Abstract
Serological cross-reactivity providing cross-protective immunity between antigenically related viruses is a cornerstone of vaccination. It was the immunological basis for the first human vaccine against smallpox introduced more than 200 years ago, and continues to underpin modern vaccine development as has recently been shown for human papillomavirus vaccines, which confer cross-protection against other oncogenic papillomavirus types not present in the vaccine. Here, we review the feasibility of cross-protective vaccination against an antigenic group of clinically important viruses belonging to the Japanese encephalitis serocomplex in the Flaviviridae family. We will discuss evidence suggesting that ‘new generation’ flavivirus vaccines may provide effective cross-protective immunity against heterologous Japanese encephalitis serocomplex viruses, and appraise potential risks associated with cross-reactive vaccine immunity. The review will also focus on the structural and mechanistic basis for cross-protective immunity among this group of flaviviruses, which is predominantly mediated by antibodies against a single viral surface protein.
Keywords: adjuvant, ChimeriVax™ technology, cross-protection, flavivirus, Japanese encephalitis virus, JE-VAX®, neutralizing antibody, vaccines, West Nile virus
Emergence, global distribution & cocirculation of clinically important viruses belonging to the Japanese encephalitis serocomplex
The Japanese encephalitis (JE) serocomplex is comprised of nine genetically and antigenically related viruses [1] within the flavivirus genus of the Flaviviridae family. Flaviviruses are a group of small, enveloped, positive-strand RNA viruses with yellow fever virus as the prototype member (reviewed in [2]) and the dengue viruses as the most clinically relevant members in terms of worldwide incidence of human disease (reviewed in [3]). Four JE serocomplex viruses are associated with epidemic outbreaks of encephalitis in humans: Japanese encephalitis virus (JEV), West Nile virus (WNV), Murray Valley encephalitis virus (MVEV) and Saint Louis encephalitis virus (SLEV) (reviewed in [4]). Their geographic distribution includes all continents except Antarctica (Table 1). All members of the serocomplex are maintained in a zoonotic cycle between primarily Culex mosquitoes and avian hosts. Although not part of the natural transmission cycle, human and equine infection with viruses of the JE serocomplex can result in severe and sometimes fatal neurological disease. JEV is the most important member of the serocomplex in terms of disease incidence: it is the leading cause of viral encephalitis in Asia, where approximately 30,000 clinical cases and approximately 10,000 deaths are reported annually, with a high incidence of neuropsychiatric deficits among survivors (reviewed in [4,5]). JEV is also a significant pathogen of horses, causing death from encephalitis in up to 40% of clinical cases, making immunization of horses against JEV with mouse brain-derived inactivated vaccine mandatory in several Asian countries [6]. WNV causes sporadic cases of febrile disease and encephalitis in Africa, India and Australia, with the majority of infections being asymptomatic. However, since the mid-1990s a series of outbreaks of West Nile encephalitis have occurred in the Middle East and eastern Europe due to the emergence of more virulent virus strains, with the most severe outbreak in North America, where in the decade since its first appearance in 1999 more than 30,000 human cases and approximately 1400 deaths have been reported [201]. MVEV can cause severe, sometimes fatal, disease in humans and is the most common cause of viral encephalitis in tropical Australia (reviewed in [7]). The virus sporadically spreads to central and southern regions of the continent, which occasionally gives rise to epidemic outbreaks of disease. The incidence of human disease due to infection with MVEV remains low, which is probably due to the low population density in the northern parts of Australia with endemic MVEV activity, given that the estimated ratio of apparent to inapparent infection with MVEV is similar to that with JEV [4]. Most clinical cases of encephalitis due to infection with SLEV in the USA occur in the southern states from Texas across to Florida. It is estimated that since 1933, when the virus was first identified, there have been >10,000 severe infections and >1000 fatal human cases of Saint Louis encephalitis (reviewed in [8]).
Table 1.
Japanese encephalitis serocomplex flaviviruses.
| Virus | Geographic distribution | Human disease |
|---|---|---|
| Alfuy virus | Australia | Not known |
| Cacipacore virus | Brazil | Not known |
| Japanese encephalitis virus | South and southeast Asia, China, Indonesia, northern Australia | Encephalitis |
| Koutango virus | Senegal | Fever, rash |
| Murray Valley encephalitis virus | Australia, Papua New Guinea | Encephalitis |
| Saint Louis encephalitis virus | Americas | Encephalitis |
| Usutu virus | Africa, Europe | Fever, rash |
| West Nile virus including Kunjin virus | Africa, Middle East, Europe, Americas, south and southeast Asia, Australia | Meningitis, encephalitis, fever, rash, flaccid paralysis |
| Yaounde virus | Central Africa | Not known |
In the past two decades, there has been an expansion of the geographic distribution of JE serocomplex viruses. Most notable are the emergence of WNV and its subsequent establishment as an endemic pathogen in most temperate regions of North America, and the spread of JEV into southwestern India, Pakistan and the Australasian region [9]. As a consequence, the geographic overlap of virus activity of the medically important members of the serocomplex has substantially increased. Currently, WNV co-circulates with SLEV in North America, JEV in India and MVEV in Australia, while JEV cocirculates with MVEV in Indonesia, Papua New Guinea and the Torres Strait Islands of northern Australia (Table 1). While JEV has been isolated on several occasions from mosquitoes on the Cape York Peninsula (Australia) and a human case of JE has been recorded, the virus is yet to become established in natural transmission cycles on the Australian mainland; nevertheless, the latter possibility remains a concern, given the abundance of suitable hosts and vectors (reviewed in [10]). A potentially more important threat to public health would be the introduction of JEV into the Americas. The WNV experience in North America over the past 10 years, combined with uncertain consequences of global warming on arbovirus transmission [11] and the ongoing geographic spread of JEV, suggest that the likelihood of JEV emergence in new target areas is high [12]. Spread of JEV into North America or Australia and establishment of transmission would probably trigger vaccination against JEV of ‘at risk’ populations, given the availability of an internationally licensed JE vaccine. However, overlap of virus activity of two or more clinically important members belonging to the JE serocomplex poses challenges to vaccination that require urgent assessment. These range from the possibility of protection against multiple members of the JE serocomplex with one (cross-protective) vaccine to unknown risks of vaccination on infection with a heterologous flavivirus, which might include vaccine-associated disease enhancement. These issues are reviewed in the following sections.
Cross-protective immunity against JE serocomplex flaviviruses: evidence from live virus infections
It has been known for many years that live virus infection with a member of the JE serocomplex will elicit protective immunity against other viruses belonging to this group, at least in animal models. For instance, Hammon and Sather showed protection in hamsters against WNV, JEV, SLEV and MVEV [13], while in mice one injection of WNV or SLEV afforded protection against lethal challenge with JEV [14]. A more recent study in hamsters confirmed that infection with SLEV or a live JEV vaccine (SA14-14-8 strain) also protected animals against a lethal challenge with virulent lineage I WNV, and WNV viremia was much lower in hamsters that were preinfected with a heterologous virus [15]. Effective cross-protective immunity between SLEV and virulent lineage I WNV, and WNV and JEV have also been described in studies in birds [16,17]. Experiments in pigs found that preinfection with MVEV or WNV (Kunjin strain) prevented viremia following subsequent challenge with JEV [18]. The only investigation in nonhuman primates on cross-protective immunity employed an extensive vaccination schedule and severe (intranasal) challenge route, and reported protection against WNV following preinfection with JEV, but only partial protection when the reciprocal combination was tested [19]. Collectively, the reports spanning research in different species over a period of >50 years establish that immunity to one JE serocomplex virus member provides a survival advantage to animals challenged with a heterologous virus of this group. They also indicate that heterologous virus challenge of preinfected animals broadens the immune response with a trend of boosting neutralizing antibodies against the immunizing virus to a higher level than that against the challenge virus. However, in combinations where a major difference in virulence between two viruses exists, the immune response against the more virulent virus tends to be of greater magnitude, consistent with the greater antigenic load produced. Accordingly, the experimental evidence does not support a strong ‘original antigenic sin’ effect between the JEV serocomplex flaviviruses, in contrast to that postulated for sequential challenge by heterologous dengue virus [20]. What remains uncertain in existing animal models or human subjects is the durability of the cross-protective response induced by infection with one JE serocomplex member. While flavivirus infection probably conveys long-lived protection against homologous viruses, it remains unclear how rapidly the cross-protective response wanes.
