Abstract
Traditionally, the most widely used criteria for response assessment in glioblastoma have been Macdonald and the Response Evaluation Criteria In Solid Tumors (RECIST). Recently, new criteria addressing contrast enhancement and fluid-attenuated inversion recovery (FLAIR)/T2 hyperintensity have been defined (the Response Assessment in Neuro-Oncology criteria) to better evaluate the effect of antiangiogenic therapy. Whether FLAIR/T2 imaging could also be helpful to refine RECIST criteria remains unresolved. This study proposed the RECIST + F criteria and compared the 4 methods (Macdonald, RECIST, RANO, and RECIST + F) to determine their agreement in identifying response and progression of recurrent glioblastomas to irinotecan-bevacizumab. Patients with recurrent glioblastoma treated with second-line irinotecan-bevacizumab were eligible. Clinical status, corticosteroid dose, and 1-dimensional and 2-dimensional measurements of tumor contrast enhancement and FLAIR hyperintensity were retrospectively assessed. Response and progression were determined according to each set of criteria. Seventy-eight patients were included. Response rates ranged from 34.2% with RECIST + F to 44.7% with Macdonald criteria. Agreement among the 4 methods in determining response and type of progression was high (kappa statistic > 0.75). One-third of patients exhibited nonenhancing progression with stable or improved contrast enhancement. Median progression-free survival was predicted by RECIST, at 13.6 weeks; RECIST + F, 12.3; Macdonald, 12.7; and RANO, 11.7 (P = .840). Intra- and interobserver correlations were high for both contrast enhancement and FLAIR hyperintensity measurements. There was a strong concordance among the different methods in determining response and progression to irinotecan-bevacizumab. Criteria integrating FLAIR hyperintensity tended, however, to reduce response rates and progression-free survival compared with criteria considering only contrast enhancement. The 1-dimensional approach appeared to be as valid as the 2-dimensional approach.
Keywords: bevacizumab, recurrent glioblastoma, response criteria
Glioblastoma is the most frequent malignant primary brain tumor in adults. Despite recent advances in treatment, particularly the combination of radiochemotherapy at first line and the use of antiangiogenic agents at recurrence, the prognosis of glioblastoma patients remains dismal.1,2 The development of clinical trials to evaluate potential therapeutic molecules is still extremely important. Consequently, appropriate response criteria are imperative to effectively evaluate novel agents and optimize patient management in daily practice.
Since their introduction in 1990, the Macdonald criteria have been the most commonly used criteria for assessing response to therapy in malignant gliomas.3 They are based on 2-dimensional (2D) measurements of contrast enhancement in conjunction with corticosteroid dose and clinical status.
The Response Evaluation Criteria In Solid Tumors (RECIST), which were introduced in 2000 and were recently revised, use 1-dimensional (1D) measurement of the enhancing lesion to determine the response.4,5 They have become the standard response criteria for systemic cancers. Although the RECIST criteria have not been widely adopted for patients with malignant gliomas, several studies have indicated that they are equally effective in assessing response.6,7
In recent years, the widespread adoption of bevacizumab as a salvage therapy has highlighted a particular limitation of these previous criteria, the phenomenon of “pseudo-response.” It consists of improved contrast enhancement that is likely due to normalization of vascular permeability but does not necessarily reflect a true antitumor effect.8 Despite a continuing decrease in enhancement, some patients exhibit a simultaneous increase in the nonenhancing component of the tumor, as depicted on fluid-attenuated inversion recovery (FLAIR) or T2-weighted images. To reduce the impact of this limitation, the recently proposed Response Assessment in Neuro-Oncology (RANO) criteria also take into account FLAIR/T2 hyperintensity as a surrogate for the nonenhancing component of the tumor.9,10
In the present study we sought to compare the aforementioned criteria in a series of patients who received antiangiogenic therapy for recurrent glioblastoma. In addition, we proposed and included in the comparison new 1D criteria that consider both contrast enhancement and FLAIR hyperintensity (the RECIST + F criteria).
