Abstract
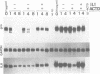
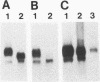
Expression of the cytokine gene gro, also known as melanoma growth stimulatory activity, is induced by inflammatory stimuli, including IL-1. To determine whether gro expression is regulated at a post-transcriptional level, the effect of IL-1 on gro mRNA stability was examined. Treatment of fibroblasts with IL-1 beta caused a dose-dependent induction of gro mRNA. When IL-1 was withdrawn, gro mRNA decayed rapidly with a half life of 1 hour. This decay occurred whether or not actinomycin D was added to block new transcription. In contrast, when IL-1 was present in the medium, the level of gro mRNA was stable over 8 hours following addition of actinomycin D. In addition, the stability of a related mRNA, IL-8, was found to be regulated by IL-1. To examine whether Northern results reflected expression of gro alpha, or of the closely related genes, gro beta and gro gamma, RNA samples were analyzed by PCR. All three genes were found to be induced by IL-1 and all mRNAs were stabilized in the presence of IL-1. Northern analysis revealed a minor species of gro mRNA which lacked poly(A). The pattern of expression of this RNA suggested that it was a decay intermediate of one or more of the gro mRNAs. The findings indicate that mRNA stabilization is an important component of IL-1 induced gene expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anisowicz A., Bardwell L., Sager R. Constitutive overexpression of a growth-regulated gene in transformed Chinese hamster and human cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7188–7192. doi: 10.1073/pnas.84.20.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisowicz A., Zajchowski D., Stenman G., Sager R. Functional diversity of gro gene expression in human fibroblasts and mammary epithelial cells. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9645–9649. doi: 10.1073/pnas.85.24.9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Yen T. J. Multiple determinants of eukaryotic mRNA stability. New Biol. 1989 Nov;1(2):121–126. [PubMed] [Google Scholar]
- Golds E. E., Mason P., Nyirkos P. Inflammatory cytokines induce synthesis and secretion of gro protein and a neutrophil chemotactic factor but not beta 2-microglobulin in human synovial cells and fibroblasts. Biochem J. 1989 Apr 15;259(2):585–588. doi: 10.1042/bj2590585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskill S., Peace A., Morris J., Sporn S. A., Anisowicz A., Lee S. W., Smith T., Martin G., Ralph P., Sager R. Identification of three related human GRO genes encoding cytokine functions. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7732–7736. doi: 10.1073/pnas.87.19.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt T. Controlling mRNA lifespan. Nature. 1988 Aug 18;334(6183):567–568. doi: 10.1038/334567a0. [DOI] [PubMed] [Google Scholar]
- Iida N., Grotendorst G. R. Cloning and sequencing of a new gro transcript from activated human monocytes: expression in leukocytes and wound tissue. Mol Cell Biol. 1990 Oct;10(10):5596–5599. doi: 10.1128/mcb.10.10.5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstein T., June C. H., Ledbetter J. A., Stella G., Thompson C. B. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science. 1989 Apr 21;244(4902):339–343. doi: 10.1126/science.2540528. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Morishita K., Yoshimura T., Lavu S., Kobayashi Y., Lew W., Appella E., Kung H. F., Leonard E. J., Oppenheim J. J. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988 Jun 1;167(6):1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser B., Clark-Lewis I., Zwahlen R., Baggiolini M. Neutrophil-activating properties of the melanoma growth-stimulatory activity. J Exp Med. 1990 May 1;171(5):1797–1802. doi: 10.1084/jem.171.5.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond A., Balentien E., Thomas H. G., Flaggs G., Barton D. E., Spiess J., Bordoni R., Francke U., Derynck R. Molecular characterization and chromosomal mapping of melanoma growth stimulatory activity, a growth factor structurally related to beta-thromboglobulin. EMBO J. 1988 Jul;7(7):2025–2033. doi: 10.1002/j.1460-2075.1988.tb03042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond A., Thomas H. G. Melanoma growth stimulatory activity: isolation from human melanoma tumors and characterization of tissue distribution. J Cell Biochem. 1988 Feb;36(2):185–198. doi: 10.1002/jcb.240360209. [DOI] [PubMed] [Google Scholar]
- Schröder J. M., Persoon N. L., Christophers E. Lipopolysaccharide-stimulated human monocytes secrete, apart from neutrophil-activating peptide 1/interleukin 8, a second neutrophil-activating protein. NH2-terminal amino acid sequence identity with melanoma growth stimulatory activity. J Exp Med. 1990 Apr 1;171(4):1091–1100. doi: 10.1084/jem.171.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder J. M., Sticherling M., Henneicke H. H., Preissner W. C., Christophers E. IL-1 alpha or tumor necrosis factor-alpha stimulate release of three NAP-1/IL-8-related neutrophil chemotactic proteins in human dermal fibroblasts. J Immunol. 1990 Mar 15;144(6):2223–2232. [PubMed] [Google Scholar]
- Schuler G. D., Cole M. D. GM-CSF and oncogene mRNA stabilities are independently regulated in trans in a mouse monocytic tumor. Cell. 1988 Dec 23;55(6):1115–1122. doi: 10.1016/0092-8674(88)90256-5. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Shyu A. B., Greenberg M. E., Belasco J. G. The c-fos transcript is targeted for rapid decay by two distinct mRNA degradation pathways. Genes Dev. 1989 Jan;3(1):60–72. doi: 10.1101/gad.3.1.60. [DOI] [PubMed] [Google Scholar]
- Stoeckle M. Y., Barker K. A. Two burgeoning families of platelet factor 4-related proteins: mediators of the inflammatory response. New Biol. 1990 Apr;2(4):313–323. [PubMed] [Google Scholar]
- Stoeckle M. Y., Hanafusa H. Processing of 9E3 mRNA and regulation of its stability in normal and Rous sarcoma virus-transformed cells. Mol Cell Biol. 1989 Nov;9(11):4738–4745. doi: 10.1128/mcb.9.11.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekamp-Olson P., Gallegos C., Bauer D., McClain J., Sherry B., Fabre M., van Deventer S., Cerami A. Cloning and characterization of cDNAs for murine macrophage inflammatory protein 2 and its human homologues. J Exp Med. 1990 Sep 1;172(3):911–919. doi: 10.1084/jem.172.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B., Mermod J. J., Vassalli P. Phagocytosis and inflammatory stimuli induce GM-CSF mRNA in macrophages through posttranscriptional regulation. Cell. 1987 Feb 27;48(4):671–679. doi: 10.1016/0092-8674(87)90245-5. [DOI] [PubMed] [Google Scholar]
- Tso J. Y., Sun X. H., Kao T. H., Reece K. S., Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985 Apr 11;13(7):2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen D. Z., Rowland A., Derynck R. Expression and secretion of gro/MGSA by stimulated human endothelial cells. EMBO J. 1989 Jun;8(6):1761–1766. doi: 10.1002/j.1460-2075.1989.tb03569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T., Treisman R. Removal of poly(A) and consequent degradation of c-fos mRNA facilitated by 3' AU-rich sequences. Nature. 1988 Nov 24;336(6197):396–399. doi: 10.1038/336396a0. [DOI] [PubMed] [Google Scholar]
- Wolpe S. D., Cerami A. Macrophage inflammatory proteins 1 and 2: members of a novel superfamily of cytokines. FASEB J. 1989 Dec;3(14):2565–2573. doi: 10.1096/fasebj.3.14.2687068. [DOI] [PubMed] [Google Scholar]