Abstract
Background
The goal of our study was to evaluate the effects of different medicinal herbs rich in polyphenol (Lemon balm, Sage, St. John's wort and Small-flowered Willowherb) used as dietary supplements on bioaccumulation of some essential metals (Fe, Mn, Zn and Cu) in different chicken meats (liver, legs and breast).
Results
In different type of chicken meats (liver, legs and breast) from chickens fed with diets enriched in minerals and medicinal herbs, beneficial metals (Fe, Mn, Zn and Cu) were analysed by flame atomic absorption spectrometry. Fe is the predominant metal in liver and Zn is the predominant metal in legs and breast chicken meats. The addition of metal salts in the feed influences the accumulations of all metals in the liver, legs and breast chicken meat with specific difference to the type of metal and meat. The greatest influences were observed in legs meat for Fe and Mn. Under the influence of polyphenol-rich medicinal herbs, accumulation of metals in the liver, legs and breast chicken meat presents specific differences for each medicinal herb, to the control group that received a diet supplemented with metal salts only. Great influence on all metal accumulation factors was observed in diet enriched with sage, which had significantly positive effect for all type of chicken meats.
Conclusions
Under the influence of medicinal herbs rich in different type of polyphenol, accumulation of metals in the liver, legs and breast chicken meat presents significant differences from the group that received a diet supplemented only with metal salts. Each medicinal herb from diet had a specific influence on the accumulation of metals and generally moderate or poor correlations were observed between total phenols and accumulation of metals. This may be due to antagonism between metal ions and presence of other chelating agents (amino acids and protein) from feeding diets which can act as competitor for complexation of metals and influence accumulation of metals in chicken meat.
Graphical abstract
Keywords: Chicken liver, Chicken legs meat, Chicken breast meat, Medicinal herbs, Beneficial metals, Feeding diets, Polyphenol
Background
Chicken meat is widely consumed worldwide. Chicken meat and meat products are important for human diet because they provide a great part of nutrients (protein, lipids), including necessary minerals and trace elements. The main source of metals in these meat foodstuffs arises from processing and manipulation of feeds. Fe, Zn, Cu and Mn are the essential metals which are required in small quantities and occur naturally in various vegetables and meat foodstuffs. However, those essential metals are given special attention due to their toxic effect in the body when their concentrations exceed limits of safe exposure. The contents of these metals in vegetables and meat foodstuffs may vary depending on the general (varieties, maturity, genetics, and age) and environmental (soils, geographical locations, season, water source and use of fertilizers) conditions of plants and animals and on methods of handling and processing. Heavy metals contaminate the environment and enter the food chain. Contamination with these metals is serious threat because of their toxicity, bioaccumulation and biomagnification in the food chain [1-4].
To avoid deficiencies that can lead to a wide variety of clinical and pathological disorders diets for livestock are supplemented with minerals [5]. Fe, Mn, Zn and Cu can be considered trace minerals with a central role in many metabolic processes throughout the body and are essential for correct growth and development of all animals, including humans. They predominantly act as catalysts in many enzyme and hormone systems which influence on growth, bone development, feathering, enzyme structure and function, and appetite. Deficiency symptoms are manifested as disturbances in metabolic processes, resulting in lower production performance, loss of appetite, reproductive disorders, and impaired immune response. Deficiencies can be caused by inadequate mineral intake or by the presence of antagonists in the diet, which interfere with or unbalance minerals uptake [2,6,7].
Generally, inorganic mineral salts (sulfates, oxides and carbonates) have been used within feed formulations, because they offer a cost-effective solution to meet the requirement of the animal for trace elements. Feeding studies demonstrated that chelated trace minerals are at least 30% more bioavailable than inorganic trace mineral salts when fed to broilers. However, it has yet to be conclusively proven that mineral chelates are better absorbed in the monogastric enterocyte. Several papers reported on metal binding to proteins in the cells [8,9]. The higher availability of chelates may be linked to the shielding of the minerals positive charge during chelation. This allows the mineral to withstand the binding activity of the negatively charged mucin layer and results in lower competition between minerals of similar charge in their resorption from the gut and transfer to the enterocyte. These phenomena, combined with lower complex formation in the intestinal lumen with compounds such as phytate, may contribute to the higher absorption of minerals from the gut. Feeding trials in mammalian species have shown that organic complexed trace minerals have higher relative bioavailability than inorganic ones and provide alternative pathways for absorption, thus leading to a reduction in the excretion of minerals [10-12].
Natural feed additives of plant origin are generally believed to be safer, healthier and less subject to hazards for humans and animals. Spice and many medicinal herbs and plant extracts have appetite- and digestion-stimulating properties, antioxidant and antimicrobial activities, influence the poultry productivity and health mainly by stabilization of normal gut microflora and prevention of pathogens colonization. Also they have beneficial effects on digestive enzymes production, improve and exert certain immunological consequences in bird's body. Mixtures of complex compounds, vitamins and minerals found in plants tend to work together synergistically. These combinations were more effective than they were each used in isolated form. These beneficial effects make them useful as potential natural animal feed additives [13-16].
Antioxidant effect of aromatic plants is due to the presence and specific arrangement of hydroxyl groups in their phenolic compounds. These polyphenol also have important biological activities in vitro such as anti-tumour, chemo-preventive and anti-inflammatory activities. It has been proposed that polyphenol from some medicinal plants may greatly increase the functionality of food in terms of health and wellness [17-19].
Antioxidant properties of compounds from some medicinal herbs can result from their free radical scavenging activity but their ability to chelate transition metal ions, especially Fe (II) and Cu (II), also plays an important role [20]. The metal chelating ability of polyphenol is related to the presence of ortho-dihydroxy polyphenol, i.e., molecules bearing catechol or galloyl groups and condensed tannins. Also since metal chelation can occur at physiological pH it has a physiological significance [21,22].
