Abstract
The neuropeptide alpha-melanocyte stimulating hormone (α-MSH) plays an important role in immune privilege by its suppression of inflammation, and its induction of regulatory T cells. This finding led us to test the possibility that we can use α-MSH to suppress autoimmune diseases, and promote re-establishment of immune tolerance to autoantigens. To test this possibility, SJL mice with experimental autoimmune encephalomyelitis (EAE) were injected with α-MSH at the first signs of paralysis. The α-MSH-treated mice in comparison with untreated EAE mice had a profound diminishment in the severity and tempo of EAE. The spleen cells in α-MSH-treated EAE produced TGF-β in response to PLP-antigen stimulation in contrast to untreated mice spleen cells that produced IFN-γ. When the α-MSH-treated EAE mice were reimmunized there was a delay of a week before the second episode of EAE. Although this delay maybe because of the induction of TGF-β-producing spleen cells by the α-MSH-treatment, it was not adequate to suppress IFN-γ-production by PLP-antigen stimulated spleen cells from untreated mice, nor able to suppress the eventual second episode of EAE. Therefore, the injection of α-MSH at the onset of paralysis is extremely effective in diminishing the severity and tempo of EAE, and the subsequent induction of potential PLP-specific Treg cells suggests that an α-MSH therapy could be attempted as part of a therapeutic regiment to impose immunoregulation and immunosuppression on an autoimmune disease of the central nervous system.
Keywords: Alpha-melanocyte stimulating hormone, Autoimmune disease, Experimental autoimmune encephalomyelitis, Immunosuppression, Neuroimmunology, Neuroimmunomodulation, Neuropeptide therapy, Regulatory immunity
1. Introduction
Certain tissue microenvironments called immune privileged uniquely regulate the immunogenic response by having a threshold to induce inflammation and immunity within their microenvironment that is higher than conventional tissues such as the skin. One of these immune privileged tissues is the eye (Streilein, 2003a). The ocular microenvironment has evolutionarily adapted several molecular mechanisms to suppress the induction of inflammation and promote the activation of regulatory immunity. Our current understanding of the molecular mechanisms of ocular immune privilege has suggested the development of guided interventions and therapies with the molecules that mediate ocular immune privilege to (re)establish tolerance to self antigens (Streilein, 2003b; Taylor, 2007). An important mediator of immunoregulation and immunosuppression within the ocular microenvironment is the neuropeptide alpha-melanocyte stimulating hormone (α-MSH) (Taylor, 2003a).
The neuropeptide α-MSH is a 13 amino acid long cleavage product of pro-opiomelanocortin hormone (Chakraborty et al., 1996; Lee et al., 1961). The neuropeptide is expressed in various body tissues, fluids, and blood (Bohm et al., 2005; Holdeman et al., 1985; Taylor and Streilein, 1996; Taylor et al., 1992). It has an important role in metabolic, pigmentary, and immune homeostasis (Bohm and Luger, 2004; Butler and Cone, 2002; Lipton and Catania, 1997; Rees, 2003; Tung et al., 2006). The effects of α-MSH on immunity are well documented (Lipton and Catania, 1997). The neuropeptide suppresses the pro-inflammatory activity of both innate and adaptive immunity. In macrophages through the melanocortin 1 and 3 receptors, α-MSH suppresses the activation of NF-κB and p38MAP-kinase by IL-1, TNF-α, and endotoxin (Brzoska et al., 1999; Manna and Aggarwal, 1998; Taherzadeh et al., 1999; Yoon et al., 2003). The α-MSH suppression of endotoxin mediated inflammation is the result of blocking toll like receptor-4 (TLR4) signaling in macrophages either through suppression of CD14 expression or by translocation of IRAK-M to the endotoxin-engaged TLR4 intracellular complex (Sarkar et al., 2003; Taylor, 2005). While α-MSH suppresses the activity of pro-inflammatory cytokines and cells, it can induce its own production and enhances its own receptor expression on macrophages potentially creating a self-perpetuating anti-inflammatory loop (Star et al., 1995; Taherzadeh et al., 1999).
Most of our understanding of the effects of α-MSH on T cells has been though characterizing the role of α-MSH in the immunosuppression and immunoregulation of Th1 cell activation within the ocular microenvironment (Taylor, 2003b). At its ocular physiological concentration of 30 pg/ml, α-MSH suppresses IFN-γ production by primed T cells that were either activated by antigen presenting cells or by cross-linking the T cell receptor (TCR) with anti-CD3 antibodies (Taylor et al., 1992, 1994). This suppression is through the melanocortin 5 receptor expressed on the CD4+ T cells (Taylor and Namba, 2001). Although this activity of α-MSH is sufficient to suppress immunogenic inflammation, the α-MSH-treated, TCR-activated, primed T cells continue to proliferate, and produce TGF-β (Nishida and Taylor, 1999). Further characterization of these T cells has revealed that they are CD25+ CD4+ Treg cells (Taylor and Namba, 2001). If these Treg cells are generated against a specific retinal autoantigen, they can be adoptively transferred to suppress experimental autoimmune uveitis (EAU) in mice (Namba et al., 2002; Taylor and Namba, 2001). The α-MSH-induced Treg cells require specific antigen in the target tissue to activate their suppressive activity; however, any antigen-activated T cell with in the target tissue can be suppressed by the α-MSH-induced Treg cells. Moreover, the antigen-specificity of these Treg cells does not need to be the disease associated autoantigen.
Because of the immunoregulatory and immunosuppressive activity of α-MSH, we decided to see if it was possible to use α-MSH to re-impose immunosuppression on an eye that has diminished immune privilege, and is suffering from autoimmune uveitis. Others have shown that a single systemic injection of α-MSH at the time of an acute phase response induced by endotoxin suppressed the inflammation in the infected tissue site and returned body temperature to normal (Glyn and Lipton, 1981; Martin and Lipton, 1990; Villar et al., 1991). When we made a similar systemic injection of α-MSH in mice with EAU there was an accelerated resolution of ocular inflammation (Taylor et al., 2000). Recently we have found that α-MSH may have an important role in the natural recovery of mice from EAU by promoting induction of retinal autoantigen-specific Treg cells that we find in the post-EAU spleen, and in minimizing retinal tissue damage during an episode of uveitis (Kitaichi et al., 2005).
The effectiveness of systemically injecting α-MSH to suppress an autoimmune disease in the eye suggested to us that it maybe possible to conduct the same treatment on other T cell mediated autoimmune diseases, such as multiple sclerosis. In this manuscript we describe the effects of a systemic injection of α-MSH peptide on PLP-antigen induced experimental autoimmune encephalomyelitis (EAE) in SJL mice.
