Abstract
Background
Prior studies have suggested that BRCA-related epithelial ovarian cancer (EOC) conveys improved survival compared to sporadic EOC, but few studies have studied differences between BRCA genotypes. We compared characteristics and outcome by genotype in BRCA-associated EOC.
Methods
BRCA-associated EOC patients, between 01/30/1981 and 12/30/2008, were retrospectively identified through IRB-approved registry studies. Clinical characteristics, including event-free (EFS) and overall survival (OS), for BRCA1 vs. BRCA2 were examined.
Results
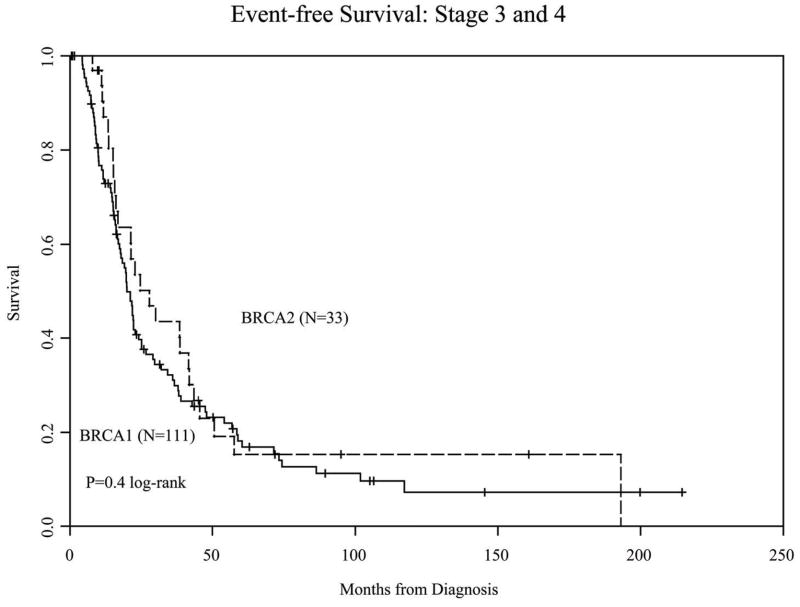
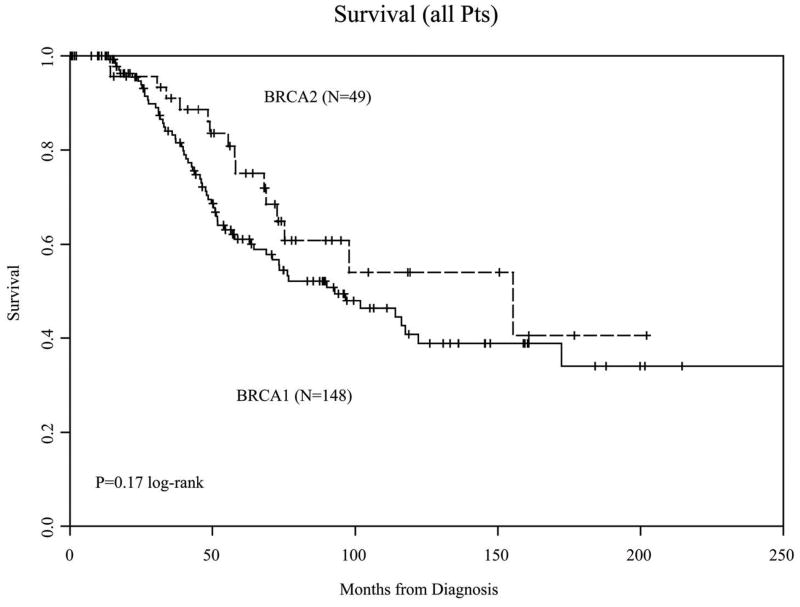
197 cases were identified (148 BRCA1; 49 BRCA2); median follow-up was 63 months. BRCA2 patients were older (55.4 vs. 51.1 years; p<0.01) and had fewer poorly-differentiated tumors (67% vs. 82%; p<0.05). No difference in EFS was observed. OS at 5-years was 75% in BRCA2 vs. 61% in BRCA1 patients; this was not statistically significant. A non-significant trend towards improved OS was observed in BRCA2 patients with advanced-stage disease (HR = 0.59, 95% CI 0.32–1.08).
Conclusions
Age and grade differed significantly between BRCA1 and BRCA2 carriers in our study population. While no overall differences in EFS or OS were observed, there was a trend towards improved OS in BRCA2 carriers with advanced-stage disease. This may reflect important differences between BRCA genotypes and should be validated in larger studies.
Keywords: BRCA1, BRCA2, Ovarian carcinoma, Genetics, Cancer
Introduction
Germline mutations in either BRCA1 or BRCA2 occur in approximately 10% of unselected women with epithelial ovarian cancer (EOC) (1). In women with an Ashkenazi Jewish background, the rate of germline mutations in BRCA1 or BRCA2, typically in one of the three “founder” mutations (185delAG and 5382insC in BRCA1; 6174delT in BRCA2), may be as high as 41% (2). The lifetime risk of developing EOC in women who carry a germline BRCA mutation can be up to 66% for BRCA1 and 27% for BRCA2 (3), with a meta-analysis of 22 studies with over 8,000 probands estimating the penetrance for ovarian cancer to be approximately 39% for BRCA1 and 11% for BRCA2 (4).
