Abstract
Gall-formers are parasitic organisms that manipulate plant traits for their own benefit. Galls have been shown to protect their inhabitants from natural enemies such as predators and parasitoids by various chemical and mechanical means. Much less attention, however, has been given to the possibility of defense against microbial pathogens in the humid and nutrient-rich gall environment. We found that the large, cauliflower-shaped, galls induced by the aphid Slavum wertheimae on buds of Pistacia atlantica trees express antibacterial and antifungal activities distinct from those found in leaves. Antibacterial activity was especially profound against Bacillus spp (a genus of many known insect pathogen) and against Pseudomonas aeruginosa (a known plant pathogen). Antifungal activity was also demonstrated against multiple filamentous fungi. Our results provide evidence for the protective antimicrobial role of galls. This remarkable antibacterial and antifungal activity in the galls of S. wertheimae may be of agricultural and pharmaceutical value.
Keywords: antibacterial, antifungal, antimicrobial, gall, multiple defenses, pathogens, Pistacia, Secondary metabolites, Slavum wertheimae
Introduction
Galling habits have evolved convergently among and within numerous insect lineages indicating that the phenomenon is highly adaptive. Galls are formed by manipulation of plant tissues by gall-forming insects to their own benefit.1,2 Galls provide their inhabitants with better nutrition and protection from harsh abiotic conditions such as temperature, precipitation and radiation. Galls also provide defense against natural enemies including predators and parasitoids.1,3-5 Pathogens may be an important mortality agent of galling insect.6,7 Fungi for example, which were isolated from galls, were recognized as a main indirect source of mortality of gall-forming insects as they destroy gall tissues.8-11 Accordingly, galled tissue was found to contain higher levels of defensive secondary metabolites compared with ungalled plant tissues.12,13 It is reasonable to expect that accumulation of such metabolites in the galls would provide multilevel protection against variable natural enemies, but its role in protection against microbial pathogens received little attention.
In the Levant, leaves and buds of wild pistachio (Pistacia spp) trees and shrubs serve as hosts for several gall-forming aphid species (Homoptera: Fordinae).14 The galls are long-lasting (from spring to fall), and in some species support up to thousands of aphids. Leaves of plants of the genus Pistacia, and especially their aphid-induced galls, contain high levels of secondary metabolites such as mono- and sesquiterpenes and pathogenesis-related (PR) proteins.15-17
In the present study, we focus on the gall-forming aphid Slavum wertheimae, which produces large cauliflower-shaped galls (Fig. 1) on the lateral buds of P. atlantica trees.5 These sealed galls may contain up to thousands of aphids for several months. The high humidity and availability of carbohydrates (including aphids’ secretions) inside the gall should make the aphids highly vulnerable to microbial and fungal infection. We examined and compared the activities of S. wertheimae galls and ungalled P. atlantica leaf tissues against various bacteria and fungi.
Figure 1.
Slavum wertheimae galls on Pistacia atlantica tree. Notice the characteristic starting of reddening at the tips of the gall.
Results
Antibacterial Activity
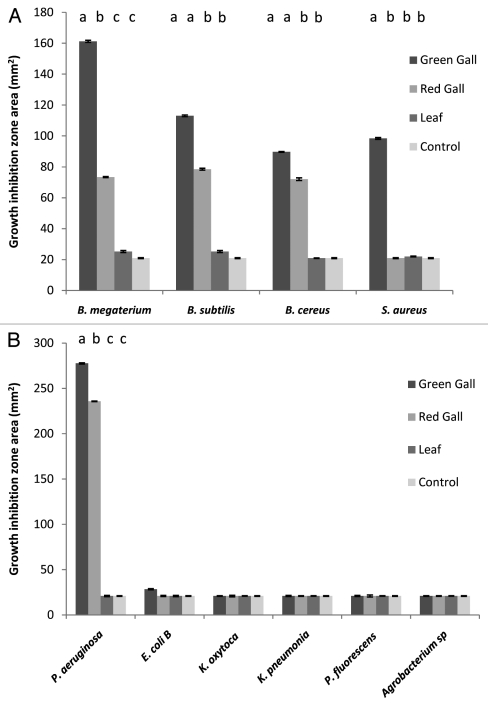
Since no specific bacterial pathogens of S. wertheimae or P. atlantica are known, we tested the effect of galls and leaf powder aqueous extract on representative bacteria including known plant (Pseudomonas. aeruginosa),18,19 and insect (Bacillus spp) pathogens.20 Both green and red galls but not leaf extracts demonstrated antibacterial activity against the Bacillus species tested (Fig. 2A). Green gall extract showed higher activity in all cases, and indeed was the only extract effective against S. aureus, but the difference was significant only for B. megaterium and S. aureus (Fig. 2A). Of the gram-negative bacteria, gall extract antibacterial activity was demonstrated only for P. aeruginosa with a small but significant difference between green and red gall extract (Fig. 2B).
