Abstract
Elevation of leaf auxin (indole-3-acetic acid; IAA) levels in intact plants has been consistently found to inhibit leaf expansion whereas excised leaf strips grow faster when treated with IAA. Here we test two hypothetical explanations for this difference in growth sensitivity to IAA by expanding leaf tissues in vivo versus in vitro. We asked if, in Arabidopsis, IAA-induced growth of excised leaf strips results from the wounding required to excise tissue and/or results from detachment from the plant and thus loss of some shoot or root derived growth controlling factors. We tested the effect of a range of exogenous IAA concentrations on the growth of intact attached, wounded attached, detached intact, detached wounded as well as excised leaf strips. After 24 h, the growth of intact attached, wounded attached, and detached intact leaves was inhibited by IAA concentrations as little as 1 µM in some experiments. Growth of detached wounded leaves and leaf strips was induced by IAA concentrations as low as 10 µM. Stress, in the form of high light, increased the growth response to IAA by leaf strips and reduced growth inhibition response by intact detached leaves. Endogenous free IAA content of intact attached leaves and excised leaf strips was found not to change over the course of 24 h. Together these results indicate growth induction of Arabidopsis leaf blade tissue by IAA requires both substantial wounding as well as detachment from the plant and suggests in vivo that IAA induces parallel pathways leading to growth inhibition.
Keywords: auxin, IAA, leaf expansion, wound response
Introduction
The plant hormone auxin (i.e., indole-3-acetic acid; IAA) controls or influences most aspects of plant development and physiology.1 Concerning leaf development alone, its morphogenic effects include control of the initiation of leaf primordia,2 control of vascular differentiation,3 as well as control of leaf expansion during both the cell division leaf growth phase,4 and the cell enlargement leaf growth phase.5
The post-cell division cell-enlargement phase of leaf growth accounts for most final leaf size as cell division is generally complete when leaves have reached no more than 20 percent of their final surface area.6,7 Tissue auxin content (both in the active free form or in various inactive conjugated forms8) varies greatly in plant tissues with the highest levels in apical meristematic tissues and young leaves. As leaves expand auxin levels fall.9,10 It has been suggested that cell expansion in leaves is promoted by auxin only at lower concentrations.5 Indeed, in the intact plant sustaining high levels of leaf auxin by various means has consistently resulted in inhibition of leaf expansion of expanding leaves. For example, Petunia transformed to constitutively overproduce IAA has smaller leaves.11 The sur and yucca mutants of Arabidopsis also have elevated IAA levels and smaller leaves.12 Application of the auxin transport inhibitor NPA (1-N-naphthylphthalamic acid) to expanding Phaseolus leaf petioles increased leaf auxin, slowed growth, and decreased final leaf size.10 Though application of aqueous auxin solutions directly to expanding Phaseolus leaves does initially increase growth for up to several hours,13 growth rate subsequently slows so that after 24 h treated leaves are significantly smaller than controls.14
This auxin-induced growth inhibition of leaf expansion in intact plants contrasts with the response of isolated tissues. Leaf strips excised from expanding Nicotiana leaves15-18 and from expanding Phaseolus leaves14 grew faster in a range of auxin concentrations compared with non-auxin controls over 24 or 48 h periods. This growth begins within 60 min of treatment.16 Auxin-induced leaf strip growth is always epinastic (downward curving) as the adaxial (upper) blade surface responds more than the abaxial (lower) surface.
Reported here are the results of an investigation into why auxin both induces increased growth in excised leaf lamina tissues but also acts to slow growth in same tissues in the intact plant. Two hypothetical explanations for this difference were considered. First we asked if auxin-induced growth of excised tissues is dependent on the changed gene expression environment produced by the wounding required to excise tissue. We also asked if auxin-induced growth of excised tissues results from separation or detachment from the plant and thus loss of some shoot or root derived growth controlling factors that otherwise condition expanding leaf cells to respond to auxin with slower growth. For our experiments we chose to experiment with young soil-grown Arabidopsis seedlings. This system offered the same advantage as young Phaseolus plants used in previous studies10,14 as the paired first and second true leaves in both plant species develop simultaneously allowing opposite untreated leaves to serve as a within plant control. Unlike Phaseolus, however, the first and second true leaves emerge nearly flat allowing for nondestructive initial sampling of leaf size. The results of the experiments described here suggest that both wounding and detachment are required for expanding leaf cells to show auxin-induced growth.
Results
To test whether leaf blade growth induction, as opposed to growth inhibition, by IAA treatment depends upon leaf wounding or upon leaf detachment from the plant we conducted a series of experiments in which the effect of applied IAA on the growth (increase in surface area) of leaf blade tissue was tested across a range of IAA concentrations with excised leaf strips, intact attached leaves, wounded attached leaves, detached (but otherwise intact) leaves, and wounded detached leaves.
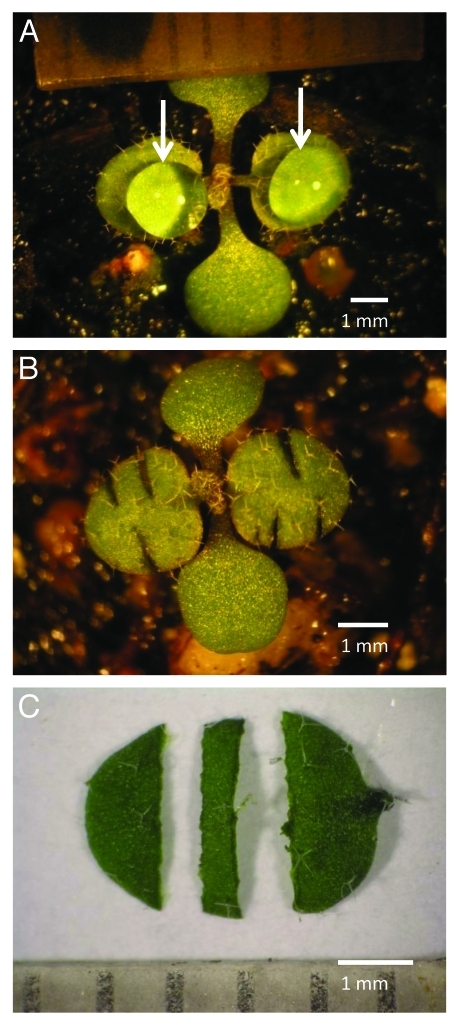
Figure 1 illustrates the conduct of the various growth studies in this investigation. For all experiments soil grown Arabidopsis seedlings were selected with approximately equal sized 1st and 2nd true leave pair, each leaf blade measuring 2.7 - 3.3 mm across the lamina at mid-blade (Fig. 1A). By this point in their development the cells of these two leaves have ceased dividing with the continuing rapid leaf growth due entirely to cell expansion.7 In order to eliminate the effect of between plant variance in growth from our results, for all experiments, leaves were treated and compared pair-wise with one randomly chosen leaf of the 1st and 2nd true leaf pair on each plant serving as a control and the other as the experimental leaf. Figure 1A also shows leaves as treated as intact attached leaves. In those experiments the control was administered a 5 µl droplet of control solution. The experimental leaf blade received the same solution augmented with IAA. For wounded attached leaves (Fig. 1B), the leaves were wounded with three incisions administered as shown from leaf margin to the midrib before the application of droplets the control or IAA containing solutions (not shown). For detached leaf experiments, both leaves of the true leaf pair were excised in the distal portion of the petiole and floated, one of each leaf pair, on the same control solution and one on the IAA containing solution (not shown). Excised leaf strips were prepared 0.7 mm wide with a double-bladed cutter from the central portion of each leaf blade as shown (C) of both first and second leaves. The excised leaf strips, one from each leaf pair, were floated on control or IAA containing solutions as with detached leaves.