A number of studies have now investigated whether the presence of a BRCA1 or BRCA2 germline mutation affects the prognosis or survival rate of women with EOC. Although some studies have observed no significant difference (5, 6), several studies have now demonstrated that BRCA1 or BRCA2 germline mutation confers an improved prognosis in terms of overall survival compared to sporadic disease (7–15). As both BRCA1 and BRCA2 are known to play roles in the repair of DNA by homologous recombination, one possibility is that the improved survival observed in BRCA-deficient EOC is secondary to increased sensitivity to platinum-based therapy, since homologous recombination is critical to the repair of DNA-damage caused by the cross-linking effects of platinum. In support of this theory, in a retrospective case control study, Tan, et al., found higher rates of response to first-line platinum-based therapy and continued platinum sensitivity at the time of subsequent recurrences in BRCA mutation carriers compared to sporadic EOC patients (14).
Although BRCA1 and BRCA2 both play critical roles in the repair of double-strand DNA breaks through homologous recombination, they also have non-overlapping functions. Differences in the development and biology of cancers occurring in BRCA1 compared to BRCA2 germline mutation carriers have been well-documented. The overall penetrance of BRCA2-related cancers appears to be lower than that of BRCA1 (4), and BRCA2 carriers who develop EOC appear to do so at a later age (1). Sixty percent to 80% of BRCA1-associated breast cancers are triple-negative or basal-like, while 80% of BRCA2- associated breast cancers are estrogen and progesterone positive, luminal A tumors (16). No specific differences in histological subtypes have been characterized between BRCA1- or 2-associated EOC (5, 15, 17, 18). However, the clinical differences in the presentation of cancers between BRCA1 and BRCA2 carriers raises the possibility that additional differences may be present in terms of natural history and overall prognosis.
Few studies have addressed potential differences in outcomes between BRCA1- and BRCA2-associated EOC. Data from a limited number of studies that compare BRCA1- and BRCA2-associated EOC separately with sporadic EOC suggest a trend towards improved outcome in BRCA2 mutation carriers (11, 13, 15, 18). In this study, we therefore asked whether clinical characteristics or outcomes, including event-free survival and overall survival, differed between BRCA1- and BRCA2-associated EOC in a collection of BRCA mutation carriers.
Patients and Methods
Patient Identification
One hundred and ninety-seven patients with BRCA-associated invasive epithelial ovarian cancer (EOC), diagnosed between 01/30/1981 and 12/30/2008, were identified through registries of BRCA mutation carriers (City of Hope, Duarte, CA; Dana-Farber Cancer Institute, Boston, MA/Beth Israel Deaconess Medical Center, Boston, MA; and University of California Irvine, Irvine, CA/the University of Utah, Salt Lake City, UT) under IRB-approved protocols, and informed consent was obtained per institutional IRB guidelines. The study was restricted to women with a demonstrated deleterious BRCA1 or BRCA2 mutation on standard sequencing. Most women in the registries were identified by genetic testing following a diagnosis of ovarian or breast cancer and subsequent referral for clinical cancer genetics consultation. A limited number of registry participants were initially identified after a prior family member had been identified as a known mutation carrier.
Clinical Characteristics
We abstracted clinical data and pathological information, including age at diagnosis, BRCA genotype, date of primary surgery, surgical stage, grade, histologic cell type, and presumed site of primary tumor, from chart review of patient records. Stage was confirmed by review of operative and pathology reports. Treatment history, event-free survival (EFS) following initial chemotherapy, and clinical status at last follow-up were also obtained from clinic and hospital records. Overall survival data (OS) was obtained from the National Death Index, medical records, or family member report. If the report was from a family member, a search for an obituary or the National Death Index was utilized to confirm the date of death.
Statistical Analysis
The primary objectives of the study were to retrospectively evaluate the clinical features of ovarian cancer in BRCA1 and BRCA2 mutation carriers and to compare these features as well as EFS and OS between the two groups. We evaluated for statistical differences in clinical characteristics between the two groups using exact tests for categorical classifications (site, histology, differentiation, stage). Differences in age were evaluated by both Wilcoxon test (for the median) and t-test (for the mean). EFS was defined as the time between ovarian cancer diagnosis and onset of first recurrence, death, or another significant event. Differences in EFS and OS for BRCA1 vs. BRCA2 mutation were examined for statistical significance using the log-rank test in the Kaplan-Meier analysis. Multivariable survival analysis was conducted using Cox regression. Since a primary goal of the study was to evaluate survival differences between BRCA1 and BRCA2 mutation carriers, this variable was included in all of the models. Additional variables were included in the model if they were statistically significant (forward stepwise regression), or already known to be independent predictors of outcomes (age, stage, and grade). As a result, the final multivariable analysis only included these latter factors. Significance was evaluated by the Wald-test. Center effects were evaluated for their influence on the results of the BRCA1 vs. BRCA2 comparison by including them as stratification factors in the multivariable analysis, and evaluated for their influence on survival by including them as independent variables in the model. Center effects were negligible in both cases (data not shown), and the results presented are from the unstratified analysis.
