Abstract
Depending on the threat to a plant, different pattern recognition receptors, such as receptor-like kinases, identify the stress and trigger action by appropriate defense response development.1,2 The plant immunity system primary response to these challenges is rapid accumulation of phytohormones, such as ethylene (ET), salicylic acid (SA), and jasmonic acid (JA) and its derivatives. These phytohormones induce further signal transduction and appropriate defenses against biotic threats.3,4 Phytohormones play crucial roles not only in the initiation of diverse downstream signaling events in plant defense but also in the activation of effective defenses through an essential process called signaling pathway crosstalk, a mechanism involved in transduction signals between two or more distinct, “linear signal transduction pathways simultaneously activated in the same cell.”5
Keywords: cantharidin, crosstalk, jasmonic acid, phytohormone signaling, protein phosphatase
Specific inhibitors of serine/threonine protein phosphatases (PPP) such as okadaic acid and cantharidin and mutations of PPP have been used to probe the role of PPP in various processes connected to different types of stress such as pathogens, wounding, cold, and drought.6-11 However, until the publication of our recent paper on the effects of cantharidin on the entire transcriptome of Arabidopsis,12 there were no studies demonstrating the full capacity of a PPP inhibitor to impact gene transcription. The microarray experiment described in this paper revealed the breadth of the role of PPP in responses to biotic and abiotic stresses. Arabidopsis response to cantharidin mimicked the response to a pathogen infection. Gene sets, which are recognized as key players in the regulation of the phytohormone signaling pathways, were regulated by PPPs.
Cantharidin Treatments Affect the Jasmonate Biosynthetic Pathway
The JA response is induced by herbivores and various types of necrotrophic pathogens.4,13 PPPs are proposed to be involved in the signal transduction pathway of this response.10,11 Our microarray analysis by the MIPS FunCat database supported this hypothesis by revealing that 30 four cantharidin-affected genes of Arabidopsis belong to wounding-response genes. Fourteen additional genes were directly or indirectly involved in JA biosynthesis: two AOC (allene oxide cyclase), two ACX (acyl-CoA oxidase), and two PEX14 (peroxisome defective 2), seven genes of the JAZ family (jasmonate-ZIM-domain protein), and PDF1.2 (plant defensin 1.2). The pool of affected genes also includes genes (hormone-responsive) which are expressed in the presence of JA and genes that are JA-independent wounding markers (Table 1). In addition, up to 80 six percent of these genes were strongly upregulated by cantharidin at one or more of the time points after treatment (Fig. 1). This observation lends additional support to the idea that PPPs might act as a negative regulator of JA signaling.
Table 1. Selected genes involved in signaling pathways in response to cantharidin treatment.
| Array Element | Locus Identifier | Signal Log Ratio 2 h | Signal Log Ratio 10 h | Signal Log Ratio 24 h | Annotation |
|---|---|---|---|---|---|
| 257769_at |
AT3G23050 |
|
|
-1.4 |
Auxin resistant 2 (IAA7) |