Figure 1.
View of young Arabidopsis thaliana plant with intact attached leaves as used in this study (A), a similar plant shown with wounded attached leaves (B), and a detached leaf illustrating preparation of leaf strips (C). For all experiments, soil grown plants were selected with a partially expanded, similarly sized, pair of the first and second true leaves (each leaf measuring between 2.7 and 3.3 mm across). Shown in A are leaves as treated as “intact attached leaves,” with 5 µl droplets (indicated by arrows) of control and of IAA containing solution administered to the first and second leaves of each plant. For “wounded attached leaves” (B), each of the first and second leaves were wounded with three incisions administered as shown from leaf margin to close to the midrib before the application of droplets (not shown). For “detached leaf” experiments, both leaves were excised in the distal portion of the petiole and floated, adaxial side down in most experiments, one of each leaf pair, on the control and on the IAA containing solution (not shown). Excised “leaf strips” were prepared 0.5 mm wide with a double-bladed cutter from the central portion of each leaf blade as shown (C) of both first and second leaves. The excised leaf strips, one from each leaf pair, were floated either on control or IAA containing solutions as with detached leaves.
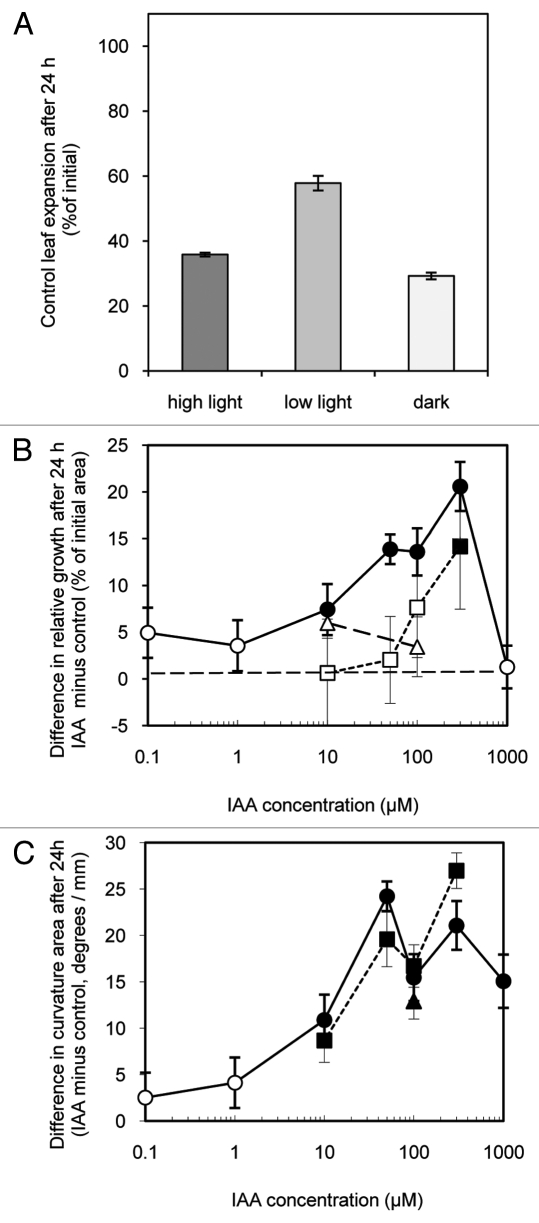
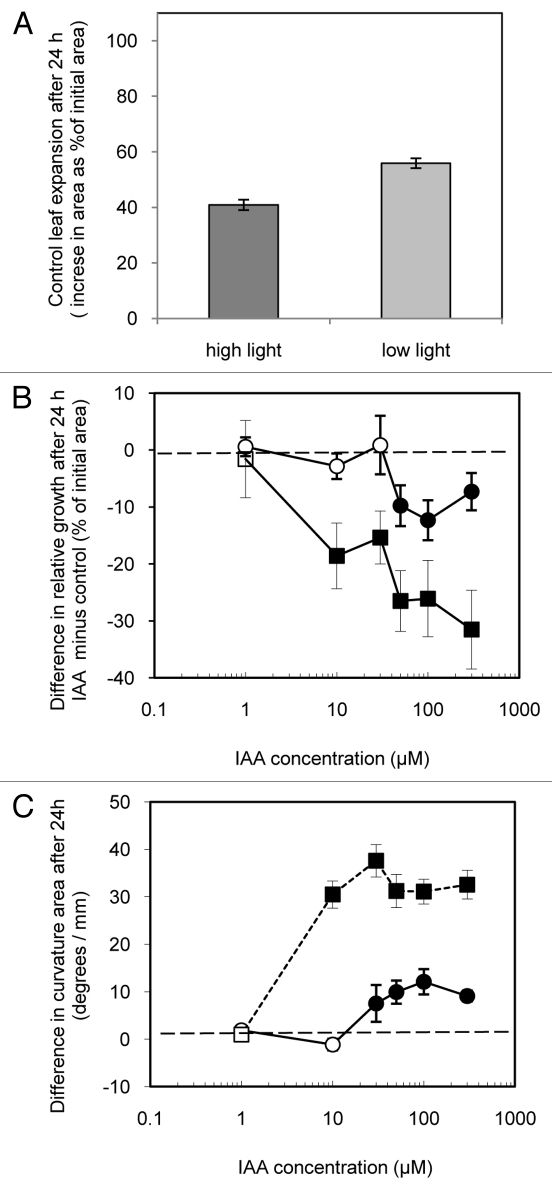
Figure 2 shows the effect of light conditions and of IAA on growth of excised leaf strips over 24 h. Control strips floated 24 h on a nutrient solution in continuous high light (275 µmol s−1 m−2), low light (135 µmol s−1 m−2) or in darkness grew significantly (Fig. 2A). Others have shown that growth of excised leaf tissues is enhanced by light.19 Here as well, growth was significantly greater in lower light and higher light conditions than in darkness. The high light treatment was evidently supra optimal for growth as these strips grew less than those in low light. Also, conspicuous anthocyanin accumulation, a hallmark of plant stress,20 occurred in the abaxial epidermis of the high light strips. Strips floated on a nutrient solution also including IAA tended to grow more than control strips (Fig. 2B). The growth of strips in the apparently stressful high light was most sensitive to IAA as strips treated with between 10 and 300 µM IAA grew significantly more than control strips. Strips treated with IAA in low light only grew significantly faster than controls in the highest concentration tested (300 µM), while strips treated in darkness showed no significant response at either of the tested IAA concentrations 10 and 100 µM). These results show that, as in Phaseolus and Nicotiana, IAA induces increased growth of Arabidopsis leaf strips though with a clear sensitivity to light conditions.
Figure 2.
Effect of light intensity and of IAA on the growth and epinastic curvature of leaf strips cut from expanding Arabidopsis leaves floated on either a control or IAA containing solutions in either high light (275 µmol s−1 m−2), low light (135 µmol s−1 m−2) or dark. Shown in A is growth (increase in area as % of initial area) of pooled control leaf strips. B shows the difference in relative growth between IAA treated and control leaf strips (i.e., the growth of each IAA treated strip minus that of the control strip from the same plant expressed as % of initial area) over 24 h in high light (●,○) low light (■,□) and dark (▲,Δ). C shows the difference (IAA treated minus control) in epinastic curvature (measured as the angle created by the tangent of terminal portion of each strip viewed in profile) expressed in degrees per mm of the same pairs of strips shown in B. Data are means +/− standard errors (n = 24). Significant differences (Student’s paired t-test) between IAA treated and controls leaf growth and curvature are indicated by filled symbols. Open symbols indicate no significant differences.