Results
Patient and Tumor Characteristics
We identified a total of 197 cases of invasive EOC in this retrospective series, which included 148 patients carrying a pathogenic mutation in BRCA1 and 49 patients with a pathogenic mutation in BRCA2. Clinical characteristics of patients in these two groups are reported in Table 1. Histology was unclassified or unknown in 9 of 197 cases (<5%); 13 of 197 cases (<7%) had an unknown primary site; and 1 of 197 (<1%) had a missing stage. The median age at presentation was significantly higher for the subgroup of patients with a BRCA2 mutation (55.4 years for BRCA2 vs. 51.1 for BRCA1, p< 0.01). No significant difference was noted in the proportion of patients with advanced-stage EOC, defined as stage 3 or 4 (75% for BRCA1 vs. 67.3% for BRCA2), but fewer patients with poorly differentiated or high-grade tumors were identified in women with a BRCA2 mutation (67% vs. 82% for BRCA2 vs. BRCA1, p< 0.05).
Table 1.
Patient Characteristics
| BRCA1 | BRCA2 | |
|---|---|---|
| Number of patients | 148 | 49 |
| Median age at diagnosis (p <0.01) | 51.1 (30.5–81.5) | 55.4 (40.3–79.1) |
| Poorly-differentiated or high-grade (p <0.05) | 82% (122/148) | 67% (33/49) |
| Stage | ||
| 1 | 20 (14%) | 6 (12%) |
| 2 | 16 (11%) | 10 (20%) |
| 3 | 96 (65%) | 31 (63%) |
| 4 | 15 (10%) | 2 (4%) |
| Unknown | 1 | 0 |
| Histology | ||
| Serous | 108 (73%) | 33 (67%) |
| Endometrioid | 14 (9%) | 6 (12%) |
| Adenocarcinoma | 6 (4%) | 2 (4%) |
| Clear cell | 3 (2%) | 2 (4%) |
| Mucinous | 2 (1%) | 0 |
| Mixed serous/mucinous | 1 (1%) | 0 |
| Unclassified/unknown | 5 (3%) | 4 (8%) |
| Transitional | 1 (1%) | 0 |
| Undifferentiated | 8 (5%) | 2 (4%) |
| Primary Site* | ||
| Ovary | 123 (83%) | 36 (73%) |
| Fallopian | 4 (3%) | 5 (10%) |
| Ovary/Fallopian | 2 (1%) | 2 (4%) |
| Primary peritoneal | 11 (7%) | 1 (2%) |
| Unknown | 8 (5%) | 5 (10%) |
A statistically significant difference was observed in the profile of the sites of disease (exact test, p<0.05).
Patients in both groups received similar ovarian cancer treatment, typically including primary cytoreductive surgery followed by chemotherapy. Most patients received post-operative combination chemotherapy with a platinum/paclitaxel regimen (72.5%). Other observed regimens of chemotherapy included platinum/cyclophosphamide (10.6%), carboplatin/docetaxel (0.5%), carboplatin/liposomal doxorubicin (0.5%), or unknown (15.9%).
Event-Free and Overall Survival
The median follow-up of live patients from diagnosis of the primary tumors was 63 months (range <1 month to 309 months); only 10 patients of the 197 patients were followed for less than 1 year. No significant difference in EFS was observed between the study groups (Figure 1A); this was also true when only patients with advanced stage at diagnosis (n=144) were included in the analysis (Figure 1B). Multivariable analysis with Cox regression, adjusting for age and poor differentiation or high grade, also did not reveal any significant difference in the hazard ratio for an event between the two groups in either the whole population or patients with advanced-stage tumors only (Table 2).
Figure 1.
Figure 1A. Event-free survival in all patients
Figure 1B. Event-free survival in advanced-stage patients
Table 2.
Survival Analysis
| Event-free HR (95% CI) | Overall survival HR (95% CI) | |
|---|---|---|
| BRCA2 vs. BRCA1 (all stages; N=197) | 0.84 (0.57–1.24) | 0.68 (0.40–1.19) |
| Adjusted for age | 0.82 (0.55–1.23) | 0.60 (0.34–1.08) |
| Adjusted for age and stage | 0.92 (0.61–1.39) | 0.64 (0.35–1.17) |
| Adjusted for age, stage, and grade | 0.95 (0.62–1.45) | 0.68 (0.37–1.25) |
| BRCA2 vs. BRCA1 (advanced stage; N=144) | 0.83 (0.53–1.28) | 0.59 (0.32–1.08) |
| Adjusted for age | 0.88 (0.55–1.41) | 0.53 (0.28–1.03); p = 0.06 |
| Adjusted for age and stage* | 0.97 (0.60–1.56) | 0.57 (0.30–1.12) |
| Adjusted for age, stage, and grade* | 1.03 (0.64–1.68) | 0.62 (0.31–1.23) |
Stage adjusted for stage 3 vs 4
OS at 5 years in the 49 BRCA2 mutation carriers was 75% (95% CI 63–90) vs. 61% (95% CI 53–71) for the 148 BRCA1 carriers (Figure 2A). When only patients with advanced-stage disease at diagnosis were included in the analysis, there was a trend towards improved outcome in BRCA2-associated cancers (Figure 2B, p = 0.08).
Figure 2.