Materials and Methods
Patient Selection
We performed a retrospective review of the records of 102 consecutive adult patients with recurrent glioblastoma who were treated with irinotecan-bevacizumab between May 2007 and January 2010 at our institution. All patients had received prior standard first-line treatment with radiotherapy and temozolomide. At progression, patients received bevacizumab (10 mg/kg) and irinotecan (340 mg/m2 or 125 mg/m2 depending on whether they were on enzyme-inducing antiepileptic drugs) every 2 weeks.
Eligible patients were included in the study if acceptable MRI data could be retrieved for scans performed within 2 weeks prior to initiating therapy and at least every 4 weeks thereafter until progression. Interpretable FLAIR and pre- and postgadolinium T1-weighted images (T1WIs) of the entire brain were required for each time point. Available clear documentation of sequential clinical data during the treatment period, including neurologic status, KPS, and corticotherapy, was also required for patient selection.
MRI Tumor Measurement
Using a standard approach, the largest diameter of the contrast-enhancing lesion on axial postgadolinium T1WI and its maximum perpendicular diameter in the same plane were measured. These values were used to obtain the largest 1D and 2D measurements (the maximal cross-sectional area resulting from the product of the 2 largest perpendicular diameters) of the contrast-enhancing lesion. To quantitatively assess the infiltrative nonenhancing disease, similar 1D and 2D measurements of FLAIR hyperintensity suggestive of an infiltrating tumor were also performed in the axial planes, as previously described.11 In the absence of other potential explanations, features considered to be suggestive of an infiltrating tumor were mass effect, cortical ribbon infiltration, and location outside the field of radiation.9
In cases where there was a gradient of enhancement, a point at which there was a clearly visible transition from nonenhancement to enhancement was selected to begin the measurement. The same procedure was used when there was a gradient of increased FLAIR signal. When a cystic cavity was present within a surrounding area of contrast enhancement, the same criteria of measurement were maintained.
Most patients (72%) had only a single tumoral lesion. In cases of multiple lesions, the 1D measurement was the largest single diameter of the lesions in the axial plane, and the 2D measurement was the sum of the maximal cross-sectional areas of the lesions in the axial plane. When only 1 or 2 of the multiple lesions were increasing in size, the enlarging lesions were considered the target lesions for response evaluation.
The MRI scans were reviewed by a neurologist (J.G.P.-L.) who was blinded to the patients’ outcomes. He performed all 1D and 2D measurements on postgadolinium T1WI and FLAIR sequences. On a subset of 100 randomly chosen scans, measurements were independently repeated by J.G.P.-L. and a neuroradiologist (M.L.) to determine intraobserver and interobserver variability, respectively.
Response Assessment
Tumor response was evaluated at each time point according to the published response assessment criteria for high-grade gliomas: RECIST, Macdonald, and RANO. We proposed additional criteria (RECIST + F) that considered the largest single diameter not only of the enhancing tumor but also of the nonenhancing component depicted on FLAIR images. In all of the criteria, response evaluation was made by taking into account not only the corresponding radiological changes but also corticosteroid use and changes in neurologic and performance status.
Radiological response was determined by comparison with the baseline scan, whereas progression was determined by comparison with the prior scan that had the smallest tumor measurement. Complete response (CR) represented disappearance of all contrast-enhancing disease. Partial response (PR) represented a 30% or more decrease in the largest single diameter of the contrast-enhancing disease (1D criteria) or a 50% or more decrease in the maximal cross-sectional area of the enhancing disease (2D criteria). Radiological response required a confirmatory scan obtained at least 4 weeks later. Progressive disease (PD) represented a 20% or more increase in the largest single diameter of the enhancing lesion (1D criteria), a 25% or more increase in the maximal cross-sectional tumor area (2D criteria), or the appearance of any new enhancing lesion. For criteria taking into account the FLAIR sequence, stability or improvement of FLAIR hyperintensity was required for classification as response or stability. Indeed, a significant increase (20% or more in the largest single diameter or 25% or more in the maximal cross-sectional area) in the nonenhancing lesions or the appearance of new lesions was considered as PD, provided that the patient was on stable or increasing doses of corticosteroids and that the increase in FLAIR signal could not be attributed to comorbid events such as effects from surgery and chemoradiation, ischemia, epilepsy, or infection. All other conditions were defined as stable disease (SD).
