Abstract
Treatment-related myelodysplastic syndrome (t-MDS) and treatment-related acute myelogenous leukemia (t-AML) represent rare secondary events in patients with primary tumors of the nervous system and predominantly affect those treated with alkylating agents or topoisomerase II inhibitors. Temozolomide has become the standard chemotherapeutic agent for malignant gliomas. The emergence of this alkylating agent with little acute toxicity or cumulative myelosuppression has led to off-label protracted chemotherapy for many patients with malignant and even low-grade infiltrative gliomas, raising concern for increased risk of t-MDS/t-AML in the few long-term survivors. On the basis of an extensive literature search, we provide a discussion of epidemiology, pathogenesis, clinical presentation, diagnosis, and therapy of these disorders. t-MDS/t-AML remain rare complications of chemotherapy in patients with primary brain tumors, and the vast majority of patients die of their primary neoplasm. Prospective randomized studies with long-term follow-up are required to accurately assess the risk of t-MDS/t-AML; however, unless survival in the most common gliomas substantially increases, t-MDS/t-AML incidence will likely remain low in this patient population.
Keywords: brain, chemotherapy, glioma, leukemia, myelodysplastic syndromes
Myelodysplastic syndromes (MDS) are a heterogenous group of clonal hematopoietic stem cell disorders. MDS is distinguished from acute myelogenous leukemia by a peripheral or marrow myeloblast count of less than 20%. Although the majority of cases occur sporadically, 10%–15% are considered to be iatrogenic (t-MDS) in the context of therapy with alkylating agents, topoisomerase II inhibitors, or ionizing radiation (Fig. 1).
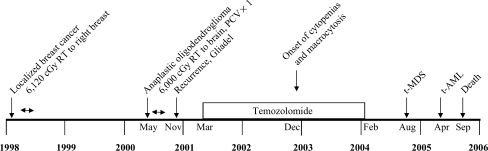
Fig. 1.
Timeline of events from initial diagnosis of cancer to the onset of acute leukemia in a patient with anaplastic oligodendroglioma (see Table 1, No. 33 for details of case). PCV, procarbazine, lomustine, vincristine; RT, radiation therapy.
Because of their ability to penetrate the blood-nervous system barrier and their intrinsic activity against a wide variety of nervous system neoplasms, alkylating chemotherapy agents have been a mainstay of nervous system tumor therapy. Temozolomide is an orally administered analogue of dacarbazine for which activity is mediated primarily via DNA methylation at the O6 position of guanine.1 It was approved by the US FDA for use in patients with relapsed anaplastic astrocytoma in 1999. On the basis of the results of a randomized, prospective trial, the drug is now considered to be the standard of care in conjunction with external beam radiotherapy after surgical tumor resection in patients with glioblastoma multiforme.2 In addition, temozolomide is increasingly used off-label for patients with tumors associated with long-term survival, such as anaplastic oligodendrogliomas and clinically or radiographically progressive low-grade gliomas.3–7 Because the risk of t-MDS/t-AML after alkylating chemotherapy is dependent on the total dose, there is concern that, with increasing and prolonged use of temozolomide or other alkylating agents, especially in patients with more favorable outcomes, more cases of t-MDS/t-AML will emerge.
We provide a review of t-MDS/t-AML in patients with tumors of the nervous system treated with alkylator chemotherapy and discuss epidemiology, pathogenesis, clinical presentation, diagnosis, and therapy of these disorders.
Methods
We performed a comprehensive search of the PubMed database of the US National Library of Medicine with use of various combinations of the following search terms: myelodysplastic, glioma, glioblastoma, leuk(a)emia, myelogenous, t-MDS, MDS, brain neoplasm, temozolomide, AML, t-AML, and treatment complication. We identified well-documented case reports and small case series of patients who developed t-MDS and t-AML during or after treatment with alkylating chemotherapy for a primary brain neoplasm. We recorded the type of alkylating and other chemotherapy agents used, dose, concomitant or sequential irradiation, genetic predisposition, type of myelogenous tumor, cytogenetic findings, latency between completion of chemotherapy and diagnosis of t-MDS/t-AML, treatment, and outcome.
Epidemiology
Primary MDS is a disease that occurs in the older population (median age at diagnosis, 76 years), whereas t-MDS/t-AML affects younger adults. The overall incidence of primary MDS is estimated at 3–20 cases per 100 000 population. Among patients more than70 years old, the risk is increased by approximately10-fold, compared with the risk in individuals less than 60 years old.8 No population-based data are available for t-MDS/t-AML, but it is estimated that 10%–15% of all MDS cases arise in patients exposed to chemo- or radiation therapy administered for other tumors. The risk of t-MDS is therefore low but not negligible. t-MDS/t-AML arises substantially earlier than do other secondary malignancies. In clinical trials of alkylating therapy, the incidence has been 0.25%–1% per year beginning 2 years after the start of therapy and decreasing 7 years after the completion of therapy. Whether the specific therapy provided for the primary cancer is the main contributor or primary diagnosis is an independent risk factor for the development of t-MDS/t-AML remains unclear.