Therefore, the aim of our study was to evaluate, for the first time in the field, the effect of different medicinal herb rich in polyphenol (Lemon balm, Sage, St. John's wort and Small-flowered Willowherb) used as dietary supplements on bioaccumulation of some essential metals (Fe, Mn, Zn and Cu) in chicken liver and chicken meat from legs and breast.
Results and discussions
The total phenols contents, determined by Folin-Ciocalteu method, of experimental feeding diets are shown in Table 1.
Table 1.
Total phenol in feeding diets (a, b, c: different letters within the same column indicate significant differences among levels (p ≤ 0.05)
| Feeding diet | Trial code |
Total phenols, mg gallic acid/g fodder |
Relative to control | |
|---|---|---|---|---|
| Average | ± 95% confidence | % | ||
| Basal diet, minerals and 2% Lemon balm | Le | 2.89a | 0.15 | 146 |
| Basal diet, minerals and 2% Sage | Sa | 2.73a | 0.15 | 138 |
| Basal diet, minerals and 2% St. John's wort | Jo | 2.39b | 0.10 | 121 |
| Basal diet, minerals and 2% Small-flowered Willowherb | Wi | 2.31b | 0.11 | 117 |
| Basal diet and minerals | Min | 1.98c | 0.10 | 100 |
| Only basal diet (control) | B | 1.98c | 0.10 | 100 |
Although the addition of medicinal herbs in the basic diet was the same (2%), due to various polyphenol contents of medicinal herbs [23], a different content of polyphenol in the final diet was identified. Higher content in polyphenol was identified in feeding diet supplemented with Lemon balm and Sage (Le, Sa), followed by feeding diets supplemented with St. John's wort and Small-flowered Willowherb (Jo, Wi). Relative to control feeding diet, content of polyphenol ranged between 117-121% for Jo and Wi diets and between 138-146% for Le and Sa feeding diets.
Except Small-flowered Willowherb all other herbs used in the experiment contain volatile oils consist mostly of volatile compounds of mono, tri- and sesquiterpenes. Besides different content of total polyphenol, herb and flowers of these plants contain various types of polyphenol.
In Lemon balm herb a lot of polyphenol were identified: flavonoids such as luteolin, quercetin, apigenin and kaempferol; phenylpropanoids including hydroxycinnamic acid derivatives (caffeic and chlorogenic acids) and in particular rosmarinic acid and other various condensed tannins [24].
In Sage herb were identified a lot of flavonoids, formed from flavones and their glycosides, phenolic glycosides, benzoic acid derivate, hydroxycinnamic acid derivate composed from free caffeic acid, caffeic acid dimer (rosmarinic acid), caffeic acid trimmers, caffeic acid tetramer, phenolic diterpenes [25].
St. John's wort herb and flowers contain various polyphenol: flavonoids (epigallocatechin, rutin, hyperoside, isoquercetin, quercitrin, quercetin, amentoflavone, astilbin, miquelianin), phenolic acids (chlorogenic acid, 3-O-coumaroylquinic acid), and various naphtodianthrones: (hypericin, pseudohypericin, protohypericin, protopseudohypericin), phloroglucinols (hyperforin, adhyperforin). The naphthodianthrones hypericin and pseudohypericin along with the phloroglucinol derivative hyperforin are thought to be the active components [26,27].
Willowherb (Small-flowered Willowherb, Epilobium parviflorum) species contain flavonoids, especially derivatives of kaempferol, quercetin, and myricetin. In E. parviflorum and E. angustifolium, β-sitosterol, various esters of sitosterol, and sitosterol glucoside have been detected. Gallic-acid derivatives may be present. Two macrocyclic ellagitannins, oenothein A and oenothein B, have been identified as the main constituents responsible for the inhibition of 5-alpha-reductase and aromatase enzymes [28]. According to Kohlert et al. [29], most active principles of plant extract are absorbed in the intestine by enterocytes, and readily metabolized by the body. The products of this metabolism are transformed into polar compounds by conjugation with glucuronate and excreted in the urine. As the active compounds are readily metabolized and have a short half-life, the risk of tissue accumulation is minimal [30].
The diverse composition of medicinal herb is reflected in a complex influence on bioaccumulation of essential metals Fe, Mn, Zn and Cu in chicken meats. The content and correlation of the investigated metals of different chicken meats is shown in Table 2, Table 3, Table 4, Table 5, Table 6 and Table 7.
Table 2.
Metals contents (mg/kg dry matter) in chicken liver, fed with different diets
| Metals/ Feeding diet | Feeding diet code | Fe | Mn | Zn | Cu | ||||
|---|---|---|---|---|---|---|---|---|---|
| Average | ± Std | Average | ± Std | Average | ± Std | Average | ± Std | ||
| Basal diet, minerals and 2% Lemon balm | Le | 45.28 | 4.43 | 6.13 | 0.58 | 22.75 | 2.13 | 4.39 | 0.52 |
| Basal diet, minerals and 2% Sage | Sa | 58.65 | 6.01 | 7.35 | 0.81 | 28.09 | 3.22 | 7.35 | 0.81 |
| Basal diet, minerals and 2% St. John's wort | Jo | 47.76 | 4.39 | 5.08 | 0.49 | 23.70 | 2.17 | 4.16 | 0.37 |
| Basal diet, minerals and 2% Small-flowered Willowherb | Wi | 34.00 | 4.50 | 4.93 | 0.54 | 16.62 | 2.01 | 4.50 | 0.52 |
| Basal diet and minerals | Min | 45.80 | 3.99 | 3.78 | 0.43 | 17.31 | 1.57 | 4.62 | 0.44 |
| Only basal diet (control) | B | 31.00 | 3.11 | 2.69 | 0.25 | 13.64 | 1.16 | 2.96 | 0.36 |
Table 3.