Figure 2A. Overall survival in all patients
Figure 2B. Overall survival in advanced-stage patients
Discussion
In this study, we sought to examine whether characteristics or outcomes differed between patients with EOC who harbor BRCA1 or BRCA2 germline mutations. Consistent with prior observations, we observed that the median age at diagnosis was younger for BRCA1 carriers than for BRCA2 carriers. However, few trials have further characterized potential differences between EOC occurring in BRCA and BRCA2 mutation carriers. Although no significant differences in stage or histology were observed, we observed that a higher proportion of BRCA1 mutation carriers in our study had poorly-differentiated or high-grade tumors, as compared with BRCA2 carriers. We observed a non-significant trend towards an improved OS for BRCA2 patients with advanced-stage disease.
Only one prior study has sought to directly compare BRCA1- and BRCA2-associated EOC (15). In this study, which included 99 BRCA1 carriers, 13 BRCA2 carriers, and 222 sporadic EOC patients, Vencken, et al., observed no significant differences between the two groups in clinical characteristics, including grade, stage, and histology. Of note, the study had significantly fewer patients in the BRCA2 carrier group (N=13), which may have affected the power to observe differences between these groups. Our findings regarding OS outcomes parallel those observed in the Vencken study, where a non-significant trend towards improved OS was observed in BRCA2 patients compared to BRCA1 patients with EOC. Additionally, the overall five-year survival rates of 60% and 85% in BRCA1 and BRCA2 patients, respectively, is consistent with our results of 61% in the BRCA1 and 75% in the BRCA2 populations. Interestingly, Vencken, et al., observed a trend for longer progression-free survival in the BRCA2 group in comparison to BRCA1 patients (5.6 vs. 2.1 years, respectively, p = 0.05), with 5-year progression-free survival remaining high with BRCA2-associated tumors (54%) as compared to 28% in BRCA1-linked cancers. In contrast, in our study, we did not note a significant difference in EFS between these populations, but this could be related to measurement error. As noted above, these differences may be, in part, secondary to the small number of BRCA2 patients in the Vencken study, where single-patient outcomes may therefore significantly skew the observed results.
Certain limitations are present in our study. Although data across four separate cancer centers were included, the overall number of patients incorporated in the analysis remains limited, given the limited eligibility criteria. By concentrating on clinical outcomes in the advanced-stage patient group, where virtually all patients received standard adjuvant chemotherapy, we sought to eliminate variability and focus on genotype and chemosensitivity. Within this population we observed a trend towards improved outcome in OS for BRCA2 patients as compared to BRCA1. The inclusion of patients treated across several decades introduces the issues of changes in treatments over this time period, including the incorporation of taxanes as a standard of care therapy in the late 1990s and the introduction of intraperitoneal chemotherapy as a standard adjuvant regimen in 2006. Additionally, we were unable to further explore whether specific chemotherapies or mutations contributed to the potential differences in survival. The retrospective nature of the study also introduces the possibility of survival bias, such that patients who had longer survivals were more likely to be screened and enrolled in BRCA mutation carrier registries, while patients with limited response to chemotherapy or shorter survival might be less likely to be captured in our registries. The high 5-year survival rates observed in our population support the likelihood that survival bias is present; however, it is not clear that it would differentially affect the BRCA1 and BRCA2 populations. Furthermore, we were also not able to evaluate potential sources of referral bias between BRCA1 and BRCA2 mutation carriers that might occur due to prevalence in the community or other unknown factors, and this is also a limitation of our study.
The higher proportion of high-grade cancers and the trend towards an improved OS outcome in BRCA2 mutation carriers that we observed in our study population may suggest that BRCA1 and BRCA2-deficient EOC do represent different clinical entities. Most prior studies encompass only small numbers of patients or are limited to the three Ashkenazi founder mutations; a review of the available literature demonstrates that several trials also observed a trend towards improved outcome in the BRCA2 group (Table 3).
Table 3.
Prior trials with BRCA1 and BRCA2 mutation carrier outcomes
| Study | N | Survival results | p-value (reported) | |
|---|---|---|---|---|
| Pharoah, 1999 | BRCA1 | 127 | Median survival: 20.6 mo 5-yr OS 21% (95% CI 14–28) |
NS |
| BRCA2 | 24 | Median survival: 16.0 mo 5-yr OS 25% (95% CI 8–42) |
||
| Boyd, 2000† | BRCA1 | 67 | 5-yr OS ~45% | -- |
| BRCA2 | 21 | 5-yr OS ~47% | ||
| Ramus, 2001 | BRCA1 | 15 14 185 delAG 1 5382insC |
Median survival: 52 mo (95% CI 25–79) | NS |
| BRCA2 | 12 all 6174delT |
Median survival: 49 mo (95% CI 24–73) | ||
| Ben David, 2002 | BRCA1 | 171 152 185delAG 19 5382insC |
Median survival: 185delAG: 52 mo (95% CI 41–63) 5382insC: >72 mo (95% CI NA) |
NS |
| BRCA2 | 58 all 6174delT |
Median survival: 52 mo (95% CI 34-NA) | ||
| Cass, 2003‡ | BRCA1 | 22 | Disease-free interval: 40 mo | 0.2 |
| BRCA2 | 12 | Disease-free interval: 57 mo | ||
| Pal, 2007 | BRCA1 | 20 | 4-yr OS 37% | 0.355 |
| BRCA2 | 12 | 4-yr OS 83% | ||
| Chetrit, 2008‡ | BRCA1 | 203 141 185delAG 18 5382insC |
Median survival: 45.1 mo (95% CI 38.8–52.4) | 0.2 |
| BRCA2 | 54 all 6174delT |
Median survival: 52.5 mo (95% CI 38.7–82.6) | ||
| Vencken, 2011 | BRCA1 | 99 | Median survival: 5.9 yrs 5-yr OS 60% |
0.06 (for OS) |
| BRCA2 | 13 | Median survival: >10 yrs 5-yr OS 85% |
Overall survival is estimated graphically from survival curves
Survival results based on advanced-stage patients only
- Pharoah PDP, Easton DF, Stockton DL, Gayther S, Ponder BAJ, Group UFOCS. Survival in familial, BRCA1-associated, and BRCA2-associated epithelial ovarian cancer. Cancer Res 1999;59: 868–871.