2. Materials and methods
2.1. Peptides, mice, and antibodies
Immunizations to induce EAE used human proteolipid peptide (PLP) amino acids 139–151 synthesized for us by Invitrogen Life Technologies (Carlsbad, CA). The use of the SJL strain of mice (Jackson, Bar Harbor, ME) for EAE (Falk et al., 2000; Kono et al., 1988; Sobel et al., 1990), the mouse model of multiple sclerosis, was approved by the Institutional Animal Care and Use Committee. Complete Freund’s adjuvant, and desiccated Mycobacterium tuberculosis were purchased from Difco Laboratories (Detroit, MI). The pertussis toxin, and synthetic adjuvant MPL–TDM were from Sigma–Aldrich (St. Louis, MO). The amidated, acetylated peptide alpha-melanocyte stimulating hormone (α-MSH) was purchased from Bachem, (King of Prussa, PA). We assayed for mouse IFN-γ, and IL-4 using R&D Systems (Minneapolis, MN) ELISA kits.
2.2. Immunization to induce EAE
The EAE was induced by injecting subcutaneously the SJL mouse into two sites on the back on either side of the spine near the base of the tail 400 µg of peptide antigen PLP (139–151) emulsified in Freund’s adjuvant fortified with 4 mg/ml of M. tuberculosis (Falk et al., 2000; Kono et al., 1988; Sobel et al., 1990). The mice were then injected ip on the day of immunization and 48 h later with 200 ng of pertussis toxin. The paralysis was monitored and scored using the 5 point scale as others have reported (Falk et al., 2000; Kono et al., 1988; Sobel et al., 1990). EAE Score 0 for no paralysis; 1 for flaccid tail; 2 for moderate hind-limp paralysis; 3 for complete hind-limp paralysis; 4 for fore-limb paralysis; and 5 death. All the experiments were comparisons between EAE mice injected ip with 50 µg of α-MSH and EAE mice injected with PBS. The α-MSH injections were done on the day the mice showed the first symptoms of paralysis, which ranged from 9 to 11 days after the immunization. The results are presented by setting the first day of paralysis for each mouse to be Day 0. Some of the EAE mice were left to recover and were then reimmunized with 400 µg of PLP (139–151) peptide emulsified in a modified Ribi adjuvant consisting of monophosphoryl-lipid A (MPL) and synthetic trehalose dicorynmycolate (TDM) in squalene (synthetic adjuvant MPL–TDM) to avoid activating a necrotizing immune response by a second exposure to M. tuberculosis. The antigen was injected into two subcutaneous sites on the back above the original immunization sites. These mice were monitored and scored for paralysis over the next 23 days. In these experiments, the day the mice were reimmunized was set as Day 0.
2.3. Cytokine and proliferation assay
Spleens were collected from EAE mice treated with and without α-MSH when the α-MSH-treated mice recovered from EAE (all the mice had EAE scores of 1 or less). The spleens were passed through a nylon mesh in DPBS (Gibco BRL, Gaithersburg, MD) and made into a single cell suspension. The cells were centrifuged (400g, 10 min), resuspended in 1 ml of RBC lysing buffer (Sigma Chemical), incubated for 1 min on ice, 9 ml of DPBS was added, and the cells were spun down again. The cells were washed once, and were resuspended in serum free media: RPMI-1640, 0.1% bovine serum albumin, and 0.2% ITS+1 media supplement (Sigma Chemical). Into the wells of a 96-well flat bottom culture plate, 4 × 105 spleen cells were added with 20 µg of PLP(139–151) peptide. The cells were incubated for 48 h, and the culture supernatant was assayed for IFN-γ, and IL-4 by ELISA Kit, and for TGF-β by bioassay. To assay proliferation, the cell cultures were incubated for 68 h, 10 µl of Cell Counting Kit-8 reagent (Dojindo Molecular Technologies, Gaithersburg, MD) was added to the wells, and the cell cultures were incubated for an additional 4 h. The reagent is a water soluble tetrazolium salt that is converted to a formazan dye in living cells. The dye was detected by reading the plates with a microplate spectrophotometer at 460 nm absorbance. Since the OD was linearly relative to cell number, the OD of each group of activated spleen cells were compared to each other to indicate relative levels of proliferation.
The concentration of TGF-β in the culture supernatant was measured using the Mv1Lu (CCL-64, ATCC, Rockville, MD) mink lung epithelial cell proliferation inhibition assay as we have done before (Taylor et al., 1997). Prior to the assay, aliquots of supernatant underwent transient acidification by adding 5 µl of 1.0 N HCl to every 100 µl of supernatant lowering the pH to 2. The acid-treated supernatant was incubated for 1 h at 4 °C. The acid was neutralized by adding 10 µl of a 1:1 mixture of NaOH (0.01 M) and HEPES (0.04 M, pH 7.4). Acid neutralization was confirmed by samples of the supernatant turning pH indicator paper to the color indicating 7.4 pH. Mv1Lu cells from subconfluent cultures were incubated with a 1:2 dilution of acid-treated supernatant in 96-well flat bottom microtiter plates (Costar, Fisher Scientifis, Pittsburgh, PA) in EMEM (Gibco BRL, Gaithersburg, MD) supplemented with 0.05% fetal calf serum. Cultures were incubated for 24 h at 37 °C, 10 µl of Cell Counting Kit-8 reagent was added to the wells, and 4 h later the OD 460 nm was read for each well. Concentration of TGF-β was determined by comparing the OD of the cultures treated with supernatant to a standard curve calculated from the OD of the cell cultures treated with known amounts of TGF-β. Specificity of the assay was monitored by treating the cells with the culture supernatant in the presence of neutralizing anti-pan TGF-β-antibody (R&D Systems).
2.4. In vitro assay for regulatory activity
The regulatory activity of the antigen stimulated spleen cells from α-MSH-treated EAE mice was conducted using an in vitro assay we used before (Namba et al., 2002; Taylor and Namba, 2001). The in vitro assay for regulatory activity was done by collecting the spleen cells from the α-MSH-treated and untreated EAE mice as above. The spleen cells from the treated and untreated mice were pooled (4 × 105 cells of each) into one well of a 96-well plate, and 40 µg of PLP(139–151) peptide was added. The mixed cell cultures were incubated for 48 h and the culture supernatant was assayed for IFN-γ and TGF-β as described above.