Figure 2.
Average diameter of bacterial growth arrest halo formed around agarose disks containing green gall powder, red gall powder, or leaf powder (4.9 mg plant material powder in each disk) and control (distilled water). (A) Gram-positive bacteria; (B) Gram-negative bacteria. In all cases disk diameter = 5 mm; n = 3 for green and red gall and for control; n = 6 for leaf. Error bars denote one standard error. Different letters above bars indicate significant differences (Bonferroni Multiple Comparison Tests. p < 0.05). One-way ANOVA indicated significant differences between the four treatments for five bacteria species (B. megaterium F3,11 = 39.5, p < 0.001; B. subtilis: F3,11 = 23.7, p < 0.001; B. cereus: F3,11 = 48.4, p < 0.001); S. aureus F3,11 = 73.4, p < 0.001; P. aeruginosa F3,11 = 188.6, p < 0.001; whereas the differences in E. coli B, K. oxytoca, K. pneumonia, P. fluorescence and Agrobacterium were not significant (p > 0.05 in all cases).
Antifungal Activity
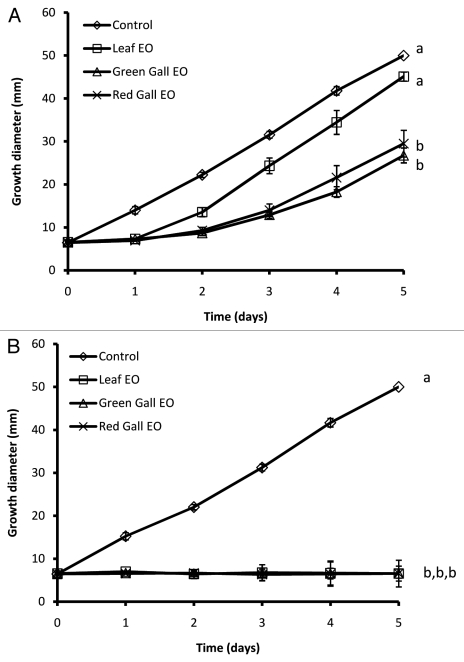
Since no specific fungal pathogens of S. wertheimae or P. atlantica are currently known, we tested fungi isolated from P. atlantica leaves as well as representative fungi. Both green and red galls had a much higher content of essential oils than leaves (~1.5% v/w for green galls, ~1.2% v/w for red galls, and ~0.08% v/w for leaves). Volatile constituents of the essential oils of galls delayed, although did not block, fungal growth in all tested fungal strains (Fig. Three and 4). Volatile constituents of leaf essential oils, on the other hand, interfered only with growth of the Penicillium sp (Fig. 3B). Although no Penicillium growth was seen during the experiment (Fig. 3B), transferring the Penicillium disks (after 5 d) to a fresh PDA plate allowed its re-growth (data not shown).
Figure 3.
Effects of volatile constituents of essential oils volatiles from Slavum wertheimae galls or from leaves on growth of two fungus strains, (A) Fomitopsis pinicola and (B) Penicillium sp isolated from P. atlantica leaves. n = 3 for each fungus-plant material combination; error bars denote one standard error; different letters indicate significant differences by day 5 (one-way ANOVA, followed by Bonferroni Multiple Comparison Tests; p < 0.05).
