Abstract
Prolyl 4-hydroxylases are ascorbate-dependent oxygenases that play key roles in a variety of eukaryotic biological processes including oxygen sensing, siRNA regulation, and collagen folding. They perform their functions by catalyzing the post-translational hydroxylation of specific proline residues on target proteins to form (2S,4R)-4-hydroxyproline. Thus far, our ability to study these post-translational modifications has been limited by the lack of a prokaryotic recombinant expression system for producing hydroxylated proteins. By introducing a biosynthetic shunt to produce ascorbate-like molecules in Eschericia coli cells that heterologously express human prolyl 4-hydroxylase (P4H), we have created a strain of Escherichia coli that produces collagenous proteins with high levels of (2S,4R)-4-hydroxyproline. Using this new system, we have observed hydroxylation patterns indicative of a processive catalytic mode for P4H that is active even in the absence of ascorbate. Our results provide insights into P4H enzymology, and create a foundation for better understanding how post-translational hydroxylation affects proteins.
Dioxygenases dependent on α-ketoglutarate play diverse roles in a variety of eukaryotic biological processes, by catalyzing the irreversible post-translational hydroxylation of proteins (1). They catalyze the insertion of oxygen atoms from molecular oxygen into methylene C–H bonds on the side chains of various amino acids in proteins, resulting in hydroxylated versions of genetically encoded amino acids at their sites of action (2). Members of this enzyme family include lysyl and asparaginyl hydroxylases as well as various prolyl 3- and 4-hydroxylases with different peptidyl proline substrate preferences, including collagen prolyl 4-hydroxylase (P4H), which is the subject of this study.
P4H plays a critical role in the maturation and folding of collagen, the major component of vertebrate connective tissue. The folding of trimeric collagen, unlike canonical protein folding, is mediated by post-translational modification (3,4). At the primary sequence level, collagens comprise long stretches of X-Y-glycine amino acid triplets, where the most common residue in the X position is proline and in the Y position of mature collagen is (2S,4R)-4-hydroxyproline (Hyp) (5). P4H-mediated post-translational modification of Pro to Hyp in the Y position allows collagen to adopt its signature triple-helical fold by changing preferred bond angles of modified proline residues through stereoelectronic effects (6,7), allowing collagen monomers to align. Despite successful transfer of human collagen biosynthesis into unicellular eukaryotes such as yeast (8–10), many details of the collagen folding pathway remain unknown, and difficult to elucidate in hosts that contains a large number of native endoplasmic reticulum enzymes and chaperones. Thus, reconstitution of collagen folding pathways in a prokaryotic host will greatly increase our understanding of how these essential biomolecules assemble. We therefore embarked on a bottom-up approach to biosynthesizing collagenous materials in Escherichia coli.
Expression and purification of properly assembled P4H tetramers in the Origami B strain of Escherichia coli was previously described by Kersteen et al. (11), opening the possibility of producing folded collagen trimers in E. coli if P4H could be activated in the cytosol. Still, because P4H is dependent on both L-ascorbate and molecular oxygen for catalysis (Figure 1, panels a and b), significant obstacles to activating P4H in the cytosol of E. coli remained. E. coli does not biosynthesize L-ascorbate, and although E. coli does harbor genes for an ascorbate transporter system, the transporter is strictly regulated for expression under anaerobic conditions (12). However, it had been demonstrated that L-ascorbate and analogs could be biosynthesized in certain strains of E. coli by heterologously overexpressing the gene for D-arabinono-1,4-lactone oxidase (ALO) from Saccharomyces cerevisae (Figure 1, panel c), and feeding the bacteria sugar 1,4-lactones as a nutrient source (13). Moreover, P4H is known to be somewhat flexible with respect to accepting reducing agents that are structurally related to L-ascorbate (14). Because E. coli harbors at least one aldose sugar dehydrogenase (15), which has the potential of producing lactones capable of acting as ALO substrates, we reasoned that ALO could be used to biosynthesize ascorbate-like molecules that are capable of acting as P4H activators from endogenous substrates in the cytosol of E. coli.