2.5. Statistical analyses
To detect statistical differences between the α-MSH-treated and untreated EAE mice, their scores over each day of the experiment were compared by two-way ANOVA. In addition, the area under the curve of the EAE score versus Day of Treatment was calculated to find the day of the maximum EAE score for each mouse group. When individual EAE scores on a specific day were compared between the α-MSH-treated and untreated groups of mice, we used the non-parametric Mann–Whitney U test. Comparisons of the concentration of cytokines between tissue culture supernatant samples were statistically analyzed by unpaired t-test. Statistical significance was indicated by P ≤ 0.05.
3. Results
3.1. The effects of α-MSH-treatment on the course and severity of EAE
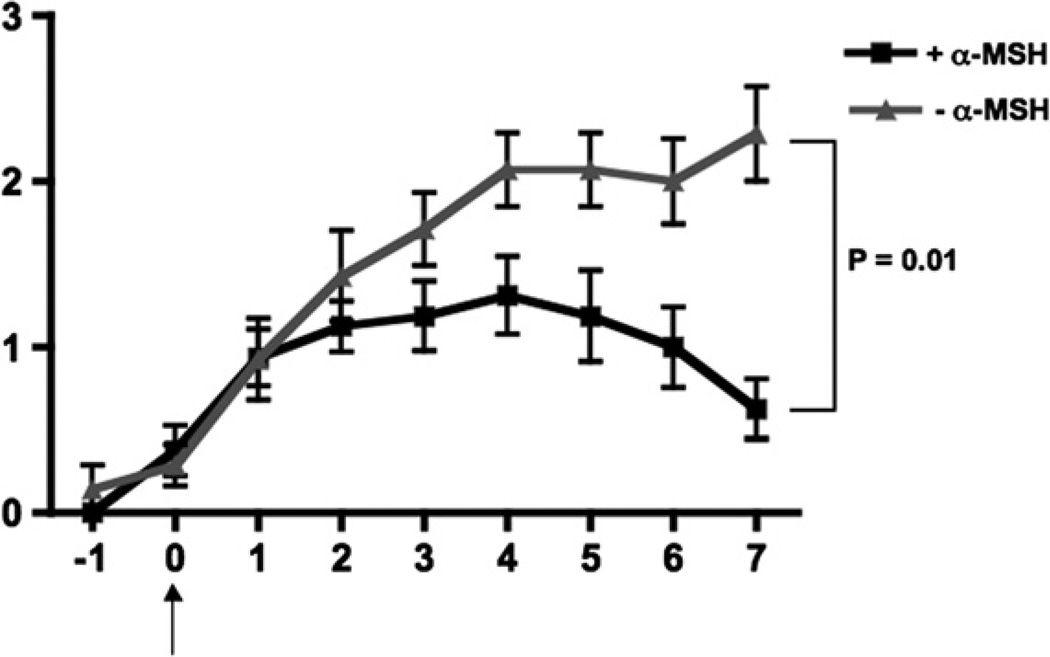
Our previous experience with α-MSH, and the multiple examples in the literature of α-MSH suppressing inflammation mediated by innate and adaptive immunity suggested that α-MSH should be an effective suppressor of T cell mediated autoimmune diseases. To test this possibility, we immunized SJL mice with the immunodominate peptide of PLP, amino acids 139–151, in complete Freud’s adjuvant plus pertussis toxin injections. We waited for the first signs of paralysis, and then injected the mice with a single dose of α-MSH (50 µg/mouse). Over the next 7 days, we scored the paralysis for each mouse. The mice that did not receive an injection of α-MSH displayed the expected progressive paralysis from flaccid tail to total hind-limb paralysis (Fig. 1). EAE was significantly diminished in the mice injected with α-MSH (Fig. 1). The peak of the area under the curve of the α-MSH-injected mice was at 4 days after the onset of paralysis with a mean score of 1.3. This was in stark contrast to the untreated EAE mice which 4 days after the onset of paralysis had an EAE score of 2.1. By 7 days after the onset of paralysis the α-MSH-treated mice were on there way toward full recovery, while there was continued progression of paralysis in the untreated EAE mice. Over these 7 days 92% of the untreated EAE mice displaying hind-limb paralysis where as only 38% of the α-MSH-treated mice had hind-limb paralysis. By 15 days after the onset of paralysis the majority of the α-MSH-treated mice had no paralysis while paralysis was still detected in the untreated mice group (see Fig. 3, Day 0 of reimmunization). Therefore, a single systemic injection of the neuropeptide α-MSH was effective in diminishing the severity and tempo of EAE.
Fig. 1.
A single treatment with α-MSH suppresses EAE. The SJL mice were immunized to induce EAE as described in the methods and were monitored every day for symptoms of paralysis. On the first day of paralysis symptoms (Day 0) one group of mice were injected with 50 µg of α-MSH ip (+α-MSH) and another group of mice were injected with PBS (−α-MSH). Both groups were monitored for another 7 days. The groups consisted of 16 mice injected with α-MSH, and 14 injected with PBS. Presented are the mean EAE scores of the group ± the SEM for each day, and are the pooled results of three separately run experiments. The α-MSH-treatment statistically (P ≤ 0.01) changed the curve of EAE scores over time.
Fig. 3.
A single treatment with α-MSH delays the onset of a second induced episode of EAE. The SJL mice were immunized to induce EAE, treated with or without α-MSH as in Fig. 1. The mice were monitored to detect the day that the α-MSH-treated mice reach almost complete recovery (Day 0), and the mice of both α-MSH-treated and untreated EAE mice were reimmunized with PLP (130–151) in synthetic adjuvant. The mice were monitored for a second episode of EAE and possible recovery. Each group consisted of 15 mice. Presented are the mean EAE scores of the group ± the SEM for each day, and are the pooled results of three separately run experiments. The α-MSH-treatment statistically (P ≤ 0.01) changed the curve of EAE scores over time with extremely significant differences (P < 0.002) before day 7 and after day 12.
