People have long speculated about the possibility of life in settings other than Earth. Only in the past few centuries, however, have we been able to conceive of the specific nature of such settings: other planets around our own sun and solar systems similar to our own elsewhere in the physical universe. Speculation on the nature of life elsewhere often has paid little heed to constraints imposed by the nature of biochemistry, however. A century of fanciful science fiction has resulted not only in social enthusiasm for the quest for extraterrestrial life, but also in fanciful notions of the chemical and physical forms that life can take, what the nature of life can be. Since the time of the Viking missions to Mars, in the mid-1970s, our view of life's diversity on Earth has expanded significantly, and we have a better understanding of the extreme conditions that limit life. Consequently, our search for extant life elsewhere in the solar system can now be conducted with broader perspective than before.
How can life be detected regardless of its nature and origin? Considering the recent spectacular advances in observational astronomy, it seems likely that the first sign of life elsewhere will be the spectroscopic detection of co-occurring nonequilibrium gases, for instance oxygen and methane, in the atmosphere of a planet around some distant star. Co-occurrence of such gases would indicate that they are replenished, perhaps most readily explained by the influence of life (1). By observation of oxygen and methane, Earth could possibly be seen as a home for life even from distant galaxies. Other potential habitats for life in this solar system, such as Mars and Europa, however, are not so obvious. The search for life on those bodies will be conducted at the level of analytical chemistry. As we undertake the detection of extraterrestrial life, it is instructive to try to put constraints on what the nature of life can be. These constraints, the requirements for life, tell us where and how to look for life, and the forms that it can take.
What Is Life?
An early question that needs to be confronted, indeed a question that in the last analysis requires definition, is: What is life? Most biologists would agree that self-replication, genetic continuity, is a fundamental trait of the life process. Systems that generally would be deemed nonbiological can exhibit a sort of self-replication, however (2). Examples would be the growth of a crystal lattice or a propagating clay structure. Crystals and clays propagate, unquestionably, but life they are not. There is no locus of genetic continuity, no organism. Such systems do not evolve, do not change in genetic ways to meet new challenges. Consequently, the definition of life should include the capacity for evolution as well as self-replication. Indeed, the mechanism of evolution—natural selection—is a consequence of the necessarily competing drives for self-replication that are manifest in all organisms. The definition based on those processes, then, would be that life is any self-replicating, evolving system.
The processes of self-replication and evolution are not reliably detectable, even in the terrestrial setting. Consequently, in the practical search for life elsewhere we need to incorporate information on the nature of the chemistries that can provide the basis for self-replication and evolution. Considering the properties of molecules likely to be needed to replicate and evolve, it is predictable that life that we encounter anywhere in the universe will be composed of organic chemicals that follow the same general principles as our own organic-based terrestrial life. The operational definition of life then becomes: Life is a self-replicating, evolving system expected to be based on organic chemistry.
Why Organic Chemistry?
The basic drive of life is to make more of itself. The chemical reactions required for the faithful propagation of a free-living organism necessarily require high degrees of specificity in the interactions of the molecules that carry out the propagation. Such specificity requires information, in the form of complex molecular structure—large molecules. The molecules that serve terrestrial organisms typically are very large, proteins and RNAs with molecular weights of thousands to millions of daltons, or even larger as in the case of genetic DNA. It is predictable that life, wherever we encounter it, will be composed of macromolecules.
Only two of the natural atoms, carbon and silicon, are known to serve as the backbones of molecules sufficiently large to carry biological information. Thought on the chemistry of life generally has focused on carbon as unique (3). As the structural basis for life, one of carbon's important features is that unlike silicon it can readily engage in the formation of chemical bonds with many other atoms, thereby allowing for the chemical versatility required to conduct the reactions of biological metabolism and propagation. The various organic functional groups, composed of hydrogen, oxygen, nitrogen, phosphorus, sulfur, and a host of metals, such as iron, magnesium, and zinc, provide the enormous diversity of chemical reactions necessarily catalyzed by a living organism. Silicon, in contrast, interacts with only a few other atoms, and the large silicon molecules are monotonous compared with the combinatorial universe of organic macromolecules.
Life also must capture energy and transform that energy into the chemistry of replication. The electronic properties of carbon, unlike silicon, readily allow the formation of double or even triple bonds with other atoms. These chemical bonds allow the capture and delocalization of electronic energy. Some carbon-containing compounds, therefore, can be highly polarized and thereby capture “resonance energy” and transform this chemical energy to do work or to produce new chemicals in a catalytic manner. The potential polarizability of organic compounds also contributes to the specificity of intermolecular interactions, because ionic and van der Waals complementarities can shift to mesh with or to repulse one another. Finally, it is critical that organic reactions, in contrast to silicon-based reactions, are broadly amenable to aqueous conditions. Several of its properties indicate that water is likely to be the milieu for life anywhere in the universe (2).