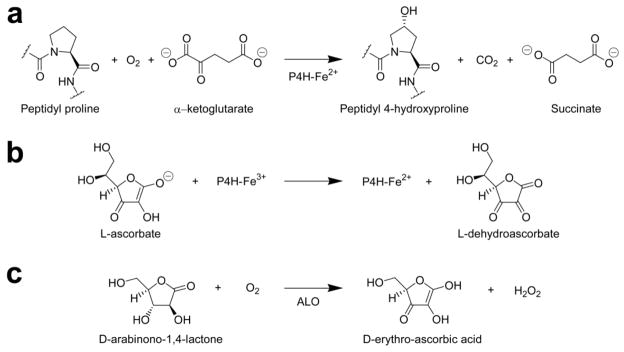
Figure 1. Reactions catalyzed by P4H and ALO.
(a) P4H catalyzes the formation of peptidyl 4-hydroxyproline from peptidyl proline and molecular oxygen. (b) In the process of catalysis, the catalytic Fe2+ ion of P4H is oxidized occasionally to Fe3+, which requires reduction by L-ascorbate for catalysis to continue. (c) ALO catalyzes the formation of ascorbate-like molecules from sugar-1,4-lactones. In the case shown, the reaction for which ALO is named, D-arabinono-1,4-lactone is reduced by ALO to D-erythro-ascorbic acid, a five-carbon analog of L-ascorbate.
To test the hypothesis that ALO could be used as an activator of P4H in E. coli, we first assembled a plasmid encoding both an expressable P4H activity reporter as well as ALO. The P4H activity reporter is composed of the P4H substrate peptide (Pro-Pro-Gly)5 fused at its C terminus to the purification tag glutathione-S-transferase, with an intervening thrombin cleavage sequence. The bifunctional plasmid (Supplementary Figure 1) was cotransformed into the Origami 2 strain of E. coli with the expression plasmid for heterotetrameric P4H previously described (11).
Cultures expressing the two plasmids were suspended in buffer and fed various sugar 1,4-lactones as a sole nutrient source, or no exogenous lactone. Resulting activity reporter peptides were purified from cell lysates by glutathione affinity, liberated from their purification tags by thrombin cleavage, and then analyzed for proline hydroxylation by liquid chromatography-mass spectrometry (LC-MS). We found that P4H was indeed cytosolically activated, and that hydroxylation levels in all samples were nearly identical, including in the sample to which no lactone was fed (Supplementary Figure 2). These data indicate not only that there is a cytosolic ALO substrate in E. coli under these conditions, but also that it is present in sufficient concentration to saturate the need of P4H when converted by ALO in the collagen expression system.
We then optimized a simple shake flask culture methodology for producing (Pro-Pro-Gly)5 repeats with high hydroxylation levels. The extent of hydroxylation observed for substrates produced in this manner was found to be strongly dependent on culture medium. We found that minimal medium yielded lower quantities of protein (Supplemental Table 1), but with higher hydroxylation levels than were obtained with rich medium (Figure 2, panels a and c–h). M9 minimal medium plus 0.4% w/v tryptone as the carbon source producing the highest hydroxylation levels at 71 ± 6 % of Y-position prolines modified. The trend of less rich medium leading to higher hydroxylation levels was seen reproducibly in experiments in which the amount of tryptone was varied, while other medium parameters were held constant (Supplementary Figure 3). On the basis of these results, we hypothesize that the hydroxylation level is modulated by concentration of nutrient source in the medium for one of two reasons: either (1) gene expression differences resulting from media composition difference can affect the production of the ALO substrate, or (2) aerobic respiration creates competiton for molecular oxygen that affects the activity of P4H and/or ALO in more nutritious medium. It is also possible that both of these factor play a role.