3.2. The effects of α-MSH-treatment on PLP immunity
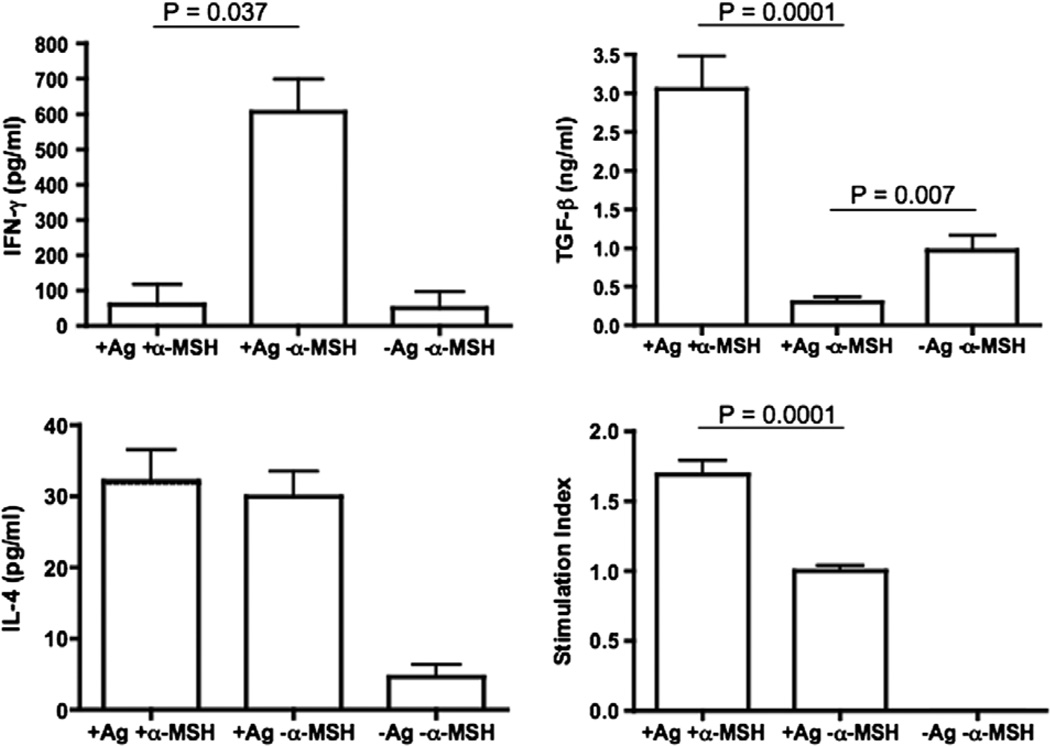
Since the α-MSH-treatment significantly diminished the severity of EAE and shortened the extent of the disease, we assayed for the effects of α-MSH-treatment on PLP-antigen specific immunity. We collected the spleen cells from EAE mice 7 days after α-MSH-treatment. The spleen cells were stimulated with PLP peptide and 48 h later the conditioned media was assayed for IFN-γ, IL-4, and TGF-β. In addition we assayed for PLP-stimulated proliferation. The PLP-antigen specific immunity of spleen cells from EAE mice not treated with α-MSH displayed an expected type 1 T cell response of high IFN-γ and low TGF-β production and proliferated (Fig. 2). There was some background detection of IL-4. In contrast the PLP-antigen specific immunity of spleen cells from EAE mice treated with α-MSH did not produce IFN-γ any more than unstimulated T cells, but produced TGF-β in abundance. These stimulated cells proliferated at a significantly higher level, but produced the same amount of IL-4 as the cells from untreated EAE mice. These results suggest that the α-MSH-treatment causes a significant switch in PLP-antigen specific immunity from a type 1 cytokine profile into a possible regulatory cytokine profile.
Fig. 2.
Treatment with α-MSH changes the cytokine profile of PLP (130–151) antigen stimulated immunity in the spleen. The mice were immunized to induce EAE, and as in Fig. 1 one group was treated with α-MSH (+α-MSH) and one group was not (−α-MSH). The mice were monitored for an additional 7 days, and their spleen cells were collected. The cultured spleen cells were stimulated by adding PLP (130–151) antigen (+Ag) to the cultures. The cultures were incubated for 48 h, and the culture media was assayed for IFN-γ, TGF-β, and IL-4. In addition the cells were assayed for proliferation 72 h after adding antigen. Presented are the mean concentration of the cytokine ± SEM of two experiments of five pooled mouse spleen cells per group in each experiment. Proliferation is presented as stimulation index ± SEM relative to antigen stimulated spleen cells from untreated EAE mice. There was a significant change in the production of IFN-γ and TGF-β with an increase in proliferation by the PLP-stimulated spleen cells from α-MSH-treated EAE mice.
3.3. The effects of α-MSH-treatment on a subsequent reimmunization with PLP peptide
Since treating mice with α-MSH caused a switch in the cytokine profile of PLP immunity that is detected in the spleen when the EAE resolves, we assayed the possibility that this change in immunity protects the mice from a second PLP-induced episode of EAE. As like before, the SJL mice were immunized with PLP 139–151 to induce EAE, and at the first sign of paralysis the mice were injected with 50 µg of α-MSH. When the majority of mice recovered from the first episode of EAE (about day 24 after the initial immunization), the mice were reimmunized with PLP (139–151) peptide in synthetic adjuvant, and scored for paralysis for the next 22 days (Fig. 3). The resulting paralysis in the α-MSH-treated mice was as sever as in the untreated EAE mice; however the onset of disease was delayed by a week, and the α-MSH-treated mice appear to resolve the second episode faster and many of the mice did not succumb to a possible third episode of paralysis like the untreated mice on days 16 through 22. There was a statistical difference (P ≤ 0.01) between the two curves in relationship to being treated with or without α-MSH during the first episode of EAE. These findings show that the α-MSH-induced changes in PLP-antigen stimulated T cell activity during the first paralysis has an effect on subsequent immunogenic responses to PLP-antigen.
3.4. In vitro assay for regulatory cell activity
One of the effects of α-MSH on primed T cells is to promote Treg cell activation (Namba et al., 2002). Since we found that post-EAE spleen cells after α-MSH-treatment are TGF-β producing cells, maybe these cells are PLP-specific regulatory immune cells? To test this possibility, we immunized two groups of the SJL mice to induce EAE. Into one group we injected α-MSH as like before. We waited 7 days for the EAE paralysis in the α-MSH-treated mice to subside, and collected the spleen cells from both groups. In culture we mixed the two types of spleen cells, cells from α-MSH-treated mice and cells from untreated mice. PLP-antigen was added to the cultures, and 48 h later we assayed the conditioned media for IFN-γ and TGF-β (Fig. 4). Although the PLP-stimulated cells from the α-MSH-treated EAE mice could not suppress IFN-γ production by the PLP-stimulated cells from untreated EAE mice, they did not contribute to any further production of IFN-γ. Moreover, there was a significant increase in TGF-β production in the cultures with spleen cells from the α-MSH-treated EAE mice. Therefore, the PLP-specific immunity in the α-MSH-treated EAE mice express the cytokine pattern of regulatory T cells; however, their target of suppressive activity must be to something other than suppressing the PLP-antigen stimulated inflammatory immunity found in the spleen.
Fig. 4.