Cross-protective immunity against JE serocomplex flaviviruses by vaccination
Studies in humans
The aforementioned findings have encouraged researchers to evaluate whether vaccination against JE serocomplex flaviviruses also elicits a cross-reactive immune response that provides some benefit in recovery from infection with a heterologous member of the serogroup. This is an important question in terms of public health in many countries, because the spread of viruses belonging to the JE serocomplex, or the emergence of variants with enhanced virulence for humans, can be rapid, allowing limited time for the production of specific vaccines, and it would potentially allow for vaccination against viruses that cause less-frequent disease and therefore for which there are no commercial interests in vaccine development. Prompted by the outbreak of WNV encephalitis in the USA, two small human vaccine studies investigated whether an internationally licensed, inactivated JE vaccine (JE-VAX®, Biken Institute, Japan) provided a protective advantage against WNV based on the detection of cross-neutralizing antibody in vaccinated individuals: while the first study yielded negative results [21], the second study, in which JE-VAX was codelivered with (live) yellow fever 17D vaccine, reported effective levels of cross-neutralizing antibody against WNV; cross-reactive humoral immunity against WNV waned in parallel with that against JEV over time, but increased sharply following a booster vaccination with JE-VAX [22]. The main difference between the two investigations was the codelivery of the live yellow fever vaccine during priming with JE-VAX, which most probably provided a potent adjuvant effect to the inactivated JE vaccine by stimulating innate immune factors critical for immunogenicity [23]. While the report by Yamshchikov et al. involved codelivery of yellow fever and JE-VAX vaccines [22], others have noted that preimmunity to yellow fever vaccine can enhance the level of durable cross-reactive neutralizing antibody against the four dengue virus serotypes in human recipients of a dengue-2 live vaccine [24]. Surprisingly, a live-attenuated JE vaccine widely used in China (SA14-14-2 strain) over the past 20 years, but not approved for international use (reviewed in [25]), showed no cross-reactive antibodies against WNV in human recipients [26]. Failure to elicit cross-reactive immunity against WNV with the live vaccine was unlikely to be a consequence of the genotype of the attenuated JEV, which was the same (genotype III) as that of the inactivated vaccine (JE-VAX) shown to produce cross-neutralizing responses in codelivery with yellow fever vaccine. In summary, this small number of human studies indicates that the immune responses elicited with current JE vaccines may be of insufficient magnitude and/or quality to promote adequate cross-protective immunity against heterologous viruses of the serocomplex; however, they also indicate that by potentiating vaccine immunogenicity (e.g., by codelivery of yellow fever vaccine), cross-neutralizing antibody titers of a magnitude thought to confer protective immunity against WNV can be achieved in human vaccine recipients.
Studies in animals
Vaccine trials in animals also support the view that relative immunogenicity is key to the induction of cross-protective immunity by vaccination; this was clearly shown in a comparison of the cross-protective value of inactivated (JE-VAX) and live (ChimeriVax™-JE, Acambis Inc., MA, USA) JE vaccines in mouse models of WNV and MVEV encephalitis [27]. ChimeriVax-JE is constructed from yellow fever virus 17D vaccine cDNA by replacement of the viral structural premembrane (prM) and envelope (E) proteins with those of an attenuated JEV strain; it has undergone Phase II and Phase III trials for safety and efficacy in humans (reviewed in [25,28,29]). While a three-dose immunization schedule with inactivated JE-VAX showed only marginal protection against lethal MVEV challenge, a single dose of ChimeriVax-JE provided complete and durable (>5 months) protective immunity against both MVEV and WNV [27]. The experiments were performed in type I interferon receptor-deficient mice, which in view of their high susceptibility to encephalitic flavivirus infection [30], present a stringent challenge model. The magnitude of the neutralizing antibody response elicited with the vaccines correlated directly with their effectiveness in cross-protection: this is exemplified by higher titers to the heterologous virus in ChimeriVax-JE-immunized mice than to the homologous virus in JE-VAX-immunized mice. A T-cell-mediated contribution to vaccine-induced cross-protection was also noted [27], although detailed antigen-specific and mechanistic studies were not performed to address the protective effects of individual CD4+ or CD8+ T-cell subsets.
Superiority of the live chimeric flavivirus vaccine approach in eliciting cross-protective immunity compared with immunization with inactivated virus has been observed in other studies. Hamsters injected with a single dose of ChimeriVax-WNV veterinary vaccine (PreveNile®, Intervet/Schering Plough, NY, USA; recalled in 2010 owing to safety concerns [202]) developed sterilizing immunity against JEV, whereas two doses of canarypox virus-vectored WNV prM/E vaccine (Recombitek, Merial Ltd, GA, USA) promoted only a marginal reduction in viremia following heterologous challenge [31]. By contrast, immunization of mice with high doses of JE-VAX provided partial cross-protection against virulent WNV in the absence of detectable cross-neutralizing antibody at the time of challenge [32,33]. This apparent difference in cross-protective efficacy of live and inactivated flavivirus vaccines, in conjunction with the efficient cross-protective immunity observed following live virus infections, raises the following questions: is induction of protective immunity against heterologous viruses of the JE serocomplex a property associated with broad immune activation following a viral infection; and can the superior immunogenicity of live vaccines be replicated with inactivated vaccines by inclusion of novel adjuvants? The traditional inactivated JE vaccine (JE-VAX) is produced from infected mouse brain, and is nonadjuvanted; it is relatively poorly immunogenic, which is reflected by the three-dose immunization regimen that is required to produce time-limited immunity in approximately 90% of individuals, with further regular boosters required for those living in nonendemic regions (reviewed in [34]). However, when formulated with an adjuvant, inactivated JE vaccines can display cross-neutralizing and cross-protective effectiveness of similar magnitude to that of the chimeric live vaccines [35]. In preclinical vaccine trials in mice and horses, adjuvanted JE-VAX or replacement cell culture-grown JE vaccine elicited neutralizing antibody titers against MVEV that were equal to or greater than those elicited with nonadjuvanted vaccine against JEV; the adjuvanted vaccines also induced neutralizing antibody titers against the more distantly related WNV, albeit at lower levels than those against MVEV, but nevertheless exceeding the threshold titer thought to be required for protection [35]. Accordingly, the protective value of the adjuvanted JE vaccine against MVEV and WNV is predicted to be similar to that of nonadjuvanted vaccine against the homologous virus. In the study, a polysaccharide-based adjuvant (Advax, Vaxine Pty Ltd, Australia) was used, which potently stimulates immunogenicity without the increased reactogenicity seen with other adjuvants, and induces balanced Th1/Th2 immune responses against the vaccine antigen [36]. Overall, the findings suggest that promising live and inactivated candidate vaccines exist that can protect humans and other susceptible animals against multiple viruses belonging to the JE serocomplex. Their utility over a longer duration, however, remains an area of active investigation; as it is plausible that responses against heterologous members of the JE serocomplex may wane more rapidly than against the homologous virus.
Risks associated with vaccination against heterologous viruses of the JE serocomplex
In contrast to the protective value against heterologous flaviviruses, cross-reactive immunity has also been associated with enhanced infection and disease in natural and laboratory settings [37-40]. Antibody-dependent enhancement (ADE) of infection is thought to account for the more severe disease that is infrequently (~0.5%) associated with secondary, heterologous dengue virus infections, and is the main obstacle for the development of a dengue vaccine (reviewed in [41]). However, in contrast to dengue viruses, there are no reports suggesting that natural infection with a JE serocomplex flavivirus predisposes the host to a risk of enhanced infection with a second member of the serocomplex. By contrast, cross-reactive antibodies are believed to restrict overlap in natural transmission of two or more members belonging to the group [42]. Only in specific laboratory settings has limited evidence for in vivo ADE with JE serocomplex members ever been described. For the JEV–MVEV pair, immunization of mice with inactivated vaccine [27,43] or passive transfer of immune serum [44,45] gave rise to a more severe infection upon subsequent challenge with the heterologous virus. The relevance for vaccine safety of a finding of disease enhancement following passive immunizations with sub-neutralizing concentrations of antiserum in highly susceptible (weanling) mice is unclear, given that the animals lack vaccine-primed memory B and T cells. However, the observation that immunization with JE-VAX can increase viremia and accelerate entry of MVEV into the brain in adult mice warrants consideration [27]. Vaccine failure with JE-VAX, which was recapitulated in the mouse experiments by using a low-dose immunization schedule (1/100th of the recommended human vaccine dose), is found in a small proportion of human vaccine recipients [34]. This suggests that in a vaccinated population a spectrum of cross-reactive immunity exists, which ranges from strong cross-protection against other members of the serocomplex to the potentially detrimental property of enhancement of infection with a heterologous virus. Nevertheless, ADE and associated enhanced disease severity among the JE serocomplex viruses probably reflects an extreme laboratory manifestation of a relatively poorly immunogenic vaccine. The phenomenon was not reproduced with an experimental MVEV prM/E-encoding DNA vaccine, which showed a high level of cross-protection against JEV [43], nor was it seen in vaccinations with a wide dose range (10–105 PFU) of ChimeriVax-JE [27], or immunizations with very low doses of Advax-adjuvanted inactivated JE vaccine [35].