Correlations (Pearson) between metal-metal in chicken liver and metals-total phenols concentrations in diets (* significance, p < 0.05)
| Compounds | Fe | Mn | Zn | Cu | Phen |
|---|---|---|---|---|---|
| Fe | 1 | 0.78182 | 0.9192* | 0.5922 | 0.57469 |
| Mn | 0.78182 | 1 | 0.91376* | 0.66654 | 0.91365* |
| Zn | 0.9192* | 0.91376* | 1 | 0.4994 | 0.80512 |
| Cu | 0.5922 | 0.66654 | 0.4994 | 1 | 0.42859 |
| Phen | 0.57469 | 0.91365* | 0.80512 | 0.42859 | 1 |
Table 4.
Metals contents (mg/kg dry matter) in meat from chicken legs, fed with different diets
| Metals/ Feeding diet | Feeding diet code |
Fe | Mn | Zn | Cu | ||||
|---|---|---|---|---|---|---|---|---|---|
| Average | ± Std | Average | ± Std | Average | ± Std | Average | ± Std | ||
| Basal diet, minerals and 2% Lemon balm | Le | 10.54 | 1.11 | 0.14 | 0.03 | 16.37 | 1.55 | 0.54 | 0.06 |
| Basal diet, minerals and 2% Sage | Sa | 15.62 | 1.73 | 0.33 | 0.05 | 15.32 | 2.02 | 0.62 | 0.07 |
| Basal diet, minerals and 2% St. John's wort | Jo | 12.49 | 1.22 | 0.17 | 0.03 | 16.09 | 1.57 | 0.33 | 0.03 |
| Basal diet, minerals and 2% Small-flowered Willowherb | Wi | 12.71 | 1.11 | 0.40 | 0.03 | 19.09 | 2.05 | 0.48 | 0.05 |
| Basal diet and minerals | Min | 12.85 | 1.11 | 0.36 | 0.03 | 11.00 | 1.51 | 0.33 | 0.03 |
| Only basal diet (control) | B | 6.94 | 0.58 | 0.15 | 0.02 | 9.00 | 1.11 | 0.25 | 0.02 |
Table 5.
Correlations (Pearson) between metal-metal in chicken legs and metals-total phenols concentrations in diets (* significance, p < 0.05)
| Compounds | Fe | Mn | Zn | Cu | Phen |
|---|---|---|---|---|---|
| Fe | 1 | 0.77136 | 0.53473 | 0.64072 | 0.39564 |
| Mn | 0.77136 | 1 | 0.40392 | 0.41003 | -0.04474 |
| Zn | 0.53473 | 0.40392 | 1 | 0.65861 | 0.64321 |
| Cu | 0.64072 | 0.41003 | 0.65861 | 1 | 0.85253* |
| Phen | 0.39564 | -0.04474 | 0.64321 | 0.85253* | 1 |
Table 6.
Metals contents (mg/kg dry matter) in chicken breast meat, fed with different diets
| Metals/ Feeding diet | Feeding diet code | Fe | Mn | Zn | Cu | ||||
|---|---|---|---|---|---|---|---|---|---|
| Average | ± Std | Average | ± Std | Average | ± Std | Average | ± Std | ||
| Basal diet, minerals and 2% Lemon balm |
Le | 4.88 | 0.55 | 0.01 | 0.001 | 7.25 | 0.82 | 0.44 | 0.04 |
| Basal diet, minerals and 2% Sage | Sa | 4.44 | 0.61 | 0.03 | 0.003 | 7.49 | 0.73 | 0.74 | 0.09 |
| Basal diet, minerals and 2% St. John's wort | Jo | 4.06 | 0.55 | 0.01 | 0.001 | 6.66 | 0.72 | 0.57 | 0.07 |
| Basal diet, minerals and 2% Small-flowered Willowherb | Wi | 3.10 | 0.43 | 0.01 | 0.001 | 5.80 | 0.65 | 0.34 | 0.03 |
| Basal diet and minerals | Min | 3.67 | 0.34 | 0.02 | 0.002 | 7.02 | 0.75 | 0.50 | 0.04 |
| Only basal diet (control) | B | 2.81 | 0.24 | 0.01 | 0.001 | 5.64 | 0.63 | 0.30 | 0.02 |
Table 7.
Correlations (Pearson) between metal-metal in chicken breast and metals-total phenols concentrations in diets (* significance, p < 0.05)
| Compounds | Fe | Mn | Zn | Cu | Phen |
|---|---|---|---|---|---|
| Fe | 1 | 0.32347 | 0.90172* | 0.48622 | 0.85683* |
| Mn | 0.32347 | 1 | 0.64476 | 0.72181 | 0.19063 |
| Zn | 0.90172* | 0.64476 | 1 | 0.79057 | 0.63427 |
| Cu | 0.48622 | 0.72181 | 0.79057 | 1 | 0.0621 |
| Phen | 0.85683* | 0.19063 | 0.63427 | 0.0621 | 1 |
For better emphasizing of the influence of different feeding diets over the bioaccumulation of metals in chicken meats, for each metal were calculated the accumulation factors (AF). This factor is the ratio between concentrations of metal in meat from experimental trial to concentration of metal in meat from control trial:
Like control trial the trial with feeding diet composed from basal diet only was selected.
Bioaccumulation of metals in chicken liver
The contents of bioactive metals Fe, Mn, Zn and Cu in chicken liver from chicks fed with different diets are presented in Table 2. In chicken liver, Fe is the predominant metal (31 to 58.65 mg/kg dry matter), followed by Zn (13.64 to 28.09 mg/kg dry matter). Mn and Cu are in the same range of concentration (3 to 7 mg/kg dry matter) significantly lower than previous. These data are in accordance with other authors who found the same contents in same meat food stuffs and are under maximum acceptable limit imposed by national and international legislation[31-34].