Spleen cells from α-MSH-treated EAE mice appear to be regulatory cells but cannot suppress IFN-γ production by PLP-antigen stimulated spleen cells from untreated EAE mice. The mice were immunized to induce EAE, and as in Fig. 1. The mice were monitored for 7 days, and the spleen cells were collected. Equal numbers of spleen cells from the α-MSH-treated EAE mice (mouse group A), and untreated EAE mice (mouse group B) were mixed in culture and stimulated by adding PLP (130–151) antigen to the spleen cell cultures. The cultures were incubated for 48 h, and the culture media was assayed for IFN-γ, and TGF-β. Presented are the mean concentration of the cytokine ± SEM of two experiments of five pooled mouse spleen cells per group in each experiment. There was significant production of TGF-β with the addition of spleen cells from the α-MSH-treated EAE mice, but there was no effect on IFN-γ production by the PLP-stimulated spleen cells from untreated EAE mice.
4. Discussion
Using a treatment procedure we used to diminish EAU in mice (Taylor et al., 2000), we found that this similar procedure diminished the severity of EAE in mice. We adapted the role of α-MSH in immune privilege to treat another T cell mediated autoimmune disease. The α-MSH-treatment changed the expected cytokine profile of autoantigen PLP (139–151) specific immunity in the spleen from IFN-γ production to TGF-β production. Such an α-MSH mediated change was seen before when primed T cells were restimulated in vitro in the presence of α-MSH (Taylor and Namba, 2001). These α-MSH-induced TGF-β producing T cells can act as regulatory T cells, and in other disease models they have been adoptively transferred to suppress delayed hypersensitivity and autoimmunity (Nishida and Taylor, 1999; Taylor and Namba, 2001). The change in cytokine profile of the PLP-stimulated immune cells in the α-MSH-treated EAE mice must be associated with the delayed induction of the second episode of EAE after PLP reimmunization. Although all the reimmunized α-MSH-treated EAE mice succumbed to a second episode of EAE, the delay prior to the first symptoms of EAE and their subsequent EAE response after the second episode were significantly different from the untreated mice. We have seen a similar delay in mice that naturally recovered from EAU and were reimmunized with retinal autoantigen to induce a second episode of uveitis (Kitaichi et al., 2005). We found that α-MSH may have a role in this phenomena, because melanocortin 5 receptor knockout EAU-convalescing mice exhibited a rapid and sever uveitic response following reimmunization that was reminiscent of an immunological memory response to the retinal autoantigen (Taylor et al., 2006). It is through the melanocortin 5 receptor on T cells that α-MSH induces regulatory activity in T cells (Taylor and Namba, 2001). From these findings it was concluded that α-MSH in the eye or systemically can promote the induction of regulatory T cells that function to prevent immunological memory to autoantigens. Therefore, the results in this manuscript with our previous findings of the effects of α-MSH on EAU demonstrated that treatment with α-MSH is extremely effective in diminishing the severity and tempo of autoimmune diseases like EAE and EAU. Also, the α-MSH-treatment changes the immune response to autoantigen that can provide some protection against reactivation of a subsequent immune response to autoantigen.
The effectiveness of an injection of α-MSH in suppressing the severity of EAE must be because α-MSH can suppress the inflammatory activity in all the immune cells involved in the autoimmune response and disease. The response of macrophages, dendritic cells, and neutrophils to pro-inflammatory cytokines is blocked by α-MSH mediated suppression of NF-κB activation within the cells (Manna and Aggarwal, 1998; Yoon et al., 2003). Macrophages and dendritic cells produce IL-10 when treated with α-MSH (Lam et al., 2006; Luger et al., 1999). Also, there is evidence that α-MSH induces its own production and expression of its own receptors on macrophages with the potential of establishing a self-perpetuating anti-inflammatory autoregulatory loop (Star et al., 1995; Taherzadeh et al., 1999). The intensity of EAE is proportional to NF-κB activation, expression of IL-1, and chemokines, and inversely proportional to the expression of IL-10 and TGF-β (Dasgupta et al., 2004; de Jong et al., 2002; Karpus and Ransohoff, 1998; Kennedy et al., 1992; Mann et al., 2002; Weinshenker et al., 2001). There is even evidence that specific polymorphisms in the melanocortin 1 receptor, a ubiquitous receptor for α-MSH on immune cells, are associated with a severe disease course in multiple sclerosis patients, but not with susceptibility to multiple sclerosis (Partridge et al., 2004). Whether α-MSH is mediating a generalized suppression of inflammation could be resolved by an examination of the pathology in the central nervous system during EAE and after α-MSH-treatment.
Autoimmune disease mediating T cells activated in the presence of α-MSH in vitro are suppressed in their production of IFN-γ (Taylor and Namba, 2001). Moreover TGF-β production in these α-MSH-treated T cells is promoted. We have found that such α-MSH-induced TGF-β-producing T cells function as regulatory T cells. Our ability to find PLP-antigen stimulated TGF-β-production in the spleens of α-MSH-treated mice suggests that, as we have seen with α-MSH-treatment of mouse autoimmune uveitis, α-MSH mediates in vivo the induction of potential Treg cells, which could be from α-MSH promoted expansion of the natural Treg cells to PLP. How such α-MSH-induced Treg cells function in vivo is unknown, and we do not know if the Treg cells are a by-product of the α-MSH-treatment or play role in the suppression of EAE. We have constantly find that their presence after the initial episode of autoimmune disease delays the onset of an induced second episode of autoimmune disease, but cannot prevent the second episode from happening (Kitaichi et al., 2005; Taylor et al., 2006). The importance of Th17 cells in autoimmunity has recently been reported in the literature (Hofstetter et al., 2005; Komiyama et al., 2006; Kroenke and Segal, 2007). There is a possibility of Treg cells supporting Th17 cell induction (Lohr et al., 2006). If this is correct then our results demonstrating a second episode of EAE after a delay may be because the α-MSH-induced Treg cells cannot suppress or may even promote Th17 cell activity.
Others have demonstrated the use of α-MSH in therapy. Therapeutic α-MSH-treatments have been tried in animal models of septic shock, contact dermatitis, rheumatoid arthritis, acute pancreatitis, toxin-induced liver failure, allergic airway inflammation, endotoxin induce uveitis, and experimental autoimmune uveoretinitis (Ceriani et al., 1994; Grabbe et al., 1996; Jahovic et al., 2004; Martin and Lipton, 1990; Nishida et al., 2004; Raap et al., 2003; Shiratori et al., 2004; Taylor et al., 2000; Wang et al., 2004). In addition, two other groups of researchers have attempted to use different forms of gene therapy to see if delivering α-MSH into the immune response of EAE will suppress the onset and subsequent pathology of EAE. One group engineered a naked DNA construct coding for the pre-cleavage product of α-MSH (Yin et al., 2003). The plasmid was injected intra-muscularly at the time of MBP immunization to induce EAE, and then injected weekly after the mice were immunized. These mice had a delay in the onset of the disease and had significantly lower mortality associated with the induction of EAE; however, the severity was only marginally lower.