The mechanism for disease enhancement by cross-reactive antibody is thought to depend on the cross-linking of virus/antibody or virus/activated complement components through interaction with cell surface molecules such as Fcγ or complement receptors, leading to enhanced infection of permissive cells such as monocytes and macrophages (reviewed in [46]), although binding of cross-reactive antibody to dengue virus may also promote the formation of virus aggregates, which could engage Fcγ receptor IIB and inhibit ADE [47]. Alternatively, Fcγ receptor-mediated uptake of flavivirus–antibody immune complexes has been suggested to modulate antiviral immune pathways by decreasing production of type I interferon, TNF-α and nitric oxide, and increasing release of IL-10 (reviewed in [48]). However, these effects could also be associated with increased viral entry and antagonism of host immune responses irrespective of signaling through Fcγ receptors [49,50]. Finally, ADE can lead to the establishment of Th2-biased immune responses. Th2 bias is a correlate of increased disease severity in secondary dengue infection (reviewed in [48]) and was associated with vaccine-enhanced paramyxovirus disease in children in the 1960s. In atypical measles and enhanced respiratory syncytial virus disease, patients had been immunized with inactivated vaccines, which failed to elicit long-lived antibody and CD8+ T-cell immunity. Following virus exposure they developed Th2-biased responses with characteristic immune complex deposition in affected tissues and eosinophilia [51]. Th2 bias also impacts humoral immune responses with the preferential switching to antibody isotypes with suboptimal effector function (see below). The inactivated measles and respiratory syncytial virus vaccines were adjuvanted with Alum, which induces a strong Th2 bias [52]. Given the above considerations, the choice of adjuvant is a critical issue in vaccine design, where a potential risk of vaccination-induced disease enhancement exists. To increase vaccine safety, Alum formulations may be supplemented or replaced with adjuvants that promote more balanced Th1/Th2 responses.
It remains unclear whether cross-reactive cellular immune responses elicited by vaccination can predispose recipients to enhanced disease following infection with a second JE serocomplex virus. While there is evidence of increased and potentially pathological T-cell activation and proinflammatory cytokine production in patients with heterologous dengue infection who develop hemorrhagic fever (reviewed in [53]), similar manifestations associated with cross-reactive T cells have so far not been documented for the JE serocomplex flaviviruses.
Immunological correlates for protective & cross-protective immunity against JE serocomplex flaviviruses
The innate and adaptive immune responses that contribute to recovery from primary infection with WNV and JEV have been extensively investigated in mouse models that recapitulate natural infection route and virus dose, and that produce clinical signs of encephalitic disease comparable to those manifested in human infections (reviewed in [54-57]). Most critical for recovery from acute infection is an intact type I interferon pathway [30,58] and a vigorous B-cell immune response with early and sustained neutralizing antibody production against the viral E protein, which requires T-cell help for switching from IgM to IgG antibody isotypes and affinity maturation [59-62]. A key role of innate and early B-cell immune responses is to restrict virus growth in extraneural tissues, and prevent virus entry into the CNS, which in turn can lead to severe encephalitic disease. While in the case of virulent WNV, CD4+ and CD8+ T cells can successfully clear virus from the infected brain [63-67] , the contribution of CD8+ T cells in recovery from JEV infection is subsidiary [60], and a disease-enhancing effect of cytotoxic T cells has been documented for MVEV in mice [68].
Antibody is also of paramount importance in vaccine-induced protection against JEV and WNV challenge. Using immunization approaches in mice that selectively induced neutralizing antibody against E protein or CD8+ T-cell immunity against other regions of the viral polyprotein, humoral immunity afforded complete protection against lethal homologous virus challenge whereas virus-specific CD8+ T-cell memory at best provided partial protection [69,70]. Similarly, a two-dose immunization schedule with an inactivated WNV vaccine licensed for veterinary use (West Nile Innovator®, Pfizer, NY, USA) induces neutralizing antibody in mice, which almost completely protects against stringent lethal intracranial WNV challenge; the humoral immunity induced with the vaccine was sufficient for protection, given that depletion of CD8+ T cells before challenge did not reduce survival rate, and vaccinated CD8+ T cell-deficient mice were equally protected in comparison to wild-type mice [71]. While these experiments highlight the importance of neutralizing antibody in vaccination-mediated protection, and suggest that protection from severe challenge with homologous virus can be achieved in the absence of a CD8+ T-cell contribution, they do not exclude the possibility that antiviral CD8+ T-cell memory responses may enhance vaccine efficacy, as well as contribute to durable resistance against re-infection following natural virus exposure. Indeed, immunization with single-chain HLA-A2 MHC trimer molecules that incorporate an immundominant WNV peptide elicited protective T cell immunity in the absence of an antibody response against lethal WNV infection [72].
The relative contribution of antibody and T cells to vaccine-induced cross-protective immunity among viruses belonging to the JE serocomplex is still unclear. Vaccine platforms that have shown cross-protective efficacy, such as prM/E protein chimeric flaviviruses [27,31], adjuvanted inactivated virus vaccine [35] and prM/E protein-based DNA vaccination [43], predominantly elicit B-cell rather than CD8+ T-cell immune responses; in the case of the ChimeriVax technology, the CD8+ T-cell responses are primarily directed against peptide determinants in the yellow fever virus backbone, which constitutes more than three quarters of the polyprotein expressed, and are not expected to significantly protect against JE serocomplex viruses (reviewed in [29]), although CD8+ T-cell determinants mapping to the prM and E proteins or cross-reactive peptides could provide cross-protection in individuals with the corresponding MHC I haplotypes [73,74]. In preliminary experiments, the Advax-adjuvanted cell culture-grown JE vaccine efficiently protected against a lethal challenge with virulent (lineage I) WNV in mice lacking CD8+ T cells, underscoring the role of vaccine-induced B-cell memory and excluding a requirement for CD8+ T cells in cross-protection against WNV with the JEV vaccine [Larena M, Prow N, Hall R, Petrovsky N, Lobigs M, Unpublished Data]. Clearly, further research designed to identify the immunological correlates for cross-protection for the different vaccination approaches is needed.
E protein epitopes important in protection against JE serocomplex flaviviruses & mechanisms for in vivo virus neutralization & clearance
Virion structure
Flaviviruses are small, approximately 50 nm spherical virus particles. Virion assembly occurs within the endoplasmic reticulum, with the capsid protein and genomic RNA associating with prM and E proteins (reviewed in [2]). Virus particles bud into the lumen of the endoplasmic reticulum as immature virions in which E and prM proteins interact to form 60 heterotrimeric spikes with icosahedral symmetry [75]. Transit of the immature virion through the trans-Golgi network triggers an extensive rearrangement on the virion surface. A low pH-induced transition causes the E proteins on immature virions to lie flat as antiparallel dimers on the surface of the virion [76], which in turn increases the susceptibility of prM to cleavage by a furin-like serine protease in the trans-Golgi network [77,78]. Release of prM occurs in the neutral pH of the extracellular space [78]. Mature flavivirus virions are relatively smooth particles that display 90 E protein dimers arranged in a herringbone pattern. While cleavage of prM is a required step in the viral lifecycle, for some flaviviruses it is an inefficient process. Moreover, partially mature flavivirus virions containing some uncleaved prM also retain infectivity [79,80].