With other species (i.e., Nicotiana15-17 and Phaseolus14) IAA-induced leaf strip growth is always epinastic. Figure 2C shows that IAA treatment also produces epinasty in Arabidopsis leaf strips. The epinastic response was more sensitive to IAA than surface area growth as all IAA treatments of 10 µM or higher concentration produced measurable epinasty regardless of light treatment.
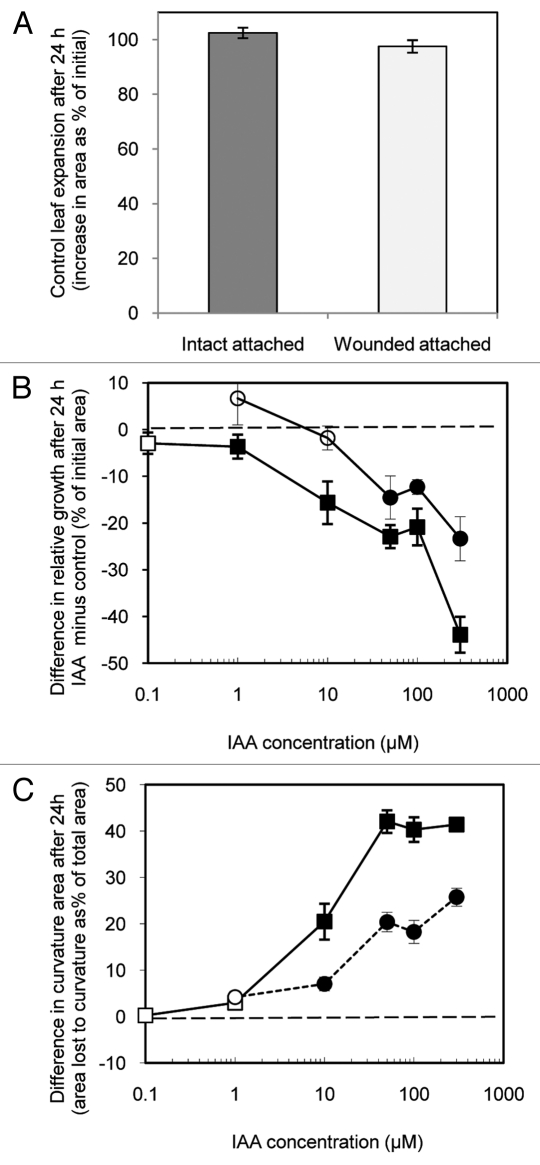
Figure 3 shows the effect of wounding and of IAA on growth and epinastic curvature of intact attached leaves (as shown in Figure 1A) and of wounded attached leaves (Fig. 1B) under low light conditions (135 µmol s−1 m−2) after 24 h. Intact attached leaves treated with control solution (on plants with an opposite IAA treated leaf) approximately doubled in size over 24 h (Fig. 3A), not significantly different for that of control leaves of plants in which both 1st and 2nd true leaves were treated with control solution (100.55 +/− 2.76%, n = 120). The leaves treated with control solution did, however, grow dramatically more than excised strips subjected to similar light conditions (Fig. 2A). Slower growth by strips might simply result from slower water uptake due to greater hydraulic resistance resulting from severed vasculature. Alternatively, altered metabolism within wounded strips might reduce the osmoregulation driving water uptake.
Figure 3.
Effects of (A) wounding on the expansion of attached Arabidopsis thaliana leaves, (B) IAA on the expansion of both intact attached and of wounded attached leaves, and (C) IAA on the curvature of both intact attached and of wounded attached leaves under low light. Shown in A is growth (increase in area as % of initial area) of the pooled values from control leaves after 24 h. B shows the difference in relative growth between IAA treated and control leaves (i.e., the growth of each IAA treated leaf minus that of the control leaf of the same plant expressed as % of initial area) over 24 h while intact attached (■,□) or while wounded attached (●,○). For the same leaf pairs, C shows the difference (IAA treated minus control) in epinastic curvature after 24 h as the difference in area before and after flattening expressed as % of the flattened area. Data are means +/− standard errors (n = 25–39). Significant differences (Student’s paired t-test) between IAA treated and controls leaf growth and curvature are indicated by filled symbols. Open symbols indicate no significant differences.
Wounding attached leaves appeared to have no consistent effect on subsequent control solution treated leaf expansion after 24 h (Fig. 3A). As well, the growth of control solution treated leaves, whether wounded or intact, did not appear to be effected by IAA treatment of the opposite leaf blade with IAA as comparison between groups differing in the IAA concentration administered to the opposite leaf blade found no consistent trends (data not shown).
IAA treatment of leaves slowed their growth relative to the control leaves on the same plants (Fig. 3B) after 24 h. This was especially true of the intact leaves which were growth inhibited by as little as 1 µM IAA. Wounded attached leaves were also growth-inhibited but only by concentrations of 50 µM and higher suggesting that wound stress decreases the growth inhibition sensitivity of leaves. Together these data indicate that, while wounding does appear to lessen the inhibiting effect of IAA on leaf blade growth, wounding alone is not sufficient to allow the IAA-induced growth seen with excised strips.
Applied at concentrations of 10 µM or more, whether to intact or wounded leaves, the growth inhibiting effect of IAA was associated with often dramatic epinastic leaf curvature after 24 h. This same effect was observed earlier with Phaseolus.10 Here (Fig. 3C) this epinastic effect (measured as the area of the flattened leaf minus the area presented by the leaf unflattened) was particularly dramatic with intact leaves treated with high concentrations of IAA.
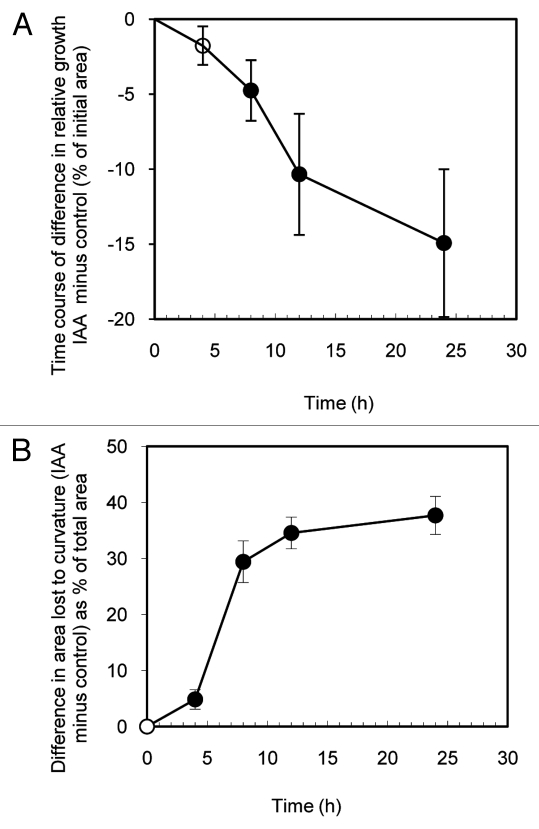
Treatment of intact attached Phaseolus leaves with IAA and other auxins results in growth inhibition and epinastic curvature within 24 h10,14 this is preceded, however, by an initial transient hyponasty. This response, which develops within two hours and lasts about six hours, has been well characterized10,21-25 and is due to an auxin-induced relative increase in abaxial surface growth13 associated with an initial surge in auxin-induced leaf growth.14Figure 4 shows that no similar response to the initial hyponastic growth surge seen in Phaseolus occurs in intact attached Arabidopsis leaves treated with 50 µM IAA. While significant IAA-induced epinasty is evident within 4 h and relatively slower growth is evident in IAA treated leaves within 8 h, no initial hyponasty is evident in these data or in casual observation of treated plants. The results suggest that the transient hyponasty seen with auxin treated Phaseolus leaves is not a general characteristic of all plant leaves.