A second group engineered an AAV α-MSH-encoded viral vector and transfected a PLP (139–151)-specific T cell line (Han et al., 2007). The transfected T cells were adoptively transferred either at the time of EAE immunization or adoptively transferred at the onset of paralysis. The mice that received α-MSH-transfected T cells at the time of immunization had a significant delay in the onset and severity of the disease. In addition adoptive transfer of α-MSH-transfected PLP-specific T cells in mice starting to show symptoms of EAE were significantly suppressed in the severity of the initial episode of EAE and were suppressed in the spontaneous remitting episode of EAE. This group’s work with α-MSH-transfected T cells had the objective of demonstrating the efficacy of having T cells that could mediate disease also be the deliverers of α-MSH to the tissue site of autoreactive T cell activation. This objective is similar as ours in this manuscript, in that by delivering α-MSH at the start of effector T cell activity in EAE we can alter the immunological response to PLP, diminish the disease, and possibly reestablish immune tolerance to the autoantigen. As with the group using α-MSH-transfected T cells, we were able to show that α-MSH therapy induces a change in the autoantigen response from a disease mediating response into a potential regulatory immune response.
We initiated this work to see if we can use the lessons of ocular immune privilege to modulate immunity in other tissues. What we have learned over the past three decades of studying the molecular mechanisms of ocular immune privilege is that the mechanisms of immune privilege activity engage the immunogenic response within the ocular microenvironment to be anti-inflammatory (Taylor, 2007). Moreover, mechanisms of immune privilege further manipulate the immunogenic response to regulate itself. Of the immunoregulatory molecules in the ocular microenvironment it is the neuropeptide α-MSH that mediates immunoregulation and immunosuppression on both the innate and adaptive immune response. The results we presented in this manuscript suggest that with α-MSH therapy we can attempt to impose immunosuppression and immunoregulation on EAE. It is possible that α-MSH therapy is a potential method to re-impose tolerance and to establish a stable autoimmune state that is no longer harmful.
Acknowledgments
We thank David Yee for his technical assistance. This work was supported by a grant from the Wadsworth Foundation.
References
- Bohm M, Luger TA. Melanocortins in fibroblast biology—current update and future perspective for dermatology. Exp. Dermatol. 2004;13(Suppl. 4):16–21. doi: 10.1111/j.1600-0625.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- Bohm M, Wolff I, Scholzen TE, Robinson SJ, Healy E, Luger TA, Schwarz T, Schwarz A. Alpha-melanocyte-stimulating hormone protects from ultraviolet radiation-induced apoptosis and DNA damage. J. Biol. Chem. 2005;280:5795–5802. doi: 10.1074/jbc.M406334200. [DOI] [PubMed] [Google Scholar]
- Brzoska T, Kalden DH, Scholzen T, Luger TA. Molecular basis of the alpha-MSH/IL-1 antagonism. Ann. NY Acad. Sci. 1999;885:230–238. doi: 10.1111/j.1749-6632.1999.tb08680.x. [DOI] [PubMed] [Google Scholar]
- Butler AA, Cone RD. The melanocortin receptors: lessons from knockout models. Neuropeptides. 2002;36:77–84. doi: 10.1054/npep.2002.0890. [DOI] [PubMed] [Google Scholar]
- Ceriani G, Diaz J, Murphree S, Catania A, Lipton JM. The neuropeptide alpha-melanocyte-stimulating hormone inhibits experimental arthritis in rats. Neuroimmunomodulation. 1994;1:28–32. doi: 10.1159/000097087. [DOI] [PubMed] [Google Scholar]
- Chakraborty AK, Funasaka Y, Slominski A, Ermak G, Hwang J, Pawelek JM, Ichihashi M. Production and release of proopiomelanocortin (POMC) derived peptides by human melanocytes and keratinocytes in culture: regulation by ultraviolet B. Biochim. Biophys. Acta. 1996;1313:130–138. doi: 10.1016/0167-4889(96)00063-8. [DOI] [PubMed] [Google Scholar]
- Dasgupta S, Jana M, Zhou Y, Fung YK, Ghosh S, Pahan K. Antineuroinflammatory effect of NF-kappaB essential modifier-binding domain peptides in the adoptive transfer model of experimental allergic encephalomyelitis. J. Immunol. 2004;173:1344–1354. doi: 10.4049/jimmunol.173.2.1344. [DOI] [PubMed] [Google Scholar]
- de Jong BA, Westendorp RG, Eskdale J, Uitdehaag BM, Huizinga TW. Frequency of functional interleukin-10 promoter polymorphism is different between relapse-onset and primary progressive multiple sclerosis. Hum. Immunol. 2002;63:281–285. doi: 10.1016/s0198-8859(02)00369-5. [DOI] [PubMed] [Google Scholar]
- Falk K, Rotzschke O, Santambrogio L, Dorf ME, Brosnan C, Strominger JL. Induction and suppression of an autoimmune disease by oligomerized T cell epitopes: enhanced in vivo potency of encephalitogenic peptides. J. Exp. Med. 2000;191:717–730. doi: 10.1084/jem.191.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glyn JR, Lipton JM. Hypothermic and antipyretic effects of centrally administered ACTH (1–24) and alpha-melanotropin. Peptides. 1981;2:177–187. doi: 10.1016/s0196-9781(81)80032-0. [DOI] [PubMed] [Google Scholar]
- Grabbe S, Bhardwaj RS, Mahnke K, Simon MM, Schwarz T, Luger TA. Alpha-melanocyte-stimulating hormone induces hapten-specific tolerance in mice. J. Immunol. 1996;156:473–478. [PubMed] [Google Scholar]
- Han D, Tian Y, Zhang M, Zhou Z, Lu J. Prevention and treatment of experimental autoimmune encephalomyelitis with recombinant adeno-associated virus-mediated alpha-melanocyte-stimulating hormone-transduced PLP139–151-specific T cells. Gene Ther. 2007;14:383–395. doi: 10.1038/sj.gt.3302862. [DOI] [PubMed] [Google Scholar]