Regardless of the response criteria, the patients should have taken the same or decreased doses of corticosteroids and should have had a stable or improved neurologic examination to be classified into the PR or SD categories. CR required that the patient was off corticosteroids and clinically stable or improved. Considered as clinical PD were (a) definite neurologic deterioration or decline in the KPS of at least 20 points not attributable to causes apart from the tumor or to a decrease in corticosteroid dose and (b) failure to return for evaluation as a result of death or deteriorating condition.
Patterns of Radiological Progression
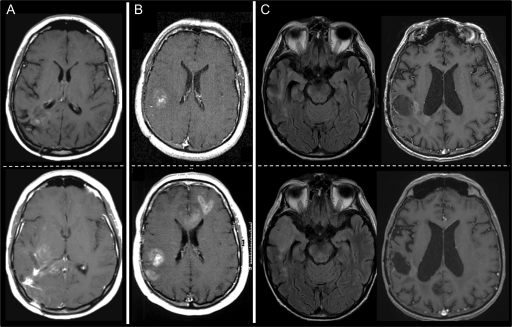
Scans showing progression based on the RECIST + F and RANO criteria were classified according to previously defined radiological recurrence patterns.11,12 When increased or new enhancement developed in contiguity with the original lesion, scans were classified as local contrast-enhanced recurrence. If there were new enhancing foci distant from the original area of enhancement, scans were defined as distant contrast-enhanced recurrence. Scans that were found to have an increase in the area of abnormal FLAIR signal (regionally or at a distance from the primary tumor site) in the absence of increased or new enhancement were classified as diffuse recurrence (Fig. 1).
Fig. 1.
Patterns of radiological progression. Local contrast-enhanced recurrence (A), distant contrast-enhanced recurrence (B), and diffuse nonenhancing recurrence (C). The top panels are baseline scans, and the bottom panels are scans at recurrence.
Statistical Analysis
Response and progression endpoints were determined according to each of the criteria. To evaluate the concordance among the criteria in assessing the best response to treatment (CR, PR, SD, or PD) and the type of progression (clinical, clinicoradiological, or radiological), the number of patients for each pair of criteria in which there was agreement was computed. The amount of agreement was summarized with the observed frequency and the kappa statistic. Cochran and McNemar tests were used to compare (globally and by pairs of criteria) the rates of objective response (CR and PR) and radiological progression without clinical deterioration. Progression-free survival (PFS) was calculated from the first day of treatment to the first documentation of progression as defined by each set of criteria. PFS distributions were estimated with the Kaplan-Meier method and compared with the log-rank test. Intra- and interobserver reproducibility of 1D and 2D measurements on FLAIR and postcontrast T1WI were calculated using Pearson's correlation coefficients. Statistical tests were 2-sided, and significance was set at P < .05. All of the analyses were performed using SPSS v15.0.
Results
Patient Characteristics
Based on the selection criteria, 78 of the 102 eligible patients were included in the study. The remainder were excluded due to incomplete clinical or neuroimaging information. All included patients (38% female and 62% male) had received prior standard treatment with radiotherapy and temozolomide (median number of adjuvant cycles, 6; range, 0 to 18), which had failed. The median age at the start of salvage therapy with irinotecan-bevacizumab was 58 years (range, 19 to 78). The median KPS was 70% (range, 30 to 90). Twenty-six patients (33.3%) had a KPS less than 70%. The median number of treatment infusions was 8 (range, 1 to 38). Patients had undergone a median of 5 MRI scans (range, 1 to 19).
Best Response
Tumor response evaluation was performed on 76 patients. Two patients in whom treatment was discontinued after the first infusion of irinotecan-bevacizumab due to pulmonary embolism were excluded from this subanalysis.