The likelihood that life throughout the universe is probably carbon-based is encouraged by the fact that carbon is one of the most abundant of the higher elements. Astronomical studies find complex organic compounds strewn throughout interstellar space. Moreover, the common occurrence of carbonaceous meteorites testifies to an organic-rich origin for our own solar system. If life indeed depends on the properties of carbon, then life is expected to occur only in association with second- or later-generation stars. This is because carbon is formed only in the hearts of former stars, so far as we know.
The Universal Nature of Biochemistry
Life as we know it builds simple organic molecules that are used as building blocks for large molecules. Amino acids are used to construct the long chains of proteins; simple sugars combine with the purine and pyrimidine bases and phosphate to construct the nucleic acids. It seems logical that the evolution of any organic-based life form would similarly result in the construction of complex molecules as repeating structures of simple subunits. Indeed, it seems likely that the basic building blocks of life anywhere will be similar to our own, in the generality if not in the detail. Thus, the 20 common amino acids are the simplest carbon structures imaginable that can deliver the functional groups used in life, with properties such as repeating structure (the peptide unit), reactivity with water, and intrinsic chirality. Moreover, amino acids are formed readily from simple organic compounds and occur in extraterrestrial bodies such as meteorites, so are likely to form in any setting that results in the development of chemical complexity necessary for life.
Similarly, the five-carbon sugars used in nucleic acids are likely to be repeated themes, perhaps in part because they are the smallest sugars that can cyclize and thereby confer spatial orientation on other molecules, for instance the purines and pyrimidines that comprise the genetic information of terrestrial organisms. Further, because of the unique abilities of purines and pyrimidines to interact with one another with particular specificity, these subunits, too, or something very similar to them, are likely to be common to life wherever it occurs. Differences in evolutionary systems likely will lie at the higher-order levels: the structures of the large molecules assembled from the simple units, and the mechanisms by which they are assembled and in which they participate.
Themes that are probably common to life everywhere extend beyond the building blocks. Energy transformation is a critical issue. The processes of life require the capture of adequate energy, from physical or chemical processes, to conduct the chemical transformations requisite for life. Based on thermodynamics there are only two such energy-capturing processes that can support “primary productivity,” the synthesis of biological materials from inorganic carbon dioxide. One process, termed lithotrophy, involves the oxidation and concomitant reduction of geochemical compounds. For instance, methanogenic organisms gain energy for growth by the use of hydrogen (H2) as a source of high-energy electrons, which are transferred to carbon dioxide (CO2), forming methane (CH4). Other microbes might use hydrogen sulfide (H2S) as an energy source, respiring with oxygen (O2), to produce sulfuric acid (H2SO4). It is thought that the earliest life on Earth relied on lithotrophic metabolism.
The second general process for obtaining energy, photosynthesis, captures light energy and converts it into energetic electrons that can be used to accomplish biochemical tasks. Photosynthesis arose early in the history of terrestrial life and probably drives most primary productivity on Earth today. The contribution of lithotrophy to terrestrial primary productivity remains unknown, however, because there currently is little information on such organisms that may be distributed throughout the Earth's crust, wherever the physical conditions permit.
Although terrestrial life and life that might arise independently of Earth are expected to use many similar, if not identical, building blocks, they also are expected to have some biochemical qualities that are unique. This expectation is based on the fact that different evolutionary lines of terrestrial evolution also have engendered novelties unique to those lines. Thus, the biochemistry of methanogenesis arose uniquely in Archaea, whereas the property of chlorophyll-based photosynthesis was invented among the phylogenetic domain Bacteria (below). The cytoskeleton, which is probably a requirement for large and complex cell structure arose in the eukaryotes. Considering the variety of Earth's life, novelty, as well as commonality, must be expected elsewhere.
