Abstract
Objectives
Optical coherence tomography, an imaging modality using near-infrared light, produces crosssectional tissue images with a lateral pixel resolution of 10 μm. However, normative data is first needed on epithelial thickness for lesion characterisation, and, to date, little exists. The purpose of our study is to measure normal laryngeal epithelial thickness by in vivo optical coherence tomography, and compare these values to those obtained from fixed ex-vivo laryngectomy specimens.
Design and Setting
Prospective at a single medical center in California, United States.
Participants
A total of 116 patients undergoing operative endoscopy.
Main outcome measures
Optical coherence tomography images of clinically normal laryngeal subsites were selected. Calibrated measurements of epithelial thickness at various laryngeal subsites were recorded. Measurements of epithelial thickness from corresponding areas were obtained using optical micrometry on histologically normal regions of 15 total laryngectomy specimens. Descriptive statistics were performed.
Results
Mean epithelial optical coherence tomography thicknesses were: true vocal cords (81 μm), false vocal cords (78 μm), subglottis (61 μm), aryepiglottic folds (111 μm), laryngeal epiglottis (116 μm) and lingual epiglottis (170 μm). Epithelial thicknesses in fixed tissues were: true vocal cords (103 μm), false vocal cords (79 μm), aryepiglottic folds (205 μm) subglottis (61 μm), laryngeal epiglottis (38 μm) and lingual epiglottis (130 μm).
Conclusions
Optical coherence tomography does not have the artifacts associated with conventional histologic techniques. The inevitable development of office-based optical coherence tomography devices will increase the precision of laryngeal measurements and contribute to the clinical application of this technology in diagnosing laryngeal disease.
Optical coherence tomography (OCT) is a new imaging modality analogous to ultrasound, with the exception that light from low-coherence sources rather than sound is used to create cross-sectional images of tissue,1 with an axial resolution approaching that of light microscopy (approximately 7 μm). This technology is already in clinical use by ophthalmologists for examination of both retina and cornea.2 Applications in the areas of cardiology, dermatology, urology, gastroenterology, and dentistry are in development.3–7 In otolaryngology, OCT has been used to image the mucosa of the upper aerodigestive tract, the middle ear, the cochlea, and the thyroid gland.8–11 OCT imaging is becoming increasingly important technology for the upper airway, especially in the larynx, where perhaps is the most promising application of this technology within the upper aerodigestive tract, because OCT can identify key structural features such as the basement membrane.8,12–14 This is particularly appealing for the management of early laryngeal cancers, as distinguishing between benign lesions and early carcinoma can be difficult without biopsy. Laryngeal microsurgery carries numerous risks including scarring and permanent vocal quality changes, and these concerns combined with the difficulty of diagnosing cancer on clinical examination alone have made the decision to pursue surgery often a considerable undertaking.
Unlike advanced solid tumors of the head and neck whose boundaries and extension to other structures can be determined or estimated using CT or MR imaging, there are no imaging techniques in clinical use that can provide surgeons with the resolution needed to identify the axial spread of early cancer through the basement membrane and into the lamina propria. At present this information on tumor behavior at the tissue structural level requires a biopsy. OCT has potential to provide a means to image with high resolution (~7 μm) the vertical extension of early cancers and other neoplasms through the basement membrane of the delicate laryngeal mucosa. The potential clinical utility of OCT in the larynx has already been demonstrated in several publications. 8,12,14
However, while detailed OCT images of both benign and malignant laryngeal disease continue to be acquired and published, little is known about the normative microstructure of the laryngeal mucosa. This is important because the dimensions of the epithelium are generally thicker in early T1 cancers, dysplastic lesions, and benign disease, which may mimic cancer on clinical examination.8 If OCT is to evolve into a viable in vivo imaging modality to provide valuable information on the cross-sectional anatomy of the larynx, then it is absolutely necessary to know the dimensions of the tissue layers in the normal larynx. Just as in previous decades when CT and MRI images were compared with gross anatomy dissections and large whole body cross-sectional slices,15 with OCT the thickness of the different layers of the laryngeal mucosa measured in vivo using OCT must be compared with measurements made in ex-vivo histological specimens. The purpose of this study is to accomplish this basic task. It is important to emphasise that although the ideal study would be to correlate the in vivo OCT images with in vivo laryngeal histological specimens, but this is unethical, because it requires performing total laryngectomies on healthy patients after in vivo OCT images were taken.
