Abstract
OBJECTIVE
To demonstrate the feasibility of performing optical coherence tomography of the human larynx on the awake patient with a novel flexible fiberoptic delivery system.
STUDY DESIGN
Prospective clinical trial.
SUBJECTS AND METHODS
Imaging was performed in 17 awake patients. A flexible optical coherence tomography probe was inserted through the nose and placed in near or gentle contact with laryngeal tissues under direct endoscopic visualization.
RESULTS
Images were successfully obtained from all laryngeal subsites and clearly identified laryngeal mucosal microanatomy. Several critical probe design modifications improved rotational and angular control of the distal tip while allowing linear translation of the probe and allowing more accurate apposition of the probe onto target tissues, which is critical for transnasal laryngeal imaging.
CONCLUSION
This study demonstrates the feasibility of awake transnasal laryngeal optical coherence tomography and identifies key instrumentation needed to obtain useful images.
The vocal fold consists of three anatomic layers, the epithelium, the lamina propria (LP), and the thyroary-tenoid muscle.1 The basement membrane is a microanatomic layer that separates the epithelium from the LP. Early laryngeal cancer is difficult to distinguish from benign disorders of the larynx, because, short of surgical biopsy under anesthesia, current examinations are unable to determine the presence of basement membrane (BM) invasion, which defines cancer.
Definitive diagnosis requires patients with questionable laryngeal lesions to bear the risks of general anesthesia, direct laryngoscopy, and biopsy. Patients may avoid the risks of surgical biopsy through the availability of office-based imaging techniques that are able to determine BM integrity.
Optical coherence tomography (OCT) is a new investigational imaging modality that uses near-infrared light to produce real-time, high-resolution (1 to 10 μm), cross-sectional images of in vivo tissue microstructure without tissue removal, cytotoxic fixation, or ionizing radiation.2 OCT is analogous to ultrasound except that, instead of acoustic properties, it relies on differences in tissue optical properties, which produce phase and intensity differences in backscattered light, to produce images. OCT systems are currently used in ophthalmology.3
In otolaryngology, it has been used to identify BM integrity in laryngeal cancer patients under general anesthesia.4 Otherwise its clinical applications have been limited in otolaryngology. It can become an indispensable clinical management tool whenever detailed information about sub-surface microstructure is essential for accurate diagnosis or treatment and when a surgical biopsy has the potential to produce significant morbidity, such as laryngeal lesions seen in the office. However, implementing OCT as an office-based screening measure for laryngeal cancer has been challenging because of its inherent optical limitations as evidenced by three reported cases in the literature to date.5 Because of its short focal length and need for steady positioning near target tissue, great control of the distal probe tip is required and has seemingly been elusive to date.
In the office setting, OCT has the potential to aid in diagnosing cancer, to monitor disease progression, and to direct biopsies. Its advantages include being performed without the need for general anesthesia or tissue removal. This study addresses the instrumentation designs necessary for reproducible imaging in this setting.
METHODS
Patient Population
Patients seen for other reasons in the office setting were examined in accordance with a University of California, Irvine IRB approved protocol.
OCT Core Instrumentation
The details of the core time-domain OCT device used in this study have been described previously6 and are summarily described here. The system in this study used a low-coherence light source (central wavelength λ = 1310 nm; JDS Uniphase, San Jose, CA).5 Raster scanned images were generated by controlled motion of the imaging fiber with a precision piezoelectric translation stage (model 663.4pr; Physik Instrumente, Tustin, CA). The optical fiber and optical elements, which comprise a small GRIN lens and prism, are enclosed by a transparent plastic tube. The axial resolution of the imaging system is determined by the coherence length of the light source and is approximately 7 μm. The lateral resolution is determined by diffraction and is approximately 10 μm. Signals were obtained up to a depth of 1.6 mm, whereas the lateral extent of each image was determined by the length over which the fiber was translated by the translation stage, typically 6 mm.
OCT Flexible Fiberoptic Delivery System
A platform was constructed to couple the piezoelectric translation stage with a commercially available fiberoptic rhinolaryngoscope (Olympus ENF-P4, Center Valley, PA). The platform allowed the position of the translation stage to be fixed anywhere alonga5cm axial distance relative to the rhinolaryngoscope. Furthermore, the platform provided for 360 degree rotation of the rhinolaryngoscope relative to the translation stage (Fig 1).
Figure 1.