A linear regression correlation test was performed to investigate correlations between metal-metal and metals-diet total phenols concentrations (Phen). The values of correlation coefficients are given in Table 3.
In chicken liver there are significant correlations between dominant metals: Fe-Zn (r = 0.9192) and Mn-Zn (0.9137) and relative good correlation between Fe-Mn (r = 0.7818). Only Mn and Zn have relative good correlations with total phenols content (r = 0.9136 and 0.8051 respectively).
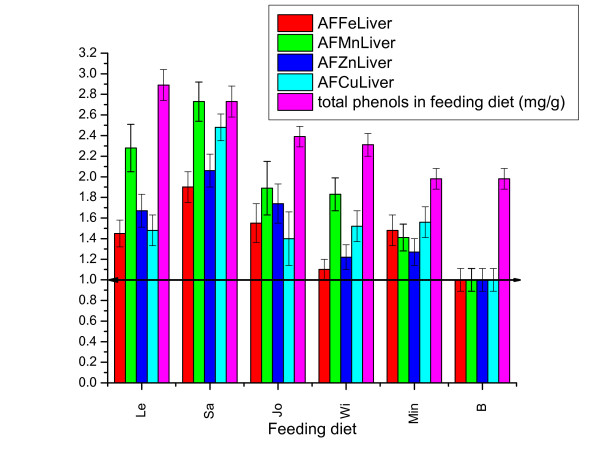
The addition of metal salts in the feed significantly influences all metals accumulations in the liver, with minor differences to the type of metal. Under the influence of medicinal herbs, accumulation of metals in the liver, however, presents significant differences from the group that received a diet supplemented only with metal salts (Min diet) (Figure 1).
Figure 1.
Metals accumulation factors (AF) in chicken liver under influence of different feeding diets (Error bar represent range for 95% confidence).
Size of AF is differentiated, depending both on the metal and diet types. Diets enriched with Lemon balm and Sage, rich in polyphenol, have the most positively pronounced effect on uptake of metals. Between the two diets, Sa diet presents the largest accumulation factors for all metals and Le diet only for Mn and Zn, comparatively with Min diet.
The lowest influences on accumulation factors of metals in the liver have diets enriched with St. John's wort and Small-flowered Willowherb, diets with lower polyphenol content (Table 1). These diets significantly positively influence only the Mn uptake. Jo diet significantly increases Zn accumulation while Wi diet significantly reduces the accumulation of Fe in chicken liver. Generally the diet enriched in Willowherb had a poor or even negative effect on metals accumulation in chicken liver, compared with other diets.
Bioaccumulation of metals in meat from chicken legs
In chicken legs meat (Table 4) Zn and Fe are the predominant metals and were found in the same range of concentration (≈ 9 to 19 mg/kg dry matter for Zn and ≈ 7 to 16 mg/kg dry matter for Fe). These are followed by Cu and Mn in the same range of concentration (≈ 0.25 to 0.64 for Cu and ≈ 0.14 to 0.40 for Mn), significantly lower than previous.
The values of correlation coefficients obtained from a linear regression test between metal-metal and metals-diet total phenols concentrations (Phen) are presented in Table 5.
Generally poor correlations between investigated metals in chicken legs meat were found. A relatively good correlation was found between: Fe-Mn (r = 0.7714) and only Cu has significant correlation with total phenols content (r = 0.8525).
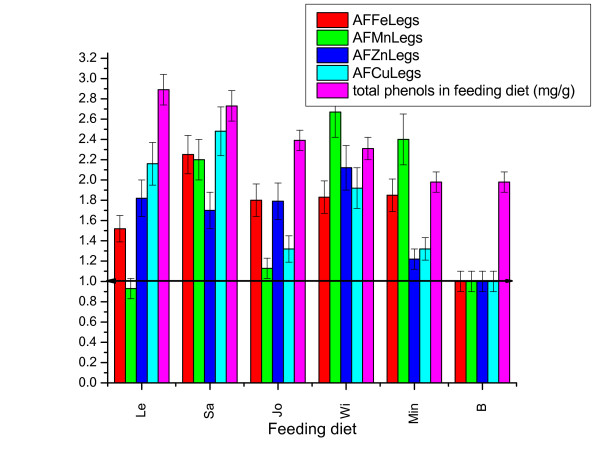
The addition of metal salts in the feed (Min diet) significantly influences the accumulation of all metals in meat from chicken legs, with significant differences of the type of metal. Mn and Fe accumulation factors were greater than Zn and Cu (Figure 2).
Figure 2.
Metals accumulation factors (AF) in meat from chicken legs under influence of different feeding diets (Error bar represent range for 95% confidence).
Under the influence of medicinal herbs, accumulation of metals in meat from chicken legs differs significantly from the group that received a diet supplemented only with metals (Min diet). Especially accumulation of Cu was positively influenced by diets rich in polyphenol (Le, Sa). The addition of Sage and Small-flowered Willowherb in diet significantly influence accumulation of Zn and Cu and less Mn and Fe. Note that addition of Lemon Balm and St. John's wort herbs in the diet have significantly negative effects on accumulation of Mn in chicken legs meat.
Bioaccumulation of metals in chicken breast meat
In chicken breast meat (Table 6), Zn is the predominant metal (≈ 5.6 to 7.5 mg/kg dry matter) followed by Fe (≈ 2.8 to 4.9 mg/kg dry matter) in the same range of concentration and Cu (≈ 0.3 to 0.74 mg/kg dry matter) at lower concentration. Mn concentration (≈ 0.01 to 0.03 mg/kg dry matter) is significantly lower than previous.