Materials and methods
Ethical considerations
This study was approved by the Institutional Review Board of the University of California, Irvine.
Subjects
Optical coherence tomography imaging was performed in 116 patients undergoing surgical endoscopy of the upper aerodigestive tract under general anesthesia at the University of California, Irvine (UCI) Medical Center. The larynxes of 83 patients were imaged with OCT. While many patients exhibited some degree of laryngeal disease, thus the clinical indication for endoscopy, not all subsites in every patient were abnormal. Only normal subsites were used for this study, and laryngeal subsites with macroscopically abnormal epithelium (e.g., clinical appearance of carcinoma, inflammation, or acute trauma produced by intubation or suspension) on endoscopic examination were excluded. Thus OCT images were analysed from only 64 patients for this study. Information obtained from OCT imaging did not alter clinical decision- making in any way during the course of the study. Six of these 64 patients later underwent total laryngectomy, which provided direct histologic comparison with corresponding OCT images. All specimens were removed from the individuals undergoing salvage surgery for squamous cell carcinoma of the larynx following radiation therapy failure.
To increase the histologic measurements database, an additional nine total laryngectomy specimens were obtained from the Department of Pathology at UCI Medical Center. Histologic preparations from tumor-uninvolved regions of these specimens as well as the six referred to above, for a total of 15, were examined with light microscopy. Table 1 outlines the characteristics of the two patient populations: one which underwent OCT, and the other from which laryngectomy specimens were procured.
Table 1.
Characteristics of patients included in study
| LM, n (%) | OCT, n (%) | |
|---|---|---|
| Number in population | 15 | 64 |
| Gender: male | 10 (71) | 38 (60) |
| Mean age (years) | 66 | 61 |
| Confirmed histological diagnosis of carcinoma (laryngeal origin or locally invasive from thyroid) | 14 (100) | 35 (56) |
LM, light microscopy; OCT, optical coherence tomography.
OCT measurements
The OCT system used and the optical principles governing its performance have been described previously, 16,17 and will only be briefly reviewed here. A low coherence light source with central wavelength of λ = 1310 nm was used (AFC BT 1020, JDS Uniphase, San Jose, CA, USA). The axial resolution of this system in tissue is 7 μm and is determined by the coherence length of the optical source. The lateral resolution is diffraction-limited (10 μm). A handheld probe containing the OCT fiber was placed through the laryngoscope bore (with or without the use of laryngeal suspension depending on the clinical need) in near, or more often, light, contact with the area of interest. Raster scanned images were generated by controlled motion of the imaging fiber using a piezoelectric stage (Model 663.4pr, Physik Instrumente, Tustin, CA, USA). In constructing these images a refractive index of 1.4, chosen to approximate that of most soft tissues in the body, was assumed.18,19 Signals were acquired up to a depth of 1.6 mm while the lateral extent of each image was determined by the length over which the fiber was translated by the stage, typically 6 mm. OCT images were acquired with the left and right side of each image representing proximal (superior) and distal (inferior) respectively. OCT imaging was performed simultaneously with endoscopic visualisation of the laryngeal subsite under investigation. Due to operating room time constraints, not all laryngeal subsites were imaged in all patients, however, for each subsite imaged, more than one image was often acquired (see Table 2).
Table 2.
Numbers of each subsite examined
| LM | OCT | |||||
|---|---|---|---|---|---|---|
| Patients | Slides | Total number of measurements | Patients | Images | Total number of measurements | |
| LarEpi | 3 | 3 | 34 | 15 | 29 | 145 |
| LinEpi | 10 | 17 | 302 | 4 | 6 | 30 |
| TVC | 5 | 8 | 45 | 46 | 136 | 680 |
| FVC | 3 | 6 | 57 | 25 | 42 | 210 |
| AEF | 7 | 15 | 201 | 6 | 10 | 50 |
| SG | 10 | 15 | 196 | 15 | 28 | 140 |
LM, light microscopy; OCT, optical coherence tomography; TVC, true vocal cord; FVC, false vocal cord; AEF, aryepiglottic fold; SG, sublgottis; LarEpi, laryngeal epiglottis; LinEpi, lingual epiglottis.