- Hofstetter HH, Ibrahim SM, Koczan D, Kruse N, Weishaupt A, Toyka KV, Gold R. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell. Immunol. 2005;237:123–130. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Holdeman M, Khorram O, Samson WK, Lipton JM. Fever-specific changes in central MSH and CRF concentrations. Am. J. Physiol. 1985;248:R125–R129. doi: 10.1152/ajpregu.1985.248.1.R125. [DOI] [PubMed] [Google Scholar]
- Jahovic N, Arbak S, Tekeli O, Alican I. Alpha-melanocyte stimulating hormone has beneficial effects on cerulein-induced acute pancreatitis. Peptides. 2004;25:129–132. doi: 10.1016/j.peptides.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Karpus WJ, Ransohoff RM. Chemokine regulation of experimental autoimmune encephalomyelitis: temporal and spatial expression patterns govern disease pathogenesis. J. Immunol. 1998;161:2667–2671. [PubMed] [Google Scholar]
- Kennedy MK, Torrance DS, Picha KS, Mohler KM. Analysis of cytokine mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J. Immunol. 1992;149:2496–2505. [PubMed] [Google Scholar]
- Kitaichi N, Namba K, Taylor AW. Inducible immune regulation following autoimmune disease in the immune-privileged eye. J. Leukoc. Biol. 2005;77:496–502. doi: 10.1189/jlb.0204114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- Kono DH, Urban JL, Horvath SJ, Ando DG, Saavedra RA, Hood L. Two minor determinants of myelin basic protein induce experimental allergic encephalomyelitis in SJL/J mice. J. Exp. Med. 1988;168:213–227. doi: 10.1084/jem.168.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke MA, Segal BM. Th17 and Th1 responses directed against the immunizing epitope, as opposed to secondary epitopes, dominate the autoimmune repertoire during relapses of experimental autoimmune encephalomyelitis. J. Neurosci. Res. 2007;85:1685–1693. doi: 10.1002/jnr.21291. [DOI] [PubMed] [Google Scholar]
- Lam CW, Perretti M, Getting SJ. Melanocortin receptor signaling in RAW264.7 macrophage cell line. Peptides. 2006;27:404–412. doi: 10.1016/j.peptides.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Lee TH, Lerner AB, Buettner-Janusch V. The isolation and structure of α- and β-melanocyte-stimulating hormones from monkey pituitary glands. J. Biol. Chem. 1961;236:1390–1394. [PubMed] [Google Scholar]
- Lipton JM, Catania A. Anti-inflammatory actions of the neuroimmunomodulator alpha-MSH. Immunol. Today. 1997;18:140–145. doi: 10.1016/s0167-5699(97)01009-8. [DOI] [PubMed] [Google Scholar]
- Lohr J, Knoechel B, Wang JJ, Villarino AV, Abbas AK. Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J. Exp. Med. 2006;203:2785–2791. doi: 10.1084/jem.20061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luger TA, Kalden D, Scholzen TE, Brzoska T. Alpha-melanocyte-stimulating hormone as a mediator of tolerance induction. Pathobiology. 1999;67:318–321. doi: 10.1159/000028089. [DOI] [PubMed] [Google Scholar]
- Mann CL, Davies MB, Stevenson VL, Leary SM, Boggild MD, Ko Ko C, Jones PW, Fryer AA, Strange RC, Thompson AJ, Hawkins CP. Interleukin 1 genotypes in multiple sclerosis and relationship to disease severity. J. Neuroimmunol. 2002;129:197–204. doi: 10.1016/s0165-5728(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Manna SK, Aggarwal BB. Alpha-melanocyte-stimulating hormone inhibits the nuclear transcription factor NF-kappa B activation induced by various inflammatory agents. J. Immunol. 1998;161:2873–2880. [PubMed] [Google Scholar]
- Martin LW, Lipton JM. Acute phase response to endotoxin: rise in plasma alpha-MSH and effects of alpha-MSH injection. Am. J. Physiol. 1990;259:R768–R772. doi: 10.1152/ajpregu.1990.259.4.R768. [DOI] [PubMed] [Google Scholar]
- Namba K, Kitaichi N, Nishida T, Taylor AW. Induction of regulatory T cells by the immunomodulating cytokines alpha-melanocyte-stimulating hormone and transforming growth factor-beta2. J. Leukoc. Biol. 2002;72:946–952. [PubMed] [Google Scholar]
- Nishida T, Miyata S, Itoh Y, Mizuki N, Ohgami K, Shiratori K, Ilieva IB, Ohno S, Taylor AW. Anti-inflammatory effects of alpha-melanocyte-stimulating hormone against rat endotoxin-induced uveitis and the time course of inflammatory agents in aqueous humor. Int. Immunopharmacol. 2004;4:1059–1066. doi: 10.1016/j.intimp.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Nishida T, Taylor AW. Specific aqueous humor factors induce activation of regulatory T cells. Invest. Ophthalmol. Vis. Sci. 1999;40:2268–2274. [PubMed] [Google Scholar]
- Partridge JM, Weatherby SJ, Woolmore JA, Highland DJ, Fryer AA, Mann CL, Boggild MD, Ollier WE, Strange RC, Hawkins CP. Susceptibility and outcome in MS: associations with polymorphisms in pigmentation-related genes. Neurology. 2004;62:2323–2325. doi: 10.1212/wnl.62.12.2323. [DOI] [PubMed] [Google Scholar]
- Raap U, Brzoska T, Sohl S, Path G, Emmel J, Herz U, Braun A, Luger T, Renz H. Alpha-melanocyte-stimulating hormone inhibits allergic airway inflammation. J. Immunol. 2003;171:353–359. doi: 10.4049/jimmunol.171.1.353. [DOI] [PubMed] [Google Scholar]
- Rees JL. Genetics of hair and skin color. Annu. Rev. Genet. 2003;37:67–90. doi: 10.1146/annurev.genet.37.110801.143233. [DOI] [PubMed] [Google Scholar]
- Sarkar A, Sreenivasan Y, Manna SK. Alpha-melanocyte-stimulating hormone induces cell death in mast cells: involvement of NF-kappaB. FEBS Lett. 2003;549:87–93. doi: 10.1016/s0014-5793(03)00797-x. [DOI] [PubMed] [Google Scholar]