The viral E protein functions in multiple steps of the virus life-cycle, including assembly, budding, attachment to target cells and viral membrane fusion (reviewed in [81]) and is also the primary target of neutralizing antibodies (reviewed in [82]). Flavivirus E protein is an elongated, type II viral fusion protein composed of three distinct domains connected by short flexible hinges (Figure 1). Domain I (DI) is an eight-stranded β-barrel located in the center of the E protein molecule. Domain II (DII) is an elongated structure that mediates dimerization of E proteins on the mature virion. A highly conserved glycine-rich loop of 13 amino acids located at the tip of DII is believed to insert into the membranes of target cells [83,84]. Domain III (DIII) adopts an immunoglobulin-like fold and is the portion of the E protein that projects farthest away from the surface of the mature virion, and is speculated to contain binding sites for cellular factors involved in virus attachment and entry [85-87].
Figure 1. Structure of the West Nile virus envelope protein.
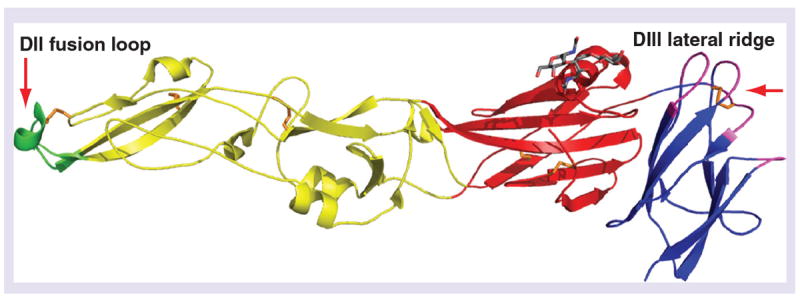
Domain I (red), DII (yellow), and DIII (blue) of the monomeric envelope protein and location of key epitopes. The fusion loop (residues 98–110, green) is located at the distal end of DII. A single carbohydrate (gray) extends from Asn154 in Domain I. The DIII-lateral ridge epitope (based on the structure of monoclonal antibody West Nile virus E16) is highlighted in magenta: the amino-terminal region (residues 302–309), the BC loop (residues 330–333), the DE loop (residues 365–368) and the FG loop (residues 389–391). The six disulfide bonds are shown in orange.
DII: Domain II; DIII: Domain III.
E protein epitopes & functional properties of the humoral immune response important in cross-protection
Antibody neutralization occurs by blocking attachment to host cells, penetration of virions into cells, and the low pH-dependent fusion of the viral and host cell membranes (reviewed in [82]). The generation and characterization of large panels of mouse and human monoclonal antibodies (mAbs) against epitopes spanning the E protein of JE serocomplex members (e.g., WNV) has enhanced our understanding of the structural correlates of the functional antibody response (Figure 1). Although mouse mAbs that bind to each of the three domains of WNV E protein have been described, the most potently inhibitory mAbs recognize the lateral ridge (LR) epitope on DIII (DIII-LR) [88-90]. However, because of the significant amino acid sequence variation in the DIII-LR epitope among JE serocomplex family members [91], DIII-LR-specific neutralizing antibodies are primarily type specific, and can neutralize homologous viruses but not closely related heterologous JE serocomplex viruses. As an example, the therapeutic DIII-LR mAb E16 neutralizes virtually all WNV strains, yet fails to bind or neutralize JEV or SLEV [89]. In comparison, the human anti-E repertoire appears more skewed to a weakly neutralizing highly cross-reactive epitope in the fusion loop of DII [92,93]. Cross-reactive polyclonal antibodies directed against the DII fusion loop epitope and derived from dengue-immune humans and hamsters confer protection against WNV in passive transfer studies in mice despite poor cross-neutralization activity in cell-based in vitro assays [94].
Humoral immunity contributes significantly to the host response to flavivirus infection. While neutralizing antibody titers correlate with protection by several flavivirus vaccines [95-97], the relationship is imperfect [98]. Antibodies may also exert protective effects via effector functions mediated by the Fc portion of the antibody molecule, including complement fixation, antibody-mediated cellular cytotoxicity and facilitating virus clearance [99,100]. These in vivo pathways for virus clearance can account for the protective value of cross-reactive antibodies against the fusion loop on DII and the observation of cross-protective humoral immunity in vaccinated animals in the absence of detectable neutralizing activity of immune sera by in vitro cell-based assays. Thus, while a cross-neutralization titer of greater than or equal to 10 between viruses belonging to the JE serocomplex predicts cross-protective immunity [101] and is most probably mediated by antibodies that recognize epitopes in DI or DIII that are conserved between the closely related viruses, the contribution of DII fusion loop-specific antibodies to cross-protection may be of greater importance but are overlooked by the in vitro measurements for vaccine efficacy. This has implications for flavivirus vaccine design, because in vivo protective value and durability of the humoral immune response may not be accurately reflected in neutralization titers in cell culture assays used traditionally as surrogate markers of protection.
Effector functions mediated by the Fc portion of the antibody molecule are critically influenced by antibody isotype, as human IgG1 and IgG3 (mouse IgG2a and IgG2b) efficiently interact with complement factor C1q [100,102] and Fcγ receptors [103], which enhance in vivo protection of antibodies, whereas binding of human IgG2 and IgG4 (mouse IgG1 and IgG3) is weaker. The cytokine milieu during induction of humoral antiviral immunity strongly affects antibody isotype switching; a balanced Th1/Th2 response promotes IgG subclass selection for subtypes that potently activate C1q and Fcγ receptor effector functions [104,105]. Therefore, live vaccines and inactivated vaccines that are formulated with adjuvants inducing balanced Th1/Th2 responses may be more effective at inducing protective immunity against homologous and heterologous flavivirus infections. Consistent with this, a recent study on human immune γ-globulin with neutralizing activity against WNV showed that IgG1 is the main subclass in naturally acquired WNV infection, and provides superior protection against WNV challenge in mice relative to the other subclasses [106].
Expert commentary & five-year view
Despite extensive experimental evidence from animal studies showing cross-protection against viruses belonging to the JE serocomplex with only rare indications of associated immunopathological complications, there remains reluctance to exploit antigenic cross-reactivity in vaccination against this group of closely related and clinically relevant flaviviruses [107]. A main reason against consideration of a cross-protective vaccine approach has been the relatively poor immunogenicity of the internationally licensed mouse brain-derived inactivated JE vaccine (JE-VAX), which was discontinued in 2005 owing to perceived safety problems and excess reactogenicity (reviewed in [25]). However, in view of the recent development of more potent candidate JE vaccines, notably those using the ChimeriVax platform or cell culture-grown inactivated JEV formulated with adjuvant (reviewed in [25,28,29,108]), the concept of cross-protective vaccination against heterologous viruses of the JE serocomplex should be revisited. There remains a major unmet need for human and veterinary vaccines against JE serocomplex viruses including: vaccines against pathogenic members of the group for which no commercial interests in vaccine development exist; vaccines for use in regions where two or more members of the group co-circulate and cause encephalitis; vaccines for use in travelers and military personnel who are exposed to infections with different members of the serocomplex; and vaccines for safeguarding against rapidly emerging and potentially more virulent outbreaks of infection with JE serocomplex viruses. While the unpredictable nature of epidemic outbreaks of encephalitic flavivirus disease is dramatically illustrated by the North American experience with WNV over the past decade, population growth in geographic regions of endemic activity of JE serocomplex viruses will inevitably increase disease incidence; for example, remote regions of tropical Australia, which are the enzootic foci for MVEV activity, will experience major industrial, agricultural and tourism development in the coming years [203], which in turn will probably result in an increase of human cases of Murray Valley encephalitis and calls for immunization of ‘at risk’ populations, although a specific MVEV vaccine is not available. A single ‘new generation’ JE serocomplex vaccine with more potent and broader immunogenicity relative to the traditional ‘first generation’ JE vaccines could meet these diverse immunization requirements. As a critical next step to address the feasibility of cross-protective immunization of humans and veterinary hosts, it will be important to evaluate cross-neutralization activity of clinical trial sera against different heterologous viruses of the serocomplex as an in vitro measurement of cross-protective efficacy and durability of the responses elicited with the novel vaccine candidates. Neutralizing antibody is an accepted surrogate for the efficacy of flavivirus vaccines where a reasonable threshold antibody level for protection is a 1:10 dilution in the 50% plaque-reduction neutralization test [101], although this value has not yet been clearly established for WNV and MVEV. Noninferiority of heterologous responses measured as percentage of seroconversion and neutralization titers relative to those obtained with the traditional licensed JE vaccine against the homologous virus could be considered as a primary end point for cross-protective efficacy testing of vaccine candidates. However, the findings should be considered with the proviso that the in vitro assay does not take into account alternative in vivo mechanisms for virus clearance mediated by the Fc portion of the antibody molecule. Recent evidence suggests that the latter may play a significant role, particularly in humans, in cross-protective immune responses against JE serocomplex viruses. While in vitro quantitation of the effector functions of antibody involving the interaction of the Fc portion with complement factors and Fc receptors on monocytes and macrophages is not simple, determination of the T-helper type immunity elicited by vaccination would provide an additional indirect read-out of cross-protective value, given that a balanced Th1/Th2 response is critical for efficient antibody-mediated protection and induction of antiviral CD8+ T-cell memory, with the relative importance of the cellular immunity in cross-protection requiring further investigation.