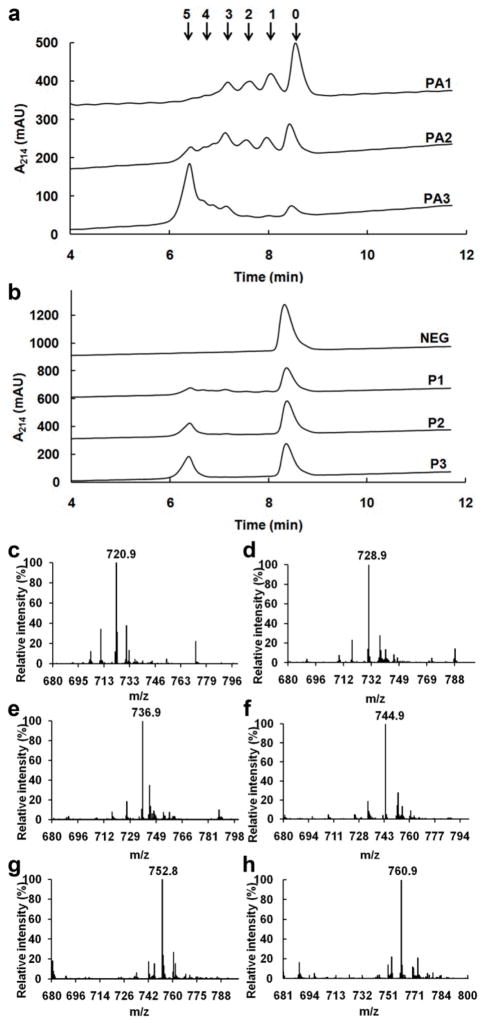
Figure 2. LC–MS analysis of (Pro-Pro-Gly)5 peptides cytosolically hydroxylated in E. coli under various conditions.
(a) UV absorbance chromatograms of (Pro-Pro-Gly)5 peptides from the cultures expressing both P4H and ALO in (“PA1”) Terrific Broth, (“PA2”) M9 minimal medium plus 0.4% w/v tryptone and 0.4% v/v glycerol, and (“PA3”) M9 minimal medium plus 0.4% w/v tryptone. (b) Cultures not expressing ALO: (“NEG”) expressing neither P4H nor ALO in Terrific Broth, (“P1”) expressing P4H only in Terrific Broth, (“P2”) expressing P4H only in M9 minimal medium plus 0.4% w/v tryptone and 0.4% v/v glycerol, and (“P3”) expressing P4H only in M9 minimal medium plus 0.4% w/v tryptone. Arrows indicate number of hydroxylated prolines in the associated peaks as determined by quadrupole mass analysis. Mass spectra of peaks with 0–5 hydroxyls are shown in parts c–h, respectively.
Cultures in which P4H was expressed without ALO in rich medium showed very low levels of hydroxylation, as expected. Yet, when the experiments were performed again using optimized minimal medium conditions, we found hydroxylation with a surprising “all-or-none” pattern. That is, the expressed (Pro-Pro-Gly)5 repeats were either nearly fully hydroxylated ((Pro-Hyp-Gly)5), or remained unhydroxylated ((Pro-Pro-Gly)5) (Figure 2, panel b). Experiments using longer P4H substrates showed a similar hydroxylation pattern (Supplementary Figure 4). These results strongly suggest the existence of a mode of processing Pro-Pro-Gly repeats by P4H that is both processive with respect to hydroxylation as well as sensitive to the ascorbate-like reducing agents produced by ALO. Still, no hydroxylation was observed in an in vitro experiment without supplemental ascorbate (Supplementary Figure 4), indicating that hydroxylation is mediated by factors present in the E. coli system that are not present in the in vitro system. Models for non-distributive processing of collagenous substrates by P4H have been postulated (16,17), but no evidence has previously been presented to support the existence of processive catalysis by P4H (18). Additionally, the notion that such an ascorbate-independent catalytic mode for human P4H could become active at low ascorbate levels has not, to our knowledge, been suggested. As ascorbate is an essential human nutrient (19,20); it is tempting to speculate that such a mode could be active in humans under conditions in which ascorbate levels are deficient. More studies will be necessary to determine what E coli. cytosolic factors mediate this newly observed phenomenon and to establish if such an ascorbate-independent mode is active in human biology.
In eukaryotes, proper hydroxylation of collagen manifests itself as an increase in thermostability. We therefore wanted to validate that the P4H-mediated hydroxylation observed in the new prokaryotic system would function for that purpose. Thus, we fused our (Pro-Pro-Gly)5 or (Pro-Pro-Gly)7 constructs to the C-terminus of the 27-amino acid T4-phage foldon domain, as was done previously for Pro-Pro-Gly synthetic peptide repeats in studies of collagen stability (21,22). The foldon domain forms a tight, obligate trimer. By keeping three individual strands associated and aligned at one end, the foldon domain overcomes the entropic barrier of stand association and stabilizes the triple helix formed by attached collagenous domains.