Figure 4.
Time course for IAA-induced growth inhibition (A) and development of epinasty (B). Shown in A is the difference in relative growth between IAA treated (50 µM) and control intact attached leaves (i.e., the growth of each IAA treated leaf minus that of the control leaf of the same plant expressed as % of initial area). For the same leaf pairs, B shows the difference (IAA treated minus control) in epinastic curvature as the difference in area before and after flattening expressed as % of the flattened area. Data are means +/− standard errors (n = 24). Significant differences (Student’s paired t-test) between IAA treated and controls leaf growth and curvature are indicated by filled symbols. Open symbols indicate no significant differences.
Figure 5 shows the effect of light conditions and of IAA on growth and epinastic curvature of detached, but otherwise intact, leaves after 24 h. Detached leaves floated on control solution differed in growth depending on light treatment as those floated in low light (135 µmol s−1 m−2) grew significantly more than those floated in high light (275 µmol s−1 m−2). Growth of detached leaves was similar to that of excised strips grown under the same light conditions (Fig. 2A) and less than that of intact attached or wounded attached leaves (Fig. 3A). IAA treatment of detached leaves slowed their growth relative to the control leaves on the same plants (Fig. 5B) after 24 h. Leaves grown in low light were especially sensitive, growing significantly slower in as little as 10 µM IAA. Detached leaves grown in high light were inhibited by 50 µM or more IAA. As with attached leaves (wounded or intact) and with excised strips IAA treatment induced epinastic curvature with the response greatest by low light grown detached leaves (Fig. 5C). The results of these experiments with intact detached leaves suggested that detachment alone cannot be responsible for the growth-induction response seen in excised strips.
Figure 5.
Effects of light intensity and IAA on the growth and curvature of detached intact Arabidopsis thaliana leaves. Shown in A is growth (increase in area as % of initial area) of the pooled values from control leaves in high light (275 µmol s−1 m−2) and in low light (135 µmol s−1 m−2). B shows the difference in relative growth between IAA treated and control leaves (i.e., the growth of each IAA treated leaf minus that of the control leaf of the same plant expressed as % of initial area after 24 h while adaxial surface down in high light (●,○) or in low light (■,□). For the same leaf pairs, C shows the difference (IAA treated minus control) in epinastic curvature after 24 h as the difference in area before and after flattening expressed as % of the flattened area. Symbols in C as in B. Data are means +/− standard errors (n = 24). Significant differences (Student’s paired t-test) between IAA treated and controls leaf growth and curvature are indicated by filled symbols. Open symbols indicate no significant differences.
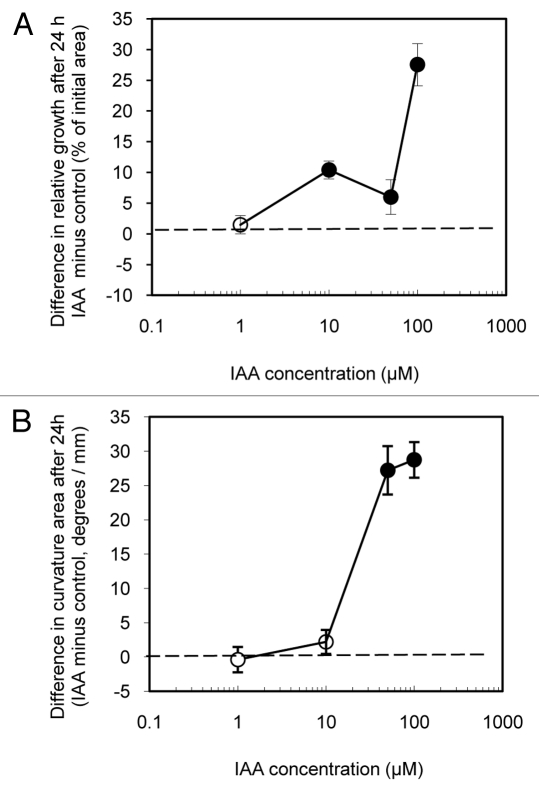
As the growth of detached leaves is generally inhibited by IAA while the growth of excised strips is increased or induced by IAA (above) it would seem that substantial wounding (i.e., more than just leaf detachment) is a requirement for the IAA growth induction response by Arabidopsis leaf blade tissue. A question then is whether wounded attached leaves as we prepared them (Fig. 1B) are sufficiently wounded to have a growth induction response when treated with IAA. Is the lack of IAA-induced growth found with those leaves due to continued attachment to the plant or due to insufficient wounding? Figure 6 shows the effect of IAA on the growth and epinastic curvature of wounded detached leaves (i.e., wounded as for wounded attached leaves then detached in the distal portion of the petiole) and floated on solutions for 24 h in low light (135 µmol s−1 m−2). Wounded detached leaves on control solutions grew 70.54 +/− 1. 26% over 24 h, less than intact attached or wounded attached leaves (Fig. 3A) but, perhaps surprisingly however, more than either control leaf strips (Fig. 2A) or intact detached leaves controls (Fig. 5A). It may be that the wound incisions, by disrupting the cuticle barrier, reduced the hydraulic resistance to water uptake thereby enhancing growth.
Figure 6.
Effects of wounding and IAA on the growth and curvature of wounded detached Arabidopsis thaliana leaves. A shows the difference in relative growth between IAA treated and control leaves (i.e., the growth of each IAA treated leaf minus that of the control leaf of the same plant expressed as % of initial area) after 24 h while adaxial surface down in high light while adaxial surface down in high light. For the same leaf pairs, B shows the difference (IAA treated minus control) in epinastic curvature after 24 h (measured as the area of the flattened leaf minus the area presented by the leaf unflattened) and expressed as the difference in area before and after flattening expressed as % of the flattened area. Data are means +/− standard errors (n = 24). Significant differences (Student’s paired t-test) between IAA treated and controls leaf growth and curvature are indicated by filled symbols. Open symbols, indicate no significant differences.
IAA treated wounded detached leaves (unlike detached intact leaves and like excised leaf strips) grew more relative to the control leaves on the same plants (Fig. 6A) over 24 h. Since the growth of wounded attached leaves is inhibited by IAA treatment while the growth of wounded attached leaves is induced by IAA, it is apparent that leaf blade detachment (separated from wounding) is a requirement for a growth induction response to IAA by Arabidopsis leaves. As with all other leaf blade preparations (i.e., strips, intact attached, wounded attached, detached intact), wounded detached leaves were made epinastic by IAA treatment over 24 h (Fig. 6B).
