Abstract
“Bulb” is a mobile and complex structure appearing in vacuolar membrane of plant cell. We recently reported new fluorescent marker lines for bulbs and bulb-less mutants. We tried multicolor visualization of vacuolar membrane to show distinct segregation of bulb-positive protein (γTIP or AtVAM3) and bulb-negative protein (AtRab75). Unexpectedly, GFP-AtRab75 resulted to localize in bulb under the condition of co-expression with TagRFP-AtVAM3. The signal intensities of GFP-AtRab75 and TagRFP-AtVAM3 were quantified and compared. The result indicates that TagRFP-AtVAM3 is concentrated in bulb than GFP-AtRab75.
Keywords: AtRab75, AtVam3, plant growth, Rab-GTPase, SNARE, vacuolar membranes, “bulb”
The plant vacuole accomplishes a variety of functions and is essential for plant growth and development. The vacuolar membrane of plant cells has a very complex three-dimensional configuration with dynamic features. We previously identified a “bulb” on the vacuolar membrane of growing Arabidopsis cotyledon.1,2 A bulb is a complex, mobile structure that forms on the continuous vacuolar membrane. Our results suggested that γ-TIP (TIP1;1) -GFP molecules were concentrated in bulbs; in contrast, GFP-AtRab75 (AtRABG3b) molecules were segregated (Figs. 1C and 2A and B). These results indicate that qualitative differences between bulbs and other vacuolar membranes (termed the “peripheral vacuolar membrane” hereafter) may exist. To date, the mechanisms underlying the biogenesis and physiological function of bulb structures remain unclear. To determine the biological significance of bulbs, we have addressed several lines of inquiry. We sought further structural evidence of bulbs; clues to its function; and direct evidence for a qualitative difference between the bulb membrane and the vacuolar membrane. Further structural evidence of bulbs has been provided from several previous studies that demonstrated the existence of bulb-like structures in a broad spectrum of tissue types and plant species.3-9 We also previously searched other markers associated with bulbs (Saito et al. 2011, Fig. 1C), based on the expression of own-promoter-driven GFP-VAM3, GFP-VTI11, and Pro35S-YFP-2xFYVE in bulbs. In addition, the cytoplasm of some bulbs was visualized with the expression of Pro35S-GFP. To elucidate the function of bulbs, we identified mutants with a bulb-less phenotype. We reasoned that a mutant with abnormally shaped vacuolar membranes may also be deficient in bulbs. Next, we found that some shoot gravitropism (sgr) mutants were nearly devoid of bulbs in broad-type tissues, based on the expression patterns of own-promoter-driven GFP-VAM3, Pro35S YFP-2xFYVE, and Pro35SGFP.10 We also provided direct evidence for a qualitative difference between the vacuolar membrane and the bulb membrane by visualizing the vacuolar membrane in a single cell with different colored markers, GFP-AtRab75 and TagRFP-Vam3. In this paper, we describe some unexpected results from this dual-color study of vacuolar membranes, the interesting conclusions that arose, and future perspectives.
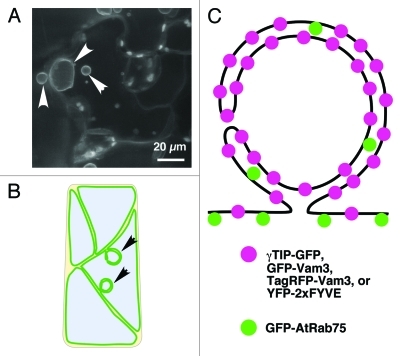
Figure 1. Structural evidence for bulbs based on the expression of other markers in plant cells. (A) Bulbs observed in Arabidopsis cotyledon. Confocal laser scanning microscopic images. White arrowheads indicate bulbs. (B) Schematic illustration of vacuolar membrane in plant cells. Black arrowheads indicate bulbs. (C) Model of a bulb.