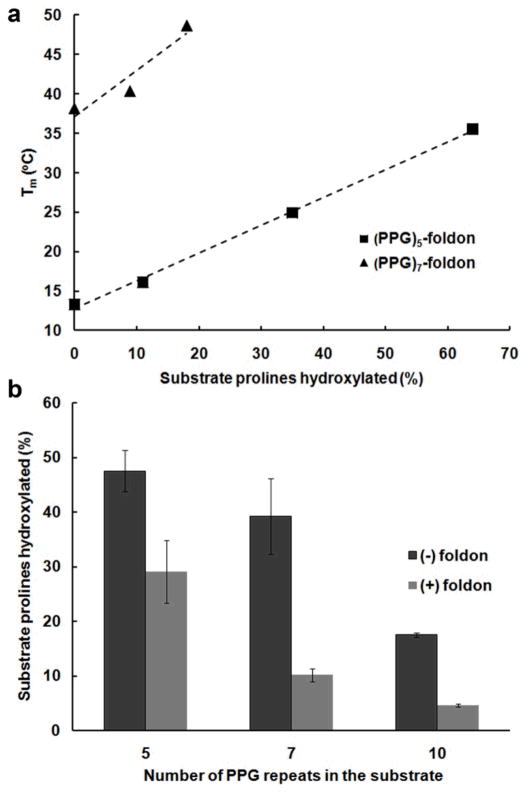
We hydroxylated the (Pro-Pro-Gly)5-foldon and (Pro-Pro-Gly)7-foldon constructs to different extents by varying culture conditions. Then, we measured the effect on thermostability by circular dichroism spectroscopy using a previously described method (23). Values of Tm, the midpoint temperature of the thermal transition between folded and unfolded states, were found to increase with hydroxylation level for both constructs (Figure 3, panel a), verifying that P4H-mediated hydroxylation in our recombinant system does indeed have the desired functional effect on collagenous material.
Figure 3. Triple helix formation by P4H-mediated hydroxylation of collagenous peptides in E. coli.
(a) Relationship between the Tm values of triple helical (Pro-Pro-Gly)5-foldon and (Pro-Pro-Gly)7-foldon, and their hydroxylation levels. Squares represent (Pro-Pro-Gly)5-foldon. Triangles represent (Pro-Pro-Gly)7-foldon. (b) Hydroxylation levels of (Pro-Pro-Gly)5, (Pro-Pro-Gly)5-foldon, (Pro-Pro-Gly)7, (Pro-Pro-Gly)7-foldon, (Pro-Pro-Gly)10, and (Pro-Pro-Gly)10-foldon constructs co-expressed with both P4H and ALO in E. coli, using M9 minimal medium plus 0.4% w/v tryptone and 0.4% v/v glycerol. Hydroxylation level is reported as the percentage of substrate prolines (proline in the Y position of X-Y-glycine repeats) that were hydroxylated.
Finally, we used the system to investigate how P4H interacts with collagen as it folds. We produced a series of (Pro-Pro-Gly)n constructs, where n = 5, 7, and 10, that were either expressed as fusions to a foldon domain or left unfused. The amino acid sequences and chain lengths of these constructs were chosen such that triple helical domains in the resulting proteins would exhibit a wide range of Tm values; unhydroxylated (Pro-Pro-Gly)10-foldon (Tm = 63 ± 2 °C) forms a triple helix with a Tm value well above that of the bacterial culture condition, while another construct, ((Pro-Pro-Gly)5) (24), is incapable of forming trimers under culture conditions, even when fully hydroxylated ((Pro-Hyp-Gly)5). We produced this series of collagenous polypeptides by co-expressing P4H and ALO in M9 minimal medium plus 0.4% w/v tryptone and 0.4% v/v glycerol. We found that foldon-fused Pro-Pro-Gly repeats consistently exhibited lower extents of hydroxylation than their unfused counterparts (Figure 3, panel b). Also, the longer Pro-Pro-Gly repeats were disproportionately less hydroxylated (Figure 3, panel b). These data indicate that, on the whole, P4H-mediated hydroxylation is dependent on the folded state of the collagenous material in the E. coli expression system. That is, as the collagenous material folds to adopt a triple-helical structure, it loses its ability to act as a P4H substrate. This phenomenon is consistent with a previous report suggesting that P4H operates on unfolded collagen strands in eukaryotic cells (25).
