Abstract
Sites of ubiquitin modification have been identified by mass spectrometry based on the increase in molecular mass of a tryptic peptide carrying two additional glycine residues from the ubiquitin moiety. However, such peptides with GG shifts have been difficult to discover. We identify 870 unique sites of ubiquitin attachment on 438 different proteins of the yeast Saccharomyces cerevisiae.
Keywords: Ubiquitin, Proteomics, Mass spectrometry
Ubiquitin is a 76 amino acid protein that is covalently attached to proteins as a post-translational modification1. Because regulation by ubiquitin modification plays a role in nearly every cellular pathway 2, it is critical to identify sites of ubiquitin attachment. Sites of ubiquitin attachment have been identified by mass spectrometry. After cleavage of a ubiquitin-modifed protein by trypsin, two glycine residues from the ubiquitin moiety remain attached to the modified lysine, increasing the mass of that peptide by 114.1 daltons 3. Recent advances in GG-peptide sequencing have been made by using faster mass spectrometers and/or a monoclonal antibody specific for the GG remnant, which was used to enrich for GG-peptides from protein lysates treated with trypsin. Thousands of sites of ubiquitination have been identified in the human using such methods 4–7. A large-scale reference for ubiquitin sites identified in yeast is still a missing resource.
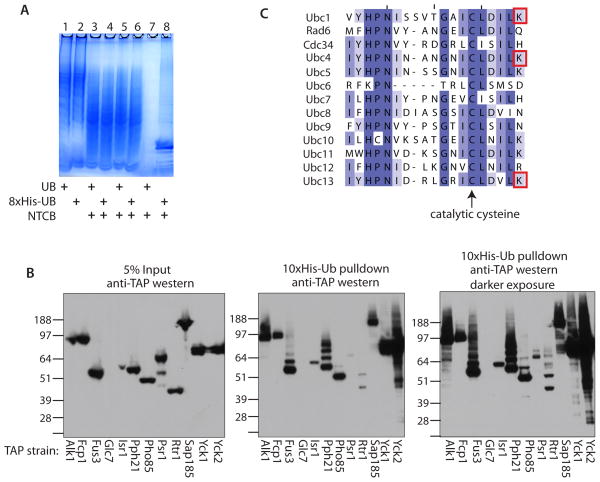
At least 1000 proteins in the yeast Saccharomyces cerevisiae and many more in human cells are be ubiquitinated at a given time 3. In the analysis of complex mixtures by tandem mass spectrometry, only the most abundant peptides are picked for fragmentation; thus, GG-peptides, in low abundance compared to unmodified peptides, are not sequenced. In theory, if the complexity of the peptide mixture were reduced, more GG-peptides could be sequenced. We attempted to reduce complexity by cleaving proteins with a chemical that does not cleave ubiquitin, such that the ubiquitin would remain intact for affinity purification. Ubiquitin does not contain any cysteine residues, allowing treatment of a protein mixture with 2-nitro-5-thiocyanobenzoic acid (NTCB), which cleaves peptide bonds at cysteines 8. Cysteine is encoded by 1.3% of yeast codons; therefore, it should appear ~6 times in an average protein of 450 amino acids. Thus, given a single site of ubiquitin attachment in a protein, cleavage by NTCB followed by digestion with trypsin could potentially enrich a tryptic GG-peptide up to 6-fold over that achieved by affinity purification alone. In practice, cleavage at cysteine prior to ubiquitin affinity purification produced only a minimal enrichment (1.5X) of sequenced GG-peptides even though the NTCB cleavage was efficient (Figure 2A and Supplementary Table 1). Therefore, we discuss the treated and untreated samples together.
Figure 2.