Discussion
The humid and carbohydrate-rich interior cavities of the S. wertheimae galls are likely to provide favorable conditions for pathogen development. A given gall may support thousands of aphids for several months, during which they secrete large amounts of honeydew,21-23 giving rise to high humidity and high sugar content that promote infestation by pathogens. Here we demonstrate that S. wertheimae galls have considerable antibacterial and antifungal properties distinct from those of intact leaves.
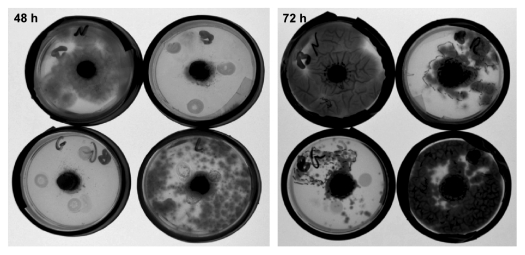
Interestingly, the antibacterial effectivity of gall extracts was especially notable against members of the Bacillus genus and against P. Aeruginosa, but not against another member of the Pseudomonas genus, P. fluorescence. Many members of the Bacillus genus are known insect pathogens,20 while P. aeruginosa is a plant pathogen.18,19 P. fluorescence, on the other hand, is known to promote plant growth by controlling pathogenic organisms.24 This suggests that the antibacterial activity is rather specific. On the fungi side, volatiles of gall essential oils had profound fungistatic effect against three filamentous fungi spp, two of which isolated from P. atlantica leaves (Figs. 3, 4). Although the exact P. atlantica originated fungi species was not determined, they have been identified as belonging to the Penicillium genus which is known to contain insect pathogenic species, and is a common gall invader,10 and the Aspergillus genus which is known to contain plant pathogenic species. The ecological and evolutionary relevancy of these findings needs further research.
Figure 4.
Representative Petri dish demonstrating the effects of volatiles constituents of essential oils from Slavum wertheimae galls and leaves on growth of Aspergillus sp isolated from P. atlantica leaves. Left, after 48 h of growth; right, after 72 h of growth. For all time points - clockwise from top left corner: control, essential oils from red gall, essential oils from leaves, essential oils from green gall. The dark circle in the middle of each plate is the seeded fungus.
It should be noted that previous studies have demonstrated antibacterial activity in organic extracts of Pistacia leaves.25,26 Although this seem to be of some contradiction to the results brought here (i.e., no detectable activity in the leaves), these studies used a much more coarse extraction methodology (e.g., very long organic solvent extracts) as opposed to our use of the more ecologically relevant water based extract.
The origin (i.e., endogenous metabolism of the galling insect vs. manipulation of host metabolism by the galling insect) was beyond the scope of the present study as was the identification of the antibacterial and antifungal compound(s). Nevertheless, aphid galls on Pistacia spp are known to accumulate large amounts of secondary metabolites compared with other plant parts, including mono-, sesqui- and triterpenes,14,16,27,28 tannins (unpublished data) and pathogenesis-related (PR) proteins.16 It is likely that a combination of these compounds provides the pronounced antimicrobial capacity of the galls.
We show that gall tissues have considerable negative effects on the growth of various bacterial and fungal pathogens. Our findings meet well with the “escape from enemy” hypothesis, which suggests that gall-inducing insects enjoy protection from various natural enemies,3 including microbial ones. These findings however, await deeper confirmation with natural S. wertheimae or Pistacia pathogens, once identified. Gall-formers are vulnerable to numerous predators (vertebrates and especially arthropods), parasitoids, and pathogens (fungi and bacteria).1,4,5,7-11,29 The presence of a variety of defensive compounds in the galls of Pistacia can provide a protective solution against this wide range of natural enemies. From a broader view, given the considerable diversity of gall forming insects,2 galls could greatly expand available sources for antimicrobial compounds for pharmaceutical and agricultural use.
Material and Methods
Host Plants and Gall-Forming Aphids
Pistacia atlantica (Anacardiaceae) is a deciduous dioecious tree of the eastern Mediterranean maquis and forests, and of the Irano-Turanian steppe. The tree serves as the exclusive host for the aphid Slavum wertheimae (Fordinae), which induces large, cauliflower-shaped galls (Fig. 1). The galls are formed on the lateral buds in the spring, and several months later in the fall, just before the galls open, they turn red. Shortly afterwards, thousands of winged aphids migrate away from the open mature galls.30 Because the gall's color transformation might be associated with changes in the chemical composition of the galls, our bioassays were performed on both green and red galls.