Key issues.
The Japanese encephalitis (JE) serocomplex is a group of antigenically related mosquito-borne flaviviruses that comprises several clinically important members for which human and/or veterinary vaccines are not available.
Expansion of the geographic distribution of JE serocomplex viruses (e.g., emergence of West Nile virus and its subsequent establishment as an endemic pathogen in North America and the spread of JE virus in India and into the Australasian region) has resulted in increased geographic overlap of virus activity of different pathogenic members of the group, with implications for vaccination.
Research in different species over a period of >50 years clearly shows that infection with one JE serocomplex virus provides protective immunity in challenges with heterologous viruses of the group, although it remains unclear how rapidly the cross-protective response wanes.
‘New generation’ candidate JE vaccines employing the ChimeriVax™ technology or using cell culture-grown inactivated virus formulated with an adjuvant can, at least in the short term, elicit protective immunity against multiple JE serocomplex viruses; notably, preclinical animal studies show that they induce cross-neutralizing antibody responses against heterologous viruses of the serocomplex, which are similar to, or exceed, those induced with the traditional JE vaccine (JE-VAX®) against the homologous virus.
While antibody-dependent enhancement of infection is thought to account for the more severe disease associated with a subset of secondary, heterologous dengue virus infections and is the main obstacle for development of a dengue vaccine, the risk of antibodydependent enhancement of infection and associated increased disease severity among the JE serocomplex viruses following vaccination appears more remote; the more potent and broader immunogenicity of new generation JE vaccines combined with the induction of balanced Th1/Th2 immune responses is likely to further reduce the risk of adverse events in cross-protective vaccination against JE serocomplex viruses.
The critical importance of humoral immunity in vaccine-mediated protection against flavivirus infection is well documented; however, the relative contribution of antibody and CD8+ T cells to cross-protective immunity against JE serocomplex viruses requires further research.
Recent research shows that effector functions of antibody involving the interaction of the Fc portion with complement factors and Fc receptors on monocytes and macrophages significantly contribute to in vivo clearance of flavivirus infection; the cross-reactive human antibody repertoire is skewed towards weakly neutralizing epitopes on the fusion loop of domain II of envelope protein, which are protective in vivo via effector functions by the Fc portion of the antibody molecule.
Acknowledgments
The authors would like to thank Kyle Austin for assistance with the preparation of Figure 1.
This work was supported by NIH grants R01-AI077955 (MS Diamond) and U54 AI057160 (the Midwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research). MS Diamond is a paid consultant for MacroGenics, Inc, which has licensed the E16 monoclonal antibody.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
-
•
of interest
-
••
of considerable interest
- 1.Cook S, Holmes EC. A multigene analysis of the phylogenetic relationships among the flaviviruses (Family: Flaviviridae) and the evolution of vector transmission. Arch Virol. 2006;151(2):309–325. doi: 10.1007/s00705-005-0626-6. [DOI] [PubMed] [Google Scholar]
- 2.Lindenbach BD, Rice CM. Flaviviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2001. pp. 991–1042. [Google Scholar]
- 3.Guzman MG, Halstead SB, Artsob H, et al. Dengue: a continuing global threat. Nat Rev Microbiol. 2010;8(Suppl. 12):S7–S16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4•.Solomon T. Flavivirus encephalitis. N Engl J Med. 2004;351(4):370–378. doi: 10.1056/NEJMra030476. Provides an overview of the pathogenesis of JE serocomplex viruses. [DOI] [PubMed] [Google Scholar]
- 5.Halstead SB, Jacobson J. Japanese encephalitis. Adv Virus Res. 2003;61:103–138. doi: 10.1016/s0065-3527(03)61003-1. [DOI] [PubMed] [Google Scholar]
- 6.Konishi E, Shoda M, Kondo T. Prevalence of antibody to Japanese encephalitis virus nonstructural 1 protein among racehorses in Japan: indication of natural infection and need for continuous vaccination. Vaccine. 2004;22(9–10):1097–1103. doi: 10.1016/j.vaccine.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Mackenzie JS, Lindsay MD, Coelen RJ, Broom AK, Hall RA, Smith DW. Arboviruses causing human disease in the Australasian zoogeographic region. Arch Virol. 1994;136(3–4):447–467. doi: 10.1007/BF01321074. [DOI] [PubMed] [Google Scholar]
- 8.Reisen WK. Epidemiology of St. Louis encephalitis virus. Adv Virus Res. 2003;61:139–183. doi: 10.1016/s0065-3527(03)61004-3. [DOI] [PubMed] [Google Scholar]
- 9•.Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10(Suppl. 12):S98–S109. doi: 10.1038/nm1144. Review summarizing geographic spread and overlap of enzootic activity of JE serocomplex viruses. [DOI] [PubMed] [Google Scholar]
- 10.Van Den Hurk AF, Ritchie SA, Mackenzie JS. Ecology and geographical expansion of Japanese encephalitis virus. Ann Rev Entomol. 2009;54:17–35. doi: 10.1146/annurev.ento.54.110807.090510. [DOI] [PubMed] [Google Scholar]
- 11.Colwell RR, Epstein PR, Gubler D, et al. Climate change and human health. Science. 1998;279(5353):968–969. doi: 10.1126/science.279.5353.963g. [DOI] [PubMed] [Google Scholar]
- 12.Nett RJ, Campbell GL, Reisen WK. Potential for the emergence of Japanese encephalitis virus in California. Vector Borne Zoonotic Dis. 2009;9(5):511–517. doi: 10.1089/vbz.2008.0052. [DOI] [PubMed] [Google Scholar]
- 13.Hammon WM, Sather GE. Immunity of hamsters to West Nile and Murray Valley viruses following immunization with St. Louis and Japanese B. Proc Soc Exp Biol Med. 1956;91(3):521–524. doi: 10.3181/00379727-91-22314. [DOI] [PubMed] [Google Scholar]
- 14.Price WH, Thind IS, O’Leary W, El Dadah AH. A protective mechanism induced by live group B arboviruses against heterologous group B arboviruses independent of serum neutralizing antibodies or interferon. Am J Epidemiol. 1967;86(1):11–27. doi: 10.1093/oxfordjournals.aje.a120716. [DOI] [PubMed] [Google Scholar]
- 15•.Tesh RB, Travassos Da Rosa AP, Guzman H, Araujo TP, Xiao SY. Immunization with heterologous flaviviruses protective against fatal West Nile encephalitis. Emerg Infect Dis. 2002;8(3):245–251. doi: 10.3201/eid0803.010238. Study in hamsters which shows that live virus infection with members of the JE serocomplex induces efficient cross-protective immunity against other viruses in the group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang Y, Reisen WK. Previous infection with West Nile or St. Louis encephalitis viruses provides cross protection during reinfection in house finches. Am J Trop Med Hyg. 2006;75(3):480–485. [PubMed] [Google Scholar]
- 17.Nemeth NM, Bosco-Lauth AM, Bowen RA. Cross-protection between West Nile and Japanese encephalitis viruses in red-winged blackbirds (Agelaius phoeniceus) Avian Dis. 2009;53(3):421–425. doi: 10.1637/8574-010109-Reg.1. [DOI] [PubMed] [Google Scholar]
- 18.Williams DT, Daniels PW, Lunt RA, Wang LF, Newberry KM, Mackenzie JS. Experimental infections of pigs with Japanese encephalitis virus and closely related Australian flaviviruses. Am J Trop Med Hyg. 2001;65(4):379–387. doi: 10.4269/ajtmh.2001.65.379. [DOI] [PubMed] [Google Scholar]
- 19.Goverdhan MK, Kulkarni AB, Gupta AK, Tupe CD, Rodrigues JJ. Two-way cross-protection between West Nile and Japanese encephalitis viruses in bonnet macaques. Acta Virol. 1992;36:277–283. [PubMed] [Google Scholar]