- Boyd J, Sonoda Y, Federici MG, Bogomolniy F, Rhei E, Maresco DL, Saigo PE, Almadrones LA, Barakat RR, Brown CL, Chi DS, Curtin JP, Poynor EA, Hoskins WJ. Clinicopathologic features of BRCA-linked and sporadic ovarian cancer. JAMA 2000;283: 2260–2265.
- Ramus SJ, Fishman A, Pharoah PD, Yarkoni S, Altaras M, Ponder BA. Ovarian cancer survival in Ashkenazi Jewish patients with BRCA1 and BRCA2 mutations. Eur J Surg Oncol 2001;27: 278–81.
- Ben David Y, Chetrit A, Hirsh-Yechezkel G, Friedman E, Beck BD, Beller U, Ben-Baruch G, Fishman A, Levavi H, Lubin F, Menczer J, Piura B, Struewing JP, Modan B. Effect of BRCA mutations on the length of survival in epithelial ovarian tumors. J Clin Oncol 2002;20: 463–6.
- Cass I, Baldwin RL, Varkey T, Moslehi R, Narod SA, Karlan BY. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer 2003;97: 2187–95.
- Pal T, Permuth-Wey J, Kapoor R, Cantor A, Sutphen R. Improved survival in BRCA2 carriers with ovarian cancer. Fam Cancer 2007;6: 113–9.
- Chetrit A, Hirsh-Yechezkel G, Ben-David Y, Lubin F, Friedman E, Sadetzki S. Effect of BRCA1/2 mutations on long-term survival of patients with invasive ovarian cancer: The National Israeli Study of Ovarian Cancer. J Clin Oncol 2008;26: 20–25.
- Vencken PM, Kriege M, Hoogwerf D, Beugelink S, van der Burg ME, Hooning MJ, Berns EM, Jager A, Collee M, Burger CW, Seynaeve C. Chemosensitivity and outcome of BRCA1- and BRCA2-associated ovarian cancer patients after first-line chemotherapy compared with sporadic ovarian cancer patients. Ann Oncol 2011;12: 12.
BRCA1 and BRCA2 share a number of commonalities, including their roles as critical players in homologous recombination, their linkage to certain cancers, including breast and ovarian cancers, and more recent findings that PARP inhibitors, which can impair single-strand break repair by inhibition of the activity of PARP-1, have synthetic lethality in BRCA1- or BRCA2-deficient tumors (19–22). The observation of improved outcomes and increased platinum and PARP-inhibitor sensitivity in both BRCA1 and BRCA2 carriers with EOC as compared to sporadic EOC has further emphasized these commonalities. However, additional differences between the molecular functions of BRCA1 and BRCA2 may also be clinically significant.
BRCA1 is a critical player in a number of cellular functions, including DNA repair, both through homologous recombination and nucleotide-excision repair, cell-cycle-checkpoint control, protein ubiquitination, and chromatin remodeling (23–25). In contrast, while the functions of BRCA2 remain incompletely explored, its dominant role appears to be in homologous recombination and recruitment of RAD51 to double-strand breaks (23, 24). These separate functions could potentially underlie clinical differences between BRCA1 and BRCA2 mutation carriers with EOC. Of note, our study did not demonstrate a difference in EFS after initial treatment between these two groups, including in patients with advanced-stage disease, suggesting that differences in response between BRCA1 and BRCA2 mutation carriers may not manifest until later phases of the disease. As the standard of care for adjuvant therapy in patients with advanced-stage disease includes a platinum-based chemotherapy, this finding would be consistent with results from previously-reported studies that initial platinum-sensitivity is high in both BRCA1- and BRCA2-associated EOC (14, 15).
A finding that BRCA1- and BRCA2-deficient EOC are not necessarily the same clinical entity could also affect other approaches to care for these patients. Recent discoveries have prompted significant excitement regarding the role of PARP-inhibitors in the treatment of BRCA-associated EOC, with multiple studies demonstrating single-agent activity of these agents, even in patients with heavily-pre-treated disease (19, 20, 22, 26, 27). However, as patients have remained on these agents for prolonged periods of time, mechanisms of resistance have also emerged. Recent studies have described the occurrence of a secondary mutation that restores the reading frame and production of a functional BRCA protein in both BRCA1- and BRCA2-deficient cells as a mechanism of resistance to both platinum and PARP-inhibitors (28–31). While these secondary mutations may occur in both BRCA1 and BRCA2 carriers, other putative mechanisms of platinum and PARP-inhibitor resistance may not be shared between these two groups. For example, recent reports have suggested that an alternative route of platinum-inhibitor resistance in BRCA1-deficient cancers is mediated through loss of the DNA-damage repair protein 53BP1 (32, 33). Because 53BP1 is active in earlier chromatin remodeling events at the time of double-strand breaks, it has a more significant effect on BRCA1-dependent functions. Accordingly, similar effects of 53BP1 loss were not demonstrated in BRCA2-deficient cells (33). These findings also support the notion that BRCA1 and BRCA2 have additional functions outside of their shared roles in double-strand break DNA repair which may have implications for novel targeted therapies, both in terms of response and resistance.