Few retrospective studies on the relative incidence of t-MDS/t-AML as secondary neoplasms in patients with primary brain tumors are available in the pediatric and only 1 in the adult oncology literature. In a Turkish series of 992 pediatric patients with brain tumors who were seen over 34 years in a single institution, MDS was encountered in 1 patient.9 In a cohort of 1283 patients with brain tumors who were less than22 years old and seen over the course of 18 years at St. Jude Children's Hospital, 2 patients developed t-MDS, and 1 patient developed t-AML.10 Three of 198 children with central nervous system tumors treated as part of a clinical trial by the Pediatric Oncology Group developed t-MDS/t-AML.11 In a meta-analysis of 7 randomized clinical trials for adult patients with brain tumors, Greene et al. identified 2 of 1628 individuals who experienced acute nonlymphocytic leukemia after carmustine chemotherapy. The risk of developing this complication was 24.6 times higher than expected, whereas in recipients of other chemotherapy, no leukemia cases were encountered.12
Overall, the t-MDS/t-AML risk among patients with brain tumors is likely to be substantially lower than in patients with other primary neoplasms.10,11,13 In addition, t-MDS/t-AML in patients with primary brain tumors is less common than in those with other secondary neoplasms.14 According to a national registry, the estimated cumulative risk of developing a secondary neoplasm within 10 years after diagnosis of the first malignancy is 1.9% among children.15 The median latency ranges from 1 to 17 years (median, 6.7 years). Hematologic malignancies are notable because of their short latency (median, 2–6 years; range, 1–11 years).16–18 The cumulative risk for developing t-AML is 0.6% at 15 years.17 t-MDS/t-AML is most frequently encountered after Hodgkin's disease, osteogenic sarcoma, acute lymphoblastic leukemia, non-Hodgkin's lymphoma, and Ewing sarcoma. In a single-institution retrospective series of 112 adult patients with t-MDS/t-AML, the median time from initial diagnosis to t-MDS was 71 months (range, 7–331 months). t-MDS occurred in 51%, 55% of whom later experienced transformation to an acute leukemia. The most common primary neoplasms were Hodgkin's disease (26%), breast cancer (18%), and ovarian cancer (13%).19 The 5-year cumulative risk of t-MDS/t-AML after autologous stem cell transplantation for Hodgkin's and non-Hodgkin's lymphoma was found to be 4.2% in one series of 230 patients.20
Pathogenesis
Evolution of a secondary neoplasm reflects a complex pathogenetic process dependent on genetic susceptibility, including polymorphisms for drug metabolism, environmental factors, and treatment (exposure to ionizing radiation and mutagenic chemotherapeutic agents).21 Studies on the individual leukemogenic potential of a treatment modality are often confounded by concomitant administration of other therapies and lack of control for risk factors. A synergistic effect of radiotherapy and chemotherapy in inducing second malignant neoplasms has been reported, but usually, the individual contribution of each treatment modality is unknown. Smith et al. found no difference in clinical presentation, latency, and cytogenetic abnormalities between recipients of radiation therapy alone and chemotherapy alone, suggesting that the mutagenicity of these treatments may be quite similar.22
Cell of Origin
Myelodysplastic syndromes are clonal hematopoietic stem cell disorders, and t-MDS/t-AML represents a distinct category of these illnesses.23 It has been suggested that marrow stem cells are particularly susceptible to the mutagenic effects of alkylating agents, because they are relatively deficient in O6-alkylguanine-alkyltransferase. About three-quarters of patients initially receive a diagnosis of t-MDS and then progress to t-AML over a median interval of 4 months.22
Chemotherapy
The chemotherapeutic agents most commonly implicated in the pathogenesis of t-MDS/t-AML are alkylating agents, such as melphalan, nitrosoureas (carmustine, lomustine, and semustine), procarbazine, or temozolomide, and topoisomerase II inhibitors (epipodophyllotoxins). Thio-TEPA, dacarbazine, dibromodulcitol, and cisplatin are also probably leukemogenic. Cyclophosphamide produces t-MDS less commonly.19,24 A higher risk appears to result from myeloablative regimens prior to stem cell transplantation, a treatment rarely used for patients with brain tumors. The risk of t-MDS/t-AML is related to the specific DNA-damaging agent, dose, duration of therapy, and increases as patients age.3,22 Alkylating agents produce t-MDS/t-AML with a latency of 5–7 years (median, 55 months) after exposure.22
t-MDS/t-AML has been described after therapy of primary brain tumors with classic alkylating agents, radiation therapy alone, or combined chemotherapy and radiation therapy. From the literature, we identified 44 cases of t-MDS/t-AML after therapy of primary brain tumors (Table 1). There were 20 male and 18 female patients (gender not listed for 6) with a median age of 25 years (range, 0.25–69 years). The most common primary tumor was anaplastic astrocytoma (9), followed by diffuse astrocytoma (7), medulloblastoma, glioblastoma (6 each), and choroid plexus papilloma. Twenty-eight patients developed t-MDS, and 1 patient received a diagnosis of preleukemia. Of those, 13 progressed to t-AML. In 14 patients, t-AML was the first hematologic diagnosis and, in 1, acute nonlymphoblastic leukemia. The median interval from completion of initial chemotherapy to diagnosis of t-MDS/t-AML was 15.5 months (range, 0–92 months). Patients received the following alkylating agents as part of their therapy: lomustine, carmustine, nimustine, procarbazine, cyclophosphamide, or nitrogen mustard. Ten recipients of temozolomide developed t-MDS/t-AML3,25–27 after 3–25 cycles. Other agents used were vincristine, prednisone, cisplatin, carboplatin, methotrexate, 2,4-diamino-5, 4-dichloro-phenyl-6-methylpyrimidine, dactinomycin, thioguanine, hydroxyurea, doxorubicin, etoposide, teniposide, and 6-mercaptopurine.