In conclusion, by introducing a biosynthetic shunt to produce ascorbate-like molecules, we have created a strain of E. coli that can produce recombinant proteins with high levels of hydroxylated proline residues. We have verified that hydroxylation of collagenous materials produced in the new system leads to an increase in thermostability and also that P4H interacts with collagenous materials as they fold in a manner similar to that of the eukaryotic system. Additionally, we present evidence for a processive mode of P4H catalysis that operates in the absence of ascorbate. Last but not least, the technology we describe should prove useful for producing and studying proteins that are hydroxylated by other ascorbate-dependent hydroxylases.
METHODS
For complete experimental methods including details of constructions of plasmids, protein expression and purification, and all sequences of DNA oligomers and peptides, please see Supporting Information.
Lactone Feeding
Plasmids expressing GST-(Pro-Pro-Gly)5, ALO, and P4H genes were co-transformed into Origami 2 (DE3) competent cells (Novagen). A starter culture was grown overnight in LB medium supplemented with 30 μg/ml Kanamycin and 200 μg/ml Ampicillin. The starter culture was used to inoculate flasks of Terrific Broth culture medium with appropriate antibiotics. The culture was incubated at 37 °C (250 rpm), and induced with 500 μM of isopropyl-1-thio-β-D-galactopyranoside (IPTG, US Biological) at OD600 = 1.6–1.8, and expressed at 23 °C (250 rpm) for 14–18 h. Cell pellets were collected, washed three times with Dulbecco’s phosphate buffered saline (DPBS), and then resuspended in DPBS for incubation with effectors.
These cell suspensions were split into 5 aliquots, and to each aliquot 250 μM Fe(II)SO4 was added, together with one of the following compounds at 10 mM: (1) D-arabinono-1,4-lactone, (2) L-galactono-1,4-lactone, (3) L-gulono-1,4-lactone, (4) L-ascorbic acid, or (5) nothing additional. The cell suspensions were incubated at 30 °C (250 rpm) for 3 hrs. Cell pellets were collected, washed three times with DPBS, resuspended in DPBS, and then lysed by sonication. The lysate supernatants were collected after centrifugation and the expressed GST-(Pro-Pro-Gly)5 was purified using glutathione affinity resin as described in “protein expression and purification” section of Supporting Information.
GST-(Pro-Pro-Gly)5 samples were then incubated with 50 U per mg-protein of thrombin to cleave GST tags from the (Pro-Pro-Gly)5 peptides at RT. After 2 h, the cleaved peptide was separated from GST by applying the proteolysis mix to a 10 kDa cut off Amicon protein concentrator (Millipore) and collecting the flow through.
Characterization of Hydroxylation
The concentrations of the purified proteins were determined by UV280 nm with a Nanodrop spectrophotometer (Thermo Scientific). In order to remove GST tags from the collagen like peptides, 4 units thrombin (MP Biomedical) was incubated with 75 μg of protein at RT for 2 h in a final volume of 60 μL in DPBS; 1 mM benzamidine (Sigma–Aldrich) was added to the mixture to stop the cleavage reaction. The samples were analyzed by LC–MS (Waters) equipped with a diode array detector as well as a quadrupole mass spectrometer, with a gradient of 5–95% Acetonitrile over 1 h. Quantitative determination of hydroxylation fraction was quantified by using the areas of extracted ion chromatograms. All relative ionization efficiencies (RIE) were set equal 1 after verification that the RIE for (Pro-Hyp-Gly)5 was greater than 80% of that for (Pro-Pro-Gly)5 by comparing the ionization peak areas with UV214 nm peak area in chromatogram in Fig. 2b. The percentage of substrate prolines hydroxylated (H.level %) was calculated using equation S1, in which n is the number of hydroxylated substrate prolines (note: only prolines in the Y position of X-Y-glycine repeats are considered as substrate prolines), nmax is the total number of substrate prolines, and An is the peak area in extracted ion chromatograms of peptide with hydroxylated proline number of n.
| (1) |
Characterization of Collagenous Material Thermostability
The proteins containing foldon were cleaved by Thrombin CleanCleave™ Kit (Sigma), and the cleaved products were separated from GST tag and uncleaved products by applying the mixture to glutathione affinity resin and collecting the flow through. The peptides were then concentrated using a 3 kDa cut off Amicon protein concentrator (Millipore), heated at 95 °C for 5 min, and applied to a 0.2 μm spin filter microcon (Millipore) to remove possible residual protein impurities. The purity of the products was checked by SDS–PAGE and analytical HPLC (Waters), and the final peptide concentration was determined by measuring UV280 nm in Nanodrop spectrophotometer and analytical HPLC.