The Physical Limits of Life: The Habitable Zone
Thought on where in the solar system life might occur was limited historically by the belief that life relies ultimately on light and warmth from the sun and, therefore, is restricted to the surfaces of planets. The inner boundary of the “habitable zone” in our solar system was considered as approximately between Earth and Venus, not so close to the sun as to be too hot for life. The outer boundary was considered to lie between Mars and Jupiter, not so far from the sun that the surface of a body would necessarily be frozen or receive too little light for efficient photosynthesis. Light probably is not directly required for life to arise, however, except as it may be involved in the formation of organic compounds during the accretion of a planetary system. On the other hand, the biological use of light energy, photosynthesis, may be a prerequisite for persistence of planetary life over billions of years. The reason for this conjecture is that light provides a continuous and relatively inexhaustible source of energy. Life that depends only on chemical energy inevitably will fail as resources diminish and cannot be renewed.
Nonetheless, we know that life occurs in Earth's crust, away from the direct influence of light, and that many organisms have metabolisms that function independently of light. Thus, the outer boundary of the potentially habitable zone extends into the far reaches of the solar system, to any rocky body with internal heating, regardless of its distance from the sun. [I specify “rocky” body to accommodate chemicals expected to be required for metabolism (below).] Life can persist in the absence of light by using inorganic energy sources, as do lithotrophic organisms, or organic sources deposited in planetary interiors during their accretion, as do heterotrophs (4). Therefore, rather than proximity to the sun, it seems more useful to define the habitable zone for life in terms of the chemical and physical conditions that are expected to be required for life. Our view of life's possible extremes currently is limited to the extremes of terrestrial life. Considering the intrinsic fragility of complex organic systems, coupled with the powerful force of natural selection, I venture that the physical limits of life are likely to be about the same anywhere in the universe. The window of chemical and physical settings that permit life are broad, however. Some important considerations are the following.
Chemical Setting.
Although the general energy requirement of life is a state of chemical disequilibrium, in which some oxidation-reduction reaction can occur, the specific thermodynamic requirements of biological energy-gathering strategies constrain the sites where life can occur. For example, a setting for lithotrophic organisms requires the occurrence of an appropriate mix of oxidized and reduced chemicals. Photosynthetic organisms require sufficient light of appropriate frequency. The light must be sufficiently energetic to support biosynthesis, but not so energetic as to be chemically destructive. These considerations constrain photosynthesis-based life to the spectral zone of about 300–1,500 nm in wavelength. (Terrestrial photosynthesis is limited to about 400–1,200 nm.) Beyond the requirements for energy metabolism and CO2 as a carbon source, terrestrial life requires only a few elements: H, N, P, O, S, and the suite of metals.
Physical Setting.
Physical constraints on life include temperature, pressure, and volume. The extreme diversity of terrestrial life probably provides an analog for life's diversity anywhere.
Temperature is a critical factor for life. Temperatures must be sufficiently high that reactions can occur, but not so high that that complex and relatively fragile biomolecules are destroyed. Moreover, because life probably depends universally on water, the temperature must be in a range for water to have the properties necessary for solute transfer. Water can be stabilized against boiling by pressure, but at too-low temperatures, water becomes crystalline and inconsistent with transport. Currently, the upper temperature record for culturable microbes is 112–113°C, held by hyperthermophilic archaeons of the genera Pyrolobus and Pyrodictium (5). Even the spectacularly durable bacterial endospore does not survive extended heating beyond ca. 120°C. The lower-temperature boundary for life is not established, but microbes are recoverable from ice, and growth of organisms has been detected in ice to −20°C (6). The physical properties of ice can allow solute diffusion at temperatures much lower than the freezing point (7). Thus, if based on aqueous organic chemistry, the temperature span for life anywhere in the universe is likely to be less than 200°C, within roughly −50° to 150°C.
Pressure and Volume.
The pressure required of a setting for life is probably limited at the lower end only by the vapor pressure required to maintain water or ice. An upper limit for pressure tolerance is unknown. Organisms on the terrestrial seafloor experience pressure over 1,000 atmospheres, and microbes recovered from deep oil wells are exposed to far higher pressures. The upper pressure limit for life probably is determined mainly by the effect of the pressure in reducing volume for occupancy. Life can be remarkably small, however. It is estimated that cells only a few hundred nanometers in diameter can contain all of the components considered necessary for life (8).
The expected commonality of chemistry in life's processes assists in life detection because it predicts that terrestrial types of biochemicals are useful targets for analysis even in an extraterrestrial setting. On the other hand, the expected similarity of terrestrial and potentially alien life complicates the interpretation of positive chemical tests for biochemicals. Thus, analyses of simple terrestrial-like biochemical compounds might not distinguish between a signal of life on one hand and an abiotically derived organic chemical, or between an alien life form and a terrestrial contaminant. Distinction between organisms with different evolutionary origins may require analysis of macromolecules and genes. Particularly, the nature and detail of the genetic information would be telling.