Wounding has been shown to induce a drop in leaf free IAA in tobacco leaves.26 One possible explanation for a wounding requirement for leaf blade growth induction by IAA could be that wounding lowers endogenous free IAA levels within leaves to below that optimal for growth. Thus exogenous IAA would restore the levels within the leaf to optimal levels and restore growth. We tested this possibility by analyzing leaf blade free IAA content in variously prepared leaves and leaf strips. Table 1 shows that the free IAA concentration in intact attached leaves treated with control solution for 0, 6, 12, or 24 h does not change significantly. The mean of sample values at the different times ranging from 20.3 to 33.2 ng free IAA / g fresh wt are consistent with other estimates of young leaf IAA levels.9,10Table 1 also shows that the free IAA content of freshly excised strips is approximately the same as that of entire leaves. Floating excised strips on control solution and subjecting them to high light (thus making them susceptible in IAA-induce growth [see above]) found the IAA content of excised strips not to change significantly after 6, 12 and 24 h. As well, the free IAA content of wounded attached leaves, detached leaves subjected to either high light or low light, wounded detached leaves subjected to low light was not significantly changed by these treatments. Only leaf strips subjected to low light for 24 h were found to have significantly different IAA content where the level was found to elevated relative to all other treatments. Together the results suggests that wounding does not lower endogenous free IAA content in Arabidopsis leaves or that the results of our growth studies can be otherwise explained by changing endogenous IAA levels. Also, it should be noted that the endogenous IAA levels measured here are low relative to the exogenous concentrations found effective for induction of growth and epinasty or inhibition of growth (i.e.10 µM in most experiments). Assuming Arabidopsis leaf blade tissue in our experiments to 95% water and that the hormone was uniformly distributed, the highest mean IAA content measured (e.g., 52.9 ng/g for excised strips in low light) would have the endogenous free IAA within the tissue at 0.32 µM. While effective permeation of the leaf by exogenous IAA in our experiments is a potential question, it would appear that the effects on leaf growth and curvature we report require raising the leaf free IAA by perhaps an order of magnitude or more.
Table 1. Effects of wounding, light treatment and detachment on leaf free IAA content of Arabidopsis thaliana leaves.
|
Leaf treatment |
Time |
|||
|---|---|---|---|---|
| 0 h | 6 h | 12 h | 24 h | |
| intact attached |
26.5 +/− 7.1 |
24.7 +/− 3.0 |
28.4 +/− 4.9 |
33.2 +/− 1.5 |
| wounded attached |
|
|
|
21.0 +/− 4.7 |
| detached (high light) |
|
|
|
24.3 +/− 2.1 |
| detached (low light) |
|
|
|
35.3 +/− 3.3 |
| wounded detached |
|
|
|
19.4 +/− 1.9 |
| strips (high light) |
27.4 +/− 6.1 |
20.2 +/− 1.4 |
23.3 +/− 4.7 |
36.2 +/− 3.4 |
| strips (low light) | 52.9 +/− 13.4 | |||
First and second true leaves of young seedlings were variously treated. Using a high-throughput method, free IAA was extracted with organic solvents and purified using two solid-phase extraction steps from flash frozen leaves/leaf strips that had been variously treated (see Figure 1) for the indicated times. Following methylation, tissue IAA was quantified by ion monitoring gas chromatography-mass spectrometry. Data are expressed as nanograms of free IAA per gram fresh weight, mean +/− SE (n = 4–19). Strips treated 24 h in low light were found to contain significantly higher IAA content than tissue from all other treatments. No other significant differences (Duncan’s multiple range test) between treatments were detected.
Discussion
The results reported here show that leaf strips excised from expanding Arabidopsis leaves (as with strips excised from leaves of Phaseolus14 and from Nicotiana15) were induced to grow faster by IAA. Also, as with Phaseolus,10 the growth of intact attached Arabidopsis leaves was inhibited by IAA (Fig. 3), although without (Fig. 4) the initial hyponastic growth surge found with Phaseolus leaves.13,14 Wounding attached Arabidopsis leaves did not reverse the effect on growth by IAA (Fig. 2), although the sensitivity of growth to inhibition by IAA is decreased by wounding. Detached intact leaves also respond to IAA treatment with inhibition of growth (Fig. 5) but wounded detached leaves are induced to grow faster by IAA (Fig. 6). Together these results indicate that growth induction of Arabidopsis leaf blade tissue by IAA requires both substantial wounding as well as detachment from the plant.
Both wounding and exogenous applications of IAA are known to induce the production of the plant hormone ethylene,27 suggesting the possibility that the growth effects seen here are induced by ethylene especially as elevated plant ethylene is associated with petiole epinasty in some plants.27 Earlier work, however, suggests ethylene induction is not responsible for either IAA-induced growth of excised leaf strips or for IAA-induced growth inhibition attached leaves. In Phaseolus, a poor correlation was found between ethylene biosynthesis and IAA-induced leaf strip growth and ethylene was unable to produce epinasty also in leaf strips.15 Growth inhibition by IAA of intact attached Phaseolus leaves was blocked by inhibition of ethylene. As well, the growth of attached leaves of ethylene insensitive Arabidopsis ein-4 mutants was inhibited by IAA no differently than was the growth of the attached leaves of wild type plants.
Light is known to induce leaf cell expansion28 with increased growth mediated by both increased photosynthesis as well as blue and red light receptors.19 In Phaseolus leaf discs light stimulated expansion is saturated by continuous white light at 100 µmol s−1 m−2. In the current study, leaf strip expansion was similarly optimal under “low light” conditions (135 µmol s−1 m−2) with “high light (275 µmol s−1 m−2) clearly supra optimal (Fig 2A).
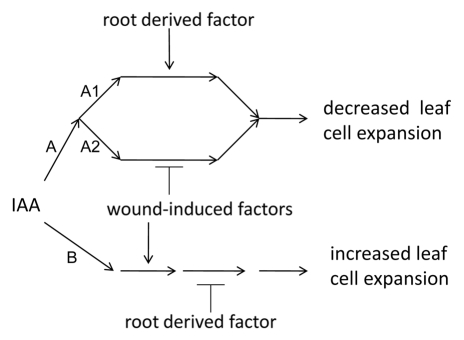
Our results suggest a model for the effect of elevated IAA on leaf blade expansion in Arabidopsis. We propose that elevation of IAA level within the leaf tissue leads to either decreased or increased cell expansion by two separate signal transduction pathways (A and B). Since leaves that are either unwounded or attached to the plant respond physiologically with decreased growth we suggest that two sub-pathways (A1 and A2) lead from IAA receptor binding to the a decrease in growth. The function of one of these sub-pathways (A1) is dependent on leaf attachment and presumably requires some continuously supplied root derived chemical factors (discussed below). The second sub-pathway (A2) is dependent on wounding and presumably requires the presence of wound-induced factors (discussed below). Since increased growth by leaf blade tissues requires both wounding as well as detachment a second signal transduction pathway leading to increased growth requires both presence of wound-induced factors and the absence of root derived factors. In our experiments, all IAA treatments that increased or decreased overall tissue expansion also produced epinastic curvature with the magnitude of the effect on growth, whether positive or negative, tightly correlated with the extent of curvature (Fig. 2C, 3C, 5C, 6B). When growth was slowed by IAA treatment (i.e., when tissue is either unwounded and/or attached to the plant), growth at the abaxial (lower) surface was slowed more than at the adaxial (upper) surface. When increased growth was induced by IAA treatment (i.e., when the tissue was both wounded and detached), growth at the adaxial (lower) surface was increased more than growth at the abaxial surface. These results suggests that pathways A and B not to be equally robust in the cells toward the adaxial and abaxial surfaces. In the adaxial epidermis (and perhaps immediately underlying cells) pathway B is apparently more sensitive to IAA than is the same pathway is in the cells of the abaxial epidermis (and underlying cells) while pathway A is more sensitive in the abaxial epidermis than in the adaxial epidermis.
The identity of an IAA receptor or receptors responsible for initiating pathways A and B (Fig. 7) are not apparent from our experiments. The principle IAA receptor proteins have been identified as TIR129,30 and related AFB1–3 proteins31 which function to accelerate ubiquitin mediated removal of specific transcription repressor proteins that, in turn regulate the expression of genes associated with auxin-response DNA domains.1 There is, however, considerable evidence for the existence additional receptors32 including auxin-binding protein 1 (ABP1). Though overexpression of ABP-1 has been shown to increase cell expansion in tobacco leaf strips18 and conditional repression of ABP-1 in Arabidopsis retards leaf expansion,5 it is not clear that this receptor is specifically associated with signal transduction leading to IAA-induced growth or that involvement of other receptors are precluded from involvement.