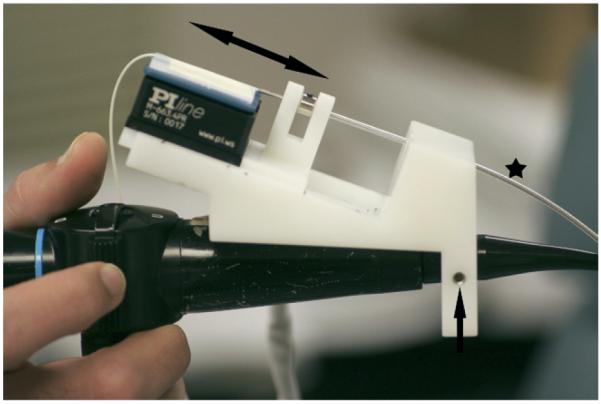
The platform used to couple the piezoelectric translation stage with the fiberoptic rhinolaryngoscope. (Double headed arrow indicates the to and fro motion of the translation stage; star, the optical plastic tube covering and the vertebrated tube that house the optical fiber; single headed arrow, the screw that allows rotation of the rhinolaryngoscope relative to the OCT probe.)
The OCT image plane is defined by the direction of light propagation, which is orthogonal to the long axis of the probe. This direction was marked just off of the distal tip of the probe. This tomographic image is analogous to the plane produced by a scalpel slicing through tissue, with the long axis of the probe represented by the handle of the scalpel, the axial translation of the probe tip represented by the to and fro movement of the blade, and the plane of light propagation represented by the angle of the scalpel on the tissue surface. Image quality is directly proportional to the proportion of reflected and back scattered light that could be detected, which required a near perpendicular plane to the tissue surface. This requirement made it necessary to accurately control the rotational orientation of the distal tip of the probe. To this end, the OCT optical fiber was passed through a flexible vertebrated tube. The distal tip of the vertebrated tube was glued to the optical fiber 5 cm removed from the prism and GRIN lens. This configuration permitted high-fidelity, accurate rotational control of the distal tip of optical fiber (inside the larynx) from a proximal location along its length (outside the nose).
Images were displayed continuously on a monitor at a frame rate of 1 Hz. By convention, increased backscatter of OCT light was depicted as increased whiteness on gray-scale imaging. OCT imaging was performed in tandem with direct endoscopic visualization, which allowed simultaneous viewing of the video and the OCT image on adjacent monitors (Fig 2). The images on the monitors were used to adjust probe position to improve image quality. To allow efficient adjustment of probe position over the tissues, two separate modifications in the delivery instrumentation were used. First, the tip of the vertebrated tube/optical probe unit (OCT probe) was coupled to the tip of the rhinolaryngoscope with a disposable, slide-on channeled endosheath (Vision Sciences 33-4401, Orangeburg, NY). This coupling allowed radial control of the distal tip of the OCT probe (Fig 3). Furthermore, the OCT probe was coupled to the translation stage with o-rings that afforded simultaneous rotational control of the distal tip of the optical OCT fiber during imaging. In essence, the o-rings functioned in a fashion similar to ball bearings. These modifications allowed multiple degrees of freedom that provided for dependable imaging in each patient.
Figure 2.
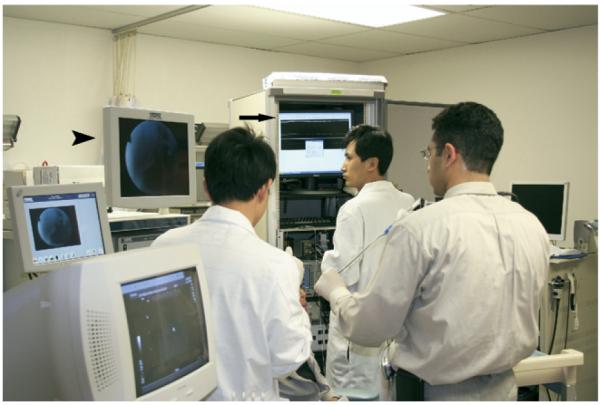
OCT imaging was performed in tandem with direct endoscopic visualization and allows simultaneous viewing of the endoscopic video (arrowhead) and the OCT image (arrow)on adjacent monitors.
Figure 3.
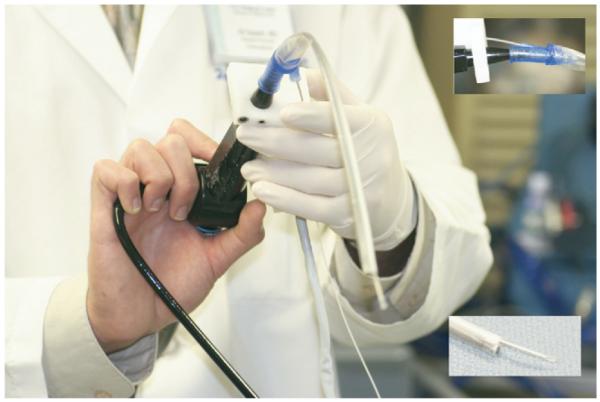
The slide-on channeled endosheath through which the rhinolaryngoscope and OCT probe were placed (top right inset) and coupled at their distal ends (bottom right inset).