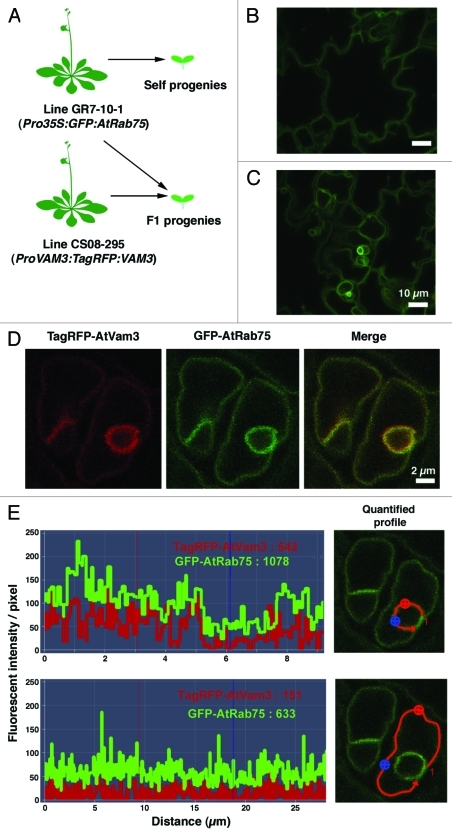
Figure 2. Dual color visualization of vacuolar membranes. (A) The generation of transgenic plants that express tagged proteins. (Top) Control self propagation is shown for the GR7–10–1 (Saito et al. 2002) plants. (Bottom) Cross-fertilization produced progeny that expressed both TagRFP:VAM3 and GFP:AtRab75. (B) Confocal laser scanning microscopic image of 3DAG cotyledon epidermal cells of plants produced by self progeny of GR7-10-1. (C) Confocal laser scanning microscopic image of 3DAG cotyledon epidermal cells of the F1 progeny of a cross between GR7-10-1 and ProVAM3:TagRFP:VAM3 marker plant lines. (D) Confocal laser scanning microscopic images of guard cells of the F1 progeny of a cross between GR7-10-1 and ProVAM3:TagRFP:VAM3 marker lines. (E) Quantification of fluorescent intensity/pixel in bulb membranes and peripheral vacuolar membranes. The areas quantified are indicated with red lines in the microscopic images on the right.
Dual-color visualization of continuous vacuolar membranes with the co-expression of GFP-AtRab75 and TagRFP-Vam3
In our original report, we showed that γ-TIP-GFP expression could be used to visualize bulbs, but GFP-AtRab75 could not. However, in plants that expressed GFP-AtRab75, electron microscopy showed that the bulb structures existed.1 To obtain direct evidence of qualitative differences between bulbs and peripheral vacuolar membrane, we introduced the ProVAM3:TagRFP:VAM3 gene11 into transgenic Pro35S-GFP-AtRab75 plants (GR7-10-1)1 by cross-fertilization (Fig. 2A, B). The F1 progenies co-expressed TagRFP-Vam3 and GFP-AtRab75 (Fig. 2C). Unexpectedly, confocal laser scanning microscopy showed that, when co-expressed with TagRFP-Vam3, GFP-AtRab75 was also detected in bulbs (Fig. 2C). We then tested other conditions; e.g., the expression γTIP-GFP and Venus-AtRab75 with a dual site gateway binary vector, or direct transformation of GR7-10-11 plants with a construct that harbored γTIP-mRFP with other drug-related marker. In those conditions, the AtRab75 signal was also detected in the bulb (data not shown). We speculated that characteristics or contents of bulb membrane may change under the condition of the overexpression of membrane proteins (e.g., TagRFP-Vam3 or γTIP-mRFP).
TagRFP-Vam3 is More Concentrated than GFP-AtRab75 in the Bulb
We noticed that the bulb membrane often appeared to be a deeper yellow than the peripheral vacuolar membrane when labeled with the dual color, TagRFP-AtVam3 and GFP-AtRab75 (Fig. 2D). This suggested that TagRFP-AtVam3 might be more concentrated in the bulb than GFP-AtRab75. To investigate this notion, we compared the fluorescence intensity/pixel of two markers that localized to the bulbs and to the peripheral vacuolar membrane (Fig. 2E). In the bulb, the average fluorescent intensity/pixel was 542 for TagRFP-AtVam3 and 1078 for GFP-AtRab75 (Fig. 2E, top). In the peripheral vacuolar membrane, the average fluorescent intensity/pixel was 151 for TagRFP-AtVam3 and 633 for GFP-AtRab75 (Fig. 2E, bottom). The ratio of the bulb: peripheral vacuolar membrane fluorescent intensity/pixel was 542/151 = 3.6 for TagRFP-AtVam3 and 1078/633 = 1.7 for GFP-AtRab75. This suggested that TagRFP-AtVam3 was more concentrated than GFP-AtRab75 in the bulb. The bulb comprised two layers of vacuolar membranes. Thus, the fluorescent molecules that were distributed evenly over the both vacuolar membranes would emit a signal with approximately twice the intensity as the molecules that were only distributed over one of the membranes. Although the conditions in this study were artificial, this suggested that TagRFP-AtVam3 was concentrated in the bulb than GFP-AtRab75.