Finally, differences in BRCA1- and BRCA2-deficient EOC might also have broader implications in the approach to sporadic EOC. A phenotype of “BRCAness,” where dysfunction of BRCA1 or BRCA2 is observed in the absence of a germline BRCA mutation, recently has been discussed with regards to sporadic breast and ovarian cancers (34, 35). This dysfunction may be mediated in several different ways. For example, aberrant methylation of the CpG-rich regulatory regions of BRCA1 can result in silencing of gene expression and resultant loss of BRCA1 activity; such hypermethylation has been observed in 5 to 31% of sporadic EOC (34). A breast cancer cell line with dense BRCA1 CpG island hypermethylation and consequent loss of BRCA1 protein expression has similar sensitivity to PARP-inhibitors when compared to a BRCA-mutant cell line (36). Similarly, an inhibitor of BRCA2, EMSY, has been observed to be amplified in up to 17% of cases of sporadic EOC(37) and may, therefore, convey a phenotype similar to BRCA2 loss. Konstantinopoulos, et al., recently identified a gene expression profile of “BRCAness” that correlated with chemotherapy outcomes in 70 patients with sporadic EOC (38); and Gelmon, et al., presented a report of activity of the PARP-inhibitor olaparib in patients with sporadic EOC (27), findings which are consistent with theory that sporadic EOC may have defects in BRCA1 or BRCA2 function or other members of the homologous recombination pathway that can convey a BRCA-like phenotype. If, however, BRCA1 and BRCA2 deficiencies do result in differing clinical outcomes, sensitivities to chemotherapy, and mechanisms of platinum- and PARP-inhibitor-resistance, these considerations may need to be incorporated in further explorations of the “BRCAness” phenotype.
In our study, we observed that BRCA1 patients were more likely to have high-grade or poorly-differentiated EOC, and a trend towards improved OS was observed in BRCA2 patients with advanced-stage disease. As a number of potential confounding factors were present, the power of our study to detect a true difference in OS in these populations was limited, given the number of patients for whom data were available. Nonetheless, the finding that outcomes differ between BRCA1 and BRCA2 carriers with EOC could significantly inform the approach to developing therapies both in BRCA mutation carriers and in sporadic EOC. A recent larger study of outcomes in BRCA1, BRCA2, and sporadic EOC patients was presented at the American Association of Cancer Research (AACR) conference in 2011 and strongly suggests that the trend observed in our study is accurate, with a hazard ratio of 0.69 for survival in BRCA2 carriers compared to BRCA1 carriers after adjustment for age at diagnosis (p = 5 × 10−4) (39). This is similar to the point estimate of the hazard ratio we observed, allowing for the limitations in power in our study given the smaller sample size. A post-hoc power analysis demonstrated that more than 3 times the number of events observed in our study (n=80 out of 197) would have been required to achieve an 80% power to observe this point estimate. Additional studies have been published exploring the potential difference in survival outcomes between BRCA1 and BRCA2 mutation carriers. Two papers have sought to analyze data from The Cancer Genome Atlas (TCGA) project with regards to BRCA alterations and effects on survival (40, 41). Of note, a limited number of cancers with BRCA1 and BRCA2 mutations are available in this data set, with a total of 37 BRCA1 mutations (27 germline, 10 somatic) and 29 BRCA2 mutations (20 germline, 9 somatic). Additionally, 34 patients with epigenetically silenced BRCA1 were identified. In both studies, patients with epigenetically-silenced BRCA1 did not have an improved survival compared to patients with no alterations in BRCA. The TCGA study demonstrated improved survival in BRCA1/2 mutated cancers compared to wild-type, but did not further compare the outcomes of BRCA1 vs. BRCA2 loss. In the Yang study, an OS benefit was described with BRCA2-mutated cancers as compared to BRCA1-mutated cancers. Interestingly, this study demonstrated no improvement in OS in BRCA1-mutated cancers as compared to wild-type, in contrast to the study from AACR. This may be due to the small number of patients or potentially the inclusion of somatic BRCA1 and BRCA2 mutations in the analysis. The results of these studies further support the need for additional investigation in future studies regarding specific chemosensitivities and/or mutations that underlie the observed survival differences between BRCA mutation carriers in order to provide better insight into the molecular mechanisms of BRCA-mediated features of EOC and guide clinical approaches to care.
Acknowledgments
Financial Support:
This research described was supported in part by the Markel Foundation; by Award Number RC4CA153828 (to JNW) from the National Cancer Institute (the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health); by the Dana Farber/Harvard Cancer Center SPORE in Breast Cancer (to JEG); and by the Friends of Dana Farber (to JL). Support was also received from Award Number R01CA74415 (to SLN) from the National Institutes of Health, and from the Morris and Horowitz Families Endowed Professorship (to SLN).