Figure 7.
Simple general model for the effect of elevated IAA on growth of expanding leaf blades of Arabidopsis. The results presented here suggest that IAA applied to leaf blade tissues initiates two signal transduction pathways. Binding of IAA to an undetermined receptor (or receptors) might initiate separate signal transduction events leading in one case to decreased leaf cell expansion (A) or to increased leaf blade cell expansion (B). Since decreased leaf growth results from IAA treatment when leaves either remained attached to the plant or when the leaf blade tissue is unwounded, pathway A would appear to branch through two parallel sub-pathways (A1 and A2). A1 is induced in some fashion by leaf attachment possible due to provision to the leaf of a root derived factor. A2 is inhibited in some fashion by leaf wounding possibly involving wound-induced factors. Pathway B leading to increased leaf blade expansion appears to be upregulated by wounding possibly through wound-induced factors and downregulated by leaf attachment possibly through removal of a root derived factor.
Leaf wounding represents a threat to plants often associated with pathogen attack that must be effectively responded to. Not surprisingly, the transcriptional response to leaf wounding in Arabidopsis involves a substantial portion of the plant’s genome.33 Interestingly, leaf wounding results in widespread downregulation of the expression of some IAA response genes.33 In tobacco, however, genes responsible for increasing the hypersensitive response to infection are upregulated following wounding by binding of a specific transcription factor to the associated auxin-response domains of these genes.34 How wounded-induced changes to the leaf gene expression environment contributes to a reversal in the growth response to IAA by expanding leaf cells is not clear.
In our results stress in the form of high light, sufficient to induce conspicuous anthocyanin expression, appeared to increase growth induction by IAA in the excised strips (Fig. 2B) and to decreased growth inhibition by IAA in detached intact leaves (Fig. 5B). It could be argued that the growth induction response to IAA by leaf strips in low light was simply less sensitive to IAA than high light treated leaf strips (Fig. 2B) because in low light control leaf strips grew much more than control strips in high light. Perhaps growth induction by IAA would be less evident in more rapidly growing tissues. Similarly, the reduced sensitivity of high light treated detached intact leaves to IAA growth inhibition compared with that of detached intact leaves in low light might be simply because control intact leaves in high light grew significantly less than control detached intact leaves in low light. This explanation is undermined by the effect of IAA on the growth of wounded detached leaves. Wounded detached leaves in low light without IAA grew more over 24 h (70.54 +/− 1. 26%) than either control excised strips (Fig. 2A) or detached leaves (Fig. 5A) yet could still evidence substantial growth enhancement treated with IAA (Fig. 6A). A more attractive explanation for our data are that light stress results in similar changes to the leaf blade gene expression environment as does wound stress. There is extensive crosstalk within the signal transduction and altered gene expression among different stress responses.35 Thus the gene expression environment induced in Arabidopsis leaves by wounding that results in reversing the physiological growth response to IAA from inhibiting growth to inducing growth would, at least in part, also be induced by high light stress. Perhaps other stresses (cold, salt, etc) could also enhance the IAA growth induction response by Arabidopsis leaves.
Perhaps the most interesting aspect of our results is the requirement that leaves be detached from the plant to experience IAA-induced growth. Implied is that an entity, continuously supplied by the rest of the plant, somehow interacts with IAA or auxin signal transduction in leaves of the intact plant to inhibit growth and preventing IAA-induced growth. Our results give few clues to the nature of such a signal. Conceivably the signal could be electrical36 but it seems unlikely that such signals would be continuously supplied. A chemical signal seems more likely. Such an entity could enter the leaf in either via phloem transport or the xylem stream. Long distance transfer of both mRNA and microRNA in the phloem is known to regulate gene expression.37,38 It seems likely, however, that the 1st and 2nd true leaves of Arabidopsis at the developmental stage we employed them in our experiments have already passed through the sink to source transition.39 If they had, a role for these molecules in controlling the IAA response of expanding leaves would seem unlikely. The xylem stream could provide a more likely source of any number of root-derived potential modulators of IAA responses including abscisic acid, cytokinins, strigolactones, as well as an as yet unidentified signal molecule(s) evidenced by the bypass mutant.40 Of these, cytokinins seem unpromising in this respect as cytokinin deficient Arabidopsis transformants have impaired leaf expansion due to both fewer as well as smaller cells.41
Wounding can affect the level of endogenous free IAA as well as the biosynthetic pathway utilized. For example, slicing potato tubers results in a doubling in tissue IAA level between 12 and 24 h.42 In tobacco, however, crushing injury to leaf blade tissue results in a two- to 3-fold drop in the IAA content of adjacent leaf tissue within 6 h. This lower IAA level is sustained through 24 h post wounding.26 This wound induced drop in leaf blade IAA levels is believed to induce both the plant defense gene encoding proteinase inhibitor II and nicotine production that occurs following wounding.43,44 In germinating bean, wounding by excision of the cotyledons resulted in a change in biosynthetic pathway from tryptophan dependent to tryptophan independent pathways and early excision doubled the rate of labeled anthranilate incorporation into IAA.45 Our analysis of Arabidopsis leaf blade IAA content (Table 1) does not support a role for changing endogenous IAA levels in explaining the results seen here as no significant changes in IAA levels were found over the course of 24 h in either intact attached leaves (which respond to exogenous IAA with decreased growth) or in excised leaf strips subjected to high light (which are induced to grow by IAA).
Materials and Methods
Plant material. Columbia (Col-0) wild type Arabidopsis thaliana L. seeds (Lehle Seeds, Round Rock, TX) were sown on moist potting medium (AIS (Enriched) Arabidopsis Growth Medium, Lehle Seeds, Round Rock, TX) in plug flats enclosed within plastic trays and clear plastic humidity domes (all from Morton’s Horticultural Products, McMinnville, TN). Following stratification at 4° for 4 d, enclosed plug flats were placed in a growth chamber (Econair Bigfoot, Winnipeg, Canada) at 19° under continuous light (approximately 135 µmol s−1 m−2). After 4 d, during which the seeds germinated and the seedlings established themselves, the humidity covers were removed and the plants were grown an additional 7–9 d.
Growth studies. For all experiments individual seedlings were selected with approximately equal sized 1st and 2nd true leaf pair, each leaf measuring 2.7 - 3.3 mm across the lamina at mid-blade (Fig. 1A). For the “intact attached” experiments, plants were first digitally imaged and then one leaf of the 1st and 2nd true leaf pair was administered a 5 µl droplet of control solution containing full strength Murishige and Skoog nutrients (Caisson Laboratories, Logan, UT) augmented with 10 mM KCl and pH 6 with 0.1 mM Mes/BTP. The osmolarity of control solution was determined by vapor pressure osmometer (Vapro 5520; Wescor, Logan, UT, USA) to be 102.4 +/− 0.6 mmol/kg. The other (treatment) leaf received the same solution but also containing IAA (prepared from IAA stock solutions brought to pH 6 with BTP) at a range of concentrations. Humidity domes replaced over the plants kept the solutions from evaporating. After 24 h both the control and experimental leaf of each plant was detached and imaged first unflattened and a second time unrolled and gently flattened by the weight of a glass microscope slide.
For wounded attached experiments plants were similarly treated as above except that before application of control and IAA droplets, 3 incisions were made to each leaf reaching from the leaf margin to close to the midvein (Fig. 1B).