Conclusion and Perspectives
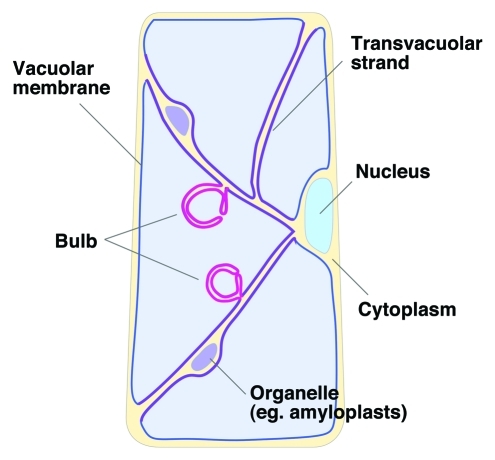
We propose that the complex configuration of vacuolar membranes may serve a specialized function. Our schematic model of the vacuolar subdomain is shown in Figure 3. In a recent report, we proposed that two independent mechanisms would be needed to maintain vacuolar integrity; this was based on the slightly different phenotypes of two bulb-less mutants, zig/atvti11 and sgr2. The zig/atvti11 mutant had no bulb structure and also had no transvacuolar strands; in contrast, sgr2–1 had no bulb structure, but maintained transvacuolar strands. This suggested that the bulb biogenesis pathway might include two critical steps, one related, and the other unrelated, to the maintenance of transvacuolar strands. Furthermore, transvacuolar strands may form a distinct subregion within the vacuolar membrane (Fig. 3), because the membrane curvature of a transvacuolar strand is clearly different from the curvatures of other vacuolar membranes. The bulb curvature is also different from the curvatures of peripheral vacuolar membranes or transvacuolar strands; thus, we also speculate that bulbs have an unknown function that depends on the recruitment of specific molecules. Further studies of the molecular basis, function, and biogenesis of bulbs will be necessary to understand what physiological roles bulbs play in the lifecycle of plants.
Figure 3.
Schematic model of the vacuolar subdomain.
Glossary
Abbreviations:
- SNARE
soluble N-ethyl-maleimide sensitive factor attachment receptor proteins
- DAG
day after germination
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18061
References
- 1.Saito C, Ueda T, Abe H, Wada Y, Kuroiwa T, Hisada A, et al. A complex and mobile structure forms a distinct subregion within the continuous vacuolar membrane in young cotyledons of Arabidopsis. Plant J. 2002;29:245–55. doi: 10.1046/j.0960-7412.2001.01189.x. [DOI] [PubMed] [Google Scholar]
- 2.Saito CA. “bulb” subregion in the vacuolar membrane. Plant Morphology. 2003;15:60–7. [Google Scholar]
- 3.Uemura T, Yoshimura SH, Takeyasu K, Sato MH. Vacuolar membrane dynamics revealed by GFP-AtVam3 fusion protein. Genes Cells. 2002;7:743–53. doi: 10.1046/j.1365-2443.2002.00550.x. [DOI] [PubMed] [Google Scholar]
- 4.Escobar NM, Haupt S, Thow G, Boevink P, Chapman S, Oparka K. High-throughput viral expression of cDNA-green fluorescent protein fusions reveals novel subcellular addresses and identifies unique proteins that interact with plasmodesmata. Plant Cell. 2003;15:1507–23. doi: 10.1105/tpc.013284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hicks GR, Rojo E, Hong S, Carter DG, Raikhel NV. Geminating pollen has tubular vacuoles, displays highly dynamic vacuole biogenesis, and requires VACUOLESS1 for proper function. Plant Physiol. 2004;134:1227–39. doi: 10.1104/pp.103.037382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vera-Estrella R, Barkla BJ, Bohnert HJ, Pantoja O. Novel regulation of aquaporins during osmotic stress. Plant Physiol. 2004;135:2318–29. doi: 10.1104/pp.104.044891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reisen D, Marty F, Leborgne-Castel N. New insights into the tonoplast architecture of plant vacuoles and vacuolar dynamics during osmotic stress. BMC Plant Biol. 2005;5:13. doi: 10.1186/1471-2229-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beebo A, Thomas D, Der C, Sanchez L, Leborgne-Castel N, Marty F, et al. Life with and without AtTIP1;1, an Arabidopsis aquaporin preferentially localized in the apposing tonoplasts of adjacent vacuoles. Plant Mol Biol. 2009;70:193–209. doi: 10.1007/s11103-009-9465-2. [DOI] [PubMed] [Google Scholar]
- 9.Mohanty A, Luo A, DeBlasio S, Ling X, Yang Y, Tuthill DE, et al. Advancing cell biology and functional genomics in maize using fluorescent protein-tagged lines. Plant Physiol. 2009;149:601–5. doi: 10.1104/pp.108.130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saito C, Uemura T, Awai C, Tominaga M, Ebine K, Ito J, et al. The occurrence of bulbs, a complex configuration of the vacuolar membrane, is affected by mutations of vacuolar SNARE and phospholipase in Arabidopsis. Plant J. 2011;68:64–73. doi: 10.1111/j.1365-313X.2011.04665.x. [DOI] [PubMed] [Google Scholar]
- 11.Uemura T, Morita MT, Ebine K, Okatani Y, Yano D, Saito C, et al. Vacuolar/pre-vacuolar compartment Qa-SNAREs VAM3/SYP22 and PEP12/SYP21 have interchangeable functions in Arabidopsis. Plant J. 2010;64:864–73. doi: 10.1111/j.1365-313X.2010.04372.x. [DOI] [PubMed] [Google Scholar]