We wish to thank Geraldine Connie, Leticia Najera, and Lacolle Robinson for assistance with data acquisition, and Tracy Sulkin at City of Hope for assistance with manuscript preparation.
Footnotes
Details of Ethics Approval:
The study was approved by the Investigational Review Board at all four participating cancer centers.
Disclosure Statement: The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Risch HA, McLaughlin JR, Cole DEC, et al. Prevalence and Penetrance of Germline BRCA1 and BRCA2 Mutations in a Population Series of 649 Women with Ovarian Cancer. Am J Hum Genet. 2001;68:700–710. doi: 10.1086/318787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moslehi R, Chu W, Karlan B, et al. BRCA1 and BRCA2 mutation analysis of 208 Ashkenazi Jewish women with ovarian cancer. Am J Hum Genet. 2000;66:1259–1272. doi: 10.1086/302853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robles-Diaz L, Goldfrank DJ, Kauff ND, et al. Hereditary ovarian cancer in Ashkenazi Jews. Fam Cancer. 2004;3:259–264. doi: 10.1007/s10689-004-9552-0. [DOI] [PubMed] [Google Scholar]
- 4.Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pharoah PDP, Easton DF, Stockton DL, et al. Survival in familial, BRCA1-associated, and BRCA2-associated epithelial ovarian cancer. Cancer Res. 1999;59:868–871. [PubMed] [Google Scholar]
- 6.Johannsson OT, Ranstam J, Borg A, et al. Survival of BRCA1 Breast and Ovarian Cancer Patients: A Population-Based Study from Southern Sweden. J Clin Oncol. 1998;16:397–404. doi: 10.1200/JCO.1998.16.2.397. [DOI] [PubMed] [Google Scholar]
- 7.Rubin SC, Ivor B, Kian B, et al. Clinical and Pathological Features of Ovarian Cancer in Women with Germ-Line Mutations of BRCA1. N Engl J Med. 1996;335:1413–1416. doi: 10.1056/NEJM199611073351901. [DOI] [PubMed] [Google Scholar]
- 8.Aida H, Takakuwa K, Nagata H, et al. Clinical features of ovarian cancer in Japanese women with germ-line mutations of BRCA1. Clin Cancer Res. 1998;4:235–240. [PubMed] [Google Scholar]
- 9.Boyd J, Sonoda Y, Federici MG, et al. Clinicopathologic features of BRCA-linked and sporadic ovarian cancer. JAMA. 2000;283:2260–2265. doi: 10.1001/jama.283.17.2260. [DOI] [PubMed] [Google Scholar]
- 10.Ben David Y, Chetrit A, Hirsh-Yechezkel G, et al. Effect of BRCA mutations on the length of survival in epithelial ovarian tumors. J Clin Oncol. 2002;20:463–466. doi: 10.1200/JCO.2002.20.2.463. [DOI] [PubMed] [Google Scholar]
- 11.Cass I, Baldwin RL, Varkey T, et al. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97:2187–2195. doi: 10.1002/cncr.11310. [DOI] [PubMed] [Google Scholar]
- 12.Majdak EJ, Debniak J, Milczek T, et al. Prognostic impact of BRCA1 pathogenic and BRCA1/BRCA2 unclassified variant mutations in patients with ovarian carcinoma. Cancer. 2005;104:1004–1012. doi: 10.1002/cncr.21276. [DOI] [PubMed] [Google Scholar]
- 13.Chetrit A, Hirsh-Yechezkel G, Ben-David Y, et al. Effect of BRCA1/2 Mutations on Long-Term Survival of Patients With Invasive Ovarian Cancer: The National Israeli Study of Ovarian Cancer. J Clin Oncol. 2008;26:20–25. doi: 10.1200/JCO.2007.11.6905. [DOI] [PubMed] [Google Scholar]
- 14.Tan DSP, Rothermundt C, Thomas K, et al. “BRCAness” Syndrome in Ovarian Cancer: A Case-Control Study Describing the Clinical Features and Outcome of Patients With Epithelial Ovarian Cancer Associated With BRCA1 and BRCA2 Mutations. J Clin Oncol. 2008;26:5530–5536. doi: 10.1200/JCO.2008.16.1703. [DOI] [PubMed] [Google Scholar]
- 15.Vencken PM, Kriege M, Hoogwerf D, et al. Chemosensitivity and outcome of BRCA1- and BRCA2-associated ovarian cancer patients after first-line chemotherapy compared with sporadic ovarian cancer patients. Ann Oncol. 2011;12:12. doi: 10.1093/annonc/mdq628. [DOI] [PubMed] [Google Scholar]
- 16.Foulkes WD, Metcalfe K, Sun P, et al. Estrogen receptor status in BRCA1- and BRCA2-related breast cancer: the influence of age, grade, and histological type. Clin Cancer Res. 2004;10:2029–2034. doi: 10.1158/1078-0432.ccr-03-1061. [DOI] [PubMed] [Google Scholar]
- 17.Ramus SJ, Fishman A, Pharoah PD, et al. Ovarian cancer survival in Ashkenazi Jewish patients with BRCA1 and BRCA2 mutations. Eur J Surg Oncol. 2001;27:278–281. doi: 10.1053/ejso.2000.1097. [DOI] [PubMed] [Google Scholar]
- 18.Pal T, Permuth-Wey J, Kapoor R, et al. Improved survival in BRCA2 carriers with ovarian cancer. Fam Cancer. 2007;6:113–119. doi: 10.1007/s10689-006-9112-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fong PC, Boss DS, Yap TA, et al. Inhibition of Poly(ADP-Ribose) Polymerase in Tumors from BRCA Mutation Carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 20.Fong PC, Yap TA, Boss DS, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010;28:2512–2519. doi: 10.1200/JCO.2009.26.9589. [DOI] [PubMed] [Google Scholar]