For detached leaf experiments, both leaf blades of the true leaf pair were excised in the distal portion of the petiole and, after initial imaging, were floated individually, side down, in 3 ml of solution in 12 well tissue culture plates mounted on a rotary shaker (75 rpm) in either continuous high light (275 µmol s−1 m−2) or low light (135 µmol s−1 m−2) at 19°. One of each leaf pair was floated on the control solution and one on the IAA containing solution for 24 h after which they were patted dry and re-imaged unflattened and then flattened.
Excised leaf strips were prepared 0.5 mm wide with a double-bladed cutter from the central portion of each leaf blade leaf margin to leaf margin of both first and second leaves (Fig. 1C). The excised leaf strips, were first imaged gently flattened and subsequently were floated, one from each leaf pair, on control or IAA containing solutions as with detached leaves. After 24 h strips were reimaged both in profile (i.e., edge on) and them flattened.
For wounded detached experiments, leaves were wounded as for wounded attached (above), then imaged and floated, as described above, one of every leaf pair on control and one on IAA containing solutions for 24 h followed by reimaged as above.
Image J (public domain image analysis software; http://rsbweb.nih.gov/ij/) was used to quantify leaf and strip area before and after the 24 h treatment period. For analysis, the growth (increase in surface area as percent of initial area) of each control leaf was subtracted from that of the IAA treated leaf of the same plant. Curvature of strips was quantified as elsewhere15,17 as the angle created by the intersection of the tangents described by the terminal portions of each strip when viewed in profile. Leaf curvature (for intact attached, wounded attached, detached, and wounded detached experiments) was quantified as loss of area to curvature which was measured as the difference in leaf area before and after gentle leaf flattening following 24 h solution treatment. Analysis leaf curvature involved subtracting the curvature of the control leaf from that of the IAA treated leaf for each plant.
IAA quantification. Leaf blade tissue IAA was purified and quantified largely using a high-throughput method described previously.46 Briefly, samples of 0.023 -0.060 g consisting of variously pre-treated, flash-frozen, and pooled leaves or leaf strips were homogenized along with an internal standard ([13C6] IAA) in extraction buffer (65% isopropanol, 35% 0.2 M imidazole, pH 7.0). Following one hour incubation on ice and centrifugation, extracts were loaded into 96-well microplates on a sample rack of an automated liquid handler programmed for sample purification. Sample purification consisted of elution first from a conditioned amino anion exchange column and then elution from a polymethymethacrylate column with the purified IAA of each sample finally in methanol.
The IAA of each sample was then methylated by combining with ethereal diazomethane containing 10% methanol from the sample, dried under N2, and dissolved in 30 µL ethyl acetate. Actual IAA quantification was by GC-MS-selected ion monitoring as previously described by Ribnicky et al.47 using a model 6890N GC/5973Network MS (Agilent Technologies, Palo Alto, CA) equipped with a HP-5MS fused silica capillary column (Agilent Technologies). Injector temperature was at 280°C and an initial oven temperature of 70°C ramping 20°C min−1 to 280°C. The monitored ions were mass-to-charge ratio 130 and 136 (quinolinium ions from sample IAA and from the 13C6-labeled internal standard, respectively) and mass-to-charge ratio 189 and 195 for the corresponding molecular ions.
Statistical analysis. Analysis of within plant leaf and leaf strip growth and curvature data was by means of Student’s paired t-tests. Between plant growth and curvature data analysis and endogenous leaf IAA content data analysis were by means of one-way analysis of variance with means separated using Duncan’s multiple range test. All analyses were conducted using SPSS 17.0 (SPSS Inc., Chicago, IL).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
AKNOWLEDGEMENTS
Support for this project by National Institutes of Health (NIH, USA) Grant Number P20 RR016741 from the IDeA Network of Biomedical Research Excellence / Biomedical Research Infrastructure Network (INBRE/BRIN) Program of the National Center for Research Resources is gratefully acknowledged. Research at the University of Minnesota was supported by the National Science Foundation (grants MCB0725149 and IOSPGRP-0923960), the Minnesota Agricultural Experiment Station, and the Gordon and Margaret Bailey Endowment for Environmental Horticulture. The publication contents are the responsibility of the authors and conclusions drawn should not be seen as official views of the funding sources.
Footnotes
Current address: University of North Dakota School of Medicine and Health Sciences; Grand Forks, ND USA
Current address: Midwestern University College of Dental Medicine; Glendale, AZ USA
Current address: Illinois College of Optometry; Chicago, IL USA
¥Current address: Monsanto Company; Saint Louis, MO USA
Previously published online: www.landesbioscience.com/journals/psb/article/18026
References
- 1.Chapman EJ, Estelle M. Mechanism of auxin-regulated gene expression in plants. Annu Rev Genet. 2009;43:265–85. doi: 10.1146/annurev-genet-102108-134148. [DOI] [PubMed] [Google Scholar]
- 2.Reinhardt D, Mandel T, Kuhlemeier C. Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell. 2000;12:507–18. doi: 10.1105/tpc.12.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sieburth LE. Auxin is required for leaf vein pattern in Arabidopsis. Plant Physiol. 1999;121:1179–90. doi: 10.1104/pp.121.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ljung K, Bhalerao RP, Sandberg G. Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J. 2001;28:465–74. doi: 10.1046/j.1365-313X.2001.01173.x. [DOI] [PubMed] [Google Scholar]
- 5.Braun N, Wyrzykowska J, Muller P, David K, Couch D, et al. Conditional repression of AUXIN BINDING PROTEIN 1 reveals that it coordinates cell division and cell expansion during postembryonic shoot development in Arabidopsis and tobacco. Plant Cell. 2008;20:2746–62. doi: 10.1105/tpc.108.059048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poethig RS, Sussex AW. The cellular parameters of leaf development in tobacco: a clonal analysis. Planta. 1985;165:170–84. doi: 10.1007/BF00395039. [DOI] [PubMed] [Google Scholar]
- 7.Beemster GTS, De Veylder L, Vercruysse S, West G, Rombaut D, Van Hummelen P, et al. Genome-wide analysis of gene expression profiles associated with cell cycle transitions in growing organs of Arabidopsis. Plant Physiol. 2005;138:734–43. doi: 10.1104/pp.104.053884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seidel C, Walz A, Park S, Cohen JD, Ludwig-Müller J. Indole-3-acetic acid protein conjugates: novel players in auxin homeostasis. Plant Biol (Stuttg) 2006;8:340–5. doi: 10.1055/s-2006-923802. [DOI] [PubMed] [Google Scholar]
- 9.Sitbon F, Sundberg B, Olsson O, Sandberg G. Free and conjugated indoleacetic acid (IAA) contents in transgenic tobacco plants expressing the iaaM and iaaH IAA biosynthesis genes from Agrobacterium tumefaciens. Plant Physiol. 1991;95:480–5. doi: 10.1104/pp.95.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keller CP, Stahlberg R, Barkawi L, Cohen JD. Long-term inhibition by auxin of leaf blade expansion in bean and Arabidopsis. Plant Physiol. 2004;134:1217–26. doi: 10.1104/pp.103.032300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klee HJ, Horsch RB, Hinchee MA, Hein MB, Hoffman NL. The effects of overproduction of two Agrobacerium tumefaciens T-DNA auxin biosynthetic gene products in transgenic petunia plants. Genes Dev. 1987;1:86–96. doi: 10.1101/gad.1.1.86. [DOI] [Google Scholar]