OCT Imaging Technique
All equipment was assembled, calibrated, and properly sterilized before data acquisition. OCT imaging was performed while patients were awake in an office setting. The patient’s nose was first anesthetized and decongested with a lidocaine-oxymetazoline spray. Then a fiberoptic rhinolaryngoscope with a slide-on channeled endosheath was inserted through the nose to the supraglottis. The patient was asked to phonate while 4 mL of 4% lidocaine was slowly infused through the working channel of the endosheath in 1 mL increments to anesthetize the larynx. The device was removed from the nose and a flexible OCT probe was inserted into the working channel. The device was reinserted through the nose and placed in near or gentle contact with areas of interest. OCT and endoscopic images were captured continuously, recorded as video, and catalogued in a database.
RESULTS
Seventeen patients (11 male, 6 female; average age, 62 years; age range, 27 to 83 years) were examined. OCT images were obtained from 34 vocal cords and from all other laryngeal sub sites; including the interarytenoid mucosa, arytenoids, aryepiglottic folds, and false vocal cords. They clearly depicted the expected microstructures of the laryngeal mucosa and included a well-defined basement membrane, epithelium, LP, blood vessels, and minor glands with ducts terminating at the mucosal surface (Fig 4).
Figure 4.
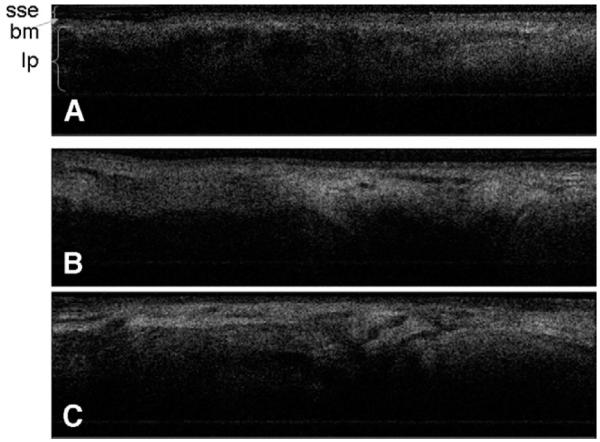
OCT images demonstrate representative laryngeal subsites. (A) Left true vocal cord; (B) right true vocal cord; (C) interarytenoid mucosa, note the increased glandular structures seen in this location. (sse, stratified squamous epithelium; bm, basement membrane; lp, lamina propria.)
The epithelium is a thin structure at the surface that demonstrates low signal intensity, which reflects less light scattering and is clinically consistent with the translucent appearance of the epithelium. In contrast, the LP demonstrates higher signal intensity because it is optically dense due to its extracellular matrix proteins (mainly collagen and elastin), blood vessels, and lymphatics.7 The heterogeneous regions of high (white) and low (black) signal intensity reflects the heterogeneity of the texture and appearance of tissue within the LP. The signal diminishes near a depth of 2 mm.
The patients tolerated the procedures well. The design modifications in the delivery system instrumentation were found to improve rotational and angular control of the distal tip while allowing linear translation of the probe (necessary for obtaining the tomographic image). The improved control allowed more accurate apposition of the probe onto target tissues and the predictability of obtaining excellent images in awake patients. The design modifications decreased total imaging time to approximately 10 minutes and decreased the equipment turnover time between patients to approximately 15 minutes (limited by the sterilization time of the transparent plastic tube used to house the optical fiber and optical elements). Once several of these tubes were made available, the turnover time decreased to approximately 10 minutes.
DISCUSSION
We present our experience in imaging normal larynges in 17 adult subjects. Present image guided therapies rely on CT, MRI, or ultrasound to diagnose or treat disease with 1 mm tissue resolution at best. OCT is unique in its ability to provide images of vocal cord microanatomy in vivo and is optimally suited to evaluate thin-layered structures such as those that line the surface of the larynx, because much laryngeal pathology is located within 1 to 2 mm of the mucosal surface. Furthermore, the images presented in Figure 4 are snapshots of tissue structure and do not demonstrate the full capabilities of the near real-time in vivo OCT imaging.
OCT will be most applicable as a useful adjunctive tool in the management of patients with potential T1 tumors of the larynx who may have dysplastic lesions or early microinvasive tumors. It is most suitable to help in their differential diagnosis. OCT does not reliably identify the integrity of the basement membrane in bulky lesions, because thick hypercellular tissues increase backscattering and limit light penetration into deeper layers of the specimen. However, this limitation is not clinically significant, as these lesions would undergo biopsy solely on clinical criteria.