| 265149_at |
AT1G51400 |
-2.2 |
|
-1.2 |
Photosystem II 5 kD protein |
| 253203_at |
AT4G34710 |
|
2.7 |
1.1 |
Arginine carboxylase 2 (ADC2) |
| 254283_s_at |
AT4G22870;AT4G22880 |
|
|
1.1 |
Leucoanthocyanidin dioxygenase, putative |
| 261564_at |
AT1G01720 |
1.7 |
|
1.3 |
Arabidopsis NAC domain containing protein 2 (ATAF1) |
| 261037_at |
AT1G17420 |
|
|
1.3 |
Lipoxygenase 3 (LOX3) |
| 258047_at |
AT3G21240 |
|
|
1.4 |
4-coumarate:CoA ligase 2 (4CL2) |
| 247213_at |
AT5G64900 |
1.9 |
|
1.5 |
Elicitor peptide 1 precursor (ATPEP1/PROPEP1) |
| 252831_at |
AT4G39980 |
|
|
1.5 |
3-deoxy-D-arabino-heptulosonate 7-phosphate synthase (DHS1) |
| 254926_at |
AT4G11280 |
3.4 |
2.4 |
1.6 |
1-aminocyclopropane-1-carboxylic acid (ACC) synthase 6 (ACS6) |
| 266168_at |
AT2G38870 |
2 |
|
1.7 |
Protease inhibitor, putative |
| 245928_s_at |
AT5G24770;AT5G24780 |
|
|
1.8 |
Vegetative storage protein 2 (VSP2) |
| 246099_at |
AT5G20230 |
2.7 |
|
2 |
Arabidopsis blue-copper-binding protein (ATBCB) |
| 251336_at |
AT3G61190 |
3.4 |
|
2 |
Bon association protein 1 (BAP1) |
| 247655_at |
AT5G59820 |
4.3 |
4.2 |
2.1 |
Responsive to high light 41 (RHL41) |
| 260391_at |
AT1G74020 |
|
2.8 |
2.3 |
Strictosidine synthase 2 (SS2) |
| 250738_at |
AT5G05730 |
1.5 |
2.8 |
2.6 |
Anthranilate synthase α subunit 1 (ASA1) |
| 256321_at |
AT1G55020 |
|
2.6 |
3.1 |
Lipoxygenase 1 (LOX1); lipoxygenase |
| 249101_at |
AT5G43580 |
1.4 |
3.3 |
3.5 |
Serine-type endopeptidase inhibitor |
| 266225_at |
AT2G28900 |
|
-1.7 |
|
Outer envelope protein 16 (OEP16) |
| 259383_at |
AT3G16470 |
|
-1.7 |
|
Jacalin lectin family protein (JR1) |
| 263539_at |
AT2G24850 |
2.3 |
-1.6 |
|
Tyrosine aminotransferase 3 (TAT3) |
| 253161_at |
AT4G35770 |
|
2.4 |
|
Dark inducible 1 (SEN1) |
| 261144_s_at |
AT1G19660;AT1G75380 |
-1.3 |
|
|
Wound-responsive family protein |
| 259764_at |
AT1G64280 |
1.3 |
|
|
Nonexpresser of PR genes 1 (NPR1) |
| 251984_at |
AT3G53260 |
1.3 |
|
|
Phenylalanine ammonia-lyase 2 (PAL2) |
| 253993_at |
AT4G26070 |
1.4 |
|
|
Mitogen-activated protein kinase kinase 1 (MEK1) |
| 267470_at |
AT2G30490 |
1.7 |
|
|
Cinnamate 4-hydroxylase (ATC4H/C4H/CYP73A5) |
| 256770_at |
AT3G13790 |
1.7 |
|
|
RNA-binding protein, putative |
| 256186_at |
AT1G51680 |
1.8 |
|
|
4-coumarate:CoA ligase 1 (4CL1) |
| 263845_at |
AT2G37040 |
1.8 |
|
|
PHE ammonia lyase 1 (PAL1) |
| 248551_at |
AT5G50200 |
2.2 |
|
|
Wound-responsive 3 (WR3) |
| 261648_at |
AT1G27730 |
2.8 |
|
|
Salt tolerance zinc finger (STZ) |
| 261506_at |
AT1G71697 |
3.8 |
|
|
Choline kinase (ATCK1) |
| 257644_at |
AT3G25780 |
2.4 |
|
|
Allene oxide cyclase 3 (AOC3) |
| 259366_at |
AT1G13280 |
|
-1.4 |
|
Allene oxide cyclase 4 (AOC4) |
| 245249_at |
AT4G16760 |
1.4 |
1.9 |
|
Acyl-CoA oxidase 1 (ACX1) |
| 246304_at |
AT3G51840 |
|
1.8 |
|
Acyl-CoA oxidase 4 (ACX4) |
| 252411_at |
AT3G47430 |
|
|
-1.2 |
Peroxin 11B (PEX11B) |
| 247422_at |
AT5G62810 |
-1.2 |
|
|
Peroxisome defective 2 (PEX14) |
| 250292_at |
AT5G13220 |
|
|
2.5 |
Jasmonate-ZIM-domain protein 10 (JAS1/JAZ10/TIFY9) |
| 256017_at |
AT1G19180 |
2.8 |
1.8 |
1.8 |
Jasmonate-ZIM-domain protein 1 (JAZ1/TIFY10A) |
| 262171_at |
AT1G74950 |
|
|
1.3 |
Jasmonate-ZIM-domain protein 2 (JAZ2/TIFY10B) |
| 261033_at |
AT1G17380 |
|
|
2.5 |