- 12.Boerjan W, Cervera MT, Delarue M, Beeckman T, Dewitte W, Bellini C, et al. Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell. 1995;7:1405–19. doi: 10.1105/tpc.7.9.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayes AM, Lippincott JA. Growth and gravitational response in the development of leaf blade hyponasty. Am J Bot. 1976;63:383–7. doi: 10.2307/2441904. [DOI] [Google Scholar]
- 14.Keller CP. Leaf expansion in Phaseolus: transient auxin-induced growth increase. Physiol Plant. 2007;130:580–9. doi: 10.1111/j.1399-3054.2007.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keller CP, Van Volkenburgh E. Auxin-induced epinasty of tobacco leaf tissues. A non-ethylene mediated response. Plant Physiol. 1997;113:604–10. doi: 10.1104/pp.113.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller CP, Van Volkenburgh E, Van Volkenburgh E Evidence that auxin-induced growth of tobacco leaf tissues does not involve cell wall acidification. Plant Physiol. 1998;118:557–64. doi: 10.1104/pp.118.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones AM, Im K-H, Savka MA, Wu M-J, DeWitt NG, Shillito R, et al. Auxin-dependent cell expansion mediated by overexpressed auxin-binding protein 1. Science. 1998;282:1114–7. doi: 10.1126/science.282.5391.1114. [DOI] [PubMed] [Google Scholar]
- 18.Chen J-G, Shimomura S, Sitbon F, Sandberg G, Jones AM. The role of auxin-binding protein 1 in the expansion of tobacco leaf cells. Plant J. 2001;28:607–17. doi: 10.1046/j.1365-313x.2001.01152.x. [DOI] [PubMed] [Google Scholar]
- 19.Van Volkenburgh E, Cleland RE. Light-stimulated cell expansion in bean (Phaseolus vulgaris L.) leaves. I. Growth can occur without photosynthesis. Planta. 1990;182:72–6. doi: 10.1007/BF00239986. [DOI] [PubMed] [Google Scholar]
- 20.Winkel-Shirley B. Molecular genetics and control of anthocyanin expression. Adv Bot Res. 2002;37:75–94. doi: 10.1016/S0065-2296(02)37044-7. [DOI] [Google Scholar]
- 21.Lippincott BB, Lippincott JA. Auxin-induced hyponasty of the leaf blade of Phaseolus vulgaris. Am J Bot. 1971;58:817–26. doi: 10.2307/2441559. [DOI] [Google Scholar]
- 22.Hayes AB. Developmental aspects of leaf blade hyponasty. Bot Gaz. 1977;138:52–5. doi: 10.1086/336898. [DOI] [Google Scholar]
- 23.Hayes AB. Auxin-cytokinin effects in leaf blade hyponasty. Bot Gaz. 1978;139:385–9. doi: 10.1086/337015. [DOI] [Google Scholar]
- 24.Hayes AB. The interaction of auxin and ethylene in the maintenance of leaf bladeform in Phaseolus vulgaris L. var. Pinto. Am J Bot. 1981;68:733–40. doi: 10.2307/2443178. [DOI] [Google Scholar]
- 25.Hayes AM, Lippincott JA. The timing of and effect of temperature on auxin-induced hyponastic curvature of the bean primary leaf blade. Am J Bot. 1981;68:305–11. doi: 10.2307/2442766. [DOI] [Google Scholar]
- 26.Thornburg RW, Li X. Wounding Nicotiana tabacum leaves causes a decline in endogenous indole-3-acetic acid. Plant Physiol. 1991;96:802–5. doi: 10.1104/pp.96.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abeles FB, Morgan PW, Saltveit ME Jr. Ethylene in Plant Biology, 2nd ed. San Diego, California: Academic Press, 1992. [Google Scholar]
- 28.Van Volkenburgh E. Leaf expansion – an integrating plant behavior. Plant Cell Environ. 1999;22:1463–73. doi: 10.1046/j.1365-3040.1999.00514.x. [DOI] [Google Scholar]
- 29.Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;435:441–5. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]
- 30.Kepinski S, Leyser O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature. 2005;435:446–51. doi: 10.1038/nature03542. [DOI] [PubMed] [Google Scholar]
- 31.Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, et al. Plant development is regulated by a family of auxin receptor F box proteins. Dev Cell. 2005;9:109–19. doi: 10.1016/j.devcel.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 32.Shishova M, Lindberg S. A new perspective on auxin perception. J Plant Physiol. 2010;167:417–22. doi: 10.1016/j.jplph.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 33.Cheong YH, Chang H-S, Gupta R, Wang X, Zhu T, Luan S. Transcriptional profiling reveals novel interactions between wounding, pathogen, abiotic stress, and hormonal responses in Arabidopsis. Plant Physiol. 2002;129:661–77. doi: 10.1104/pp.002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung K-M, Sano H. Transactivation of wound-responsive genes containing the core sequence of the auxin-responsive element by a wound-induced protein kinase-activated transcription factor in tobacco plants. Plant Mol Biol. 2007;65:763–73. doi: 10.1007/s11103-007-9240-1. [DOI] [PubMed] [Google Scholar]
- 35.Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, et al. Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol. 2006;9:436–42. doi: 10.1016/j.pbi.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 36.Stahlberg R, Cosgrove DJ. The propagation of slow wave potentials in pea epicotyls. Plant Physiol. 1997;113:209–17. doi: 10.1104/pp.113.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lucas WJ, Ham B-K, Kim J-Y. Plasmodesmata - bridging the gap between neighboring plant cells. Trends Cell Biol. 2009;19:495–503. doi: 10.1016/j.tcb.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Chuck G, Candela H, Hake S. Big impacts by small RNAs in plant development. Curr Opin Plant Biol. 2009;12:81–6. doi: 10.1016/j.pbi.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 39.Turgeon R. Phloem loading: how leaves gain their independence. Bioscience. 2006;56:15–24. doi: 10.1641/0006-3568(2006)056[0015:PLHLGT]2.0.CO;2. [DOI] [Google Scholar]
- 40.Van Norman JM, Frederick RL, Sieburth LE. BYPASS1 negatively regulates a root-derived signal that controls plant architecture. Curr Biol. 2004;14:1739–46. doi: 10.1016/j.cub.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 41.Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmülling T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell. 2003;15:2532–50. doi: 10.1105/tpc.014928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fabbri AA, Fanelli C, Reverberi M, Ricelli A, Camera E, Urbanelli S, et al. Early physiological and cytological events induced by wounding in potato tuber. J Exp Bot. 2000;51:1267–75. doi: 10.1093/jexbot/51.348.1267. [DOI] [PubMed] [Google Scholar]
- 43.Kernan A, Thornburg RW. Auxin levels regulate the expression of a wound-inducible proteinase inhibitor II-chloramphenicol acetyl transferase gene fusion in vitro and in vivo. Plant Physiol. 1989;91:73–8. doi: 10.1104/pp.91.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi Q, Li C, Zhang F. Nicotine synthesis in Nicotiana tabacum L. induced by mechanical wounding is regulated by auxin. J Exp Bot. 2006;57:2899–907. doi: 10.1093/jxb/erl051. [DOI] [PubMed] [Google Scholar]
- 45.Sztein AE, Ilić N, Cohen JD, Cooke TJ. Indole-3-acetic acid biosynthesis in isolated axes from germinating bean seeds: The effect of wounding on the biosynthetic pathway. Plant Growth Regul. 2002;136:201–7. doi: 10.1023/A:1016586401506. [DOI] [Google Scholar]
- 46.Barkawi LS, Tam Y-Y, Tillman JA, Normanly J, Cohen JD. A high-throughput method for the quantitative analysis of auxins. Nat Protoc. 2010;5:1609–18. doi: 10.1038/nprot.2010.118. [DOI] [PubMed] [Google Scholar]
- 47.Ribnicky DM, Ilic N, Cohen JD, Cooke TJ. The effects of exogenous auxins on endogenous indole-3-acetic acid metabolism: the implications for carrot somatic embryogenesis. Plant Physiol. 1996;112:549–58. doi: 10.1104/pp.112.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]