- 21.Tutt A, Robson M, Garber JE, et al. Oral poly (ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 22.Audeh MW, Carmichael J, Penson RT, et al. Oral poly (ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376:245–251. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 23.Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4:665–676. doi: 10.1038/nrc1431. [DOI] [PubMed] [Google Scholar]
- 24.O’Donovan PJ, Livingston DM. BRCA1 and BRCA2: breast/ovarian cancer susceptibility gene products and participants in DNA double-strand break repair. Carcinogenesis. 2010;31:961–967. doi: 10.1093/carcin/bgq069. [DOI] [PubMed] [Google Scholar]
- 25.Quinn JE, Carser JE, James CR, et al. BRCA1 and implications for response to chemotherapy in ovarian cancer. Gynecol Oncol. 2009;113:134–142. doi: 10.1016/j.ygyno.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Sandhu SK, Wenham RM, Wilding G, et al. First-in-human trial of a poly(ADP-ribose) polymerase (PARP) inhibitor MK-4827 in advanced cancer patients (pts) with antitumor activity in BRCA-deficient and sporadic ovarian cancers. ASCO Meeting Abstracts. 2010;28:3001. [Google Scholar]
- 27.Gelmon KA, Hirte HW, Robidoux A, et al. Can we define tumors that will respond to PARP inhibitors? A phase II correlative study of olaparib in advanced serous ovarian cancer and triple-negative breast cancer. ASCO Meeting Abstracts. 2010;28:3002. [Google Scholar]
- 28.Edwards SL, Brough R, Lord CJ, et al. Resistance to therapy caused by intragenic deletion in BRCA2. Nature. 2008;451:1111–1115. doi: 10.1038/nature06548. [DOI] [PubMed] [Google Scholar]
- 29.Sakai W, Swisher EM, Karlan BY, et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature. 2008;451:1116–1120. doi: 10.1038/nature06633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swisher EM, Sakai W, Karlan BY, et al. Secondary BRCA1 Mutations in BRCA1-Mutated Ovarian Carcinomas with Platinum Resistance. Cancer Res. 2008;68:2581–2586. doi: 10.1158/0008-5472.CAN-08-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakai W, Swisher EM, Jacquemont C, et al. Functional Restoration of BRCA2 Protein by Secondary BRCA2 Mutations in BRCA2-Mutated Ovarian Carcinoma. Cancer Res. 2009;69:6381–6386. doi: 10.1158/0008-5472.CAN-09-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bunting SF, Callén E, Wong N, et al. 53BP1 Inhibits Homologous Recombination in BRCA1-Deficient Cells by Blocking Resection of DNA Breaks. Cell. 2010;141:243–254. doi: 10.1016/j.cell.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouwman P, Aly A, Escandell JM, et al. 53BP1 loss rescues BRCA1 deficiency and is associated with triple-negative and BRCA-mutated breast cancers. Nat Struct Mol Biol. 2010;17:688–695. doi: 10.1038/nsmb.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turner N, Tutt A, Ashworth A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 2004;4:814–819. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 35.Bast RC, Jr, Mills GB. Personalizing Therapy for Ovarian Cancer: BRCAness and Beyond. J Clin Oncol. 2010;28:3545–3548. doi: 10.1200/JCO.2010.28.5791. [DOI] [PubMed] [Google Scholar]
- 36.Veeck J, Ropero S, Setien F, et al. BRCA1 CpG island hypermethylation predicts sensitivity to poly(adenosine diphosphate)-ribose polymerase inhibitors. J Clin Oncol. 2010;28:e563–564. doi: 10.1200/JCO.2010.30.1010. author reply e565–566. Epub 2010 Aug 2012. [DOI] [PubMed] [Google Scholar]
- 37.Brown LA, Irving J, Parker R, et al. Amplification of EMSY, a novel oncogene on 11q13, in high grade ovarian surface epithelial carcinomas. Gynecol Oncol. 2006;100:264–270. doi: 10.1016/j.ygyno.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 38.Konstantinopoulos PA, Spentzos D, Karlan BY, et al. Gene Expression Profile of BRCAness That Correlates With Responsiveness to Chemotherapy and With Outcome in Patients With Epithelial Ovarian Cancer. J Clin Oncol. 2010;28:3555–3561. doi: 10.1200/JCO.2009.27.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolton K. BRCA2 Mutations Associated with Improved Survival for Ovarian Cancer; AACR 102nd Annual Meeting 2011; Orlando, FL: AACR; 2011. [Google Scholar]
- 40.Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang D, Khan S, Sun Y, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA. 2011;306:1557–1565. doi: 10.1001/jama.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]