- Shiratori K, Ohgami K, Ilieva IB, Koyama Y, Yoshida K, Ohno S. Inhibition of endotoxin-induced uveitis and potentiation of cyclooxygenase-2 protein expression by alpha-melanocyte-stimulating hormone. Invest. Ophthalmol. Vis. Sci. 2004;45:159–164. doi: 10.1167/iovs.03-0492. [DOI] [PubMed] [Google Scholar]
- Sobel RA, Tuohy VK, Lu ZJ, Laursen RA, Lees MB. Acute experimental allergic encephalomyelitis in SJL/J mice induced by a synthetic peptide of myelin proteolipid protein. J. Neuropathol. Exp. Neurol. 1990;49:468–479. doi: 10.1097/00005072-199009000-00002. [DOI] [PubMed] [Google Scholar]
- Star RA, Rajora N, Huang J, Stock RC, Catania A, Lipton JM. Evidence of autocrine modulation of macrophage nitric oxide synthase by alpha-melanocyte-stimulating hormone. Proc. Natl. Acad. Sci. USA. 1995;92:8016–8020. doi: 10.1073/pnas.92.17.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streilein JW. Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. J. Leukoc. Biol. 2003a;74:179–185. doi: 10.1189/jlb.1102574. [DOI] [PubMed] [Google Scholar]
- Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat. Rev. Immunol. 2003b;3:879–889. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- Taherzadeh S, Sharma S, Chhajlani V, Gantz I, Rajora N, Demitri MT, Kelly L, Zhao H, Ichiyama T, Catania A, Lipton JM. Alpha-MSH and its receptors in regulation of tumor necrosis factor-alpha production by human monocyte/macrophages. Am. J. Physiol. 1999;276:R1289–R1294. doi: 10.1152/ajpregu.1999.276.5.R1289. [DOI] [PubMed] [Google Scholar]
- Taylor A. A review of the influence of aqueous humor on immunity. Ocul. Immunol. Inflamm. 2003a;11:231–241. doi: 10.1076/ocii.11.4.231.18269. [DOI] [PubMed] [Google Scholar]
- Taylor A, Namba K. In vitro induction of CD25+ CD4+ regulatory T cells by the neuropeptide alpha-melanocyte stimulating hormone (alpha-MSH) Immunol. Cell Biol. 2001;79:358–367. doi: 10.1046/j.1440-1711.2001.01022.x. [DOI] [PubMed] [Google Scholar]
- Taylor AW. Modulation of regulatory T cell immunity by the neuropeptide alpha-melanocyte stimulating hormone. Cell Mol. Biol. (Noisy-le-grand) 2003b;49:143–149. [PubMed] [Google Scholar]
- Taylor AW. The immunomodulating neuropeptide alpha-melanocyte stimulating hormone (a-MSH) suppresses LPS-stimulated TLR4 with IRAK-M in macrophages. J. Neuroimmunol. 2005;162:43–50. doi: 10.1016/j.jneuroim.2005.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AW. Ocular immunosuppressive microenvironment. Chem. Immunol. Allergy. 2007;92:71–85. doi: 10.1159/000099255. [DOI] [PubMed] [Google Scholar]
- Taylor AW, Alard P, Yee DG, Streilein JW. Aqueous humor induces transforming growth factor-beta (TGF-beta)-producing regulatory T-cells. Curr. Eye Res. 1997;16:900–908. doi: 10.1076/ceyr.16.9.900.5043. [DOI] [PubMed] [Google Scholar]
- Taylor AW, Kitaichi N, Biros D. Melanocortin 5 receptor and ocular immunity. Cell Mol. Biol. 2006;52:141–147. [PubMed] [Google Scholar]
- Taylor AW, Streilein JW. Inhibition of antigen-stimulated effector T cells by human cerebrospinal fluid. Neuroimmunomodulation. 1996;3:112–118. doi: 10.1159/000097235. [DOI] [PubMed] [Google Scholar]
- Taylor AW, Streilein JW, Cousins SW. Identification of alpha-melanocyte stimulating hormone as a potential immunosuppressive factor in aqueous humor. Curr. Eye Res. 1992;11:1199–1206. doi: 10.3109/02713689208999545. [DOI] [PubMed] [Google Scholar]
- Taylor AW, Streilein JW, Cousins SW. Alpha-melanocyte-stimulating hormone suppresses antigen-stimulated T cell production of gamma-interferon. Neuroimmunomodulation. 1994;1:188–194. doi: 10.1159/000097167. [DOI] [PubMed] [Google Scholar]
- Taylor AW, Yee DG, Nishida T, Namba K. Neuropeptide regulation of immunity. The immunosuppressive activity of alpha-melanocyte-stimulating hormone (alpha-MSH) Ann. NY Acad. Sci. 2000;917:239–247. doi: 10.1111/j.1749-6632.2000.tb05389.x. [DOI] [PubMed] [Google Scholar]
- Tung YC, Piper SJ, Yeung D, O’Rahilly S, Coll AP. A comparative study of the central effects of specific POMC-derived melanocortin peptides on food intake and body weight in Pomc null mice. Endocrinology. 2006 doi: 10.1210/en.2006-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar M, Perassi N, Celis ME. Central and peripheral actions of alpha-MSH in the thermoregulation of rats. Peptides. 1991;12:1441–1443. doi: 10.1016/0196-9781(91)90231-d. [DOI] [PubMed] [Google Scholar]
- Wang CH, Jawan B, Lee TH, Hung KS, Chou WY, Lu CN, Liu JK, Chen YJ. Single injection of naked plasmid encoding alpha-melanocyte-stimulating hormone protects against thioacetamide-induced acute liver failure in mice. Biochem. Biophys. Res. Commun. 2004;322:153–161. doi: 10.1016/j.bbrc.2004.07.091. [DOI] [PubMed] [Google Scholar]
- Weinshenker BG, Hebrink D, Kantarci OH, Schaefer-Klein J, Atkinson E, Schaid D, McMurray CM. Genetic variation in the transforming growth factor beta1 gene in multiple sclerosis. J. Neuroimmunol. 2001;120:138–145. doi: 10.1016/s0165-5728(01)00424-6. [DOI] [PubMed] [Google Scholar]
- Yin P, Luby TM, Chen H, Etemad-Moghadam B, Lee D, Aziz N, Ramstedt U, Hedley ML. Generation of expression constructs that secrete bioactive alpha MSH and their use in the treatment of experimental autoimmune encephalomyelitis. Gene Ther. 2003;10:348–355. doi: 10.1038/sj.gt.3301902. [DOI] [PubMed] [Google Scholar]
- Yoon SW, Goh SH, Chun JS, Cho EW, Lee MK, Kim KL, Kim JJ, Kim CJ, Poo H. Alpha-melanocyte-stimulating hormone inhibits lipopolysaccharide-induced tumor necrosis factor-alpha production in leukocytes by modulating protein kinase A, p38 kinase, and nuclear factor kappa B signaling pathways. J. Biol. Chem. 2003;278:32914–32920. doi: 10.1074/jbc.M302444200. [DOI] [PubMed] [Google Scholar]