Abstract
Import of nuclear encoded proteins into chloroplast is an essential and well-regulated mechanism. The cytosolic kinases STY8, STY17 and STY46 have been shown to phosphorylate chloroplast preprotein transit peptides advantaging the binding of a 14-3-3 dimer. Analyses of sty8 sty17 sty46 mutant plants revealed a role for the kinases in chloroplast differentiation, possibly due to lack of transit peptide phosphorylation. Moreover we could show that not only phosphorylation but also transit peptide dephosphorylation appears to be required for the fine regulation of the back-transport of nuclear encoded proteins to the chloroplast.
Keywords: chloroplast, phosphatase, phosphorylation, protein import, STY kinase
Chloroplasts originated from an endosymbiotic event in which an ancestral photosynthetic cyanobacterium was engulfed by a heterotophic host cell. During evolution about 95% of the genetic information was transferred from the chloroplast to the nuclear genome, thus requiring an efficient and well regulated back-transport of nuclear encoded proteins to the chloroplast.1 Several cytosolic players govern the targeting of preproteins to the chloroplast. Most chloroplast precursor proteins display the feature to bind the heat shock chaperone protein Hsp70.2,3 Beside Hsp70, 14-3-3 proteins can bind a subset of chloroplast preproteins.4 A 14-3-3 dimer interacts with many precursor proteins in their transit peptide, forming together with Hsp70 the so-called “guidance complex” which enhances the import rate of the preproteins.2 The affinity of 14-3-3 for the substrate increases when the binding site on the substrate is phosphorylated.2 In Arabidopsis thaliana the responsibility for the transit peptide phosphorylation was attributed to three high homologous protein kinases: STY8, STY17 and STY46.5
Three Cytosolic Kinases Influence Chloroplast Differentiation
We have recently shown that the so called dual specificity kinases STY8, STY17 and STY46 play an important role in the transition from etioplast to chloroplast.6 STY8 appears to be activated by an intramolecular autophosphorylation event on a conserved threonine residue within the kinase activation segment. We demonstrated that although STY8 harbors typical motifs for tyrosine phosphorylation, only serine and threonine residues were phosphorylated on the kinase itself and posttranslationally on kinase substrates. Thus, we speculate on the possibility that tyrosine phosphorylation activity might have been lost during evolution due to the lack of tyrosine residues on the transit peptide of chloroplast precursor proteins.
In an in vivo analysis we observed a significantly delayed greening process in sty8 sty17 sty46 Arabidopsis triple mutant lines, displayed by a slower accumulation of chlorophyll, a lower photosynthetic performance of PSII and degenerated plastids on an ultrastructural level. At a molecular level we demonstrated that during the greening process nuclear-encoded phosphorylated proteins accumulate slower in the chloroplasts of the sty8 sty17 sty46 mutant. We therefore hypothesize that our results identify phosphorylation as an important regulatory mechanism for the cytosolic targeting of precursor proteins to the chloroplast (Fig. 1). In this report, we further show that not only transit peptide phosphorylation but also subsequent dephosphorylation acts as a regulatory mechanism for preproteins import into chloroplast.
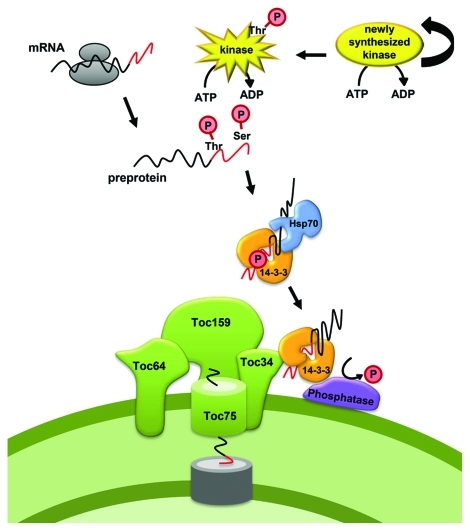
Figure 1.
Working model of cytosolic phosphorylation of chloroplast transit peptides. The cytosolic kinases are phosphorylated by an intramolecular event resulting in active kinase molecules, which can subsequently phosphorylate chloroplast transit peptides on serine and threonine residues. Phosphorylation and 14-3-3 binding enhances association with the Toc receptor, from where the proteins are imported after dephosphorylation by an as yet unidentified protein phosphatase.
Transit Peptide Dephosphorylation is Essential for Import into Chloroplasts
A first indication that chloroplast preproteins need to be dephosphorylated to be efficiently imported into the chloroplast was obtained performing import experiment in the presence of the phosphatase inhibitor NaF. The addition of the unspecific phosphatase inhibitor NaF prohibits import of phosphorylated preproteins. Furthermore, import is hindered by introduction of a thiophosphate group which is significantly slower dephosphorylated, suggesting that the activity of a phosphatase might be required for successful import.7 Following this notion, to investigate whether the dephosphorylation of the transit peptide is a prerequisite for efficient import, we generated a phospho-mimicry mutant of the model substrate pSSU by exchanging the two phosphorylated serines at position 31 and 34 to aspartic acid (pSSU S31/34D). Next, we performed import experiments with isolated chloroplasts from Pisum sativum and observed a severe reduction of the import efficiency for pSSU S31/34D as compared with pSSU WT (Fig. 2A). Moreover, we transiently expressed pSSU and pSSU S31/34D fused to GFP in Arabidopsis mesophyll protoplasts. We observed a longer permanence of pSSU S31/34D in the cytosol pointing at a slowed import (Fig. 2B). The amount of GFP-fused SSU located in the cytosol (pSSU) and in the chloroplasts (mSSU) was analyzed by immunoblotting with GFP antisera (Fig. 2C), showing significantly less mature SSU.