Jasmonate-ZIM-domain protein 5 (JAZ5/TIFY11A) |
| 266901_at |
AT2G34600 |
|
|
1.6 |
Jasmonate-ZIM-domain protein 7 (JAZ7/TIFY5B) |
| 256159_at |
AT1G30135 |
|
3.4 |
3.7 |
Jasmonate-ZIM-domain protein 8 (JAZ8/TIFY5A) |
| 260205_at |
AT1G70700 |
|
|
1.1 |
Jasmonate-ZIM-domain protein 9 (JAZ9/TIFY7) |
| 249052_at | AT5G44420 | 2.3 | Low-molecular-weight cysteine-rich 77 (PDF1.2) |
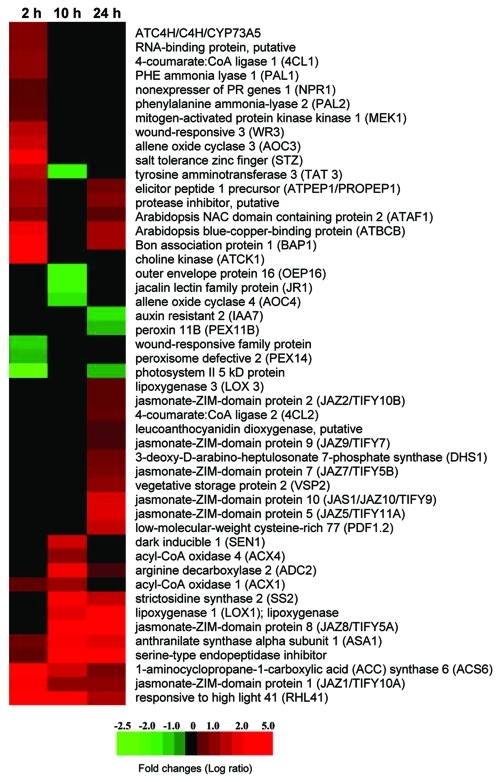
Figure 1.
Heat-map showing expression profiles of wounding-responsive genes of Arabidopsis at three time points after treatment with 200 µM cantharidin.
JA biosynthesis occurs in two cellular compartments: chloroplasts and peroxisomes. Genes encoding lipoxygenase (LOX) and AOC, which are involved in the synthesis of cis-(+)-12-oxophytodienoic acid from α-linolenic acid (C18:3) in the chloroplast, are either up- or downregulated at one or more of the time points after cantharidin treatment (Table 1). In the LOX gene family, the upregulated LOX1 (AT1G55020) and LOX3 (AT1G17420) encode lipoxygenase enzymes involved in biosynthesis of oxylipins.14 LOX3 belongs to 13-lipoxygenases and is responsible for the biosynthesis of JA while LOX1 has 9-lipoxygenase activity, and its role is still unclear.15 Four AOC homologs encoding AOC1, 2, 3, and 4 were identified in the Arabidopsis genome.16 Two of them were affected by cantharidin treatment with AOC3 upregulated (2.4 fold at 2 h) and AOC4 downregulated (-1.4 fold at 10 h). The functional role of PPP on the regulation of these AOC genes remains to be elucidated. Recent research on four genes of the JA biosynthetic pathway (LOX2, AOS, AOC2 and OPR3) revealed that, like most JA responsive genes, they are downregulated by SA.17
Regulation of the Expression of JA-Responsive Genes
The jasmonate ZIM-domain repressor family (JAZ) consists of 12 members that control the expression of JA-responsive genes by interacting with transcription factors such as AtMYC2.18,19 Not all JAZ proteins have empirically confirmed repressor functions (Chico et al., 2008).18 JA accumulation induces JAZ repressor interactions with ubiquitin ligase SCFCOI1 and subsequent 26S proteosome degradation.3,20,21 It is worth mentioning that JAZ and ligase SCFCOI1 interaction depends on bioactive JA derivatives, particularly JA-isoleucine (JA-Ile) conjugate.3,22 The JAZ proteins encoded by JA-responsive genes are involved in control of the JA pathway by negative feedback mechanisms.18 Their expression takes place within minutes after wounding at both local and systemic levels.3,21 Chung et al. observed significant differences in timing of JAZ expression in response to herbivory compared with mechanical wounding.21 JAZ-response to feeding by the insect Spodoptera exigua was rapid and long lasting, while the response to mechanical wounding was immediate and short-lived. Their experiment elegantly shows how fine tuned the timing of the plant immune system is for different challenges. In our experiment, the expression of up to seven JAZ genes increased after 24 h (Fig. One and Table 1). Only JAZ1 was upregulated at all time points, and JAZ8 had the highest expression among all induced JAZ genes. In our experiment, the transcription pattern of particular JAZ members was similar to that caused by herbivory feeding.