A linear regression correlation test was performed to investigate correlations between metal-metal and metals- diet total phenols concentrations (Phen). The values of correlation coefficients are given in Table 7.
There are significant correlations between Fe-Zn concentrations (r = 0.90172) in chicken breast meat and relative good correlations were found between: Zn-Cu (r = 0.7906) and Mn-Cu (r = 0.7218). Only Fe has significant correlation with total phenols content (r = 0.8568).
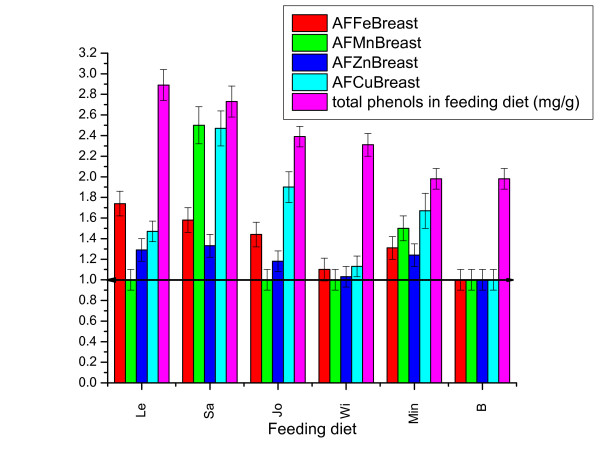
The addition of metal salts in the feed significantly influences the accumulation of all metals in meat from chicken legs, with significant differences from the type of metal. In Min diet, Mn and Cu uptake factors were larger than Zn and Fe (Figure 3). Under the influence of medicinal plants, metals accumulation in chicken breast differ significantly from the group that received a diet supplemented only with metals salts (Figure 3).
Figure 3.
Metals accumulation factors (AF) in chicken breast meat under influence of different feeding diets (Error bar represent range for 95% confidence).
Fe accumulation is significantly positively influenced by Lemon balm and Sage diets, rich in polyphenol, and less affected or even reduced in diets with St. John's herb and Small-flowered Willowherb, which has a lower concentration of polyphenol. Accumulation of Mn and Cu is more positively influenced by Sage diet; the other diets even cause a significant reduction of Mn accumulation. Accumulation of Zn in chicken breast meat is generally less influenced by all the diets used. Sage diet (Sa) clearly differ from other diets having the greatest positive influence on the accumulation of Mn, Cu and Fe and less on Zn.
Conclusions
The contents of essential metals (Fe, Mn, Zn and Cu) in chicken meats are different, depending mainly on the type of meat. So Fe is the predominant metal in liver and Zn is the predominant metal in legs and breast chicken meat. The addition of metal salts in the feed influences accumulations of all metals in the liver, legs and breast chicken meat with specific difference to the type of metal and meat. The greatest influences were observed in legs meat for Fe and Mn. However, under the influence of medicinal herbs (Lemon balm, Sage, St. John's wort and Small-flowered Willowherb) rich in different type of polyphenol (flavonoids, phenols acids, benzoic acid derivate, phenylpropanoids derivate, condensed tannins), accumulation of metals in the liver, legs and breast chicken meat presents significant differences from the group that received a diet supplemented only with metals salts. Each medicinal herb from diet had a specific influence on the metals accumulation and generally moderate or poor correlations were observed between total phenols and metal accumulation factors. Great influence on all metal accumulation factors was observed in diet enriched with sage, which had only significantly positive effect for all type of chicken meat. The effects of medicinal herb in the metals biochemistry of broiler are still unclear and complex. The differences regarding metals accumulations could appear due to the antagonism between metal ions or to the chemical composition of chicken diets, which comprise other chelating agents like amino acids and protein from soybean and corn. This fact leads to a competition between polyphenols and amino acids for metals complexation and bioaccumulation. Although some effects have already been demonstrated, the mechanisms involved in bioaccumulation of these are still widely unknown. More investigations on the action of the active compounds and their effects in vivo are required and significant improvement of animal performance must be shown before medicinal herbs or plants extracts are effectively adopted as feed additives.
Experimental
In our research the national and international behaviour codes were complied regarding the experiment with animals.
Experimental design
A total of 180 mixed one-day-old broiler chicks (Ross 308) were purchased from a local hatchery, weighed on arrival and randomly allocated to 18 pens (1 × 1 m) of 10 birds each, with equal numbers of male and females (tree replicates per each treatment) in a ventilated broiler house containing wood shavings as litter material. Water and feed were available ad libitum. All chickens were fed the similar starter (weeks 0-3 of age) and grower (weeks 4-6 of age) diets (Table 8), but receive different diet defined as: Le, Sa, Jo, Wi, Min and B treatments, respectively. Ingredients and nutrients composition of diets are shown in Table 8 and 9. Medicinal herbs, Lemon balm (Melissae folium), Sage herb (Salvia officinalis), St. John's wort (Hypericum perforatum) and Small-flowered Willowherb (Epilobium parviflorum), purchased from local specialized market, have been dried and ground to pass through a 2-mm screen. Minerals salts, p.a. purity (Merck Chemicals, Germany) used for metal sources were: Mohr salt as a source of iron, MnSO4 4H2O as a source of manganese, ZnSO4 7H2O as a source of zinc and CuSO4 5H2O as a source of copper. Due to low metal uptake from medicinal plants [35], its contribution to experimental diets has not been taken into account. Experimental diets were prepared weekly by a special feed factory. Diets were formulated to meet or exceed the requirements of the National Research Council (NRC) [36] for broilers at this age. Composition of basal diet is presented in Table 9.
Table 8.