The OCT images were digitally captured and catalogued in a computer database into one of six laryngeal subsites: true vocal cords, false vocal cords, aryepiglottic folds, laryngeal epiglottis, lingual epiglottis and subglottis. Digital morphometry was performed using Adobe Photoshop (Adobe System, San Jose, CA, USA) to obtain measurements of epithelial thickness. For each image, five measurements were made at 1 mm intervals and mean epithelial thickness was calculated. Measurements were not performed in laryngeal subsites in which the demarcation between epithelium and underlying lamina propria could not be identified conclusively.
Histological measurements
Laryngectomy specimens were prepared for histological exam according to standard protocol of the Department of Pathology at our institution which consists of immersion in neutral buffered 10% formalin solution for 48 h, followed by embedding in paraffin, sectioning, and staining with hematoxylin and eosin. Each specimen was examined under light microscopy and epithelial thickness measured by dropping a line perpendicular to the lumen surface and measuring a distance to the basement membrane, including any cilia. This was repeated every 1 mm. Histologically abnormal areas were not measured. Areas in which the quality of the specimen was judged inadequate to accurately determine epithelial thickness (e.g., tissue layers had separated or relevant structures could not be discerned) were also excluded. In order to standardise the artifact from preparation of the specimens, only permanent sections were measured; frozen sections were excluded. Due to the policies of the Department of Pathology regarding the retention /archival of slides in the specimen storage facility, not all subsites were available for examination on every patient (the remaining slides were destroyed prior to the time of this study). However, of the slides available, more than one section per subsite per patient was often present (see Table 2).
Optical coherence tomography morphometry and light microscopy morphometry, or measurement of distances on each image / slide, were performed by two separate researchers working independently of one another. Neither researcher was privy to the findings of the other until the time of statistical analysis.
Epithelial thickness values were average by subsite for each patient in both OCT and histologic measurements. If several histology slides were examined of a single subsite for a particular patient, averages per slide were in turn averaged to produce one mean per subsite per patient. These values were then used to calculate an overall mean per subsite for all patients in each arm. Standard errors for each value were also derived.
Results
Figure 1 shows a representative histological coronal section of a true vocal cord in the axial plane as viewed by light microscopy. The bracket marks the extent of the epithelium, with an arrow demonstrating the locus of the basement membrane. Figure 2 shows an OCT image in the coronal plane of the true vocal cords of the same patient. Again, the epithelium and its basement membrane are plainly evident and are marked with a bracket and an arrow respectively. In this patient, average thickness of the true vocal cord epithelium is 153 μm by light microscopy and 161 μm by OCT.
Fig. 1.

Histological coronal section of a true vocal cord, as seen by light microscopy. The bracket marks the extent of the epithelium, with an arrow indicating the basement membrane.
Fig. 2.
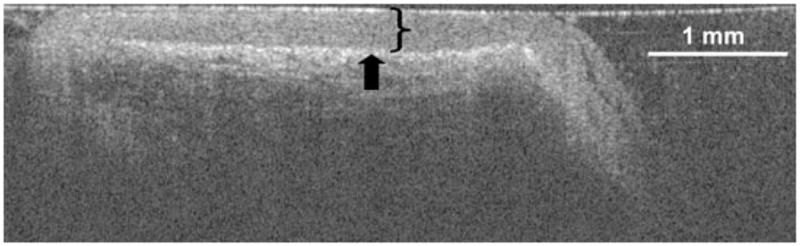
Optical coherence tomography image in the coronal plane of the true vocal cords of the same patient as in Fig. 1. The bracket marks the extent of the epithelium, with an arrow indicating the basement membrane. A length of 1 mm is shown for reference.