The spectra of the peptides (55 μM in DPBS buffer) were acquired in Jasco J-815 CD spectrometer with a 1 mm pathlength quartz cell. The ellipticity at 210 (13) was then monitored from −10 °C to 80 °C as the temperature was increased at a rate of 1 °C per min. The thermal transition curve was defined as three phases: folded state, melting state, and unfolded state, which were each linearly fit. The value of Tm was determined as the temperature at the midpoint of the intersections.
Supplementary Material
Acknowledgments
We thank Professor Jennifer Cochran and Sarah Moore of Stanford University’s Department of Bioengineering for providing S. cerevisae cells used in this work. S.D. acknowledges the support of Crary Fellowship. A.E.B. acknowledges funding from her W.M. Keck chaired associate professorship in bioengineering for the support of this work. R.T.R. is grateful for support from grant R01 AR044276 (NIH).
Footnotes
Supporting Information. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Ozer A, Bruick RK. Non-heme dioxygenases: cellular sensors and regulators jelly rolled into one? Nat Chem Biol. 2007;3:144–153. doi: 10.1038/nchembio863. [DOI] [PubMed] [Google Scholar]
- 2.Loenarz C, Schofield CJ. Expanding chemical biology of 2-oxoglutarate oxygenases. Nat Chem Biol. 2008;4:152–156. doi: 10.1038/nchembio0308-152. [DOI] [PubMed] [Google Scholar]
- 3.Berg RA, Prockop DJ. The thermal transition of a non-hydroxylated form of collagen. Evidence for a role for hydroxyproline in stabilizing the triple-helix of collagen. Biochem Biophys Res Commun. 1973;52:115–120. doi: 10.1016/0006-291x(73)90961-3. [DOI] [PubMed] [Google Scholar]
- 4.Uitto J, Prockop DJ. Intracellular hydroxylation of non-helical protocollagen to form triple-helical procollagen and subsequent secretion of the molecule. Eur J Biochem. 1974;43:221–230. doi: 10.1111/j.1432-1033.1974.tb03403.x. [DOI] [PubMed] [Google Scholar]
- 5.Ramshaw JA, Shah NK, Brodsky B. Gly-X-Y tripeptide frequencies in collagen: a context for host-guest triple-helical peptides. J Struct Biol. 1998;122:86–91. doi: 10.1006/jsbi.1998.3977. [DOI] [PubMed] [Google Scholar]
- 6.Bretscher LE, Jenkins CL, Taylor KM, DeRider ML, Raines RT. Conformational stability of collagen relies on a stereoelectronic effect. J Am Chem Soc. 2001;123:777–778. doi: 10.1021/ja005542v. [DOI] [PubMed] [Google Scholar]
- 7.Hodges JA, Raines RT. Stereoelectronic and steric effects in the collagen triple helix: toward a code for strand association. J Am Chem Soc. 2005;127:15923–15932. doi: 10.1021/ja054674r. [DOI] [PubMed] [Google Scholar]
- 8.Myllyharju J, Nokelainen M, Vuorela A, Kivirikko KI. Expression of recombinant human type I-III collagens in the yeast Pichia pastoris. Biochem Soc Trans. 2000;28:353–357. [PubMed] [Google Scholar]
- 9.Olsen D, Yang C, Bodo M, Chang R, Leigh S, Baez J, Carmichael D, Perala M, Hamalainen ER, Jarvinen M, Polarek J. Recombinant collagen and gelatin for drug delivery. Adv Drug Deliv Rev. 2003;55:1547–1567. doi: 10.1016/j.addr.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Vuorela A, Myllyharju J, Nissi R, Pihlajaniemi T, Kivirikko KI. Assembly of human prolyl 4-hydroxylase and type III collagen in the yeast Pichia pastoris: formation of a stable enzyme tetramer requires coexpression with collagen and assembly of a stable collagen requires coexpression with prolyl 4-hydroxylase. EMBO J. 1997;16:6702–6712. doi: 10.1093/emboj/16.22.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kersteen EA, Higgin JJ, Raines RT. Production of human prolyl 4-hydroxylase in Escherichia coli. Protein Expr Purif. 