Even with the current device, there is a definite learning curve to the procedure. It takes practice to navigate the probe, manipulate it efficiently, position it correctly, and hold it steady while looking at the endoscopic and OCT monitors all in an awake patient. Motion artifact can be problematic, especially in awake patients. Patient motion and instrument motion affect image quality because this system operates at 1 frame per second. Improvements in the design of the delivery system have improved the ability to obtain quality images anywhere in the larynx and decreased the time and patient discomfort needed to do so by improving probe stability and control. Future high-speed systems with faster image acquisition rates will help eliminate motion artifact. OCT has potential use to assist the diagnosis and staging of early laryngeal cancers, to determine the presence or absence of invasion, to select sites for biopsy in diffuse lesions that may have areas of invasion, as a guide for margin selection with resections, and to monitor treatment effect and surveillance for recurrence.
CONCLUSION
We present the largest study of office-based awake OCT imaging of the larynx published to date, thus demonstrating the feasibility of this technique. This is the first report of instrumentation designs and imaging techniques necessary for reproducible and predictable laryngeal OCT imaging on awake patients in the office, including correct positioning on target tissue, reorienting the angle or image plane to obtain other views, and reducing motion artifact. Office-based, fiberoptic trans-nasal OCT of the larynx in awake patients has the potential to aid in diagnosing laryngeal lesions, to provide more targeted biopsy of suspicious lesions, and to allow more objective noninvasive monitoring of disease progression and response to therapeutic interventions. Its advantages include being performed without the need for general anesthesia or tissue removal. In order to define the specificity of OCT in this setting, a larger cohort of patients with lesions clinically suspicious for early cancer is required.
ACKNOWLEDGMENTS
We would like to acknowledge the National Institutes of Health (DC 006026, CA 91717, EB 00293, RR 01192, M01-RR00827-28), Flight Attendant Medical Research Institute (32456), State of California Tobacco Related Disease Research Program (12RT-0113), and Air Force Office of Scientific Research (F49550-04-1-0101).
The U.S. Government is authorized to reproduce and distribute reprints for Governmental purposes notwithstanding any copyright notation. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of the Air Force Research Laboratory of the U.S. Government.
FINANCIAL DISCLOSURE
This work was supported by the National Institutes of Health (DC 006026, CA 91717, EB 00293, RR 01192, M01-RR00827-28), Flight Attendant Medical Research Institute (32456), State of California Tobacco Related Disease Research Program (12RT-0113), Air Force Office of Scientific Research (FA9550-04-1-0101). The sponsors did not play a role in study design, collection, analysis, and interpretation of data; writing of the report; and decision to submit the paper for publication.
Footnotes
Presented at the Annual Meeting of the American Academy of Otolaryngology–Head and Neck Surgery, Washington, DC, September 16-19, 2007.
AUTHOR CONTRIBUTIONS Ali Sepehr, wrote manuscript and performed imaging; William B. Armstrong, provided patients and expertise on laryngeal endoscopy; Shuguang Guo, provided assistance with optical system; Jianping Su, provided assistance with optical system; Jorge Perez, provided assistance with the physical instrumentation; Zhonping Chen, provided assistance with optical system; and Brian J.F. Wong, provided patients and expertise on laryngeal endoscopy, and directed intrument design.
REFERENCES
- 1.Hirano M. Phonosurgery: basic and clinical investigations. Otologica (Fukuoka) 1975;21:239–442. [Google Scholar]
- 2.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–81. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voo I, Mayrofrides EC, Puliafito CA. Clinical applications of optical coherence tomography for the diagnosis and management of macular diseases. Ophthalmol Clin North Am. 2004;17:21–31. doi: 10.1016/j.ohc.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong WB, Ridgway JM, Vokes DE, et al. Optical coherence tomography of laryngeal cancer. Laryngoscope. 2006;116:1107–13. doi: 10.1097/01.mlg.0000217539.27432.5a. [DOI] [PubMed] [Google Scholar]
- 5.Klein AM, Pierce MC, Zeitels SM, et al. Imaging the human vocal folds in vivo with optical coherence tomography: a preliminary experience. Ann Otol Rhinol Laryngol. 2006;115:277–84. doi: 10.1177/000348940611500405. [DOI] [PubMed] [Google Scholar]
- 6.Ren H, Ding Z, Zhao Y, et al. Phase-resolved functional optical coherence tomography: simultaneous imaging of in situ tissue structure, blood flow velocity, standard deviation, birefringence, and Stokes vectors in human skin. Optics Lett. 2002;27:1702–4. doi: 10.1364/ol.27.001702. [DOI] [PubMed] [Google Scholar]
- 7.Ridgway JM, Jackson RP, Guo S, et al. In vivo optical coherence tomography of the oral cavity and oropharynx. Arch Otolaryngol Head Neck Surg. 2006;132:1074–81. doi: 10.1001/archotol.132.10.1074. [DOI] [PubMed] [Google Scholar]


