Abstract
We have recently identified a new class III chitinase from pomegranate seeds (PSC). Interestingly, this new chitinase naturally binds calcium ions with high capacity and low affinity, suggesting that PSC is a Ca-storage protein. Analysis of the amino acid sequence showed that this enzyme is rich in acidic amino acid residues, especially Asp, which are responsible for calcium binding. Different from other known chitinases, PSC is located in the stroma of amyloplasts in pomegranate seeds. Transmission electron microscopy (TEM) analysis indicated that the embryonic cells of pomegranate seeds are rich in calcium ions, most of which are distributed in the stroma and the starch granule of the amyloplasts, consistent with the above idea that PSC is involved in calcium storage, a newly non-defensive function.
Keywords: Amino acid sequence, Amyloplast, Calcium storage, Chitinase, Ultrastructure
Plants correspond to pathogen attacks by inducing the expression of a large number of genes that encode diverse proteins, many of which are believed to have a function in defense. Chitinases are kinds of these induced proteins, and play significant roles in the interaction between plants and pathogenic fungi.1-7 Chitinases (EC 3.2.1.14) could hydrolyze chitin, a linear homopolymer of β-1,4-linked N-acetylglucosamine (GlcNAc) residues, to protect the plants from pathogen attacks.8,9 In contrast to the roles of chitinases in defense, much less attention was paid to their non-defensive functions.10 We have purified the chitinase from the pomegranate seeds (PSC) which has been classified into the chitinase III according to the structural properties and amino acid sequences.11-14 Differently, this enzyme is mainly distributed in the amyloplasts of the pomegranate seeds, and naturally binds calcium ions with a stoichiometry of approximately ten moles Ca2+ per mole of protein,14 suggesting that PSC may be the Ca-storage protein. The content of this enzyme decreases with time during seed germination.14 All these findings implied that PSC could play a non-defensive role in seeds. In this addendum, some new results about predicted three-dimensional structure of PSC and calcium distribution in amyloplasts were reported, which advances our understanding of the structure and function of this new enzyme.
Prediction of three dimensional structure of chitinase III in pomegranate seeds
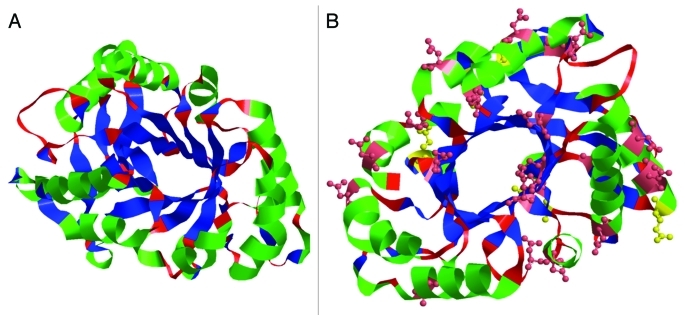
Sequence comparison indicates that PSC is highly homologous to a rubber tree chitinase whose three-dimensional structure has been determined by X-ray crystallography as a stable (βα)8 barrel fold with three pairs of disulfide linkage.15 Three-dimensional structure of the PSC was predicted by homology modeling using the crystal structure of the rubber tree chitinase as a template (Fig. 1A). Accordingly, eight alternating β-strands and α-helices were predicted in the PSC with six conserved cysteine residues presumably formed three pairs of disulfide bonds. The predicted active site is located in the loop regions connecting the carboxyl ends of the β-strands and amino ends of α-helices on top of the simulated three dimensional structure. Glu 127 is considered as one of residues consisting of this active site, which was identified in the loop sequence connected to the 4th β-strand (Fig. 1B).10 One distinctive structural feature of PSC from its analogs is that PSC contains 21 acidic amino acid residues (Glu and Asp) (Fig. 0.1B), representing the highest amount among all known class III chitinases.8,16 This result is in good agreement with its isoelectric point (4.6). Furthermore, MALDI-TOF-MS and X-ray diffraction (XRD) analyses showed that these acidic amino acid residues in PSC are responsible for the binding of 10 calcium ions14 with a similar way to reported Ca-storage proteins.8,17
Figure 1.
Predicted structure of PSC. (A) The predicted three dimensional structure of the PSC using the homology modeling contains eight alternating β-strands (blue) and α-helices (green); (B) Possible calcium binding sites (glutamic acid in yellow and aspartic acid in pink).