Table 1 shows the rates of best response as determined by the different criteria. Objective responses were observed in 30 patients (39.5%) according to the RECIST criteria, in 26 (34.2%) according to RECIST + F, in 34 (44.7%) according to Macdonald, and in 31 (40.8%) according to RANO (P = .060). Globally, response rates tended to be slightly lower with 1D criteria compared with 2D criteria and with criteria taking into account the FLAIR hyperintensity compared with criteria that evaluated only the contrast enhancement. Further comparisons of pairs of criteria showed differences only between the RECIST + F and Macdonald criteria (P = .008).
Table 1.
Rates of best response to therapy as determined by the response criteria
| Complete Response,n (%) | Partial Response, n (%) | Objective Response, n (%) | Stable Disease, n (%) | Progressive Disease, n (%) | |
|---|---|---|---|---|---|
| RECIST | 7 (9) | 23 (30) | 30 (39) | 34 (45) | 12 (16) |
| RECIST + F | 6 (8) | 20 (26) | 26 (34) | 36 (48) | 14 (18) |
| Macdonald | 7 (9) | 27 (36) | 34 (45) | 30 (39) | 12 (16) |
| RANO | 6 (8) | 25 (33) | 31 (41) | 31 (41) | 14 (18) |
Table 2 illustrates the percentage of agreement among the different criteria in assessing the best response and the kappa statistics for the agreement between pairs of criteria. In general, there was a strong concordance among all 4 criteria, with percentages of agreement ranging from 87% to 95% and kappa estimates above 0.8 in all cases. The strongest agreements were found between pairs of 1D criteria and pairs of 2D criteria.
Table 2.
Concordance among the different criteria in assessing the best response during the treatment period
| RECIST | RECIST + F | Macdonald | RANO | |
|---|---|---|---|---|
| RECIST | 0.92 (0.85–0.99) | 0.88 (0.79–0.97) | 0.81 (0.69–0.92) | |
| RECIST + F | 72/76 (95%) | 0.81 (0.69–0.92) | 0.83 (0.72–0.93) | |
| Macdonald | 70/76 (92%) | 66/76 (87%) | 0.92 (0.85–0.99) | |
| RANO | 66/76 (87%) | 67/76 (88%) | 72/76 (95%) |
Upper values represent the kappa statistic and its 95% confidence interval. Lower values represent the observed frequency and percent.
Type of Progression
Ten patients were excluded from the analysis because treatment was discontinued before disease progression due to venous thromboembolism (n = 6), ischemic colitis (n = 1), cerebrovascular disease (n = 2), or pneumocystis pneumonia (n = 1).
Patients who progressed to irinotecan-bevacizumab, which was defined based on clinical and radiological data, were classified into 3 groups for each set of evaluation criteria: clinical, clinicoradiological, and radiological. The numbers of patients considered to have tumor progression because of clinical deterioration without imaging confirmation or in discordance with neuroimaging was 14 (20.6%), 12 (17.6%), 13 (19.1%), and 10 (14.7%) for the RECIST, RECIST + F, Macdonald, and RANO criteria, respectively. Radiological progression without clinical deterioration was observed in 31 patients (45.6%) according to the RECIST criteria, in 38 (55.9%) according to RECIST + F, in 31 (45.6%) according to Macdonald, and in 39 (57.4%) according to RANO. Radiological progression was more frequent according to criteria that addressed FLAIR hyperintensity compared with the criteria that measured only the contrast enhancement (RANO vs RECIST, P = .008; RANO vs Macdonald, P = .008; RECIST + F vs RECIST, P = .016; RECIST + F vs Macdonald, P = .016). No significant differences were found between the RECIST and Macdonald criteria (P = 1.00) or the RECIST + F and RANO criteria (P = .100). In some patients (7 [10%] according to the RECIST + F criteria and 8 [12%] according to the RANO criteria], progression on FLAIR sequences preceded the clinical deterioration determined by the other respective criteria.
We also evaluated the concordance among the abilities of the different criteria to assess the type of progression. Percentages of agreement ranged from 85% to 98.5%, and kappa estimates ranged from 0.76 to 0.98, which indicated a strong concordance among all of the sets of criteria (Table 3).