Ingredients and nutrient composition of experimental diets
| Nr. | Diets | Diet code |
|---|---|---|
| 1 | Basal diet + 2% Lemon Balm + minerals from salts, at NRC level (80 mg/kg Fe, 60 mg/kg Mn, 40 mg/kg Zn and 8 mg/kg Cu) |
Le |
| 2 | Basal diet + 2% Sage herb + minerals from salts, at NRC level (80 mg/kg Fe, 60 mg/kg Mn, 40 mg/kg Zn and 8 mg/kg Cu) |
Sa |
| 3 | Basal diet + 2% St. John's wort + minerals from salts, at NRC level (80 mg/kg Fe, 60 mg/kg Mn, 40 mg/kg Zn and 8 mg/kg Cu) |
Jo |
| 4 | Basal diet + 2% Small-flowered Willowherb + minerals from salts, at NRC level (80 mg/kg Fe, 60 mg/kg Mn, 40 mg/kg Zn and 8 mg/kg Cu) | Wi |
| 5 | Basal diet + minerals from salts, at NRC level (80 mg/kg Fe, 60 mg/kg Mn, 40 mg/kg Zn and 8 mg/kg Cu) |
Min |
| 6 | Basal diet only (kept as control) | B |
Table 9.
Ingredients and nutrient composition of basal diet
| Composition | Stage 0-3 weeks (%) |
Stage 4-6 weeks (%) |
|---|---|---|
| Corn | 56.5 | 61,62 |
| Soybean meal | 34 | 31 |
| Fish meal | 5 | 2 |
| Sunflower oil | 1,5 | 2,5 |
| CaCO3 | 0,8 | 1 |
| Dicalcium phosphate | 1,2 | 1 |
| NaCl | 0,2 | 0,2 |
| DL Metionine | 0,3 | 0,18 |
| Vitaminic Premix | 0,5 | 0,5 |
| Nutritive characteristic | ||
| E M (kcal/kg feed) | 3204 | 3214 |
| Crude protein (%) | 22,91 | 20,03 |
| Lizine (%) | 1,27 | 1,06 |
| Metionine + cistine (%) | 0,95 | 0,72 |
| Calcium (%) | 1,03 | 0,89 |
| Total Phosphor (%) | 0,73 | 0,62 |
| Crude Celuloze (%) | 3,16 | 3,08 |
Samples collection and preparation
Meat samples
At days 43 of age, 6 birds per pen were selected, weighed and slaughtered to obtain the meat samples. Meat samples were first dried in oven at 105°C for 24 hours and then digested in the Muffle furnace by stepwise increase of the temperature up to 550°C till the white ash formed. The ash was dissolved in 0.5 N HNO3 and filtered through ash-free filter paper before analysis. Each sample solution was made up with dilute HNO3 (0.5 N) to a final volume of 50 mL and analysed by flame atomic absorption spectrometry. Necessary dilutions were made[2,3].
Metals analysis and quality control
The concentrations of Fe, Mn, Zn, Cu in the solutions were determined using a flame atomic absorption spectrophotometer with high resolution continuum source (Model ContrAA 300, Analytik Jena, Germany), fitted with a specific conditions of particular metal using appropriate drift blanks. Mix standard solutions of heavy metals (1000 mg/L), namely iron, manganese, zinc and copper - ICP Multielement Standard solution IV CertiPUR, were purchased from Merck Germany. Solutions of varying concentrations were prepared for all the metals by diluting the standards. HPLC water was used for the preparation of reagents and standards. All chemicals were trace metal grade (Suprapur). Concentrate nitric acid (HNO3 65%), were obtained from Merck Germany. All glassware was treated with Pierce solution 20% (v/v), rinsed with cold tap water followed by 20% (v/v) nitric acid and then rinsed with double-distilled water. For quality control purposes, blanks and duplicates samples were analysed during the procedure. NCS Certified Reference Material-DC 85104a and 85105a (China National Analysis Center for Iron&Steel), was analysed for quality assurance. Per cent recovery means were: Fe (93%), Mn (94%), Zn (105%), and Cu (103%). The variation coefficients were below 10%. Detection limits (μg/mL) were determined by the calibration curve method: Fe (0.14), Mn (0.18), Zn (0.45), Cu (0.11). The blank reagent and standard reference soil materials were included in each sample batch to verify the accuracy and precision of the digestion procedure and also for subsequent analyses.
Feed analysis for total polyphenol
The most used extraction solvent of polyphenol from vegetable matrix is methanol or ethanol and water-methanol/ethanol solutions [37]. The hydro-ethanol extracts for total polyphenol contents determination were prepared as following: 1 g feed samples, grounded and sieved at 0.3 mm was mixed with 20 mL 50% ethanol (v/v). The obtained hydro-ethanol extracts were rested for 30 minutes and finally filtered. The total polyphenol content was determinate using Folin & Ciocalteu method [38]. In this reagent Molybdenum is in superior oxidation state (+6) and has a yellow colour. The polyphenol are responsible for the reduction of Mo 6+ to the inferior state of oxidation (Mo 5+, Mo 4+) with blue colour. It was prepared 2.0 M Folin-Ciocalteu phenol reagent, 10 mM gallic acid standard solutions (50% ethanol) and 7.5% carbonate solutions. All chemicals and reagents were analytical grade or purest quality purchased from Merck and Fluka. For the preparation of calibration curve 0.5 mL aliquot of 0.2, 0.3, 0.4, 0.8 and 1.2 μM/mL gallic acid solution were mixed with 2.0 mL Folin-Ciocalteu reagent (diluted 1/10) and 2.0 mL sodium carbonate solution. The absorbance of solutions was read after 2 h at 750 nm using UV-VIS spectrophotometer SPECORD 205 by Analytik Jena. All determinations were realized in triplicate for each sample. Total polyphenol content was expressed like mg gallic acid/g feed. Squared correlation coefficient (R2) for calibration curve was 0.994 [39,40].