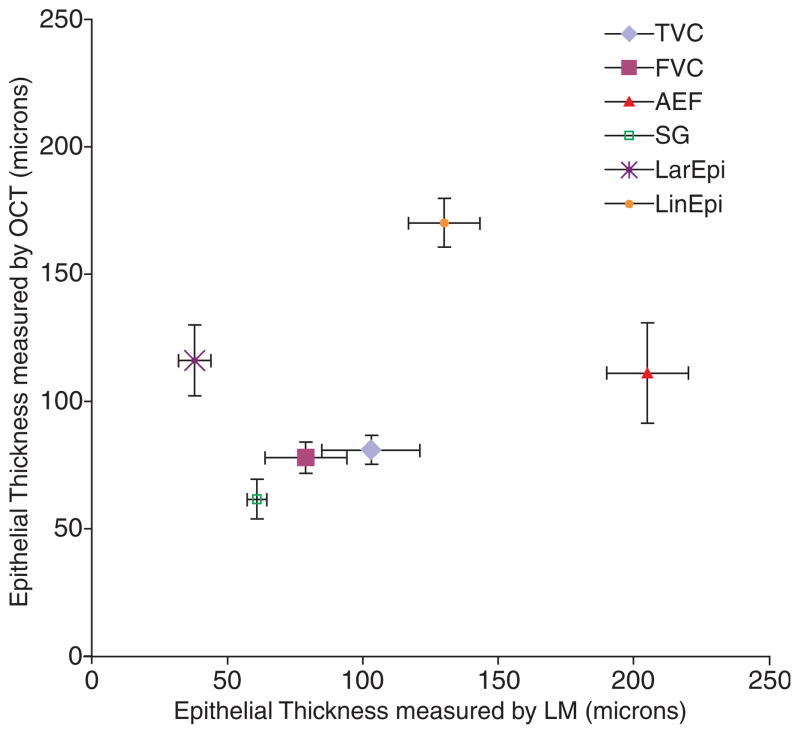
Table 3 gives the mean epithelial thickness values for each subsite by both modalities and the standard error of the mean associated with each value. Figure 3 graphically depicts the mean thickness at each subsite obtained by light microscopy (x-axis) versus the mean thickness obtained by OCT (y-axis) with their respective standard errors for each subsite shown as cross-bars.
Table 3.
Mean epithelial thicknesses (μm)
| LM (SEM) | OCT (SEM) | |
|---|---|---|
| TVC | 103 (18.2) | 80.9 (5.6) |
| FVC | 79 (15.2) | 77.8 (6.2) |
| AEF | 205 (15.2) | 111 (19.7) |
| SG | 61 (3.6) | 61.5 (7.7) |
| LarEpi | 38 (6) | 116 (13.8) |
| LinEpi | 130 (13.2) | 170 (9.5) |
LM, light microscopy; OCT, optical coherence tomography; TVC, true vocal cord; FVC, false vocal cord; AEF, aryepiglottic fold; SG, sublgottis; LarEpi, laryngeal epiglottis; LinEpi, lingual epiglottis.
Fig. 3.
Mean epithelial thickness at each laryngeal subsite measured by light microscopy (LM) and optical coherence tomography (OCT), with standard errors. TVC, true vocal cords; FVC, false vocal cords; AEF, aryepiglottic folds; SG, sublgottis; LarEpi, laryngeal epiglottis; LinEpi, lingual epiglottis.
Discussion
Synopsis key /new findings
Previous OCT studies done in the larynx have largely focused on ex vivo qualitative identification of histological features.12,13,20 Our study is the only to date to establish a quantitative comparison between epithelial thickness in vivo with OCT, and ex vivo with light microscopy in the larynx.
Comparisons with other studies
Optical coherence tomography accurately depicts glands, vasculature, tissue layers and other structures.8,13,14,20 In contrast, we have compared quantitative measurements obtained by both OCT and light microscopy in the upper aerodigestive tract. Similar studies have been done elsewhere in the body, particularly the eye. Chauhann and Marshall found that the total thickness of both fixed and fresh bovine retinas as determined by OCT had a strong linear correlation to measurements obtained by light microscopy, although this work has been criticised as describing artifact.21 Wirbelauer et al. found that the thickness of diseased human corneas measured by in vivo OCT was directly proportional to that measured by light microscopy of the same specimens when fixed ex vivo.22 In the upper aerodigestive tract, Wong et al. measured epithelial thickness by OCT, but did not correlate these findings with histology.8
Strengths of the study
Histology is the gold standard for tissue diagnosis. However, the use of histological slides examined with light microscopy for measuring normal epithelial thickness (as well as that of neoplasms) is not without its disadvantages. For microscopic examination, tissue must first be removed and then subjected to fixation, sectioning and staining, which may result in artifacts. Johnson et al. found that canine labiobuccal mucosal margins on histological slides were reduced to half their in vivo size, and tongue mucosal margins on histological slides similarly shrunk by one-third.23 Formalin fixation shrinks laryngeal tissues grossly by 9–42% (in the false vocal cord and true vocal cord respectively) relative to freshly excised normal canine larynxes.24 Further manipulation for histologic preparation such as embedding and sectioning may result in up to 20% additional reduction in size.24 Moreover, different laryngeal subsites vary in the degree to which they are affected, creating distortion.24 In contrast, OCT images areas of interest in vivo and in real time, without these artifacts produced by histologic preparation.