(A) Analysis of NTCB treatment and his-tag purifications of protein lysates from yeast strains either expressing 8xHis-ubiquitin or untagged ubiquitin by SDS-PAGE and coomassie staining. Lanes 1 and 2- whole cell lysates; lanes 3 and 4- protein fragments after treatment with NTCB; lanes 5 and 6- protein fragments post dialysis; lanes 7 and 8- metal affinity purified Ub-fragments. Yeast strains as indicated. (B) Validation of ubiquitinated protein kinases and phosphatases. The TAP strain for each of the indicated kinases and phosphatases was transformed with a 10xHis-ubiquitin expression plasmid and grown to mid-log phase. The yeast were lysed in denaturing buffer. 5% of the lysate was analyzed by anti-TAP western blot (left panel) and the 10xHis-ubiquitinated proteins were purified from the remaining lysate using metal affinity resin. The resin was boiled in sample buffer + ∀-mercaptoethanol and analyzed by anti-TAP western blot (middle panel; darker exposure in the right panel). Molecular weights are indicated. (C) The 13 yeast E2 ubiquitin conjugating enzymes aligned by ClustalW and colored according to percent identity, darker purple = higher conservation. The catalytic cysteine is highlighted with an arrow and the red boxes indicate the lysines that were identified as having a ubiquitin remnant mass shift in this study.
We cultured 2L of yeast that express only 8xHistidine-tagged ubiquitin from a constitutive promoter in rich media to mid-log phase (detailed protocols in Supplementary Material). The cells were lysed in 6 M guanidine-HCl, 1 M glycine, pH to 9.0 by bead beating. The soluble fraction was reduced with TCEP and one half was mock treated and the other treated with NTCB. The lysates were dialyzed into a buffer suitable for His-tag purification (6 M guanidine-HCl, 50 mM sodium phosphate, 300 mM NaCl, pH 8.0). His-tagged proteins were collected using cobalt resin. The concentration of guanidine was stepped down to 1.4 M guanidine during wash steps. Bound proteins were eluted twice with 0.6 ml 50 mM EDTA, 1 M imidazole, 1.4 ml 2 M guanidine-HCl, 50 mM sodium phosphate and 300 mM NaCl at pH 8.0. The eluates were dialyzed into 50 mM ammonium bicarbonate, pH 7.4 in a 20K MWCO cassette. This His-tag purification was pure as visualized by coomassie staining (Figure 2A). After dialysis their volume was reduced to 1 ml in a speed-vac. To reduce disulfide bonds, we treated the dialyzed eluates with 5 mM TCEP for 15 minutes at room temperature followed by alkylation of cysteines with 10mM chloroacetamide at room temperature in the dark 9. The reduced and alkylated samples were incubated with sequencing-grade trypsin at a ratio of protein:trypsin of 30:1 at 37°C with mild shaking for 2.5 hours. The peptides were dried to completion and stored at −20°C.
In order to segregate GG-peptides from unmodified peptides, we separated peptides by charge. Most tryptic peptides have a charge of +2 at low pH, due to the amino-terminal +1 charge and the carboxy-terminal lysine or arginine +1 charge (histidine, if present, adds +1). By contrast, GG-peptides have an extra amino-terminus due to the two glycines that remain attached after tryptic digestion, and therefore a net charge of at least +3. We performed strong cation exchange at pH 3. The trypsinized peptides were resuspended in 100 μl buffer A (10mM potassium phosphate, 25% acetonitrile, pH 3) + 0.1% formic acid. Peptides were injected and separated on a Polysulfoethyl column using an Agilent HPLC. Buffer B (10 mM potassium phosphate, 500 mM KCl, 25% acetonitrile, pH 3) was added in a gradient from 0–50% over 30 minutes at 1ml/min. We collected 1 ml fractions and pooled the first five and last five fractions. Fractions were desalted on C18 cartridges. The peptides were resuspended in 21 μl 0.1% formic acid.
Each fraction was split in half, subjected twice to LC-ESI-MS/MS using homemade 75 μm diameter fused-silica emitter tip columns packed with 25–30 cm of 5 μm C18 beads over a 90 minute linear gradient (1% acetonitrile, 0.1% formic acid to 35% acetonitrile, 0.1% formic acid) on a nano-UPLC in line with a Thermo Scientific LTQ-OrbitrapXL mass spectrometer. The parent ions were scanned in the OrbitrapXL and the 5 most intense ions per scan were fragmented by CID. The resulting fragment ions were scanned in the LTQ. Dynamic exclusion was used to exclude ions with the same m/z from being picked for fragmentation for 3 minutes.