2004;38:279–291. doi: 10.1016/j.pep.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Aboulwafa M, Smith MH, Saier MH., Jr The ascorbate transporter of Escherichia coli. J Bacteriol. 2003;185:2243–2250. doi: 10.1128/JB.185.7.2243-2250.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee BH, Huh WK, Kim ST, Lee JS, Kang SO. Bacterial production of D-erythroascorbic acid and L-ascorbic acid through functional expression of Saccharomyces cerevisiae D-arabinono-1,4-lactone oxidase in Escherichia coli. Appl Environ Microbiol. 1999;65:4685–4687. doi: 10.1128/aem.65.10.4685-4687.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tschank G, Sanders J, Baringhaus KH, Dallacker F, Kivirikko KI, Gunzler V. Structural requirements for the utilization of ascorbate analogues in the prolyl 4-hydroxylase reaction. Biochem J. 1994;300(Pt 1):75–79. doi: 10.1042/bj3000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Southall SM, Doel JJ, Richardson DJ, Oubrie A. Soluble aldose sugar dehydrogenase from Escherichia coli: a highly exposed active site conferring broad substrate specificity. J Biol Chem. 2006;281:30650–30659. doi: 10.1074/jbc.M601783200. [DOI] [PubMed] [Google Scholar]
- 16.de Jong L, van der Kraan I, deWaal A. The kinetics of the hydroxylation of procollagen by prolyl 4-hydroxylase. Proposal for a processive mechanism of binding of the dimeric hydroxylating enzyme in relation to the high kcat/Km ratio and a conformational requirement for hydroxylation of -X-Pro-Gly- sequences. Biochim Biophys Acta. 1991;1079:103–111. doi: 10.1016/0167-4838(91)90030-4. [DOI] [PubMed] [Google Scholar]
- 17.de Waal A, de Jong L. Processive action of the two peptide binding sites of prolyl 4-hydroxylase in the hydroxylation of procollagen. Biochemistry. 1988;27:150–155. doi: 10.1021/bi00401a023. [DOI] [PubMed] [Google Scholar]
- 18.Gorres KL, Raines RT. Prolyl 4-hydroxylase. Crit Rev Biochem Mol Biol. 45:106–124. doi: 10.3109/10409231003627991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandl J, Szarka A, Banhegyi G. Vitamin C: update on physiology and pharmacology. Br J Pharmacol. 2009;157:1097–1110. doi: 10.1111/j.1476-5381.2009.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pauling L. Evolution and the need for ascorbic acid. Proc Natl Acad Sci USA. 1970;67:1643–1648. doi: 10.1073/pnas.67.4.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frank S, Kammerer RA, Mechling D, Schulthess T, Landwehr R, Bann J, Guo Y, Lustig A, Bachinger HP, Engel J. Stabilization of short collagen-like triple helices by protein engineering. J Mol Biol. 2001;308:1081–1089. doi: 10.1006/jmbi.2001.4644. [DOI] [PubMed] [Google Scholar]
- 22.Jenkins CL, Raines RT. Insights on the conformational stability of collagen. Nat Prod Rep. 2002;19:49–59. doi: 10.1039/a903001h. [DOI] [PubMed] [Google Scholar]
- 23.Boudko S, Frank S, Kammerer RA, Stetefeld J, Schulthess T, Landwehr R, Lustig A, Bachinger HP, Engel J. Nucleation and propagation of the collagen triple helix in single-chain and trimerized peptides: transition from third to !rst order kinetics. J Mol Biol. 2002;317:459–470. doi: 10.1006/jmbi.2002.5439. [DOI] [PubMed] [Google Scholar]
- 24.Persikov AV, Ramshaw JA, Brodsky B. Prediction of collagen stability from amino acid sequence. J Biol Chem. 2005;280:19343–19349. doi: 10.1074/jbc.M501657200. [DOI] [PubMed] [Google Scholar]
- 25.Walmsley AR, Batten MR, Lad U, Bulleid NJ. Intracellular retention of procollagen within the endoplasmic reticulum is mediated by prolyl 4-hydroxylase. J Biol Chem. 1999;274:14884–14892. doi: 10.1074/jbc.274.21.14884. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.