Table 3.
Concordance among the different criteria in assessing the type of progression
| RECIST | RECIST + F | Macdonald | RANO | |
|---|---|---|---|---|
| RECIST | 0.83 (0.72–0.95) | 0.98 (0.93–1.00) | 0.76 (0.62–0.92) | |
| RECIST + F | 61/68 (90%) | 0.81 (0.69–0.93) | 0.92 (0.84–1.00) | |
| Macdonald | 67/68 (99%) | 60/68 (88%) | 0.78 (0.65–0.91) | |
| RANO | 58/68 (85%) | 65/68 (96%) | 59/68 (87%) |
Upper values represent the kappa statistic and its 95% confidence interval. Lower values represent the observed frequency and percent.
Patterns of Radiological Progression
Fifty-six and 58 patients demonstrated radiological progression Based on the RECIST + F and RANO criteria, 56 and 58 patients demonstrated radiological progression, respectively. According to the RECIST + F criteria, 28 patients (50%) had local contrast-enhanced recurrence, 11 (19.7%) had new foci of enhancement distant from the original area of enhancement, and 17 (30.3%) had diffuse recurrence that was visible on only FLAIR sequences. Similarly, the pattern of progression according to the RANO criteria was local enhancement in 28 patients (48.3%), distant enhancement in 10 (17.2%), and a diffuse nonenhancing tumor in 20 (34.5%).
Progression-free Survival
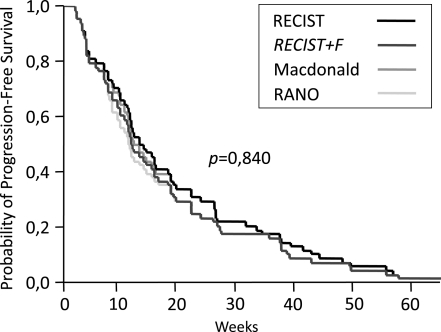
The 68 patients who experienced PD were included in this subanalysis. PFS estimates were determined for each of the response criteria (Fig. 2). Median PFS was 13.6 weeks (95% confidence interval [CI], 9.5–17.6) for RECIST, 12.3 weeks (95% CI, 9.4–15.2) for RECIST + F, 12.7 weeks (95% CI, 9.7–15.7) for Macdonald, and 11.7 weeks (95% CI, 9.3–14.2) for RANO. The 6-month PFS rates were 29.4% (95% CI, 18.6–40.1) for the RECIST and Macdonald criteria and 23.5% (95% CI, 13.5–33.5) for the RECIST + F and RANO criteria. Although PFS times determined by the RECIST + F and RANO criteria were slightly shorter than those determined by the RECIST and Macdonald criteria, the differences did not reach significance (P = .840). When analyzing only the patients who experienced diffuse nonenhancing progression, the RECIST + F and RANO criteria shortened PFS times by a median of 39 and 33 days (P = .256), respectively, compared with the RECIST and Macdonald criteria. All PFS times of all 4 criteria similarly correlated with overall survival (all 4 correlation coefficients, 0.9; P < .001). No statistically significant differences were found in overall-survival times between patients exhibiting only FLAIR progression and the others.
Fig. 2.
Kaplan-Meier estimates of progression-free survival according to the different criteria.
Measurement Reproducibility
Intraobserver correlation coefficients were 0.97 (P < .001) and 0.98 (P < .001) for 1D measurements on FLAIR and post-contrast T1WI, respectively. For 2D measurements, Pearson's correlation coefficients were 0.97 (P < .001) and 0.98 (P < .001), respectively. Interobserver correlation coefficients were 0.86 (P < .001) and 0.96 (P < .001) for 1D FLAIR and contrast enhancement measurements, respectively, and 0.93 (P < .001) and 0.95 (P < .001) for the respective 2D measurements.