Table 2:
Patients with t-MDS/t-AML after treatment for a primary central nervous system neoplasm. t-MDS/t-AML diagnosis, treatment and outcome.
| No. | Type of myelogenous tumor | Cytogenetics | Latency [months after completion of initial chemotherapy or as stated] | Treatment | Outcome | Survival [months] | Cause of Death |
|---|---|---|---|---|---|---|---|
| 1 | tMDS | monosomy 5, 7 | 15 | tAML | U | ||
| 2 | pre-leukemia' | 45, XY, -7, t(3;3)(q23; q29) | 23 | Ara-C, daunomycin; vincristine, prednisone, L-asparaginase, daunomycin | biphenotypic leukemia | U | leukemia |
| 3 | tMDS (RAEB) | unknown | 25 (after initial tumor dx) | chemotherapy, [allo SCT] | death | U | PD |
| 4 | tMDS with myelofibrosis | unknown | 102 (after initial tumor dx) | chemotherapy, [allo SCT] | death | U | PD |
| 5 | tMDS | unknown | 17 | methylprednisolone, etoposide, mitoxantrone | death | U | unknown |
| 6 | tMDS | complete or partial loss of chr. 5 and 7 | 24 | supportive | death | 8 | PD |
| 7 | tMDS | complete or partial loss of chr. 5 and 7 | 28 | allo SCT | death | 10 | allo SCT |
| 8 | tMD | complete or partial loss of chr. 5 and 7 | 16 | supportive | tAML; death | 4 | tAML |
| 9 | tMDS | complete or partial loss of chr. 5 and 7 | 22 | supportive | death | 12 | PD |
| 10 | tMDS | complete or partial loss of chr. 5 and 7 | 31 | supportive | alive | 10 + | – |
| 11 | tMDS | complete or partial loss of chr. 5 and 7 | 3 | allo SCT | tAML; death | 2 + | – |
| 12 | tMDS | complete or partial loss of chr. 5 and 7 | 31 | supportive | tAML; alive | 0.5 | tAML |
| 13 | AMML | 14 | daunorubicin, Ara-C | death | 3 | tAMML, phlegmasia coerulea dolens | |
| 14 | tMDS | 10 | daunorubicin, Ara-C | tAMML; death | 4 | tAMML, candida PNA | |
| 15 | tMDS | monosomy 7 | 92 | death | U | ||
| 16 | tMDS | monosomy 7 | 57 | death | U | ||
| 17 | tAML | 33 | death | U | |||
| 18 | tMDS | partial deletion long arm chr 4 | 3 | supportive | death | 1 | tMDS |
| 19 | tMDS (RAEB) | – | 18 | daunorubicin, Ara-C (tAMML) | tAMML (myelomonocytic) | 3 | hemoperitoneum (tAMML) |
| 20 | tAML (promyelocytic) | – | 5 | rubidomycin, Ara-C | death | 0.75 | tAML |
| 21 | tAML | – | > 24 | – | death | U | PD malignant brain tumor |
| 22 | tAML (FAB M5b) | – | 3.5 | U | |||
| 23 | tAML (FAB M2) | – | 18.5 | U | |||
| 24 | tMDS | structural abnormalities chr. 7, 10, 17, 21 | 19 | -; mitoxantrone, Ara-C, 6-thioguanine (for AML) | tAML (FAB M4) 8 months later | 19 | tAML |
| 25 | tMDS | monosomy 7 | 5 | – | alive 8 months after diagnosis of tMDS | 8 + | – |
| 26 | erythroleukemia | 50,XY,+ X,+ del(1)(p21), + 10, + 11,-12, + der(12)t(12:?)(q?:?) | 0 | – | death | 3 (estimated; 6 months after dx with germ cell tumor) | ICH |
| 27 | ANLL' | – | 48 | – | death | < 1 month | ICH |
| 28 | tMDS | del 5q, MLL amplification | 24 (after completion of last chemotherapy) | – | death | 2 | fungal sepsis |
| 29 | tAML (M6, erythroleukemia) | – | 3 | – | death | 1 | ICH (shortly after shunt placement; known to have PD of brain tumor; plt 75) |
| 30 | tAML (M4, myelomonocytic leukemia) | – | 8 | daunorubicin, Ara-C, thioguanine | death | 2 | tAML |
| 31 | tCMML | 46, XY, der(11)t(1;11)(q21;q14) | – | hydroxyurea | tAML | U | – |
| 32 | tAMML | llp to 12p translocation, deletion of chromosome 16q | 28 | – | death | 2 | tAMML |
| 33 | tMDS (RAEB); AML | del chr. 1, 5, 6, 7, 11, 16; rearrangement of chr. 3, 9, 11, 12 | 0 | idarubicin, cytarabine; for AML: FLAG; mitoxantrone, etoposide; HD cytarabine with G-CSF | tAML | 13 | tAML |
| 34 | tAML (M2) | 46, XY[6]/46, XY, del(7)(q22)[14]) | 2 | Ara-C, daunorubicin | alive, undergoing treatment | U | – |
| 35 | tMDS (‘hypocellular marrow with 10 % blasts) | – | 2 | yes | tAML (M1); death | 7 | complications during induction chemotherapy |
| 36 | tMDS (RAEB) | 46, XX, -7, +mar/45, XY, -7 | 19 (from initial brain tumor diagnosis) | no | death | 16 | – |
| 37 | tAML | – | 2 | – | death | 0 | pneumonia |
| 38 | tMDS (RAEB) | 45 XX, -7 | 53 (after diagnosis of brain tumor) | HD busulfan/cyclophosphamide, MRD allo SCT, it cytarabine x 1 | alive, in remission | 35 + | – |
| 39 | tMDS(CMML) | 46,XX,t(11;16)(q23;p13)[17]/46,idem,i(17)(q10)[2]/46,XX[1] | 19 | bone marrow transplant | ? | U | ? |
| 40 | tMDS (RAEB) | 46 XX, del (3)(q11.1) | 1 | thalidomide; 3 + 7 regimen (idarubicin, cytarabine) | mixed lineage acute leukemia | 5 weeks after initiation of induction therapy for leukemia | acute leukemia |
| 41 | tMDS (RAEB) | 47, XY, +8, der(15)t(1;15)(p10:q10) | 76 | – | tAML | 15 | tAML |
| 42 | tMDS (RAEB) | 46, XX, +1, der(1;7)(q10;p10), i(21)(q10) | 31 (after salvage therapy) | – | death | 3 | acute renal failure, DIC |
| 43 | tAMML | 0 (after last chemotherapy) | Ara-C, daunorubicin | death | 2 | sepsis, tAML | |
| 44 | acute promyelocytic leukemia | – | 0 | – | death | 0 | ICH |
Abbreviations: allo SCT, allogeneic stem cell transplant; Ara-C, cytarabine; DIC, disseminated intravascular coagulopathy; FLAG, fludarabine, cytarabine; G-CSF, HD, high-dose; ICH, intracranial hemorrhage; MRD, matched related donor; PD, progression of brain tumor; RAEB, refractory anemia with excess blasts; tAML, treatment-related myelogenous leukemia; tCMML, chronic myelomonocytic leukemia; tMDS, treatment-related myelodysplastic syndrome; tAMML, treatment-related acute myelomonocytic leukemia; U, unknown.
Table 1.
Patients with t-MDS/t-AML after treatment for a primary central nervous system neoplasm identified through a PubMed search. Exposure to chemo- and radiation therapy.