Given that histologic preparation generally results in tissue shrinkage, our findings are somewhat surprising in that the OCT measurements are similar or even smaller than light microscopy measurements for several subsites. Wirbelauer et al. found that thickness of corneas measured in vivo by OCT was, in fact, greater than the thickness of the same corneas measured ex vivo by light microscopy, by about 9%.22 This is keeping with a setting of histologic shrinkage. However, Chauhann and Marshall, working only with ex vivo bovine retinas, found the opposite: that light microscopy measurements were often larger than OCT by a comparable factor.21 Our study appears to more closely reflect the findings of Chauhann. Chief among the reasons for this is likely the specific technique of OCT image acquisition. The in vivo study was performed on awake patients via slit lamp, with no direct contact to the tissues measured, and thus no pressure exerted, whereas the ex vivo study did not have to avoid direct contact given that there would be no concern for irritation of the cornea and patient comfort.21,22 Although our work was performed in vivo, it frequently involved direct contact of the probe with the tissue under investigation. Even slight pressures created by the probe may be sufficient to alter the measurements, and it is impossible to gauge the amplitude of pressures exerted by the hand-held probe.
There are several other points to bear in mind when analyzing these results. First, the study populations in the two arms are different. The OCT group is a heterogeneous population that includes individuals both with and without a history of laryngeal cancer, smoking (the biggest risk factor for laryngeal cancer), and radiation therapy (which may cause permanent damage in the tissues irradiated). In contrast, every patient in the light microscopy arm of this study underwent total laryngectomy for carcinoma, which recurred or persisted following radiation treatment. It is possible that their disease and previous therapies produced an increase in epithelial thickness via edema, inflammation, or fibrosis.25 In the subsites of the larynx not affected by cancer, these alterations may be difficult to appreciate clinically but nonetheless present; differences of 10–20 mm are imperceptible to the naked eye. As noted in the methods, areas with histologically obvious abnormalities such as dysplasia or inflammation on microscopy were excluded from measurements in an attempt to reduce the potential impact of these effects. Ideally, we could measure the epithelial thickness by light microscopy of larynxes taken from young healthy individuals, but, of course, this is unethical because it will require that healthy individuals without any laryngeal disease underwent total laryngectomy for further histological examination. So what it is feasible to measure are larynxes from patients that underwent total laryngectomy for malignant disease of the larynx, although these larynxes are usually distorted due to radiation or surgical artifact.
Another source of possible error in the use of OCT concerns variability of the refractive index. In processing the backscattering optical signal, we assume that the refractive index of laryngeal soft tissue is uniformly 1.4. While practical use of the OCT device demands this assumption be made, it is, nonetheless, a broad assumption as tissue is heterogeneous and will have actual refractive index variations due to glands, ducts, dense connective tissue, and different degrees of hydration. For example, Knuttel and Boehlau-Godau found that the presence of an epidermal sweat gland containing a significant amount of water may lower the refractive index at that point to 1.37, relative to 1.43 in the surrounding tissues. 19 This is relevant to our study in a variety ways. Pressures exerted intraoperatively by instrumentation, such as the endotracheal tube or laryngoscope, may result in altered tissue perfusion. Post-radiation changes may vary from edema to fibrosis.25 Either may, in turn, significantly dynamically affect the hydration level of the tissue, and thus the refractive index. For instance, significant fibrosis and decreased tissue perfusion would both result in an increased refractive index. Underestimating the refractive index would result in an artificially thinned epithelium as measured by OCT, and may, in part, explain our data. Likewise, trauma from intubation or instrumentation (such as the forces exerted on tissue during suspension) could also produce the opposite effect, swelling, and lead to an over-estimation of epithelial thickness. Such changes when obvious were excluded from our analysis, however subtle changes may have been difficult to detect clinically.
Finally, OCT measurements are subject to a certain degree of bias as a consequence of imaging being performed in areas most accessible to the probe. This is due to clinical limitations on the amount of time available to image patients while under anesthesia. Hence, there is a preponderance of true and false vocal cord measurements in contrast to measurements of the lingual surface of the epiglottis which would generally require repositioning of the laryngoscope. In contrast, laryngectomy renders all regions of the organ readily accessible to the probe.