Statistical treatment of data
Measurements of total polyphenol and metals contents in feed respectively meat samples were expressed in terms of means and standard deviation. The whole data were subjected to a statistical analysis and correlation matrices were produced to examine the inter-relationships between the investigated metals concentrations of the meat samples and between these metals and total polyphenol from different diets, Student's t-test was employed to estimate the significance of values. Statistical significance was computed using Pair-Samples T-Test, with a significance level of p < 0.05. The data were statistically analysed and plotted using a statistical package Origin Pro 8.5.1.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
DSS and IG contributed equally to the study design, collection of data, development of the animals and vegetables sampling, analyses, interpretation of results and preparation of the paper. Both authors read and approved the final manuscript.
Contributor Information
Ducu Sandu Stef, Email: ducu_stef@yahoo.com.
Iosif Gergen, Email: igergen@yahoo.com.
Acknowledgements
This work was supported by CNCSIS - UEFISCSU, project number 1116/2009 PNII - IDEI, code 896/2008.
References
- Sabir SM, Khan SW, Hayat I. Effect of environmental pollution on quality of meat in district Bagh, Azad Kashmir. Pakistan Journal of Nutrition. 2003;2:98–101. [Google Scholar]
- Birla Singh K, Taneja SK. Concentration of Zn, Cu and Mn in vegetables and meat foodstuffs commonly available in Manipur: a North Eastern state of India. EJEAFChe. 2010;9:610–616. [Google Scholar]
- Harmanescu M, Alda LM, Bordean DM, Gogoasa I, Gergen I. Heavy metals health risk assessment for population via consumption of vegetables grown in old mining area; a case study: Banat County Romania. Chem Centr J. 2011;5:64. doi: 10.1186/1752-153X-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murariu M, Gradinaru RV, Mihai M, Jurcoane S, Drochioiu G. Unexpected effect of nickel complexes of some histidine-containing peptides on Escherichia coli. Romanian Biotechnological Letters. 2011;16(3):6242–6246. [Google Scholar]
- Drinceanu D, Julean C, Simiz E, Ştef L, Luca I, Sofian D. Effects of mineral supplements on bioproductive results in egg-laying hens farmed in organic systems. Scientific Papers: Animal Science and Biotechnologies. 2011;44:30–36. [Google Scholar]
- Abdulkarimi R, Abdullahi A, Amini M. Mentha extract consumption (Mentha piperita L) reduced blood iron concentration and increased TIBC levels in broiler chickens. Journal of American Science. 2011;7:494–500. [Google Scholar]
- Okoye COB, Ibeto CN, Ihedioha JN. Assessment of heavy metals in chicken feeds sold in south eastern, Nigeria. Advances in Applied Science Research. 2011;2:63–68. [Google Scholar]
- Murariu M, Dragan ES, Drochioiu G. Electrospray ionization mass spectrometric approach of conformationally-induced metal binding to oligopeptides. Eur J Mass Spectrom. 2010;16:511. doi: 10.1255/ejms.1092. [DOI] [PubMed] [Google Scholar]
- Drochioiu G, Manea M, Dragusanu M, Murariu M, Dragan ES, Petre BA, Mezo G, Przybylski M. Interaction of beta-amyloid(1-40) peptide with pairs of metal ions: An electrospray ion trap mass spectrometric model study. Biophys Chem. 2009;144(1-2):9–20. doi: 10.1016/j.bpc.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Bao YM, Choct M, Iji PA, Bruerton K. Effect of organically complexed Copper, Iron, Manganese and Zinc on broiler performance, mineral excretion and accumulation in tissues. J Appl Poult Res. 2007;16:448–455. [Google Scholar]
- Nollet L, van der Klis JD, Lensing M, Spring P. The effect of replacing inorganic with organic trace minerals in broiler diets on productive performance and mineral excretion. J Appl Poult Res Winter. 2007;16:592–597. doi: 10.3382/japr.2006-00115. [DOI] [Google Scholar]
- Abdallah AG, El-Husseiny OM, Abdel-Latif KO. Influence of some dietary organic mineral supplementations on broiler performance. International Journal of Poultry Science. 2009;8:291–298. [Google Scholar]
- Hernandez F, Madrid J, Garcıa V, Orengo J, Megıas MD. Influence of Two Plant Etracts on Broilers Performance, Digestibility, and Digestive Organ Size. Poultry Sci. 2004;83:169–174. doi: 10.1093/ps/83.2.169. [DOI] [PubMed] [Google Scholar]
- Stef L, Dumitrescu G, Drinceanu D, Stef D, Mot D, Julean C, Tetileanu R, Corcionivoschi N. The effect of medicinal plants and plant extracted oils on broiler duodenum morphology and immunological profile. Rom Biotechnol Lett. 2009;14:4606–4614. [Google Scholar]
- Khaligh F, Sadeghi G, Karimi A, Vaziry A. Evaluation of different medicinal plants blends in diets for broiler chickens. Journal of Medicinal Plants Research. 2011;5:1971–1977. [Google Scholar]
- Zaki AA Elbarawy AM Darwish AS Biochemical studies on the effect of Nasturtium Officinalis plant extract in chickens fed raw soya bean meals Aust J Basic & Appl Sci 20115755–761.10649322 [Google Scholar]
- Jang A, Liu XD, Shin MH, Lee BD, Lee SK, Lee JH, Jo C. Antioxidative potential of raw breast meat from broiler chicks fed a dietary medicinal herb extract mix. Poultry Sci. 2008;87:2382–2389. doi: 10.3382/ps.2007-00506. [DOI] [PubMed] [Google Scholar]