| No. | Reference | Age at Diagnosis of Brain Tumor [years] | Sex | Primary Tumor | Alkylating Chemotherapy Agents | Topisomerase II Inhbitor | Total Dose | No. of Cycles | Other Chemotherapy Agents | XRT | Dose [Gy] | Genetic Predisposition |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Akyuz | 15 | U | medulloblastoma | CCNU, PCB | – | – | – | vincristine | CST | – | – |
| 2 | Blatt | 3 | M | medulloblastoma | CCNU | – | – | – | vincristine, prednisone | CST | 36 Gy neuraxis, 54 Gy tumor bed | – |
| 3 | Broniscer | 0.4 | U | CPC | high cumulative dose’ | – | – | – | unknown | CST | unknown | TP53 mutation |
| 4 | Broniscer | 0.5 | U | CPC | high cumulative dose’ | – | – | – | unknown | – | unknown | TP53 mutation |
| 5 | Buyulpamukcu | 6.9 | M | ependymoblastoma | CCNU, PCB | – | – | 6 | vincristine | CST | unknown | – |
| 6 | Chamberlain | 51 | M | AA | CCNU, PCB; TMZ | – | – | 6; 12 | vincristine; - | IFXRT | 60 | – |
| 7 | Chamberlain | 44 | F | LGA | TMZ | – | – | 24 | – | IFXRT | 54 | – |
| 8 | Chamberlain | 69 | F | AA | TMZ; CCNU, PCB | – | – | 6; 6 | -; vincristine | IFXRT | 60 | – |
| 9 | Chamberlain | 38 | M | AA | TMZ; CCNU, PCB | – | – | 18; 6 | -; vincristine | IFXRT | 60 | – |
| 10 | Chamberlain | 34 | M | LGA | TMZ; CCNU, PCB | – | – | 24; 6 | -; vincristine | IFXRT | 54 | – |
| 11 | Chamberlain | 34 | F | AOA | TMZ; TMZ | – | – | 4; 24 | -; vincristine | IFXRT; gamma knife | 59.4 | – |
| 12 | Chamberlain | 59 | M | AO | CCNU, TMZ | – | – | 6, 5 | -; - | – | – | – |
| 13 | Cohen | 56 | F | angioblastic meningioma | BCNU; CCNU | -; -; teniposide | 720 mg/m2; 700 mg/m2; 10,000 mg/m2 | 3; 5; (2 yrs) | – | WBRT | ||
| 14 | Cohen | 60 | F | oligodendroglioma | meCCNU | – | 2700 mg/m2 | – | – | – | – | |
| 15 | Duffner | 1.92 | U | CPC | CYC(A) | etoposide (B) | 26 (alternating AAB/AAB) | vincristine (A)/cisplatin (B) | CST | 25.6 BR, 38.4 PF, 24 SP | – | |
| 16 | Duffner | 0.58 | U | desmoplastic infantile ganglioglioma | CYC(A) | etoposide (B) | 25 (alternating AAB/AAB) | vincristine (A)/cisplatin (B) | – | – | – | |
| 17 | Duffner | 1 | U | ependymoma | CYC(A) | etoposide (B) | 24 (alternating AAB/AAB) | vincristine (A)/cisplatin (B) | CST | 35.2, 53.2, PF | – | |
| 18 | Dufour | 11 | M | leptomeningeal oligodendrogliomatosis | PCB, CYC; -; TMZ | etoposide; etoposide; - | 2.25 g/m2 (etopopside) | 1; 4; 3 | cisplatin, vincristine, carboplatin; carboplatin; - | CST | 54 (PF), 36 (neuraxis) | – |
| 19 | Genot | 45 | F | anaplastic astrocytoma | CCNU; PCB; CCNU | teniposide, doxorubicin (added after a few months); teniposide | 1350 mg/m2 (CCNU) | 7 ?; 8 | – | – | – | – |
| 20 | Genot | 30 | F | GBM | CCNU; CCNU; PCB | teniposide; teniposide; teniposide | 1000 mg/m2 (CCNU) | 2; 1 (?); 9 | – | IFXRT | 60 | – |
| 21 | Goffman | 5 | M | grade III ependymoma | ; BCNU; - | – | 600 mg/m2 (BCNU), 720 mg/m2 (cisplatin) | ; 6; 6 | HDMTX; -; cisplatin | CST | 36 BR, 51.6 PF, 24 SP | – |
| 22 | Greene | 59 | M | GBM | BCNU | – | 1158 mg | 3 | – | IFXRT | 59.85 | – |
| 23 | Greene | 23 | M | LGA | BCNU | – | 3792 mg | 9 | – | IFXRT | 40 | – |
| 24 | Hayani | 1 | F | medulloblastoma | nitrogen mustard,PCB | – | – | 24 | vincristine, prednisone | – | – | – |
| 25 | Hayani | 7 | F | medulloblastoma | nitrogen mustard,PCB | – | – | 12 | vincristine, prednisone | CST | 36 (neuraxis), 18 (boost to PF), 3.6 (boost to T-spine) | – |
| 26 | Heimdal | 24 | M | mixed germ cell tumor (immature teratoma, endodermal sinus tumor, seminoma) | – | – | 3 | vincristine, cisplatin, bleomycin | IFXRT ? | 19.8 | – | |
| 27 | Hildebrand | 35 | M | L frontal astrocytoma | CCNU | teniposide | 2350 mg (teniposide), 2940 mg (CCNU) | 13 (10 full, 3 reduced dose) | -; 2,4-diamino-5-4′ -dichloro-phenyl-6-methylpyrimidine (DDMP), 75 mg/sq m, with leucovorin calcium (citrovorum factor) | IFXRT | 60 | – |
| 28 | Karajannis | 1.8 | M | chiaasmatic astrocytoma, grade II | -; -; procarbazine, CCNU | -; -; - | 18 months; 3; 6 | vincristine, carboplatin; vincristine, dactinomycin (?); thioguanine | – | – | – | |
| 29 | Kempin | 20 | F | medulloblastoma | CCNU, PCB | – | – | 5 | vincristine | CST | 30 Gy (neuraxis) + 20 Gy boost to posterior fossa | – |
| 30 | Kempin | 29 | F | R parietal GBM | BCNU (x 2), CCNU | – | – | CCNU Q6-8 wks for 4 yrs | hydroxyurea during XRT | WBRT | 60 | |
| 31 | Muroi | 19 | M | pontine glioma | ACNU | – | 2894 mg | 12 years | – | – | – | – |
| 32 | Nora | 5 | M | cerebellar astrocytoma, grade III | BCNU (x2), then CCNU | – | dosage appears to be incorrectly stated (120 mg/kg) | 2.5 years | – | IFXRT | 52.5 | |
| 33 | Noronha | 66 | F | AO | PCB, CCNU; BCNU-impregnated wafers; TMZ | – | – | 1 (excessive myelotoxicity); 1; 25 | vincristine | IFXRT | 60 | – |
| 34 | Perry | 39 | M | AA | PCB, CCNU | – | PCB 28,300 mg, CCNU 1500 mg | 9 | vincristine | IFXRT | 60 | – |
| 35 | Perry | 26 | F | GBM | BCNU, mitomycin-C | BCNU 600 mg, mitomycin-C 25 mg/m2; cisplatin 525 mg, etoposide 1400 mg iv, 600 mg po | 3; 5 | 6-mercaptopurine; etoposide, cisplatin (i.a.) | WBRT | 44 Gy (BR), 18 Gy boost to tumor bed | suspected Li-Fraumeni syndrome | |
| 36 | Pui | 1.4 | M | brainstem astrocytoma | PCB, nitrogen mustard | – | – | – | – | ? | ? | – |
| 37 | Robustelli | 28 | M | GBM | CCNU | – | 3030 mg | 12 | – | WBRT | 55 | – |
| 38 | Rogers | 34 | F | AA | PCB, CCNU | – | – | 6 | hydroxyurea (during radiation), vincristine | IFXRT + SRS | 55.8 + 18 × 2 | – |
| 39 | Rowley | 16 | F | PNET | – | etoposide, doxorubicin | 2950 mg/m2, 375 mg/m2 | – | – | – | – | – |
| 40 | Su | 44 | F | AA | ACNU; TMZ | – | – | 4; during SRT (5 days), then 6 cycles | interferon; -; thalidomide | IFXRT ?; SRT | 57 (40 to ‘ventricles’ and 17 to tumor); 24 | – |
| 41 | Sugiyama | 0.25 | M | medulloblastoma | ACNU | – | ACNU 650 mg, tegafur 50 g, MTX 6.8 g | tegafur, MTX | CST | 30 (posterior fossa), 10 (neuraxis) | ||
| 42 | Sugiyama | 29 | F | AA | MCNU; ACNU | – | MCNI 1425 mg, ACNU 740 mg, CDDP 100 | 2 during XRT, 11 thereafter; 5 + (?) | -; cisplatin | IFXRT | 54 | – |
| 43 | Vogl | 4 | F | GBM | BCNU | -; etoposide; HDMTX | – | during XRT; 8 wks; 10 wks | it MTX, vincristine | WBRT | 33 (WB), 10 (L hemisphere) | – |
| 44 | Wiernik | 31 | M | malignant glioma | – | tenisposide | – | 12 | – | IFXRT | 56 | NF1 |
Abbreviations: F, female; M, male; AA, anaplastic astrocytoma; ACNU, nimustine; AO, anaplastic oligodendroglioma; AOA, anaplastic oligoastrocytoma; BCNU, carmustine; BR, brain; CCNU, lomustine; CPC, choroid plexus papilloma; CSI, craniospinal radiation; CYC, cyclophosphamide; GBM, glioblastoma multiforme; HDMTX, high-dose methotrexate; IFXRT, involved field radiation therapy; LGA, low-grade astrocytoma; MCNU, ranimustine; meCCNU, senimustine; PCB, procarbazine; PF, partial field; PNET, primitive neuroektodermal tumor; SP, spine; SRT, stereotactic radiation therapy; SRS, stereotactic radiosurgery; TMZ, temozolomide; WBRT, whole brain radiation therapy.