- 20.Midgley CM, Bajwa-Joseph M, Vasanawathana S, et al. An in-depth analysis of original antigenic sin in dengue virus infection. J Virol. 2011;85(1):410–421. doi: 10.1128/JVI.01826-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanesa-Thasan N, Putnak JR, Mangiafico JA, Saluzzo JE, Ludwig GV. Short report: absence of protective neutralizng antibodies to West Nile virus in subjects following vaccination with Japanese encephalitis or dengue vaccines. Am J Trop Med Hyg. 2002;66(2):115–116. doi: 10.4269/ajtmh.2002.66.115. [DOI] [PubMed] [Google Scholar]
- 22.Yamshchikov G, Borisevich V, Kwok CW, et al. The suitability of yellow fever and Japanese encephalitis vaccines for immunization against West Nile virus. Vaccine. 2005;23(39):4785–4792. doi: 10.1016/j.vaccine.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 23.Pulendran B. Learning immunology from the yellow fever vaccine: innate immunity to systems vaccinology. Nat Rev Immunol. 2009;9(10):741–747. doi: 10.1038/nri2629. [DOI] [PubMed] [Google Scholar]
- 24.Guirakhoo F, Kitchener S, Morrison D, et al. Live attenuated chimeric yellow fever dengue type 2 (ChimeriVax-DEN2) vaccine: Phase 1 clinical trial for safety and immunogenicity: effect of yellow fever pre-immunity in induction of cross neutralizing antibody responses to all 4 dengue serotypes. Hum Vaccin. 2006;2(2):60–67. doi: 10.4161/hv.2.2.2555. [DOI] [PubMed] [Google Scholar]
- 25.Beasley DW, Lewthwaite P, Solomon T. Current use and development of vaccines for Japanese encephalitis. Exp Opin Biol Ther. 2008;8(1):95–106. doi: 10.1517/14712598.8.1.95. [DOI] [PubMed] [Google Scholar]
- 26.Tang F, Zhang JS, Liu W, et al. Failure of Japanese encephalitis vaccine and infection in inducing neutralizing antibodies against West Nile virus, People’s Republic of China. Am J Trop Med Hyg. 2008;78(6):999–1001. [PubMed] [Google Scholar]
- 27••.Lobigs M, Larena M, Alsharifi M, Lee E, Pavy M. Live chimeric and inactivated Japanese encephalitis virus vaccines differ in their cross-protective values against murray valley encephalitis virus. J Virol. 2009;83(6):2436–2445. doi: 10.1128/JVI.02273-08. Provides proof-of-concept data for cross-protective vaccination with ChimeriVax-JE against other JE serocomplex viruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Appaiahgari MB, Vrati S. IMOJEV((R)): a yellow fever virus-based novel Japanese encephalitis vaccine. Expert Rev Vaccines. 2010;9(12):1371–1384. doi: 10.1586/erv.10.139. [DOI] [PubMed] [Google Scholar]
- 29•.Guy B, Guirakhoo F, Barban V, Higgs S, Monath TP, Lang J. Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses. Vaccine. 2010;28(3):632–649. doi: 10.1016/j.vaccine.2009.09.098. Comprehensive review of development of yellow fever virus-based chimeric vaccines. [DOI] [PubMed] [Google Scholar]
- 30.Lobigs M, Mullbacher A, Wang Y, Pavy M, Lee E. Role of Type 1 and Type 2 interferon responses in recovery from infection with an encephalitic flavivirus. J Gen Virol. 2003;84(Pt 3):567–572. doi: 10.1099/vir.0.18654-0. [DOI] [PubMed] [Google Scholar]
- 31.Bosco-Lauth A, Mason G, Bowen R. Pathogenesis of Japanese encephalitis virus infection in a golden hamster model and evaluation of flavivirus cross-protective immunity. Am J Trop Med Hyg. 2011;84(5):727–732. doi: 10.4269/ajtmh.2011.11-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim CK, Takasaki T, Kotaki A, Kurane I. Vero cell-derived inactivated West Nile (WN) vaccine induces protective immunity against lethal WN virus infection in mice and shows a facilitated neutralizing antibody response in mice previously immunized with Japanese encephalitis vaccine. Virology. 2008;374(1):60–70. doi: 10.1016/j.virol.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 33.Takasaki T, Yabe S, Nerome R, Ito M, Yamada K, Kurane I. Partial protective effect of inactivated Japanese encephalitis vaccine on lethal West Nile virus infection in mice. Vaccine. 2003;21(31):4514–4518. doi: 10.1016/s0264-410x(03)00507-3. [DOI] [PubMed] [Google Scholar]
- 34.Monath TP. Japanese encephalitis vaccines: current vaccines and future prospects. Curr Top Microbiol Immunol. 2002;267:105–138. doi: 10.1007/978-3-642-59403-8_6. [DOI] [PubMed] [Google Scholar]
- 35•.Lobigs M, Pavy M, Hall RA, et al. An inactivated Vero cell-grown Japanese encephalitis vaccine formulated with Advax, a novel inulin-based adjuvant, induces protective neutralizing antibody against homologous and heterologous flaviviruses. J Gen Virol. 2010;91(Pt 6):1407–1417. doi: 10.1099/vir.0.019190-0. Preclinical study in mice and horses showing that formulation of inactivated JE vaccine with an adjuvant elicits cross-protective neutalizing antibody against heterologous JE serocomplex viruses that are similar or exceed those induced against the homologous virus with nonadjuvanted vaccine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrovsky N. Freeing vaccine adjuvants from dangerous immunological dogma. Expert Rev Vaccines. 2008;7(1):7–10. doi: 10.1586/14760584.7.1.7. [DOI] [PubMed] [Google Scholar]
- 37.Barrett AT, Gould EA. Antibody-mediated early death in vivo after infection with yellow fever virus. J Gen Virol. 1986;67:2539–2542. doi: 10.1099/0022-1317-67-11-2539. [DOI] [PubMed] [Google Scholar]
- 38.Gould EA, Buckley A. Antibody-dependent enhancement of yellow fever and Japanese encephalitis virus neurovirulence. J Gen Virol. 1989;70:1605–1608. doi: 10.1099/0022-1317-70-6-1605. [DOI] [PubMed] [Google Scholar]
- 39.Hawkes RA. Enhancement of the infectivity of arboviruses by specific antisera produced in domestic fowls. Aust J Exp Biol Med Sci. 1964;42:465–482. doi: 10.1038/icb.1964.44. [DOI] [PubMed] [Google Scholar]
- 40.Peiris JS, Porterfield JS. Antibody-dependent plaque enhancement: its antigenic specificity in relation to Togaviridae. J Gen Virol. 1982;58:291–296. doi: 10.1099/0022-1317-58-2-291. [DOI] [PubMed] [Google Scholar]
- 41.Murphy BR, Whitehead SS. Immune response to dengue virus and prospects for a vaccine. Ann Rev Immunol. 2011;29:587–619. doi: 10.1146/annurev-immunol-031210-101315. [DOI] [PubMed] [Google Scholar]
- 42.Reisen WK, Lothrop HD, Wheeler SS, et al. Persistent West Nile virus transmission and the apparent displacement St. Louis encephalitis virus in southeastern California, 2003–2006. J Med Entomol. 2008;45(3):494–508. doi: 10.1603/0022-2585(2008)45[494:pwnvta]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lobigs M, Pavy M, Hall RA. Cross-protective and infection-enhancing immunity in mice vaccinated against flaviviruses belonging to the Japanese encephalitis virus serocomplex. Vaccine. 2003;21:1572–1579. doi: 10.1016/s0264-410x(02)00743-0. [DOI] [PubMed] [Google Scholar]
- 44.Broom AK, Wallace MJ, Mackenzie JS, Smith DW, Hall RA. Immunization with γ globulin of Murray Valley encephalitis virus and with an inactivated Japanese encephalitis virus vaccine as prophylaxis against Australian encephalitis: evaluation in a mouse model. J Med Virol. 2000;61:259–265. doi: 10.1002/(sici)1096-9071(200006)61:2<259::aid-jmv13>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 45.Wallace MJ, Smith DW, Broom AK, et al. Antibody-dependent enhancement of Murray Valley encephalitis virus virulence in mice. J Gen Virol. 2003;84(Pt 7):1723–1728. doi: 10.1099/vir.0.18980-0. [DOI] [PubMed] [Google Scholar]
- 46.Takada A, Kawaoka Y. Antibody-dependent enhancement of viral infection: molecular mechanisms and in vivo implications. Rev Med Virol. 2003;13(6):387–398. doi: 10.1002/rmv.405. [DOI] [PubMed] [Google Scholar]