Discussion
Over the past decades, several proposals have been made in the attempt to overcome the successive limitations of response assessment in malignant gliomas. In the present study, we compared the Macdonald, RECIST, and RANO criteria in a series of patients with recurrent glioblastoma who received salvage therapy with irinotecan-bevacizumab. We also proposed and included in the comparison an additional 1D criteria that addressed both contrast enhancement and FLAIR hyperintensity (the RECIST + F criteria). Because radiological response and PFS are the most commonly used endpoints in clinical trials for recurrent glioblastoma,13 we focused our analysis on these issues.
Overall, agreement in determining the best response to treatment was high. The objective response rates, which ranged between 34% and 45%, were comparable to previous studies using the same therapeutic regimen.2,14 The rates observed with the criteria that integrated FLAIR hyperintensity, however, were slightly lower than those obtained with the criteria that measured only contrast enhancement. Adding the FLAIR criteria reduced the response rates by approximately 5%, which appeared to be due to the phenomenon of “pseudo-response” (an increase in the infiltrating nonenhancing component of the tumor with stable or decreased contrast enhancement).8 Both the RANO and RECIST + F criteria were equally effective at detecting pseudo-responses.
Agreement among all 4 criteria in determining radiological progression was also high. In this study, PFS was lower than previously reported with the irinotecan-bevacizumab regimen,2,14,15 which may be explained by the inclusion of patients with lower performance status (one-third had a KPS less than 70%) and very close (monthly) MRI monitoring, allowing early detection of radiological progression.
Despite overall agreement among the tested criteria, and consistent with previous studies,11,16,17 it is worth noting that in approximately one-third of patients the RANO and RECIST + F criteria detected an infiltrating nonenhancing recurrence pattern, which preceded by about one month the detection of progression by the classic Macdonald and RECIST criteria. The RANO and RECIST + F criteria were equally efficient in detecting radiological progression prior to clinical deterioration compared with the Macdonald and RECIST criteria. Thus, the rates of radiological progression without clinical deterioration increased from 45% to 57% when FLAIR hyperintensity was taken into account. In this situation, the clinician faces the dilemma of whether to discontinue bevacizumab, even with the lack of effective salvage therapy and the risk of a “rebound effect” after its withdrawal,18 or to pursue the treatment despite objective radiological evidence of progression in the hope of delaying clinical deterioration.19
In general, our results were consistent with previous studies that showed that 1D measurements of contrast enhancement are comparable to 2D measurements for response assessment in high-grade gliomas.6,7 Furthermore, our study suggested that 1D and 2D measurements of FLAIR hyperintensity are also comparable and reproducible, with good intra- and interobserver agreement. Measuring and interpreting changes in FLAIR hyperintensities in treated glioblastoma patients is challenging and requires careful exclusion of nontumoral causes, the most frequent being radiation-induced white matter changes. However, some radiological features, such as sulcal effacement, ventricular compression, thickening of the corpus callosum, cortical ribbon infiltration, and location outside the field of radiation, suggest a tumoral nature.9 Our study supported these observations, and the retrospective analysis of the images had the advantage of facilitating such an interpretation. Undoubtedly, the integration of advanced imaging tools, such as MR perfusion, diffusion, and spectroscopy, is mandatory for prospective studies addressing this issue.20
Despite these limitations, our results suggest an agreement among the different criteria used to assess the response to bevacizumab-containing salvage regimens for recurrent glioblastoma. Even if it concerns only a minority of patients and does not dramatically change the overall results, adding a FLAIR hyperintensity evaluation to the contrast-enhanced measures appears to provide a surrogate for nonenhancing infiltrating tumors that avoids overestimation of radiological responses and PFS in some patients. Interestingly, criteria based on a 1D measurement approach (RECIST + F) seem comparable and as reliable as those based on a 2D approach, but simpler to implement.
Acknowledgments
We thank Dr. F. Laigle-Donadey, Dr. S. Taillibert, Dr. D. Psimaras, Dr. C. Dehais, Dr. A. Idbaih, and Dr. N. Martin-Duverneuil for their clinical assistance and Dr. M. Fiorelli for critical review of this article.