It appears that patients with primary brain tumors treated with nitrosoureas develop t-MDS/AML at a shorter latency period than do those with other cancers, suggesting a synergistic effect with radiation therapy or a unique property of these alkylating agents.3,26,28 Latency appears to be even shorter in children.28 However, this may also simply reflect a selection bias (patients with normal latency die from the brain tumor prior to clinical manifestation of MDS).
Radiation Therapy
Radiation therapy adds to the risk of developing MDS conferred by chemotherapy.29 Our literature review revealed the use of adjuvant irradiation in 34 patients who later developed myelodysplasia (partial [18], craniospinal [11], whole brain irradiation [5], stereotactic radiosurgery [3], and unknown [1]) (Table 1). t-MDS/t-AML has also been described in individuals exposed to radiation only.22,30,31 For example, Detourmignies et al. described a 12-year-old boy who developed acute promyelocytic leukemia after radiation for cerebral astrocytoma.32 Risk may be correlated to the volume of exposed marrow. However, in Loening et al.'s series, the incidence of t-AML was independent from radiation exposure, whereas the overall risk of secondary neoplasms was higher in recipients of both whole brain radiation therapy and chemotherapy for childhood leukemia, compared with chemotherapy alone.17
Cytogenetics, Molecular Findings
There is a strong association between previous genotoxic exposure and karyotypic features. Unique cytogenetic changes of chromosomes 5 and 7 are present in 43%–87% of cases and particularly in patients treated with alkylating agents (Fig. 2). These chromosomal abnormalities are detected in only 18% of patients with sporadic leukemia.19,22,33–36 Several subsets of genetic changes are known and include loss of the entire or part of the long arm of the chromosome. The critical region of the chromosome 5 deletion appears to include bands q23 to q32,36 but target genes are unknown. The target of the chromosome 7 deletion is inactivation of the AML1 tumor suppressor gene. Abnormalities affecting chromosome 5 and 7 have been identified in patients treated with radiation therapy alone.22,37
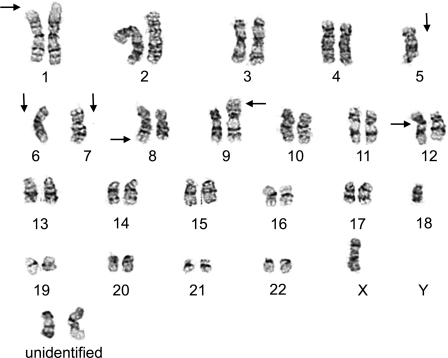
Fig. 2.
Cytogenetic analysis of a bone marrow specimen. Multiple complex cytogenetic abnormalities were noted, including deletions of 1p and 3p (not shown); losses of chromosomes 5, 6, and 7; additional materials of unknown origin onto 8q, 9p, and 12p; and the presence of 2–4 marker chromosomes. The composite karyotype was designated as 40-45,XX,del(1)(p36.1),del(3)(p13p25),-5,-6,-7, add(8)(q24), add(9)(p24), add(12)(p13), +2-4mar[cp6] (image courtesy of Dr. Peining Li, Department of Genetics, Yale University School of Medicine).
The most common cytogenetic abnormalities identified in our literature review were structural alterations affecting chromosome 5 or 7. Balanced rearrangements involving chromosome 11 were reported in 4 patients (see also Table 1).
Genetic Predisposition
Second malignant neoplasm in patients with brain tumor may be based on genetic predisposition. Genetic abnormalities were found in 29% of patients afflicted with t-MDS/t-AML in the St. Jude series.10 Examples for hereditary predisposition are Li-Fraumeni syndrome, mismatch repair deficiency based on biallelic mutations, neurofibromatosis type 1, Fanconi anemia, and Down syndrome. In our literature review, a genetic tumor predisposition syndrome might have played a role in the pathogenesis of t-MDS/t-AML in 4 patients (Li-Fraumeni syndrome confirmed in 2 and suspected in 1; neurofibromatosis, type I, in 1).
Various genetic polymorphisms of enzymes involved in the metabolism of chemotherapeutic agents have been implicated in the pathogenesis of t-MDS/t-AML (glutathione S-transferase [GSTP1 –Val], cytochrome P450 3A [protective], NAD[P]H:quinine oxidoreductase, and thiopurine methyl-transferase).13 In addition, polymorphisms of genes involved in DNA repair (XRCC1) may increase susceptibility to t-MDS/t-AML.38 A polymorphism in the gene encoding glutathione S-transferase π (GSTP1; codon 105, heterozygous isoleucin-valine, or valine – valine) increases the risk of t-AML after chemotherapy by 2–4-fold, especially when GSTP1 substrates (exampleg, cyclophosphamide, and adriamycin) are used.39
Clinical Presentation and Diagnosis
The clinical features of t-MDS/t-AML are a result of progressive bone marrow failure. Symptomatic anemia is the most common presentation, but easy bruising reflecting thrombocytopenia and repeated infections may also be prominent. Progressive macrocytosis may be an early indicator of MDS, but this finding is also seen during chemotherapy without t-MDS/t-AML. Organomegaly is an infrequent finding.