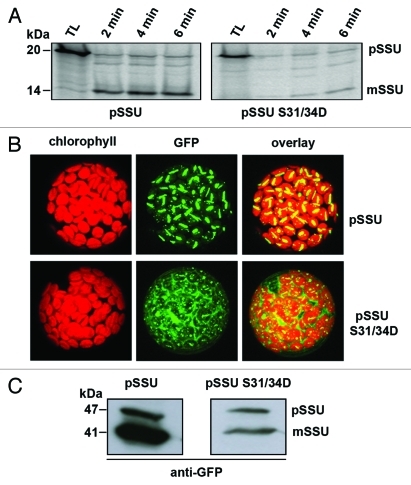
Figure 2.
Dephosphorylation is important for efficient import of preproteins into chloroplasts. (A) pSSU and pSSU S31/34D were translated in wheat germ lysate and imported into chloroplasts as described.8 TL shows 10% of the translation input. The precursor form (pSSU) and the processed mature form (mSSU) are indicated. (B) Arabidopsis protoplasts were isolated and transformed with pSSU and pSSU S31/34D C-terminal GFP-fusion proteins as described.9 Pictures were obtained by confocal laser scanning microscopy. Chlorophyll fluorescence is shown in red and GFP in green. (C) Transformed protoplasts were collected 16 h after transformation, lysed in a buffer containing 2% mM Tris7HCl pH 7.7, 10 mM MgCl2, 15 mM EGTA, 150 mM NaCl, 15 mM β-glycerophosphate, 0.1% Tween-20, 10% glycerol, 1 mM DTT, 1 mM NaF, 0.5 mM PMSF, 1x complete protease inhibitor cockatil (Roche) and subjected to SDS-PAGE and decorated with GFP antisera (Roche). Again the precursor of SSU and the mature form are shown.
Taken together these results indicate that dephosphorylation of precursor proteins is an essential step for efficient import, implying that not only phosphorylation but also dephosphorylation by a yet unidentified phosphatase regulates import of nuclear encoded proteins into the chloroplast. We therefore speculate that these modifications of chloroplast transit peptides are an important developmental mechanism to regulate chloroplast biogenesis on a posttranslational level allowing the plant to react dynamically in response to environmental changes.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18127
References
- 1.Gould SB, Waller RF, McFadden GI. Plastid evolution. Annu Rev Plant Biol. 2008;59:491–517. doi: 10.1146/annurev.arplant.59.032607.092915. [DOI] [PubMed] [Google Scholar]
- 2.May T, Soll J. 14-3-3 proteins form a guidance complex with chloroplast precursor proteins in plants. Plant Cell. 2000;12:53–64. doi: 10.1105/tpc.12.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ivey RA, Subramanian C, Bruce BD. Identification of a Hsp70 recognition domain within the rubisco small subunit transit peptide. Plant Physiol. 2000;122:1289–99. doi: 10.1104/pp.122.4.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fellerer C, Schweiger R, Schongruber K, Soll J, Schwenkert S. Cytosolic HSP90 cochaperones HOP and FKBP interact with freshly synthesized chloroplast preproteins of Arabidopsis. Mol Plant. 2011 doi: 10.1093/mp/ssr037. In press. [DOI] [PubMed] [Google Scholar]
- 5.Martin T, Sharma R, Sippel C, Waegemann K, Soll J, Vothknecht UC. A protein kinase family in Arabidopsis phosphorylates chloroplast precursor proteins. J Biol Chem. 2006;281:40216–23. doi: 10.1074/jbc.M606580200. [DOI] [PubMed] [Google Scholar]
- 6.Lamberti G, Gugel IL, Meurer J, Soll J, Schwenkert S. The cytosolic kinases STY8, STY17 and STY46 are involved in chloroplast differentiation in Arabidopsis thaliana. Plant Physiol. 2011 doi: 10.1104/pp.111.182774. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waegemann K, Soll J. Phosphorylation of the transit sequence of chloroplast precursor proteins. J Biol Chem. 1996;271:6545–54. doi: 10.1074/jbc.271.11.6545. [DOI] [PubMed] [Google Scholar]
- 8.Stengel A, Benz P, Balsera M, Soll J, Bolter B. TIC62 redox-regulated translocon composition and dynamics. J Biol Chem. 2008;283:6656–67. doi: 10.1074/jbc.M706719200. [DOI] [PubMed] [Google Scholar]
- 9.Duy D, Wanner G, Meda AR, von Wiren N, Soll J, Philippar K. PIC1, an ancient permease in Arabidopsis chloroplasts, mediates iron transport. Plant Cell. 2007;19:986–1006. doi: 10.1105/tpc.106.047407. [DOI] [PMC free article] [PubMed] [Google Scholar]