- Brenes A, Viveros A, Goñi I, Centeno C, Saura-Calixto F, Arija I. Effect of grape seed extract on growth performance, protein and polyphenol digestibilities, and antioxidant activity in chickens. Span J Agric Res. 2010;8:326–333. [Google Scholar]
- Polat U, Yesilbag D, Eren M. Serum biochemical profile of broiler chickens fed diets containing Rosemary and Rosemary volatile oil. J Biol Environ Sci. 2011;5:23–30. [Google Scholar]
- Karamać M. Chelation of Cu(II), Zn(II), and Fe(II) by tannin constituents of selected edible nuts. Int J Mol Sci. 2009;10:5485–5497. doi: 10.3390/ijms10125485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokhar S, Apenten RKO. Iron binding characteristics of phenolic compounds: some tentative structure-activity relations. Food Chem. 2003;81:133–140. doi: 10.1016/S0308-8146(02)00394-1. [DOI] [Google Scholar]
- Andjelković M, Van Camp J, De Meulenaer B, Depaemelaere G, Socaciu C, Verloo M, Verhe R. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006;98:23–31. doi: 10.1016/j.foodchem.2005.05.044. [DOI] [Google Scholar]
- Vargias L, Harmanescu M, Gergen I. Mineral Content and Antioxidant Capacity of Aqueous and Methanolic Extract from Epilobium Hirsutum. Scientifical Researches. Agroalimentary Processes and Technologies. 2005;XI(1):199–204. [Google Scholar]
- Safra J, Pospísilová M, Honegr J, Spilková J. Determination of selected antioxidants in Melissae herba by isotachophoresis and capillary zone electrophoresis in the column-coupling configuration. J Chromatogr A. 2007;1171:124–132. doi: 10.1016/j.chroma.2007.09.024. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency. Assessment report on Salvia Officinalis L., Folium and Salvia Officinalis L., Aetheroleum. 2010. http://www.emea.europa.eu/docs/en_GB/document_library/Herbal_-_HMPC_assessment_report/2010/02/WC500070850.pdf
- Franchi GG, Nencini C, Collavoli E, Massarelli P. Composition and antioxidant activity in vitro of different St. John's Wort (Hypericum perforatum L.) extracts. Journal of Medicinal Plants Research. 2011;5:4349–4353. [Google Scholar]
- Orčić DZ, Mimica-Dukić NM, Francišković MM, Petrović SS, Jovin EĐ. Antioxidant activity relationship of phenolic compounds in Hypericum perforatum L. Chem Centr J. 2011;5:34. doi: 10.1186/1752-153X-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valimareanu S Deliu C Polymorphism detection of in vitro cultivated Epilobium (Onagraceae) species using RAPD method Contribuţii Botanice 2008XLII113–120.17415859 [Google Scholar]
- Kohlert C, Van Rensen I, Marz R, Schindler G, Graefe EU, Veit M. Bioavailability and pharmokinetics of natural volatile terpenes in animal and humans. Planta Medica. 2000;66:495–505. doi: 10.1055/s-2000-8616. [DOI] [PubMed] [Google Scholar]
- Barreto MSR, Menten JFM, Racanicci AMC, Pereira PWZ, Rizzo PV. Plant extracts used as growth promoters in broilers. Brazilian Journal of Poultry Science. 2008;10:109–115. [Google Scholar]
- Iwegbue CMA, Nwajei GE, Iyoha EH. Heavy metal residues of chicken meat and gizzard and turkey meat consumed in Southern Nigeria. Bulgarian Journal of Veterinary Medicine. 2008;11:275–280. [Google Scholar]
- Uluozlu OD, Tuzen M, Mendil D, Soylak M. Assessment of trace element contents of chicken products from Turkey. Journal of Hazardous Materials. 2009;163:982–987. doi: 10.1016/j.jhazmat.2008.07.050. [DOI] [PubMed] [Google Scholar]
- Akan JC, Abdulrahman FI, Sodipo OA, Chiroma YA. Distribution of heavy metals in the liver, kidney and meat of beef, mutton, caprine and chicken from Kasuwan Shanu market in Maiduguri Metropolis, Borno State, Nigeria. Research Journal of Applied Sciences, Engineering and Technology. 2010;2:743–748. [Google Scholar]
- Ordinance 975/16 XII 1998 of Romanian Ministry of Health. http://www.unilab.ro/downloads/O%20975%20norme%20igienico-sanitare%20-%20marieta.pdf
- Harmanescu M. Heavy Metals Determination in Selected Medicinal Plants. Annals of the Faculty of Engineering Hunedoara - Journal of Engineering. 2007;V(1):63–68. [Google Scholar]
- Subcommittee on Poultry Nutrition, National Research Council. Nutrient Requirements of Poultry. Ninth Revised. The National Academies Home, 500 Fifth St. N.W. Washington, D.C; 1994. 20001 http://www.nap.edu/catalog.php?record_id=2114. [Google Scholar]
- Ignat I, Volf I, Popa VI. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011;126:1821–1835. doi: 10.1016/j.foodchem.2010.12.026. [DOI] [PubMed] [Google Scholar]
- Folin O, Ciocalteu V. On tyrosine and tryptophane determination in proteins. Journal of Biological Chemistry. 1927;27:627–650. [Google Scholar]
- Harmanescu M, Moisuc A, Radu F, Dragan SI, Gergen I. Total polyphenol content determination in complex matrix of medicinal plants from Romania by NIR spectroscopy. Bulletin UASVM Agriculture. 2008;65:123–128. [Google Scholar]
- Moigradean D Poiana MA Gogoasa I Harmanescu M Gergen I Lazureanu A The correlations between total antioxidant capacity and total polyphenol content established for tomatoes Lucrări stiinłifice medicină veterinară 2007XL486–489.22122896 [Google Scholar]