It may be valuable in the future to image a larynx during surgery, and then immediately after each step in the histologic processing cascade to determine the effect perfusion, formalin fixation, ethanol dehydration and paraffin embedding on the epithelial thickness measured. Nevertheless, morphometry of histological specimens will always be subject to highly variable shrinkage artifacts, and these can vary considerably depending on the nature of the tissue (e.g., lamina propria versus epithelium) and the details of the chemical processing route. This is a contributing factor to the large histologic measurement error bars in Fig. 3. In contrast, in vivo OCT may more accurately represent the true morphology of the targeted region since no chemical process (fixation) or volume contraction (dehydration) is involved. Our findings suggest that the impact of the probe contacting the tissue surface still remains a potential source of artifact, but we are presently working on the development and evaluation of a clinical OCT device mounted on a surgical microscope which may eliminate this effect. Likewise, the placement of the laryngoscope or endotracheal tube may cause subtle swelling in tissue; we are therefore currently engaged in the design of an OCT device that works in tandem with a rigid videostroboscopy system, though this is a formidable optomechanical engineering task.
Clinical applicability of the study
Regardless, the clinical impact of OCT in laryngology will be its ability to interrogate and image the epithelium and its demarcation with the superficial lamina propria. Knowledge of the normal thickness of these layers is critically important as they thicken in pathologic conditions, in particular dysplasia and chronic inflammation. Previous decades saw the verification of CT and MR imaging as clinically useful tools through the rigorous comparison of these modalities to macroscopic anatomy; OCT images must be likewise legitimised by reference to histology.
Conclusion
This study reports the in vivo thickness of the laryngeal epithelium in six distinct subsites measured using OCT, a non-invasive imaging modality. OCT does not have the artifacts associated with conventional histologic techniques and can be performed in living tissues. Knowledge of the normal thickness of the epithelium may be the first sign of early malignancy. The inevitable development of office-based OCT devices will increase the demand for precise measurements of the laryngeal microstructure and its correlation with histopathology. This innovation will likely accelerate the application of the OCT technology in both diagnosing laryngeal disease and monitoring its progressions.
Acknowledgments
This work was supported by the National Institutes of Health (DC 006026, A 91717, EB 00293, RR 01192, RR00827), Flight Attendant Medical Research institute (32456), State of California Tobacco Related Disease Research Program (12RT-0113), Air Force Office of Scientific Research (F49620_00_1_0371), and the Arnold and Mabel Beckman Foundation.
Footnotes
This work was presented at the Middle/Western Section of the Triological Society Meeting, February 4, 2006 in San Diego, California.
Conflict of interest
None to declare.
References
- 1.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirano K, Ito Y, Suzuki T, et al. Optical coherence tomography for the noninvasive evaluation of the cornea. Cornea. 2001;20:281–289. doi: 10.1097/00003226-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Regar E, Schaar JA, Mont E, et al. Optical coherence tomography. Cardiovasc Radiat Med. 2003;4:198–204. doi: 10.1016/j.carrad.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Welzel J. Optical coherence tomography in dermatology: a review. Skin Res Technol. 2001;7:1–9. doi: 10.1034/j.1600-0846.2001.007001001.x. [DOI] [PubMed] [Google Scholar]
- 5.Tearney GJ, Brezinski ME, Southern JF, et al. Optical biopsy in human urologic tissue using optical coherence tomography. J Urol. 1997;157:1915–1919. [PubMed] [Google Scholar]
- 6.Sivak MV, Jr, Kobayashi K, Izatt JA, et al. High-resolution endoscopic imaging of the GI tract using optical coherence tomography. Gastrointest Endosc. 2000;51(4 Pt 1):474–479. doi: 10.1016/s0016-5107(00)70450-0. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Otis L, Piao D, et al. Characterization of dentin, enamel, and carious lesions by a polarization-sensitive optical coherence tomography system. Appl Opt. 2005;44:2041–2048. doi: 10.1364/ao.44.002041. [DOI] [PubMed] [Google Scholar]