After 24 exposure to cantharidin, the expression of JA marker genes such as PDF1.2 and VSP (vegetative storage protein) increased 2.3- and 1.8-fold, respectively (Table 1). We observed an upregulation of ATPEP1/PROPEP1and PEPR1which encode proteins responsible for amplification of JA-dependent responses. The protein PROPEP1 is a precursor of the elicitor peptide 1 (ATPEP1), and PEPR1 is its receptor.23 ATPEP1 belongs to the so called Damage Associated Molecular Patterns (DAMPS), and its presence induces PDF1.2 expression.23 Also, exogenous SA or induction of SA-dependent responses causes downregulation of PDF1.2 and VSP2 genes.24 Interestingly, Rojo et al.11 showed that the protein phosphatase inhibitor okadaic acid does not induce VSP gene expression, while JA and wounding do. Although genes JR1 (jacalin lectin family protein), WR3 (wound responsive gene 3), and ATCK (choline kinase) were regulated in the same way in our and Rojo’s experiments, the expression of both WR3 and ATCK is wounding but not JA-dependent.
Crosstalk of Signaling Pathways in Response to Cantharidin Treatments
Plants activate a response system upon pathogen or insect attack by producing phytohormonal signals such as SA, JA, and ET. The interactions between these induced defense-signaling pathways are documented as positive and negative crosstalk.25,26 Mediators of this extraordinarily complicated network and signal markers which facilitate the identification of the dominating signal pathway are both important aspects of research on crosstalk. Several such genes were affected by cantharidin treatment.12 For example, gene NPR1 (Nonexpressor of PR genes 1) slightly increased expression at the 2 h time point (1.3)(Table1). NPR1 functions as a sensor of redox status and controls SA and JA signal transduction pathways by negatively regulating SA signaling, and positively modulating the expression of JA-dependent genes during herbivore attack.25 The situation became even more complicated when Leon-Reyes et al. discovered that NPR1-mediated SA/JA crosstalk is modulated by one more factor– ET.26 Also, the role of NPR1 varies, depending on its cellular localization, nuclear or cytosolic. Two other proteins located in the crosstalk downstream of NPR1 were proposed as SA/JA interaction regulators: glutaredoxin (GRX480) and WRKY70, a transcription factor of SA-dependent genes. The roles of GRX480 were discovered because of its strong interaction with TGA transcription factors.27 In our experiment, GRX480 was strongly induced by cantharidin (2.4 at 10 h time point and 1.3 at 24 h time point) while the transcript level of WRKY70 decreased (-1.3 at 10 h and -1.2 at 24 h).12 The role of this transcription factor in regulation of SA-dependent signaling was described in a study where its accumulation caused a decrease of MeJA-dependent PDF1.2 expression.28 This indirectly agreed with our data: downregulation of WRKY70 and upregulation of PDF1.2. Results presented above might suggest PPPs’ critical role in negative regulation of plant defenses, where PPP inhibition prevents the establishment of appropriate crosstalk between signaling pathways. Moreover, cantharidin seems to have major effect on the JA signaling pathway. The most characteristic signal markers (PDF1.2 and VSP) showed strong expression changes (Table 1). The expression of the very commonly used SA signal marker gene PR1 (Pathogenesis Related gene 1), as well as other members of Pathogenesis-Related family (such as PR2), were downregulated and only one (PR5) upregulated at the 24 h time point.12 Taken together, these data suggest that cantharidin treatment might favor both JA-dependent and -independent wound responses. This hypothesis will need additional empirical confirmation.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18146