- 47.Chan KR, Zhang SL, Tan HC, et al. Ligation of Fc γ receptor IIB inhibits antibody-dependent enhancement of dengue virus infection. Proc Natl Acad Sci USA. 2011;108(30):12479–12484. doi: 10.1073/pnas.1106568108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ubol S, Halstead SB. How innate immune mechanisms contribute to antibody-enhanced viral infections. Clin Vaccin Immunol. 2010;17(12):1829–1835. doi: 10.1128/CVI.00316-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boonnak K, Dambach KM, Donofrio GC, Tassaneetrithep B, Marovich MA. Cell type specificity and host genetic polymorphisms influence antibody-dependent enhancement of dengue virus infection. J Virol. 2011;85(4):1671–1683. doi: 10.1128/JVI.00220-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kou Z, Lim JY, Beltramello M, et al. Human antibodies against dengue enhance dengue viral infectivity without suppressing type I interferon secretion in primary human monocytes. Virology. 2011;410(1):240–247. doi: 10.1016/j.virol.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 51.Polack FP. Atypical measles and enhanced respiratory syncytial virus disease (ERD) made simple. Pediatr Res. 2007;62(1):111–115. doi: 10.1203/PDR.0b013e3180686ce0. [DOI] [PubMed] [Google Scholar]
- 52.Marichal T, Ohata K, Bedoret D, et al. DNA released from dying host cells mediates aluminum adjuvant activity. Nat Med. 2011;17(8):996–1002. doi: 10.1038/nm.2403. [DOI] [PubMed] [Google Scholar]
- 53.Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol. 2011;11(8):532–543. doi: 10.1038/nri3014. [DOI] [PubMed] [Google Scholar]
- 54.Klein RS, Diamond MS. Immunological headgear: antiviral immune responses protect against neuroinvasive West Nile virus. Trends Mol Med. 2008;14(7):286–294. doi: 10.1016/j.molmed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 55.Diamond MS, Mehlhop E, Oliphant T, Samuel MA. The host immunologic response to West Nile encephalitis virus. Front Biosci. 2009;14:3024–3034. doi: 10.2741/3432. [DOI] [PubMed] [Google Scholar]
- 56.Samuel MA, Diamond MS. Pathogenesis of West Nile virus infection: a balance between virulence, innate and adaptive immunity, and viral evasion. J Virol. 2006;80(19):9349–9360. doi: 10.1128/JVI.01122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Larena M, Lobigs M. Immunobiology of Japanese encephalitis virus. In: Ruzek D, editor. Flavivirus Encephalitis. InTech Open Access Publisher: Rijeka, Croatia; 2011. pp. 339–382. [Google Scholar]
- 58.Samuel MA, Diamond MS. α/β interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. J Virol. 2005;79(21):13350–13361. doi: 10.1128/JVI.79.21.13350-13361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Diamond MS, Shrestha B, Marri A, Mahan D, Engle M. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J Virol. 2003;77(4):2578–2586. doi: 10.1128/JVI.77.4.2578-2586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Larena M, Regner M, Lee E, Lobigs M. Pivotal role of antibody and subsidiary contribution of CD8+ T cells to recovery from infection in a murine model of Japanese encephalitis. J Virol. 2011;85(11):5446–5455. doi: 10.1128/JVI.02611-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sitati EM, Diamond MS. CD4+ T-cell responses are required for clearance of West Nile virus from the central nervous system. J Virol. 2006;80(24):12060–12069. doi: 10.1128/JVI.01650-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diamond MS, Sitati EM, Friend LD, Higgs S, Shrestha B, Engle M. A critical role for induced IgM in the protection against West Nile virus infection. J Exp Med. 2003;198(12):1853–1862. doi: 10.1084/jem.20031223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shrestha B, Diamond MS. Role of CD8+ T cells in control of West Nile virus infection. J Virol. 2004;78(15):8312–8321. doi: 10.1128/JVI.78.15.8312-8321.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shrestha B, Samuel MA, Diamond MS. CD8+ T cells require perforin to clear West Nile virus from infected neurons. J Virol. 2006;80(1):119–129. doi: 10.1128/JVI.80.1.119-129.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brien JD, Uhrlaub JL, Nikolich-Zugich J. Protective capacity and epitope specificity of CD8+ T cells responding to lethal West Nile virus infection. Eur J Immunol. 2007;37(7):1855–1863. doi: 10.1002/eji.200737196. [DOI] [PubMed] [Google Scholar]
- 66.Brien JD, Uhrlaub JL, Nikolich-Zugich J. West Nile virus-specific CD4 T cells exhibit direct antiviral cytokine secretion and cytotoxicity and are sufficient for antiviral protection. J Immunol. 2008;181(12):8568–8575. doi: 10.4049/jimmunol.181.12.8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Purtha WE, Myers N, Mitaksov V, et al. Antigen-specific cytotoxic T lymphocytes protect against lethal West Nile virus encephalitis. Eur J Immunol. 2007;37(7):1845–1854. doi: 10.1002/eji.200737192. [DOI] [PubMed] [Google Scholar]
- 68.Licon Luna RM, Lee E, Müllbacher A, Blanden RV, Langman R, Lobigs M. Lack of both Fas ligand and perforin protects from flavivirus-mediated encephalitis in mice. J Virol. 2002;76:3202–3211. doi: 10.1128/JVI.76.7.3202-3211.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Konishi E, Ajiro N, Nukuzuma C, Mason PW, Kurane I. Comparison of protective efficacies of plasmid DNAs encoding Japanese encephalitis virus proteins that induce neutralizing antibody or cytotoxic T lymphocytes in mice. Vaccine. 2003;21(25–26):3675–3683. doi: 10.1016/s0264-410x(03)00382-7. [DOI] [PubMed] [Google Scholar]
- 70•.Pan CH, Chen HW, Huang HW, Tao MH. Protective mechanisms induced by Japanese encephalitis virus DNA vaccine: requirement for antibody but not CD8+ cytotoxic T-cell responses. J Virol. 2001;75:11457–11463. doi: 10.1128/JVI.75.23.11457-11463.2001. Important contribution to the understanding of immunological correlates of protection against JE virus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shrestha B, Ng T, Chu HJ, Noll M, Diamond MS. The relative contribution of antibody and CD8+ T cells to vaccine immunity against West Nile encephalitis virus. Vaccine. 2008;26(16):2020–2033. doi: 10.1016/j.vaccine.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim S, Li L, McMurtrey CP, et al. Single-chain HLA-A2 MHC trimers that incorporate an immundominant peptide elicit protective T cell immunity against lethal West Nile virus infection. J Immunol. 2010;184(8):4423–4430. doi: 10.4049/jimmunol.0903955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Monath TP, Liu J, Kanesa-Thasan N, et al. A live, attenuated recombinant West Nile virus vaccine. Proc Natl Acad Sci USA. 2006;103(17):6694–6699. doi: 10.1073/pnas.0601932103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith HL, Monath TP, Pazoles P, et al. Development of antigen-specific memory CD8+ T cells following live-attenuated chimeric West Nile virus vaccination. J Infect Dis. 2011;203(4):513–522. doi: 10.1093/infdis/jiq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Y, Corver J, Chipman PR, et al. Structures of immature flavivirus particles. EMBO J. 2003;22(11):2604–2613. doi: 10.1093/emboj/cdg270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuhn RJ, Zhang W, Rossmann MG, et al. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell. 2002;108(5):717–725. doi: 10.1016/s0092-8674(02)00660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li L, Lok SM, Yu IM, et al. The flavivirus precursor membrane-envelope protein complex: structure and maturation. Science. 2008;319(5871):1830–1834. doi: 10.1126/science.1153263. [DOI] [PubMed] [Google Scholar]
- 78.Yu IM, Zhang W, Holdaway HA, et al. Structure of the immature dengue virus at low pH primes proteolytic maturation. Science. 2008;319(5871):1834–1837. doi: 10.1126/science.1153264. [DOI] [PubMed] [Google Scholar]