t-MDS is usually diagnosed after a bone marrow aspirate and biopsy are performed documenting the presence of dysplasia in the marrow (Fig. 3). MDS is categorized into several entities depending on the number of cell lineages affected and the percentage of blasts present. The key morphologic entities encountered in t-MDS correspond to refractory cytopenia with multilineage dysplasia (RC-MD), refractory anemia with excess blasts-1 (RAEB-1; 5 to 9% blasts), and refractory anemia with excess blasts-2 (RAEB-2; 10 to 19% blasts).23 Most t-MDS cases are of the RAEB type.19
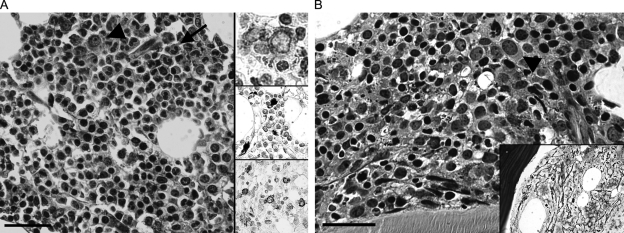
Fig. 3.
(A) Treatment-related MDS (refractory anemia with excess blasts, type 2; see Table 1, patient 33). The overall mildly hypocellular marrow is characterized by dysgranulopoeiesis (nuclear hypolobulation (pseudo Pelger-Huet nuclei; arrow), cytoplasmic hypogranularity) and dyserythropoiesis (hematoxylin & eosin (H&E), bar 50 µm). Immunohistochemistry for CD34 and c-kit demonstrate an increase in the number of immature marrow cells (approximately 10% of nucleated marrow cells; upper and lower insert, respectively; see also arrowhead in H&E stain). The middle insert shows iron deposition within marrow cells. (B) The patient progressed to acute myelogenous leukemia. Bone marrow biopsy revealed an overall hypoplastic specimen with little evidence for maturation in granulopoiesis. There is an increase in the relative number of immature myeloid elements (“blasts,” arrowhead) accounting for approximately 30% of the total marrow's nucleated forms (hematoxylin & eosin, bar 50 µm). The reticulin stain (insert) demonstrates marrow fibrosis.
Patients with 20% or more blasts in the marrow or in the peripheral blood are categorized as having t-AML.40 M1 and M2 AML are the most common therapy-related leukemia subtypes (French-American-British classification) and are associated with chromosome 5 and 7 abnormalities. M5 AML is associated with 11q23 (MLL) translocations.
Treatment and Prognosis
Patients who develop t-MDS/t-AML are treated with supportive care, including transfusion of blood products and administration of antibiotics; 5-azacytidine, decitabine, and lenalidomide are approved for the treatment of selected patients with MDS in the United States. Although the complete response rates with these compounds are relatively low, the agents have been associated with decreased transfusion requirements in specific populations of patients.41 The available chemotherapy agents are not curative, however, and the only treatment with such potential is allogeneic hematopoietic stem cell transplantation.42,43 Studies of transplantation suggest a 20%–40% chance of long-term, disease-free survival. In performing allogeneic stem cell transplantation, total body irradiation is avoided as a conditioning regimen in prior recipients of ionizing radiation.44 The optimal timing for initiation of such therapy and the optimal conditioning regimen and graft-versus-host-disease prophylaxis for transplant remain unknown. However, early transplantation may be advantageous in t-MDS/t-AML, particularly because of the relatively short duration of remission after conventional therapy. Remission induction and the inherent transplant-related mortality represent the biggest challenges. In a large single-institution series of patients with t-MDS/t-AML after systemic cancer therapy, disease-free survival at 4 years was 41%.44 Options for patients without matched related donors include a matched unrelated donor, cord blood transplantation,42 or haploidentical (mismatched family member) transplantation. New approaches to treatment include incorporation of histone deacetylase inhibitors (valproic acid or vorinostat) and combination of agents such as 5-azacytidine and lenalidomide.45
Despite these interventions, the median survival with t-MDS is 9 months and 7 months for t-AML. Most patients die of bone marrow failure or progression of MDS into leukemia. Patients with chromosome 5 and 7 abnormalities have a worse prognosis than those without these cytogenetic abnormalities.
In our literature review, we found 12 patients with central nervous system tumors with t-MDS/t-AML who were treated with chemotherapy only and 6 who underwent allogeneic stem cell transplantation. Median overall survival was 3 months (range, 0 to >35 months).
Conclusion
Protracted administration of an alkylating agent must be undertaken with an understanding of the risk of long-term treatment complications. At present, this is most relevant for patients with anaplastic oligodendrogliomas displaying deletion of chromosome 1p and IDH1 or IDH2 mutation and low-grade gliomas whose median survival exceeds the latency period for myelodysplasia. There is variation in the risk of t-MDS/t-AML based on the specific therapeutic agent used and the adjuvant administration of radiotherapy. t-MDS/t-AML appears to be a rare event in patients with nervous system neoplasms, but at present, incidence data from prospective randomized trials are unavailable for any agent or ionizing radiation. Our comprehensive literature search has not yielded any data to suggest that the number of t-MDS/t-AML cases has been increasing in recent years. The major cause of premature death in patients with infiltrative brain tumors remains progression of their primary cancer. For long-term survivors, the risk of direct or indirect tumor complications (permanent neurologic deficit, seizures, intratumoral hemorrhage, and deep venous thrombosis) or short latency adverse reactions to treatment (myelosuppression, opportunistic infection, steroid myopathy, and radiation encephalopathy) are much higher than the t-MDS/t-AML risk. Thus, a change in current practice patterns, even if not always based on prospective randomized studies, does not appear to be warranted. However, it would seem to be timely to test the hypothesis that prolonged use of alkylating chemotherapy until tumor progression or unacceptable toxicity is superior to treatment with a fixed number of cycles.
Conflict of interest statement. None declared.