References
- 1.Boller T, He SY. Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science. 2009;324:742–4. doi: 10.1126/science.1171647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert M, Jehle AK, Lipschis M, Mueller K, Zeng Y, Felix G. Regulation of cell behaviour by plant receptor kinases: Pattern recognition receptors as prototypical models. Eur J Cell Biol. 2010;89:200–7. doi: 10.1016/j.ejcb.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Koo AJ, Gao X, Jones AD, Howe GA. A rapid wound signal activates the systemic synthesis of bioactive jasmonates in Arabidopsis. Plant J. 2009;59:974–86. doi: 10.1111/j.1365-313X.2009.03924.x. [DOI] [PubMed] [Google Scholar]
- 4.De Vos M, Van Oosten VR, Van Poecke RM, Van Pelt JA, Pozo MJ, Mueller MJ, et al. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol Plant Microbe Interact. 2005;18:923–37. doi: 10.1094/MPMI-18-0923. [DOI] [PubMed] [Google Scholar]
- 5.Wang KL, Li H, Ecker JR. Ethylene biosynthesis and signaling networks. Plant Cell. 2002;14(Suppl):S131–51. doi: 10.1105/tpc.001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacKintosh C, Lyon GD, MacKintosh RW. Protein phosphatase inhibitors activate anti-fungal defence responses of soybean cotyledons and cell cultures. Plant J. 1994;5:137–47. doi: 10.1046/j.1365-313X.1994.5010137.x. [DOI] [Google Scholar]
- 7.Facchini PJ, Johnson AG, Poupart J, de Luca V. Uncoupled defense gene expression and antimicrobial alkaloid accumulation in elicited opium poppy cell cultures. Plant Physiol. 1996;111:687–97. doi: 10.1104/pp.111.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin H, Shin HS, Guo Z, Blancaflor EB, Masson PH, Chen R. Complex regulation of Arabidopsis AGR1/PIN2-mediated root gravitropic response and basipetal auxin transport by cantharidin-sensitive protein phosphatases. Plant J. 2005;42:188–200. doi: 10.1111/j.1365-313X.2005.02369.x. [DOI] [PubMed] [Google Scholar]
- 9.País SM, González MA, Téllez-Iñón MT, Capiati DA. Characterization of potato (Solanum tuberosum) and tomato (Solanum lycopersicum) protein phosphatases type 2A catalytic subunits and their involvement in stress responses. Planta. 2009;230:13–25. doi: 10.1007/s00425-009-0923-5. [DOI] [PubMed] [Google Scholar]
- 10.Dammann C, Rojo E, Sánchez-Serrano JJ. Abscisic acid and jasmonic acid activate wound-inducible genes in potato through separate, organ-specific signal transduction pathways. Plant J. 1997;11:773–82. doi: 10.1046/j.1365-313X.1997.11040773.x. [DOI] [PubMed] [Google Scholar]
- 11.Rojo E, Titarenko E, León J, Berger S, Vancanneyt G, Sánchez-Serrano JJ. Reversible protein phosphorylation regulates jasmonic acid-dependent and -independent wound signal transduction pathways in Arabidopsis thaliana. Plant J. 1998;13:153–65. doi: 10.1046/j.1365-313X.1998.00020.x. [DOI] [PubMed] [Google Scholar]
- 12.Bajsa J, Pan Z, Duke SO. Transcriptional responses to cantharidin, a protein phosphatase inhibitor, in Arabidopsis thaliana reveal the involvement of multiple signal transduction pathways. Physiol Plant. 2011;143:188–205. doi: 10.1111/j.1399-3054.2011.01494.x. [DOI] [PubMed] [Google Scholar]