- 79.Junjhon J, Edwards TJ, Utaipat U, et al. Influence of pr-M cleavage on the heterogeneity of extracellular dengue virus particles. J Virol. 2010;84(16):8353–8358. doi: 10.1128/JVI.00696-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nelson S, Jost CA, Xu Q, et al. Maturation of West Nile virus modulates sensitivity to antibody-mediated neutralization. PLoS Pathog. 2008;4(5):e1000060. doi: 10.1371/journal.ppat.1000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mukhopadhyay S, Kuhn RJ, Rossmann MG. A structural perspective of the flavivirus life cycle. Nat Rev Microbiol. 2005;3(1):13–22. doi: 10.1038/nrmicro1067. [DOI] [PubMed] [Google Scholar]
- 82.Pierson TC, Diamond MS. Molecular mechanisms of antibody-mediated neutralisation of flavivirus infection. Expert Rev Mol Med. 2008;10(12):e12. doi: 10.1017/S1462399408000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bressanelli S, Stiasny K, Allison SL, et al. Structure of a flavivirus envelope glycoprotein in its low-pH-induced membrane fusion conformation. EMBO J. 2004;23(4):728–738. doi: 10.1038/sj.emboj.7600064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Modis Y, Ogata S, Clements D, Harrison SC. Structure of the dengue virus envelope protein after membrane fusion. Nature. 2004;427(6972):313–319. doi: 10.1038/nature02165. [DOI] [PubMed] [Google Scholar]
- 85.Bhardwaj S, Holbrook M, Shope RE, Barrett AD, Watowich SJ. Biophysical characterization and vector-specific antagonist activity of domain III of the tick-borne flavivirus envelope protein. J Virol. 2001;75(8):4002–4007. doi: 10.1128/JVI.75.8.4002-4007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chu JJ, Rajamanonmani R, Li J, Bhuvanakantham R, Lescar J, Ng ML. Inhibition of West Nile virus entry by using a recombinant domain III from the envelope glycoprotein. J Gen Virol. 2005;86(Pt 2):405–412. doi: 10.1099/vir.0.80411-0. [DOI] [PubMed] [Google Scholar]
- 87.Lee E, Lobigs M. Substitutions at the putative receptor-binding site of an encephalitic flavivirus alter virulence and host cell tropism and reveal a role for glycosaminoglycans in entry. J Virol. 2000;74(19):8867–8875. doi: 10.1128/jvi.74.19.8867-8875.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beasley DW, Barrett AD. Identification of neutralizing epitopes within structural domain III of the West Nile virus envelope protein. J Virol. 2002;76(24):13097–13100. doi: 10.1128/JVI.76.24.13097-13100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oliphant T, Engle M, Nybakken GE, et al. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat Med. 2005;11(5):522–530. doi: 10.1038/nm1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sanchez MD, Pierson TC, Mcallister D, et al. Characterization of neutralizing antibodies to West Nile virus. Virology. 2005;336(1):70–82. doi: 10.1016/j.virol.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 91.Nybakken GE, Oliphant T, Johnson S, Burke S, Diamond MS, Fremont DH. Structural basis of West Nile virus neutralization by a therapeutic antibody. Nature. 2005;437(7059):764–769. doi: 10.1038/nature03956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oliphant T, Nybakken GE, Austin SK, et al. Induction of epitope-specific neutralizing antibodies against West Nile virus. J Virol. 2007;81(21):11828–11839. doi: 10.1128/JVI.00643-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Throsby M, Geuijen C, Goudsmit J, et al. Isolation and characterization of human monoclonal antibodies from individuals infected with West Nile Virus. J Virol. 2006;80(14):6982–6992. doi: 10.1128/JVI.00551-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94•.Vogt MR, Dowd KA, Engle M, et al. Poorly neutralizing cross-reactive antibodies against the fusion loop of West Nile virus envelope protein protect in vivo via Fc-γ receptor and complement-dependent effector mechanisms. J Virol. 2011;85(22):11567–11580. doi: 10.1128/JVI.05859-11. First study to show protective mechanism in vivo of poorly neutralizing Domain II-fusion loop antibodies against West Nile virus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heinz FX, Holzmann H, Essl A, Kundi M. Field effectiveness of vaccination against tick-borne encephalitis. Vaccine. 2007;25(43):7559–7567. doi: 10.1016/j.vaccine.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 96.Monath TP, Nichols R, Archambault WT, et al. Comparative safety and immunogenicity of two yellow fever 17D vaccines (ARILVAX and YF-VAX) in a Phase 3 multicenter, double-blind clinical trial. Am J Trop Med Hyg. 2002;66(5):533–541. doi: 10.4269/ajtmh.2002.66.533. [DOI] [PubMed] [Google Scholar]
- 97.Van Gessel Y, Klade CS, Putnak R, et al. Correlation of protection against Japanese encephalitis virus and JE vaccine (IXIARO(R)) induced neutralizing antibody titers. Vaccine. 2011;29(35):5925–5931. doi: 10.1016/j.vaccine.2011.06.062. [DOI] [PubMed] [Google Scholar]
- 98.Blaney JE, Jr, Matro JM, Murphy BR, Whitehead SS. Recombinant, live-attenuated tetravalent dengue virus vaccine formulations induce a balanced, broad, and protective neutralizing antibody response against each of the four serotypes in rhesus monkeys. J Virol. 2005;79(9):5516–5528. doi: 10.1128/JVI.79.9.5516-5528.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schlesinger JJ, Foltzer M, Chapman S. The Fc portion of antibody to yellow fever virus NS1 is a determinant of protection against YF encephalitis in mice. Virology. 1993;192:132–141. doi: 10.1006/viro.1993.1015. [DOI] [PubMed] [Google Scholar]
- 100.Mehlhop E, Nelson S, Jost CA, et al. Complement protein C1q reduces the stoichiometric threshold for antibody-mediated neutralization of West Nile virus. Cell Host Microbe. 2009;6(4):381–391. doi: 10.1016/j.chom.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hombach J, Solomon T, Kurane I, Jacobson J, Wood D. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2–3 September, 2004. Vaccine. 2005;23(45):5205–5211. doi: 10.1016/j.vaccine.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 102.Dowd KA, Pierson TC. Antibody-mediated neutralization of flaviviruses: a reductionist view. Virology. 2011;411(2):306–315. doi: 10.1016/j.virol.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310(5753):1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 104••.Mehlhop E, Ansarah-Sobrinho C, Johnson S, et al. Complement protein C1q inhibits antibody-dependent enhancement of flavivirus infection in an IgG subclass-specific manner. Cell Host Microbe. 2007;2(6):417–426. doi: 10.1016/j.chom.2007.09.015. Demonstrates the critical role of antibody isotype in in vivo clearance of flavivirus infection by an effector mechanism involving the Fc portion of antibody. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nimmerjahn F, Ravetch JV. Fcγ receptors as regulators of immune responses. Nat Rev Immunol. 2008;8(1):34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 106.Hofmeister Y, Planitzer CB, Farcet MR, et al. Human IgG subclasses: in vitro neutralization of and in vivo protection against West Nile virus. J Virol. 2011;85(4):1896–1899. doi: 10.1128/JVI.02155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Monath TP. Editorial: Jennerian vaccination against West Nile virus. Am J Trop Med Hyg. 2002;66(2):113–114. doi: 10.4269/ajtmh.2002.66.113. [DOI] [PubMed] [Google Scholar]
- 108.Halstead SB, Thomas SJ. New Japanese encephalitis vaccines: alternatives to production in mouse brain. Expert Rev Vaccines. 2011;10(3):355–364. doi: 10.1586/erv.11.7. [DOI] [PubMed] [Google Scholar]
Websites
- 201. [12 November 2011];CDC Division of Vector-Borne Diseases, West Nile virus. www.cdc.gov/ncidod/dvbid/westnile/surv&control.htm.
- 202. [12 November 2011];Intervet Schering-Plough announces PreveNile® West Nile Virus vaccine recall. www.avma.org/news/wnv_vaccine_recall.asp.
- 203. [12 November 2011];Kimberley Development Commission. www.kdc.wa.gov.au.