References
- 1.Brada M, Judson I, Beale P, et al. Phase I dose-escalation and pharmacokinetic study of temozolomide (SCH 52365) for refractory or relapsing malignancies. Br J Cancer. 1999;81(6):1022–1030. doi: 10.1038/sj.bjc.6690802. doi:10.1038/sj.bjc.6690802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. doi:10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Chamberlain MC, Raizer J. Extended exposure to alkylator chemotherapy: delayed appearance of myelodysplasia. J Neurooncol. 2009;93(2):229–232. doi: 10.1007/s11060-008-9764-5. doi:10.1007/s11060-008-9764-5. [DOI] [PubMed] [Google Scholar]
- 4.Hoang-Xuan K, Capelle L, Kujas M, et al. Temozolomide as initial treatment for adults with low-grade oligodendrogliomas or oligoastrocytomas and correlation with chromosome 1p deletions. J Clin Oncol. 2004;22(15):3133–3138. doi: 10.1200/JCO.2004.10.169. doi:10.1200/JCO.2004.10.169. [DOI] [PubMed] [Google Scholar]
- 5.Quinn JA, Reardon DA, Friedman AH, et al. Phase II trial of temozolomide in patients with progressive low-grade glioma. J Clin Oncol. 2003;21(4):646–651. doi: 10.1200/JCO.2003.01.009. doi:10.1200/JCO.2003.01.009. [DOI] [PubMed] [Google Scholar]
- 6.van den Bent MJ, Taphoorn MJ, Brandes AA, et al. Phase II study of first-line chemotherapy with temozolomide in recurrent oligodendroglial tumors: the European Organization for Research and Treatment of Cancer Brain Tumor Group Study 26971. J Clin Oncol. 2003;21(13):2525–2528. doi: 10.1200/JCO.2003.12.015. doi:10.1200/JCO.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 7.van den Bent MJ, Keime-Guibert F, Brandes AA, et al. Temozolomide chemotherapy in recurrent oligodendroglioma. Neurology. 2001;57(2):340–342. doi: 10.1212/wnl.57.2.340. [DOI] [PubMed] [Google Scholar]
- 8.Rollison DE, Howlader N, Smith MT, et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood. 2008;112(1):45–52. doi: 10.1182/blood-2008-01-134858. doi:10.1182/blood-2008-01-134858. [DOI] [PubMed] [Google Scholar]
- 9.Buyukpamukcu M, Varan A, Yazici N, et al. Second malignant neoplasms following the treatment of brain tumors in children. J Child Neurol. 2006;21(5):433–436. doi: 10.1177/08830738060210050901. [DOI] [PubMed] [Google Scholar]
- 10.Broniscer A, Ke W, Fuller CE, Wu J, Gajjar A, Kun LE. Second neoplasms in pediatric patients with primary central nervous system tumors: the St. Jude Children's Research Hospital experience. Cancer. 2004;100(10):2246–2252. doi: 10.1002/cncr.20253. doi:10.1002/cncr.20253. [DOI] [PubMed] [Google Scholar]
- 11.Duffner PK, Krischer JP, Horowitz ME, et al. Second malignancies in young children with primary brain tumors following treatment with prolonged postoperative chemotherapy and delayed irradiation: a Pediatric Oncology Group study. Ann Neurol. 1998;44(3):313–316. doi: 10.1002/ana.410440305. doi:10.1002/ana.410440305. [DOI] [PubMed] [Google Scholar]
- 12.Greene MH, Boice JD, Jr, Strike TA. Carmustine as a cause of acute nonlymphocytic leukemia. N Engl J Med. 1985;313(9):579. [PubMed] [Google Scholar]
- 13.Barnard DR, Woods WG. Treatment-related myelodysplastic syndrome/acute myeloid leukemia in survivors of childhood cancer–an update. Leuk Lymphoma. 2005;46(5):651–663. doi: 10.1080/10428190500051042. doi:10.1080/10428190500051042. [DOI] [PubMed] [Google Scholar]
- 14.Hijiya N, Hudson MM, Lensing S, et al. Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia. JAMA. 2007;297(11):1207–1215. doi: 10.1001/jama.297.11.1207. doi:10.1001/jama.297.11.1207. [DOI] [PubMed] [Google Scholar]
- 15.Westermeier T, Kaatsch P, Schoetzau A, Michaelis J. Multiple primary neoplasms in childhood: data from the German Children's Cancer Registry. Eur J Cancer. 1998;34(5):687–693. doi: 10.1016/s0959-8049(97)00326-2. doi:10.1016/S0959-8049(97)00326-2. [DOI] [PubMed] [Google Scholar]
- 16.Kimball DV, Gelber RD, Li F, Donnelly MJ, Tarbell NJ, Sallan SE. Second malignancies in patients treated for childhood acute lymphoblastic leukemia. J Clin Oncol. 1998;16(8):2848–2853. doi: 10.1200/JCO.1998.16.8.2848. [DOI] [PubMed] [Google Scholar]
- 17.Loning L, Zimmermann M, Reiter A, et al. Secondary neoplasms subsequent to Berlin-Frankfurt-Munster therapy of acute lymphoblastic leukemia in childhood: significantly lower risk without cranial radiotherapy. Blood. 2000;95(9):2770–2775. [PubMed] [Google Scholar]
- 18.Neglia JP, Friedman DL, Yasui Y, et al. Second malignant neoplasms in five-year survivors of childhood cancer: childhood cancer survivor study. J Natl Cancer Inst. 2001;93(8):618–629. doi: 10.1093/jnci/93.8.618. doi:10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 19.Kantarjian HM, Keating MJ, Walters RS, et al. Therapy-related leukemia and myelodysplastic syndrome: clinical, cytogenetic, and prognostic features. J Clin Oncol. 1986;4(12):1748–1757. doi: 10.1200/JCO.1986.4.12.1748. [DOI] [PubMed] [Google Scholar]
- 20.Howe R, Micallef IN, Inwards DJ, et al. Secondary myelodysplastic syndrome and acute myelogenous leukemia are significant complications following autologous stem cell transplantation for lymphoma. Bone Marrow Transplant. 2003;32(3):317–324. doi: 10.1038/sj.bmt.1704124. doi:10.1038/sj.bmt.1704124. [DOI] [PubMed] [Google Scholar]
- 21.Bhatia S, Sklar C. Second cancers in survivors of childhood cancer. Nat Rev Cancer. 2002;2(2):124–132. doi: 10.1038/nrc722. doi:10.1038/nrc722. [DOI] [PubMed] [Google Scholar]
- 22.Smith SM, Le Beau MM, Huo D, et al. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: the University of Chicago series. Blood. 2003;102(1):43–52. doi: 10.1182/blood-2002-11-3343. doi:10.1182/blood-2002-11-3343. [DOI] [PubMed] [Google Scholar]
- 23.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951. doi: 10.1182/blood-2009-03-209262. doi:10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 24.Neglia JP, Meadows AT, Robison LL, et al. Second neoplasms after acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;325(19):1330–1336. doi: 10.1056/NEJM199111073251902. doi:10.1056/NEJM199111073251902. [DOI] [PubMed] [Google Scholar]