Using the Sequest algorithm, 10, 11 we matched spectra to peptides encoded by the Saccharomyces cerevisiae genome and to those present in a concatenated scrambled decoy database. Ubiquitin remnants were identified by searching for a dynamic modification of +114.042928 daltons on lysine residues. NTCB-dependent cysteine modifications were identified by searching for a static modification of +24.995249 daltons on cysteine residues. Cysteines not modified by NTCB but alkylated by chloroacetamide were identified by searching for a dynamic modification of +32.026215 daltons. The output from Sequest was analyzed using the Trans-Proteomics Pipeline12, 13. For peptides discussed herein, the false discovery rate (FDR) as calculated by Peptide Prophet is 1%. At this cutoff, inspection of the remaining peptides showed that decoy identifications represent 0.3% of the total peptides and 2% of the GG-peptides. Peptides with a GG modification on the carboxy-terminal lysine were excluded from analysis.
In total, we identified nearly 110,000 peptides (Table 1). Most of the identified peptides had a net charge of +2 or +3. As expected, most of the sequenced GG-peptides were identified in fractions that had an average charge of ≥ +3.
Table 1.
Summary of results
| Experiment 1 | Experiment 2 | Combined | ||
|---|---|---|---|---|
| Treatment | + NTCB | Mock | Mock | |
| LTQ-Orbitrap runs | 32 | 34 | 12 | 78 |
| Total peptides | 31793 | 65023 | 12516 | 109332 |
| Total GG-peptides | 1173 | 1640 | 344 | 3157 |
| % GG-peptides | 3.7% | 2.5% | 2.7% | 2.9% |
| Unique GG-peptides | 508 | 711 | 117 | 984 |
| Total proteins identified | 2530 | 3256 | 1247 | 3491 |
| Total GG-proteins | 308 | 333 | 83 | 438 |
| % GG-proteins | 9.4% | 10.2% | 6.7% | 12.5% |
In total, we found 870 unique sites of ubiquitin attachment on 438 yeast proteins (Supplementary Table 2). Fifty percent of these sites were identified more than once, suggesting that the approach is robust. We repeated the entire protocol, and obtained a second dataset, albeit smaller in scale than the original one. However, the second dataset showed that the protocol had high reproducibility: 70% of the GG-peptides found in this set had been found in the original dataset, and 99% of the proteins in which a GG peptide was found in the replicate had been identified as having a GG peptide in the original dataset (Supplementary Figure 1). Overall, we found many previously identified sites of ubiquitination, including 40 from the data set of Peng et al. 3, the 7 lysine residues used by ubiquitin conjugating enzymes to polymerize ubiquitin 14, and the site of a regulatory ubiquitination on histone H2B 15. About 95% of the sites we found were novel, with many proteins having multiple sites of ubiquitination. As expected, the ubiquitinated proteins found in this study play a role in many cellular processes, according to their annotated GO categorizations. In accord with other studies, we were unable to identify a conserved motif surrounding the site of ubiquitin attachment 3,6. Many proteins that were identified are highly expressed, such as components of the ribosome and translation elongation factors. We did not identify many known sites of ubiquitination on short-lived proteins, such as the cell cycle regulatory protein Cln2.