The localization of calcium in pomegranate seed cells
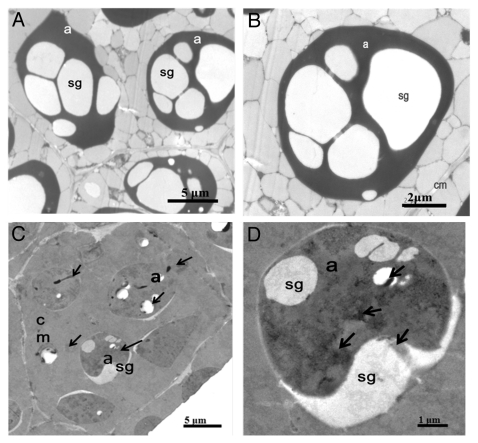
The immunoelectron microscopy results of PSC showed that it is mainly distributed in the stroma of amyloplasts of the embryonic cells of pomegranate seeds.14 On the other hand, PSC binds calcium ions with high capacity and low affinity, suggesting that PSC could be a calcium storage protein in pomegranate seeds. If this were the case, we would expect that the amyloplasts of the embryonic cells are rich in calcium ions. To shed light on the distribution of calcium, the ultrastructure of the pomegranate seeds embryos cells was observed by the transmission electron microscopy (TEM). Without treatment of potassium antimonite, no precipitate was present in the negative controls (Fig. 2A and 2B). In contrast, upon treatment with potassium antimonite as previously described,14 calcium-induced precipitates are distributed both in the stroma and starch granule of the amyloplasts (Fig. 2C and 2D), while few amount of precipitates occurs in cytosol and cell walls.18 Consistent with present observation, that calcium is also present in the amyloplasts of root-cap cells of corn.19 Thus, the present and previous findings raise the possibility that the amyloplasts could be an alternative organelle for calcium storage in plant cells. PSC might be responsible, at least partly, for calcium storage in the amyloplasts. Since PSC is compartmentalized in the amyloplasts, one of the most vital and yet least understood organelles in seedling germination and early growth, the detailed knowledge of PSC and amyloplasts presented here is beneficial to understanding the function of this organelle.
Figure 2.
Localization of calcium in the embryonic cells of pomegranate seeds by TEM. Pomegranate seed cell sections were untreated (A and B) or treated with potassium antimonate (C and D). Antimonate precipitates occur primarily in the stroma and starch granules of amyloplasts (arrows). a, amyloplast; sg, starch grain; cm, cell membrane.
Acknowledgments
This work was supported by the National Science and Technology Support Program (2011BAD23B04), and Innovation Fund for Graduate Student of China Agricultural University (KYCX201008).
Glossary
Abbreviations:
- PSC
chitinase III from pomegranate seed
- MALDI-TOF-MS
matrix-assisted laser desorption ionization-time of flight-mass spectroscopy
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18147
References
- 1.Collinge DB, Slusarenko AJ. Plant gene expression in response to pathogens. Plant Mol Biol. 1987;9:389–410. doi: 10.1007/BF00014913. [DOI] [PubMed] [Google Scholar]
- 2.Bol JF, Linthorst HJM. Plant pathogenesis-related proteins induced by virus infection. Annu Rev Phytopathol. 1990;28:113–38. doi: 10.1146/annurev.py.28.090190.000553. [DOI] [Google Scholar]
- 3.Bowles DJ. Defense-related proteins in higher plants. Annu Rev Biochem. 1990;59:873–907. doi: 10.1146/annurev.bi.59.070190.004301. [DOI] [PubMed] [Google Scholar]
- 4.Dixon RA, Harrison MJ. Activation, structure, and organization of genes involved in microbial defense in plants. Adv Genet. 1990;28:165–234. doi: 10.1016/S0065-2660(08)60527-1. [DOI] [PubMed] [Google Scholar]
- 5.Linthorst HJM, Van Loon LC. Pathogenesis-related proteins of plants. Crit Rev Plant Sci. 1991;10:123–50. doi: 10.1080/07352689109382309. [DOI] [Google Scholar]