- 8.Wong BJ, Jackson RP, Guo S, et al. In vivo optical coherence tomography of the human larynx: normative and benign pathology in 82 patients. Laryngoscope. 2005;115:1904–1911. doi: 10.1097/01.MLG.0000181465.17744.BE. [DOI] [PubMed] [Google Scholar]
- 9.Pitris C, Saunders KT, Fujimoto JG, et al. High-resolution imaging of the middle ear with optical coherence tomography: a feasibility study. Arch Otolaryngol Head Neck Surg. 2001;127:637–642. doi: 10.1001/archotol.127.6.637. [DOI] [PubMed] [Google Scholar]
- 10.Wong BJ, Zhao Y, Yamaguchi M, et al. Imaging the internal structure of the rat cochlea using optical coherence tomography at 0.827 microm and 1.3 microm. Otolaryngol Head Neck Surg. 2004;130:334–338. doi: 10.1016/j.otohns.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Pantanowitz L, Hsiung PL, Ko TH, et al. High-resolution imaging of the thyroid gland using optical coherence tomography. Head Neck. 2004;26:425–434. doi: 10.1002/hed.10392. [DOI] [PubMed] [Google Scholar]
- 12.Burns JA, Zeitels SM, Anderson RR, et al. Imaging the mucosa of the human vocal fold with optical coherence tomography. Ann Otol Rhinol Laryngol. 2005;114:671–676. doi: 10.1177/000348940511400903. [DOI] [PubMed] [Google Scholar]
- 13.Bibas AG, Podoleanu AG, Cucu RG, et al. 3-D optical coherence tomography of the laryngeal mucosa. Clin Otolaryngol Allied Sci. 2004;29:713–720. doi: 10.1111/j.1365-2273.2004.00902.x. [DOI] [PubMed] [Google Scholar]
- 14.Shakhov AV, Terentjeva AB, Kamensky VA, et al. Optical coherence tomography monitoring for laser surgery of laryngeal carcinoma. J Surg Oncol. 2001;77:253–258. doi: 10.1002/jso.1105. [DOI] [PubMed] [Google Scholar]
- 15.Friedman WH, Archer CR, Yeager VL, et al. Computed tomography of the normal larynx. Head Neck Surg. 1979;1:435–440. doi: 10.1002/hed.2890010509. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Chen Z, Saxer C, et al. Phase-resolved optical coherence tomography and optical Doppler tomography for imaging blood flow in human skin with fast scanning speed and high velocity sensitivity. Optics Lett. 2000;25:114–116. doi: 10.1364/ol.25.000114. [DOI] [PubMed] [Google Scholar]
- 17.Ren H, Ding Z, Zhao Y, et al. Phase-resolved functional optical coherence tomography: simultaneous imaging of in situ tissue structure, blood flow velocity, standard deviation, birefringence, and Stokes vectors in human skin. Optics Lett. 2002;27:1702–1704. doi: 10.1364/ol.27.001702. [DOI] [PubMed] [Google Scholar]
- 18.Knuttel A, Bonev S, Knaak W. New method for evaluation of in vivo scattering and refractive index properties obtained with optical coherence tomography. J Biomed Opt. 2004;9:265–273. doi: 10.1117/1.1647544. [DOI] [PubMed] [Google Scholar]
- 19.Knuttel A, Boehlau-Godau M. Spatially confined and temporally resolved refractive index and scattering evaluation in human skin performed with optical coherence tomography. J Biomed Opt. 2000;5:83–92. doi: 10.1117/1.429972. [DOI] [PubMed] [Google Scholar]
- 20.Nassif NA, Armstrong WB, de Boer JF, et al. Measurement of morphologic changes induced by trauma with the use of coherence tomography in porcine vocal cords. Otolaryngol Head Neck Surg. 2005;133:845–850. doi: 10.1016/j.otohns.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Chauhan DS, Marshall J. The interpretation of optical coherence tomography images of the retina. Invest Ophthalmol Vis Sci. 1999;40:2332–2342. [PubMed] [Google Scholar]
- 22.Wirbelauer C, Winkler J, Bastian GO, et al. Histopathological correlation of corneal diseases with optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2002;240:727–734. doi: 10.1007/s00417-002-0518-3. [DOI] [PubMed] [Google Scholar]
- 23.Johnson RE, Sigman JD, Funk GF, et al. Quantification of surgical margin shrinkage in the oral cavity. Head Neck. 1997;19:281–286. doi: 10.1002/(sici)1097-0347(199707)19:4<281::aid-hed6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 24.Kimura M, Tayama N, Chan RW. Geometrical deformation of vocal fold tissues induced by formalin fixation. Laryngoscope. 2003;113:607–613. doi: 10.1097/00005537-200304000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Cooper JS, Fu K, Marks J, et al. Late effects of radiation therapy in the head and neck region. Int J Radiat Oncol Biol Phys. 1995;31:1141–1164. doi: 10.1016/0360-3016(94)00421-G. [DOI] [PubMed] [Google Scholar]