There is known interplay between ubiquitin signaling and signaling by phosphorylation. We chose a set of 12 protein kinases and phosphatases that had identified ubiquitinated lysines: the kinases Alk1, Fus3, Isr1, Pho85, Yck1 and Yck2, and the phosphatases Fcp1, Glc7, Pph21, Psr1, Rtr1, and Sap185. These proteins regulate many cellular processes, including transcription, cell cycle regulation and nutrient uptake. By affinity purification and western blot, we verified that all of these proteins, except for Glc7, which was not expressed, are indeed ubiquitinated in yeast (Figure 2B). Alk1, Fus3, Pho85, Sap185, Yck1 and Yck2 were present as species representing multiple ubiquitin attachments, whereas Fcp1, Isr1, Pph21, Psr1 and Rtr1 were present as species with either only one or a few ubiquitins. Even though the yeast cultures were not treated with proteasome inhibitor prior to lysis, the ubiquitin-modified proteins were not degraded rapidly enough to escape detection. The fastest migrating bands seen in the anti-TAP western blot after His-tagged ubiquitin pulldowns (Figure 2, middle and right) co-migrated with the major bands of input TAP-tagged proteins (Figure 2, left), even though the input bands represent protein not modified by ubiquitin. Further experiments showed that ~5% of unmodified TAP-tagged proteins precipitated with the metal-affinity beads (data not shown). It is unlikely that significant ubiquitination occurred as a result of the TAP-tag, as the patterns and extents of ubiquitination varied for each protein.
Another set of proteins that we found to be ubiquitinated in yeast were the E2 ubiquitin conjugating enzymes Ubc1, Ubc4, Ubc6 and Ubc13. These ubiquitination events are of interest because Ubc1, 4 and 13 were ubiquitinated on a lysine five residues carboxy-terminal to their catalytic cysteines (Figure 2C). Ubiquitination at this site is likely to hinder the catalytic activity of the cysteine residue, and therefore of the E2 enzyme, because the lysine residue is located in the same pocket of the enzyme16–18. The catalytic domains of the E2 enzymes are conserved from yeast to man in both structure and sequence. Six of the 13 S. cerevisiae E2s and 10 of the 33 human E2s have a lysine at the +5 or +6 position after the catalytic cysteine. Ubiquitination at the cysteine +5 lysine regulates a human E2, Ube2T, causing the enzyme to no longer transfer ubiquitin to FancD2 in combination with the E3 ubiquitin ligase FancL19. Five of the 10 human E2s with a +5 lysine after the catalytic cysteine were found ubiquitinated in Kim et al. 5, including Ube2T. Taken together, these data suggest that ubiquitination at this site close to the catalytic cysteine may be a common regulatory mechanism by which E2 enzymes are regulated.
Although the enrichment of GG-peptides in this dataset due to cleavage with NTCB was not as great as anticipated, the combination of a simple SCX column and a high mass-accuracy tandem mass spectrometer resulted in the identification of a large number of ubiquitin attachment sites. Identification of these sites of ubiquitin attachment should help elucidate ubiquitin signaling in yeast.
Supplementary Material
Figure 1.
Diagram of GG-peptide enrichment strategy. (A) 8xHis-Ubiquitin is covalently attached to lysine residues of proteins. (B) Proteins are either mock treated or cleaved by addition of NTCB to the lysates. (C) Ubiquitinated proteins are purified by metal affinity resin. (D) Proteins are digested with trypsin. (E) Peptides are separated by strong cation exchange chromatography. (F) Peptides are identified by RPLC-MS/MS.
Acknowledgments
We thank Richard Gardner (University of Washington) for yeast strains. This work was supported by NIH grant P41 RR11823, NRSA fellowship F32 GM801262 to L.S., and the University of Washington’s Proteomics Resource (UWPR95794). S.F. is an investigator of the Howard Hughes Medical Institute. The authors have no competing financial interests.