- 25.Su YW, Chang MC, Chiang MF, Hsieh RK. Treatment-related myelodysplastic syndrome after temozolomide for recurrent high-grade glioma. J Neurooncol. 2005;71(3):315–318. doi: 10.1007/s11060-004-2028-0. doi:10.1007/s11060-004-2028-0. [DOI] [PubMed] [Google Scholar]
- 26.Dufour C, Da Costa L, Auger N, Jullien M, Bhangoo R, Grill J. Treatment-related myelodysplastic syndrome after temozolomide use in a child: first report. J Pediatr Hematol Oncol. 2008;30(11):857–859. doi: 10.1097/MPH.0b013e318182e74f. doi:10.1097/MPH.0b013e318182e74f. [DOI] [PubMed] [Google Scholar]
- 27.Noronha V, Berliner N, Ballen KK, et al. Treatment-related myelodysplasia/AML in a patient with a history of breast cancer and an oligodendroglioma treated with temozolomide: case study and review of the literature. Neuro Oncol. 2006;8(3):280–283. doi: 10.1215/15228517-2006-003. doi:10.1215/15228517-2006-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayani A, Mahoney DH, Jr, Taylor LD. Therapy-related myelodysplastic syndrome in children with medulloblastoma following MOPP chemotherapy. J Neurooncol. 1992;14(1):57–62. doi: 10.1007/BF00170945. [DOI] [PubMed] [Google Scholar]
- 29.Andrieu JM, Ifrah N, Payen C, Fermanian J, Coscas Y, Flandrin G. Increased risk of secondary acute nonlymphocytic leukemia after extended-field radiation therapy combined with MOPP chemotherapy for Hodgkin's disease. J Clin Oncol. 1990;8(7):1148–1154. doi: 10.1200/JCO.1990.8.7.1148. [DOI] [PubMed] [Google Scholar]
- 30.Krywicki R, Bowen K, Anderson L, et al. Mixed-lineage acute myeloid leukemia associated with a suprasellar dysgerminoma. Am J Clin Oncol. 1995;18(1):83–86. doi: 10.1097/00000421-199502000-00018. doi:10.1097/00000421-199502000-00018. [DOI] [PubMed] [Google Scholar]
- 31.Pai MR, Advani SH, Gopal R, Nair CN, Saikia T, Kamat DM. Acute leukaemia following malignant ependymoma: a case report. J Surg Oncol. 1985;29(1):1–4. doi: 10.1002/jso.2930290102. doi:10.1002/jso.2930290102. [DOI] [PubMed] [Google Scholar]
- 32.Detourmignies L, Castaigne S, Stoppa AM, et al. Therapy-related acute promyelocytic leukemia: a report on 16 cases. J Clin Oncol. 1992;10(9):1430–1435. doi: 10.1200/JCO.1992.10.9.1430. [DOI] [PubMed] [Google Scholar]
- 33.Davies SM. Therapy-related leukemia associated with alkylating agents. Med Pediatr Oncol. 2001;36(5):536–540. doi: 10.1002/mpo.1126. doi:10.1002/mpo.1126. [DOI] [PubMed] [Google Scholar]
- 34.Kushner BH, Laquaglia MP, Kramer K, Modak S, Cheung NK. Recurrent metastatic neuroblastoma followed by myelodysplastic syndrome: possible leukemogenic role of temozolomide. Pediatr Blood Cancer. 2008;51(4):552–554. doi: 10.1002/pbc.21658. doi:10.1002/pbc.21658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pedersen-Bjergaard J. Insights into leukemogenesis from therapy-related leukemia. N Engl J Med. 2005;352(15):1591–1594. doi: 10.1056/NEJMe048336. doi:10.1056/NEJMe048336. [DOI] [PubMed] [Google Scholar]
- 36.Le Beau MM, Albain KS, Larson RA, et al. Clinical and cytogenetic correlations in 63 patients with therapy-related myelodysplastic syndromes and acute nonlymphocytic leukemia: further evidence for characteristic abnormalities of chromosomes no. 5 and 7. J Clin Oncol. 1986;4(3):325–345. doi: 10.1200/JCO.1986.4.3.325. [DOI] [PubMed] [Google Scholar]
- 37.Mauritzson N, Albin M, Rylander L, et al. Pooled analysis of clinical and cytogenetic features in treatment-related and de novo adult acute myeloid leukemia and myelodysplastic syndromes based on a consecutive series of 761 patients analyzed 1976–1993 and on 5098 unselected cases reported in the literature 1974–2001. Leukemia. 2002;16(12):2366–2378. doi: 10.1038/sj.leu.2402713. doi:10.1038/sj.leu.2402713. [DOI] [PubMed] [Google Scholar]
- 38.Seedhouse C, Bainton R, Lewis M, Harding A, Russell N, Das-Gupta E. The genotype distribution of the XRCC1 gene indicates a role for base excision repair in the development of therapy-related acute myeloblastic leukemia. Blood. 2002;100(10):3761–3766. doi: 10.1182/blood-2002-04-1152. doi:10.1182/blood-2002-04-1152. [DOI] [PubMed] [Google Scholar]
- 39.Allan JM, Wild CP, Rollinson S, et al. Polymorphism in glutathione S-transferase P1 is associated with susceptibility to chemotherapy-induced leukemia. Proc Natl Acad Sci USA. 2001;98(20):11592–11597. doi: 10.1073/pnas.191211198. doi:10.1073/pnas.191211198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17(12):3835–3849. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 41.Barzi A, Sekeres MA. Myelodysplastic syndromes: a practical approach to diagnosis and treatment. Cleve Clin J Med. 2010;77(1):37–44. doi: 10.3949/ccjm.77a.09069. doi:10.3949/ccjm.77a.09069. [DOI] [PubMed] [Google Scholar]
- 42.Ballen KK. New trends in umbilical cord blood transplantation. Blood. 2005;105(10):3786–3792. doi: 10.1182/blood-2004-10-4125. doi:10.1182/blood-2004-10-4125. [DOI] [PubMed] [Google Scholar]
- 43.Anderson JE, Appelbaum FR, Fisher LD, et al. Allogeneic bone marrow transplantation for 93 patients with myelodysplastic syndrome. Blood. 1993;82(2):677–681. [PubMed] [Google Scholar]
- 44.Ballen KK, Gilliland DG, Guinan EC, et al. Bone marrow transplantation for therapy-related myelodysplasia: comparison with primary myelodysplasia. Bone Marrow Transplant. 1997;20(9):737–743. doi: 10.1038/sj.bmt.1700971. doi:10.1038/sj.bmt.1700971. [DOI] [PubMed] [Google Scholar]
- 45.Sekeres MA, List AF, Cuthbertson D, et al. Phase I Combination Trial of Lenalidomide and Azacitidine in Patients With Higher-Risk Myelodysplastic Syndromes. J Clin Oncol. 2010;28(13):2253–2258. doi: 10.1200/JCO.2009.26.0745. [DOI] [PMC free article] [PubMed] [Google Scholar]