A Genetic Signature of Terrestrial Life
All life on Earth is genetically related through an evolutionary past that extends beyond 3.8 billion years ago. We see this relatedness in the many common structural and mechanistic features that make up all cells. The relationships between different terrestrial life forms are quantitatively explicit in the now-emerging maps of the course of evolution, phylogenetic trees based on DNA sequences. Even if potentially alien organisms were to present the same biochemistry as seen in terrestrial organisms, genetic sequences could provide criteria to distinguish them if they are of different evolutionary sources.
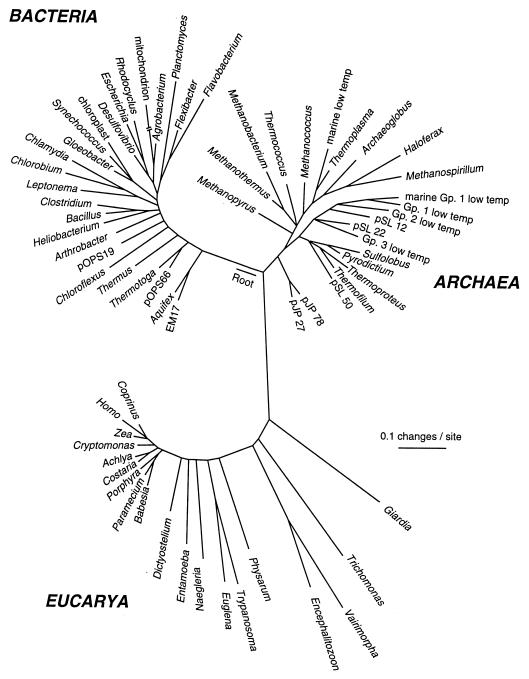
The gene sequence-based overview of terrestrial biological diversity is embodied in phylogenetic trees, relatedness diagrams such as that shown in Fig. 1 (9). The construction of a phylogenetic tree is conceptually simple. The number of differences between pairs of corresponding sequences from different organisms is taken to be some measure of the “evolutionary distance” that separates them. Pair-wise differences between the sequences of many organisms can be used to infer maps of the evolutionary paths that led to the modern-day sequences. The phylogenetic tree shown in Fig. 1 is based on small-subunit ribosomal RNA (rRNA) gene sequences, but the same topology results from comparing sequences of any other genes involved in the nucleic acid-based information-processing system of cells, the core of genetic continuity. On the other hand, phylogenetic trees based on metabolic genes, those involved in manipulation of small molecules and in interaction with the environment, sometimes are not congruent with the rRNA topology. Such genes do not offer any consistent alternative to the rRNA tree, however. Consequently, patterns that deviate from the rRNA tree probably are best interpreted to reflect lateral transfers of genes or even the intermixing of genomes in the course of evolution (10). The genome of any particular organism is comprised of genes derived evolutionarily from both vertical and lateral transmission.
Figure 1.
Universal phylogenetic tree based on small-subunit rRNA sequences. Sixty-four rRNA sequences representative of all known phylogenetic domains were aligned, and a tree was constructed by using the program fastdnaml, correcting for multiple and back mutations. That tree was modified to the composite one shown by trimming lineages and adjusting branch points to incorporate results of other analyses. Evolutionary distance (sequence difference) between the species shown is read along line segments. The scale bar corresponds to 0.1 changes per nucleotide. [Reproduced with permission from ref. 9 (Copyright 1997, American Association for the Advancement of Science).]
Phylogenetic trees are rough maps of the evolution and diversification of life on Earth. From the standpoint of both sequence divergence and complexity, most of Earth's life is seen to be microbial in nature, which is surely what we need to expect of life that might occur elsewhere in our solar system. Some of the conclusions that can be drawn from the molecular trees verify previously advanced biological hypotheses. For instance, the molecular trees confirm what was once a hypothesis, that the major organelles, mitochondria and chloroplasts, were derived from bacteria, proteobacteria and cyanobacteria, respectively. The biochemical trait of oxygenic photosynthesis arose with the cyanobacterial radiation (indicated in Fig. 1 by the lines leading to Synechococcus, Gloeobacter, and chloroplast). Because the cyanobacterial radiation is peripheral in the tree, early life must have been anaerobic and most of bacterial diversification must have happened before the availability of oxygen.