- 6.Shinya T, Hanai K, Gális I, Suzuki K, Matsuoka K, Matsuoka H, et al. Characterization of NtChitIV, a class IV chitinase induced by beta-1,3-, 1,6-glucan elicitor from Alternaria alternata 102: Antagonistic effect of salicylic acid and methyl jasmonate on the induction of NtChitIV. Biochem Biophys Res Commun. 2007;353:311–7. doi: 10.1016/j.bbrc.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Saito A, Fujii T, Shinya T, Shibuya N, Ando A, Miyashita K. The msiK gene, encoding the ATP-hydrolysing component of N,N'-diacetylchitobiose ABC transporters, is essential for induction of chitinase production in Streptomyces coelicolor A3(2) Microbiology. 2008;154:3358–65. doi: 10.1099/mic.0.2008/019612-0. [DOI] [PubMed] [Google Scholar]
- 8.Collinge DB, Kragh KM, Mikkelsen JD, Nielsen KK, Rasmussen U, Vad K. Plant chitinases. Plant J. 1993;3:31–40. doi: 10.1046/j.1365-313X.1993.t01-1-00999.x. [DOI] [PubMed] [Google Scholar]
- 9.Tyler BM. Entering and breaking: virulence effector proteins of oomycete plant pathogens. Cell Microbiol. 2009;11:13–20. doi: 10.1111/j.1462-5822.2008.01240.x. [DOI] [PubMed] [Google Scholar]
- 10.Wu CT, Leubner-Metzger G, Meins FJ, Bradford KJ. Class I beta-1,3-glucanase and chitinase are expressed in the micropylar endosperm of tomato seeds prior to radicle emergence. Plant Physiol. 2001;126:1299–313. doi: 10.1104/pp.126.3.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shinshi H, Neuhas JM, Ryals J, Meins F. Structure of a tobacco endochitinase gene: evidence that different chitinase genes can arise by transposition of sequences encoding a cysteine-rich domain. Plant Mol Biol. 1990;14:357–68. doi: 10.1007/BF00028772. [DOI] [PubMed] [Google Scholar]
- 12.Van Damme EJ, Culerrier R, Barre A, Alvarez R, Rougé P, Peumans WJ. A novel family of lectins evolutionarily related to class V chitinases: an example of neofunctionalization in legumes. Plant Physiol. 2007;144:662–72. doi: 10.1104/pp.106.087981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taira T, Hayashi H, Tajiri Y, Onaga S, Uechi G, Iwasaki H, et al. A plant class V chitinase from a cycad (Cycas revoluta): biochemical characterization, cDNA isolation, and posttranslational modification. Glycobiology. 2009;19:1452–61. doi: 10.1093/glycob/cwp119. [DOI] [PubMed] [Google Scholar]
- 14.Yang H, Zhang T, Masuda T, Lv C, Sun L, Qu G, et al. Chitinase III in pomegranate seeds (Punica granatum Linn.): a high-capacity calcium-binding protein in amyloplasts. Plant J. 2011 doi: 10.1111/j.1365-313X.2011.04727.x. In press. [DOI] [PubMed] [Google Scholar]
- 15.Terwisscha van Scheltinga AC, Hennig M, Dijkstra BW. The 1.8 Å resolution structure of hevamine, a plant chitinase/lysozyme, and analysis of the conserved sequence and structuremotifs of glycosyl hydrolase family 18. J Mol Biol. 1996;262:243–57. doi: 10.1006/jmbi.1996.0510. [DOI] [PubMed] [Google Scholar]
- 16.Fukamizo T. Chitinolytic enzymes: catalysis, substrate binding, and their application. Curr Protein Pept Sci. 2000;1:105–24. doi: 10.2174/1389203003381450. [DOI] [PubMed] [Google Scholar]
- 17.Wang S, Trumble WR, Liao H, Wesson CR, Dunker AK, Kang C. Crystal structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum. Nat Struct Biol. 1998;5:476–83. doi: 10.1038/nsb0698-476. [DOI] [PubMed] [Google Scholar]
- 18.Slocum RD, Roux SJ. An improved method for the subcellular localization of calcium using a modification of the antimonite precipitation technique. J Histochem Cytochem. 1982;30:617–29. doi: 10.1177/30.7.6179981. [DOI] [PubMed] [Google Scholar]
- 19.Chandra S, Chabot JF, Morrison GH, Leopold AC. Localization of calcium in amyloplasts of root-cap cells using ion microscopy. Science. 1982;216:1221–3. doi: 10.1126/science.216.4551.1221. [DOI] [PubMed] [Google Scholar]