Collection and Preparation of Plant Material
In the fall (October 2009), we collected fully developed green galls, red galls, and leaves from five naturally growing P. atlantica trees located near the campus of Oranim College in the Lower Galilee (Israel) (32°42′ North, 35°7′ East). Galls and leaves from all trees were pooled and frozen (at -20°C) pending analysis. Just before the beginning of the experiments, the preserved plant material was ground in liquid nitrogen to a fine powder, using mortar and pestle.
Bacterial Strains
All bacterial strains tested in this study originated from the Oranim College culture collection. Since no specific bacterial pathogens of S. wertheimae or P. atlantica are known, we tested the effect of galls and leaf powder aqueous extract on representative bacteria. The Gram positive strains used were Bacillus megaterium, Bacillus subtilis, Bacillus cereus and Staphylococcus aureus. Bacillus strains were chosen for the study because many members of this genus are known to be insect pathogens.20 The Gram negative strains used were Pseudomonas aeruginosa (a known plant pathogen),18,19 Escherichia coli B., Klebsiella pneumonia, Klebsiella oxytoca, Agrobacterium sp and Pseudomonas fluorescence.
Fungal Strains
Since no specific fungal pathogens of S. wertheimae or P. atlantica are known, we tested the effect of essential oil hydrodistillated from galls and leaves on fungi isolated from P. atlantica leaves, and on selected strains from the Oranim College culture collection. Three strains were tested: Fomitopsis pinicola (a common wood decay fungus), Aspergillus sp (a known plant pathogen), and Penicillium sp (some species are known insect pathogens). The two latter strains were isolated from P. atlantica leaves.
Determination of Antibacterial Activities
Bactericidal activity was tested by a modification of the Kirby-Bauer disk-diffusion assay.31,32 Plant material powder (2 g wet weight) was mixed with 20 ml of 2% molten agarose (at 50°C) made with sterile water (giving 1:10 final w/v final concentration). The mixture was poured into a sterile empty Petri dish and allowed to solidify. As a control, the same mixture was prepared but plant powder was replaced by 2 g of distilled water. Tested bacteria were grown at 30°C with shaking (200 rpm), in liquid Lauria Bertoni (LB) broth to O.D.600~0.6, spread on solidified LB agar medium and allowed to dry for about half an hour. Circles were collected from the solidified agar/plant powder mix using a 5 mm alcohol-sterilized cork borer, weighed and placed on the bacterial culture. Disk weight was 49 (± 5) mg. Cultures were allowed to grow for 24 to 48 h at 30°C, and growth inhibition was estimated by measuring three randomly selected diameters around the disk. All experiments were performed in triplicate, except for the experiments with leaf powder, which were done in sextuplicate.
Determination of Essential Oil Volatiles Antifungal Activity
Essential oils were extracted from the preserved and powdered galls and leaves by hydrodistillation. Green or red gall powders (50 g) were boiled in 250 ml distilled water and essential oils were collected in the side arm of a Clevenger apparatus. Leaf powder was treated similarly, but in order to obtain a sufficient quantity of essential oils we used 250 g leaf powder in one liter of water. Antifungal activity of the volatile constituents was tested as follows: fungi were seeded on potato/dextrose/agar (PDA) medium by placing agar circles (5 mm diameter) from the edge of a growing colony on a 5-cm-diameter PDA plate with the fungus facing the agar. The plates were incubated for 12 h at 30°C to ensure fungal viability, and then 15 µl of essential