128:243–52. doi: 10.1242/dev.128.2.243. [DOI] [PubMed] [Google Scholar]
- 9.Höglund AS, Rödin J, Larsson E, Rask L. Distribution of napin and cruciferin in developing rape seed embryos. Plant Physiol. 1992;98:509–15. doi: 10.1104/pp.98.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao M-J, Hegedus DD, Sharpe AG, Robinson SJ, Lydiate DJ, Hannoufa A. Isolation and characterization of a GCN5-interacting protein from Arabidopsis thaliana. Planta. 2007;225:1367–79. doi: 10.1007/s00425-006-0446-2. [DOI] [PubMed] [Google Scholar]
- 11.Parcy F, Valon C, Raynal M, Gaubier-Comella P, Delseny M, Giraudat J. Regulation of gene expression programs during Arabidopsis seed development: roles of the ABI3 locus and of endogenous abscisic acid. Plant Cell. 1994;6:1567–82. doi: 10.1105/tpc.6.11.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lotan T, Ohto M, Yee KM, West MAL, Lo R, Kwong RW, et al. Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell. 1998;93:1195–205. doi: 10.1016/S0092-8674(00)81463-4. [DOI] [PubMed] [Google Scholar]
- 13.Stone SL, Kwong LW, Yee KM, Pelletier J, Lepiniec L, Fischer RL, et al. LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proc Natl Acad Sci U S A. 2001;98:11806–11. doi: 10.1073/pnas.201413498. [DOI] [PMC free article] [PubMed] [Google Scholar]