Conflict of interest statement. Dr. Hoang-Xuan serves as an associate editor of the Revue Neurologique and serves as a consultant for Roche. Dr. Sanson serves as the Neuro-Oncology Section Editor of Current Opinion in Oncology. Dr. Delattre serves on the editorial boards of The Oncologist and the Journal of Clinical Oncology and receives research support from the Institut National du Cancer. The rest of the authors declare no conflicts of interest.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. doi:10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. doi:10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 3.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 4.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. doi:10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 5.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. doi:10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Galanis E, Buckner JC, Maurer MJ, et al. Validation of neuroradiologic response assessment in gliomas: measurement by RECIST, two-dimensional, computer-assisted tumor area, and computer-assisted tumor volume methods. Neuro Oncol. 2006;8:156–165. doi: 10.1215/15228517-2005-005. doi:10.1215/15228517-2005-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah GD, Kesari S, Xu R, et al. Comparison of linear and volumetric criteria in assessing tumor response in adult high-grade gliomas. Neuro Oncol. 2006;8:38–46. doi: 10.1215/S1522851705000529. doi:10.1215/S1522851705000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandsma D, van den Bent MJ. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol. 2009;22:633–638. doi: 10.1097/WCO.0b013e328332363e. doi:10.1097/WCO.0b013e328332363e. [DOI] [PubMed] [Google Scholar]
- 9.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-Oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. doi:10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 10.de Groot JF, Fuller G, Kumar AJ, et al. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro Oncol. 2010;12:233–242. doi: 10.1093/neuonc/nop027. doi:10.1093/neuonc/nop027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779–787. doi: 10.1212/01.wnl.0000304121.57857.38. doi:10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 12.Zuniga RM, Torcuator R, Jain R, et al. Efficacy, safety and patterns of response and recurrence in patients with recurrent high-grade gliomas treated with bevacizumab plus irinotecan. J Neurooncol. 2009;91:329–336. doi: 10.1007/s11060-008-9718-y. doi:10.1007/s11060-008-9718-y. [DOI] [PubMed] [Google Scholar]
- 13.Reardon DA, Galanis E, DeGroot JF, et al. Clinical trial end points for high-grade glioma: the evolving landscape. Neuro Oncol. 2011;13:353–361. doi: 10.1093/neuonc/noq203. doi:10.1093/neuonc/noq203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vredenburgh JJ, Desjardins A, Herndon JE, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. doi:10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 15.Xu T, Chen J, Lu Y, Wolff JE. Effects of bevacizumab plus irinotecan on response and survival in patients with recurrent malignant glioma: a systematic review and survival-gain analysis. BMC Cancer. 2010;10:252. doi: 10.1186/1471-2407-10-252. doi:10.1186/1471-2407-10-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwamoto FM, Abrey LE, Beal K, et al. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 2009;73:1200–1206. doi: 10.1212/WNL.0b013e3181bc0184. doi:10.1212/WNL.0b013e3181bc0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pope WB, Xia Q, Paton VE, et al. Patterns of progression in patients with recurrent glioblastoma treated with bevacizumab. Neurology. 2011;76:432–437. doi: 10.1212/WNL.0b013e31820a0a8a. doi:10.1212/WNL.0b013e31820a0a8a. [DOI] [PubMed] [Google Scholar]
- 18.Zuniga RM, Torcuator R, Jain R, et al. Rebound tumour progression after the cessation of bevacizumab therapy in patients with recurrent high-grade glioma. J Neurooncol. 2010;99:237–242. doi: 10.1007/s11060-010-0121-0. doi:10.1007/s11060-010-0121-0. [DOI] [PubMed] [Google Scholar]
- 19.Kesselheim JC, Norden AD, Wen PY, Joffe S. Discontinuing bevacizumab in pients with glioblastoma: an ethical analysis. Oncologist. 2011;16:1435–1439. doi: 10.1634/theoncologist.2011-0047. doi:10.1634/theoncologist.2011-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pope WB, Young JR, Ellingson BM. Advances in MRI assessment of gliomas and response to anti-VEGF therapy. Curr Neurol Neurosci Rep. 2011;11:336–344. doi: 10.1007/s11910-011-0179-x. doi:10.1007/s11910-011-0179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]