oil from the leaf or the gall preparations was placed on the inside of the plate cover (final concentration 0.1% v/v) . The plate was immediately sealed with six layers of parafilm and a layer of commercial insulation tape to prevent loss of essential oil vapor. Control plates were seeded as above but no essential oils were added. Plates were incubated at 30°C and fungal growth was estimated daily by measuring the advance of the mycelial front from the agar disk edge on three different radii (for the F. pinicola, Fusarium sp and Penicillium sp) or by coverage (for the Aspergillus sp). All experiments were performed in triplicate.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Dr. Tamar Keasar and Prof. Ido Izhaki for fruitful discussions and assistance with the statistical analysis. We also thank Dr. Shai Markman for valuable comments on the manuscript. This research was supported by The Israel Science Foundation (grant No. 940/08 to MI) and by the Oranim college seed money grant program.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18031
References
- 1.Weis AE, Walton R, Crego CL. Reactive plant tissue sites and the population biology of gall makers. Annu Rev Entomol. 1988;33:467–86. doi: 10.1146/annurev.en.33.010188.002343. [DOI] [Google Scholar]
- 2.Shorthouse JD, Rohfritsch O. The biology of insect-induced galls. Oxford, UK: Oxford University Press, 1992. [Google Scholar]
- 3.Price PW, Fernandes WG, Waring GL. Adaptive nature of insect galls. Environ Entomol. 1987;16:15–24. [Google Scholar]
- 4.Stone GN, Sconrogge K. The adaptive significance of insect gall morphology. Trends Ecol Evol. 2003;18:512–22. doi: 10.1016/S0169-5347(03)00247-7. [DOI] [Google Scholar]
- 5.Inbar M, Izhaki I, Koplovich A, Lupo I, Silanikove N, Glasser T, et al. Why do many galls have conspicuous colors? A new hypothesis. Arthropod-Plant Interact. 2010;4:1–6. doi: 10.1007/s11829-009-9082-7. [DOI] [Google Scholar]
- 6.Hawkins BA, Cornell HV, Hochberg ME. Predators, parasitoids and pathogens as mortality agents in phytophagous insect populations. Ecology. 1997;78:2145–52. doi: 10.1890/0012-9658(1997)078[2145:PPAPAM]2.0.CO;2. [DOI] [Google Scholar]
- 7.Taper ML, Zimmerman EM, Case TJ. Sources of mortality for a cynipid gall-wasp (Dryocosmus dubiosus [Hymenoptera: Cynipidae]): the importance of the tannin/fungus interaction. Oecologia. 1986;68:437–45. doi: 10.1007/BF01036752. [DOI] [PubMed] [Google Scholar]
- 8.Butin H. Effect of endophytic fungi from oak (Quercus robur L.) on mortality of leaf inhabiting gall insects. Eur J Forest Pathol. 1992;22:237–46. doi: 10.1111/j.1439-0329.1992.tb00788.x. [DOI] [Google Scholar]
- 9.Fernandes WG, Price PW. The adaptive significance of insect gall distribution: survivorship of species in xeric and mesic habitats. Oecologia. 1992;90:14–20. doi: 10.1007/BF00317803. [DOI] [PubMed] [Google Scholar]
- 10.Wilson D. Fungal endophytes which invade insect galls: insect pathogens, benign saprophytes, or fungal inquilnes? Oecologia. 1995;103:255–60. doi: 10.1007/BF00329088. [DOI] [PubMed] [Google Scholar]
- 11.Gange AC, Croft R, Wu W. Gall insect and endophytic fungal co-occurrence in a xeric and mesic site. Ecol Entomol. 2002;27:362–5. doi: 10.1046/j.1365-2311.2002.00410.x. [DOI] [Google Scholar]
- 12.Cornell HV. The secondary chemistry and complex morphology of galls formed by the Cynipinae (Hymenoptera): why and how? Am Midl Nat. 1983;110:225–34. doi: 10.2307/2425263. [DOI] [Google Scholar]
- 13.Hartley SE. The chemical composition of plant galls: are levels of nutrients and secondary compounds controlled by the gall-former? Oecologia. 1998;113:492–501. doi: 10.1007/s004420050401. [DOI] [PubMed] [Google Scholar]