Abbreviations
- Ub
ubiquitin
- UBL
ubiquitin-like protein
- TAP-tag
tandem affinity purification tag
- YEPD
yeast extract peptone dextrose growth media
- LC
liquid chromatography
- MS
mass spectrometry
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TCEP
tris(2-carboxyethyl)phosphine
- MWCO
molecular weight cut-off
- CID
collision-induced dissociation
- RPLC
reverse phase liquid chromatography
References
- 1.Pickart CM, Eddins MJ. Ubiquitin: structures, functions, mechanisms. Biochim Biophys Acta. 2004;1695:55–72. doi: 10.1016/j.bbamcr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 2.Ciechanover The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 3.Peng, et al. A proteomics approach to understanding protein ubiquitination. Nat Biotechnol. 2003;21:921–926. doi: 10.1038/nbt849. [DOI] [PubMed] [Google Scholar]
- 4.Xu G, Paige JS, Jaffrey SR. Global analysis of lysine ubiquitination by ubiquitin remnant immunoaffinity profiling. Nat Biotechnol. 2010;28:868–873. doi: 10.1038/nbt.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim W, et al. Systematic and Quantitative Assessment of the Ubiquitin-Modified Proteome. Molecular cell. 2011 doi: 10.1016/j.molcel.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danielsen JM, et al. Mass spectrometric analysis of lysine ubiquitylation reveals promiscuity at site level. Mol Cell Proteomics. 2010 doi: 10.1074/mcp.M110.003590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner SA, et al. A proteome-wide, quantitative survey of in vivo ubiquitylation sites reveals widespread regulatory roles. Mol Cell Proteomics. 2011 doi: 10.1074/mcp.M111.013284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang HY, Speicher DW. Identification of alternative products and optimization of 2-nitro-5-thiocyanatobenzoic acid cyanylation and cleavage at cysteine residues. Anal Biochem. 2004;334:48–61. doi: 10.1016/j.ab.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen ML, et al. Iodoacetamide-induced artifact mimics ubiquitination in mass spectrometry. Nature methods. 2008;5:459–460. doi: 10.1038/nmeth0608-459. [DOI] [PubMed] [Google Scholar]
- 10.Eng JK, McCormack AL, Yates JR., III An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. Journal of the American Society for Mass Spectrometry. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 11.Elias Haas W, Faherty BK, Gygi Comparative evaluation of mass spectrometry platforms used in large-scale proteomics investigations. Nat Methods. 2005;2:667–675. doi: 10.1038/nmeth785. [DOI] [PubMed] [Google Scholar]
- 12.Keller A, Eng J, Zhang N, Li XJ, Aebersold A uniform proteomics MS/MS analysis platform utilizing open XML file formats. Mol Syst Biol. 2005;1:2005.0017. doi: 10.1038/msb4100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deutsch, et al. A guided tour of the Trans-Proteomic Pipeline. Proteomics. 2010;10:1150–1159. doi: 10.1002/pmic.200900375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu P, et al. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell. 2009;137:133–145. doi: 10.1016/j.cell.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robzyk K, Recht J, Osley MA. Rad6-dependent ubiquitination of histone H2B in yeast. Science. 2000;287:501–504. doi: 10.1126/science.287.5452.501. [DOI] [PubMed] [Google Scholar]
- 16.COOK WJ, JEFFREY LC, XU YP, CHAU V. Tertiary Structures of Class-I Ubiquitin-Conjugating Enzymes Are Highly Conserved - Crystal-Structure of Yeast Ubc4. Biochemistry. 1993;32:13809–13817. doi: 10.1021/bi00213a009. [DOI] [PubMed] [Google Scholar]
- 17.Eddins MJ, Carlile CM, Gomez KM, Pickart CM, Wolberger C. Mms2-Ubc13 covalently bound to ubiquitin reveals the structural basis of linkage-specific polyubiquitin chain formation. Nature structural & molecular biology. 2006;13:915–920. doi: 10.1038/nsmb1148. [DOI] [PubMed] [Google Scholar]
- 18.Merkley N, Shaw GS. Solution structure of the flexible class II ubiquitin-conjugating enzyme Ubc1 provides insights for polyubiquitin chain assembly. The Journal of biological chemistry. 2004;279:47139–47147. doi: 10.1074/jbc.M409576200. [DOI] [PubMed] [Google Scholar]
- 19.Machida YJ, et al. UBE2T is the E2 in the Fanconi anemia pathway and undergoes negative autoregulation. Molecular cell. 2006;23:589–596. doi: 10.1016/j.molcel.2006.06.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.