- 13.Mithöfer A, Boland W. Recognition of herbivory-associated molecular patterns. Plant Physiol. 2008;146:825–31. doi: 10.1104/pp.107.113118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liavonchanka A, Feussner I. Lipoxygenases: occurrence, functions and catalysis. J Plant Physiol. 2006;163:348–57. doi: 10.1016/j.jplph.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Kilaru A, Herrfurth C, Keereetaweep J, Hornung E, Venables BJ, Feussner I, et al. Lipoxygenase-mediated oxidation of polyunsaturated N-acylethanolamines in Arabidopsis. J Biol Chem. 2011;286:15205–14. doi: 10.1074/jbc.M110.217588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stenzel I, Hause B, Miersch O, Kurz T, Maucher H, Weichert H, et al. Jasmonate biosynthesis and the allene oxide cyclase family of Arabidopsis thaliana. Plant Mol Biol. 2003;51:895–911. doi: 10.1023/A:1023049319723. [DOI] [PubMed] [Google Scholar]
- 17.Leon-Reyes A, Van der Does D, De Lange ES, Delker C, Wasternack C, Van Wees SC, et al. Salicylate-mediated suppression of jasmonate-responsive gene expression in Arabidopsis is targeted downstream of the jasmonate biosynthesis pathway. Planta. 2010;232:1423–32. doi: 10.1007/s00425-010-1265-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chico JM, Chini A, Fonseca S, Solano R. JAZ repressors set the rhythm in jasmonate signaling. Curr Opin Plant Biol. 2008;11:486–94. doi: 10.1016/j.pbi.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Chini A, Fonseca S, Fernández G, Adie B, Chico JM, Lorenzo O, et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature. 2007;448:666–71. doi: 10.1038/nature06006. [DOI] [PubMed] [Google Scholar]
- 20.Chini A, Fonseca S, Chico JM, Fernández-Calvo P, Solano R. The ZIM domain mediates homo- and heteromeric interactions between Arabidopsis JAZ proteins. Plant J. 2009;59:77–87. doi: 10.1111/j.1365-313X.2009.03852.x. [DOI] [PubMed] [Google Scholar]
- 21.Chung HS, Koo AJ, Gao X, Jayanty S, Thines B, Jones AD, et al. Regulation and function of Arabidopsis JASMONATE ZIM-domain genes in response to wounding and herbivory. Plant Physiol. 2008;146:952–64. doi: 10.1104/pp.107.115691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katsir L, Chung HS, Koo AJ, Howe GA. Jasmonate signaling: a conserved mechanism of hormone sensing. Curr Opin Plant Biol. 2008;11:428–35. doi: 10.1016/j.pbi.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krol E, Mentzel T, Chinchilla D, Boller T, Felix G, Kemmerling B, et al. Perception of the Arabidopsis danger signal peptide 1 involves the pattern recognition receptor AtPEPR1 and its close homologue AtPEPR2. J Biol Chem. 2010;285:13471–9. doi: 10.1074/jbc.M109.097394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spoel SH, Koornneef A, Claessens SM, Korzelius JP, Van Pelt JA, Mueller MJ, et al. NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell. 2003;15:760–70. doi: 10.1105/tpc.009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koornneef A, Pieterse CM. Cross talk in defense signaling. Plant Physiol. 2008;146:839–44. doi: 10.1104/pp.107.112029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leon-Reyes A, Spoel SH, De Lange ES, Abe H, Kobayashi M, Tsuda S, et al. Ethylene modulates the role of NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1 in cross talk between salicylate and jasmonate signaling. Plant Physiol. 2009;149:1797–809. doi: 10.1104/pp.108.133926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ndamukong I, Abdallat AA, Thurow C, Fode B, Zander M, Weigel R, et al. SA-inducible Arabidopsis glutaredoxin interacts with TGA factors and suppresses JA-responsive PDF1.2 transcription. Plant J. 2007;50:128–39. doi: 10.1111/j.1365-313X.2007.03039.x. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Brader G, Kariola T, Palva ET. WRKY70 modulates the selection of signaling pathways in plant defense. Plant J. 2006;46:477–91. doi: 10.1111/j.1365-313X.2006.02712.x. [DOI] [PubMed] [Google Scholar]