- 14.Wool D. Gall forming aphids. Pp. 11-58 In: Ananthakrishnan TN, ed. Biology of Gall Insects. New Delhi: Oxford & IBH, 1984, 11-58. [Google Scholar]
- 15.Caputo R, Lorenzo Mangoni L, Monaco P, Palumbo G. Triterpenes from the galls of Pistacia palaestina. Phytochem. 1979;18:896–8. doi: 10.1016/0031-9422(79)80046-1. [DOI] [Google Scholar]
- 16.Inbar M, Mayer RT, Doostdar H. Induced activity of pathogenesis related (PR) proteins in aphid galls. Symbiosis. 2003;34:293–300. [Google Scholar]
- 17.Flamini G, Bader A, Cioni PL, Katbeh-Bader A, Morelli I. Composition of the essential oil of leaves, galls, and ripe and unripe fruits of Jordanian Pistacia palaestina Boiss. J Agric Food Chem. 2004;52:572–6. doi: 10.1021/jf034773t. [DOI] [PubMed] [Google Scholar]
- 18.Elrod RP, Braun AC. Pseudomonas aeruginosa: Its rôle as a plant pathogen. J Bacteriol. 1942;44:633–45. doi: 10.1128/jb.44.6.633-645.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho JJ, Schroth MN, Kominos SD, Green SK. Ornamental plants as carriers of Pseudomonas aeruginosa. Phytopathology. 1975;65:425–31. doi: 10.1094/Phyto-65-425. [DOI] [Google Scholar]
- 20.Stahly D, Andrews RE, Yousten AA. The genus bacillus - insect pathogens. In: Stackebrandt E, Jones D, Reboli A, Farrar W, Holzapfel W, eds. The Prokaryotes, 2nd ed. New York: Springer, 2006; 563-608 [Google Scholar]
- 21.Inbar M, Schultz JC. Once again, insects worked it out first. Nature. 2001;414:147–8. doi: 10.1038/35102733. [DOI] [PubMed] [Google Scholar]
- 22.Pike N, Richard D, Foster W, Mahadevan L. How aphids lose their marbles. Proc Biol Sci. 2002;269:1211–5. doi: 10.1098/rspb.2002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wool D, Hendrixb DL, Shukrya O. Seasonal variation in honeydew sugar content of galling aphids (Aphidoidea: Pemphigidae: Fordinae) feeding on Pistacia: host ecology and aphid physiology. Basic Appl Ecol. 2006;7:141–51. doi: 10.1016/j.baae.2005.02.009. [DOI] [Google Scholar]
- 24.Ganeshana G, Kumarb MA. Pseudomonas fluorescence, a potential bacterial antagonist to control plant diseases. J Plant Interact. 2005;1:123–34. doi: 10.1080/17429140600907043. [DOI] [Google Scholar]
- 25.Benhammou N, Bekkara FA, Panovska TK. Antioxidant and antimicrobial activities of the Pistacia lentiscus and Pistacia atlantica extracts. J Pharm Pharmacol. 2008;2:22–8. [Google Scholar]
- 26.Rhouma A, Ben Daoud H, Ghanmi S. ben Salah H, Romdhane M, Demak M. Antimicrobial activities of leaf extracts of Pistacia and Schinus species against some plant pathogenic fungi and bacteria. J Plant Pathol. 2009;91:339–45. [Google Scholar]
- 27.Caputo R, Mangoni L, Monaco P, Palumbo G. Triterpenes of galls of Pistacia terebinthus: galls produced by Pemphigus utricularius. Phytochem. 1975;14:809–11. doi: 10.1016/0031-9422(75)83043-3. [DOI] [Google Scholar]
- 28.Fernández A, Camacho A, Fernández C, Altarejos J. Composition of the essential oils from galls and aerial parts of Pistacia lentiscus L. J Essent Oil Res 2000; 12: 19-23.
- 29.Abrahamson WG, Weis AE. Evolutionary ecology across three trophic levels: Goldenrods, gallmakers, and natural enemies. Princeton, New Jersey: Princeton University Press. 1997. [Google Scholar]
- 30.Wool D, Bogen R. Ecology of the gall-forming aphid, Slavum wertheimae, on Pistacia atlantica: population dynamics and differential herbivory. Isr J Zool. 1999;45:247–60. [Google Scholar]
- 31.Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–6. [PubMed] [Google Scholar]
- 32.Pitkin DH, Martin-Mazuelos E. Bioassay methods for antimicrobial and antifungal agents. In: Antimicrobial susceptibility testing protocols. Scwalbe R, Steele-Moore I, Goodwin AC, Eds. Boca Raton, USA: CRC Press, 2007: 313-40. [Google Scholar]