Other conclusions from the molecular trees clarify relationships among terrestrial life. The molecular trees show that Earth's main lines of descent fall into three relatedness groups, the “domains” of Archaea, Bacteria, and Eucarya (eukaryotes). The point of origin of the lines leading to the modern domains cannot be determined by using rRNA gene sequences alone. Comparison of gene family sequences such as the protein synthesis factors Ef-Tu and Ef-G, that diverged before the last common ancestor of life, however, indicate an origin deep on the bacterial line as shown in Fig. 1. This relationship means that Archaea and Eucarya shared common ancestry subsequent to the separation of their common ancestor from Bacteria. Biochemical properties of the organisms are consistent with this conclusion. For example, the transcription and translation machineries of modern-day representatives of Archaea and Eucarya are far more similar to one another than either is to corresponding functions in Bacteria. This result shows that the eucaryal nuclear line of descent is not a relatively recent derivative of symbiosis, rather, is as old as the line of Archaea. This result also indicates that the common textbook presentation of life as divided into two categories, prokaryote and eukaryote, is incomplete. Rather, terrestrial life is of three kinds: archaeal, bacterial, and eucaryal, distinct from one another in fundamental ways.
Gene sequences that are common to all organisms are incisive signatures of terrestrial origin. This is because organisms with independent origins are unlikely to have evolved identical genetic sequences, even if the chemical structures of the subunits that comprise the genetic information were identical. Thus, in gene sequences we can recognize terrestrial life, distinguish it from life derived from a different evolutionary origin even in the face of substantial biochemical similarity. This would become a significant issue if life—or its remains—were discovered on another body in the solar system.
Because planetary systems are formed by accretion, I think it unlikely that life on another body in the solar system arose independently of terrestrial life. It is now clear from meteorite studies that bodies can be transported from one planet to another, for instance from Mars to Earth, without excessive heating that would sterilize microbial organisms (11). Although such transfer events are now rare, they must have been far more frequent during the accretion of the planets. Large-scale infall, blasting ejecta throughout the forming solar system, probably extended until at least about 4 billion years ago and so probably overlapped with the processes that resulted in the origin of life. In principle, life, regardless of where it arose, could have survived interplanetary transport and seeded the solar system wherever conditions occur that are permissible to life. So, if we go to Mars or Europa and find living creatures there, and read their rRNA genes, we should not be surprised if the sequences fall into our own relatedness group, as articulated in the tree of life.
Acknowledgments
My research activities are supported by the National Institutes of Health, National Science Foundation, and the National Aeronautics and Space Administration Astrobiology Institute.
References
- 1.Committee on Planetary and Lunar Exploration; Space Studies Board; Commission on Physical Sciences, Mathematics and Applications; National Research Council. Strategy for the Detection and Study of Other Planetary Systems and Extrasolar Planetary Materials: 1990–2000. Washington, DC: Natl. Acad. Press; 1990. [Google Scholar]
- 2.Feinberg G, Shapiro R. Life Beyond Earth: The Intelligent Earthling's Guide to Life in the Universe. New York: Morrow; 1980. [Google Scholar]
- 3.Miller S L, Orgel L E. The Origins of Life on the Earth. Englewood Cliffs, NJ: Prentice–Hall; 1974. [Google Scholar]
- 4.Gold T. The Deep Hot Biosphere. New York: Springer; 1998. [Google Scholar]
- 5.Stetter K. FEBS Lett. 1999;452:22–25. doi: 10.1016/s0014-5793(99)00663-8. [DOI] [PubMed] [Google Scholar]
- 6.Rivkina E M, Friedmann E I, McKay C P, Gilichinsky D A. Appl Environ Microbiol. 2000;66:3230–3233. doi: 10.1128/aem.66.8.3230-3233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price B P. Proc Natl Acad Sci USA. 2000;97:1247–1251. doi: 10.1073/pnas.97.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Space Studies Board; Commission on Physical Sciences, Mathematics and Applications; National Research Council. Size Limits of Very Small Microorganisms: Proceedings of a Workshop. Washington, DC: Natl. Acad. Press; 1999. [Google Scholar]
- 9.Pace N R. Science. 1997;276:734–740. doi: 10.1126/science.276.5313.734. [DOI] [PubMed] [Google Scholar]
- 10.Woese C R. Proc Natl Acad Sci USA. 2000;97:8392–8396. doi: 10.1073/pnas.97.15.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKay D S, Gibson E K, Jr, Thomas-Keprta K L, Vali H, Romanek C S, Clemett S J, Chillier X D F, Maechling C R, Zare R N. Science. 1995;273:924–930. doi: 10.1126/science.273.5277.924. [DOI] [PubMed] [Google Scholar]