SUMMARY
The basic helix-loop-helix (bHLH) transcription factor Math5 (Atoh7) is transiently expressed during early retinal histogenesis and is necessary for retinal ganglion cell (RGC) development. Using nucleoside pulse-chase experiments and clonal analysis, we determined that progenitor cells activate Math5 during or after the terminal division, with progressively later onset as histogenesis proceeds. We have traced the lineage of Math5+ cells using mouse BAC transgenes that express Cre recombinase under strict regulatory control. Quantitative analysis showed that Math5+ progenitors express equivalent levels of Math5 and contribute to every major cell type in the adult retina, but are heavily skewed toward early fates. The Math5>Cre transgene labels 3% of cells in adult retina, including 55% of RGCs. Only 11% of Math5+ progenitors develop into RGCs; the majority become photoreceptors. The fate bias of the Math5 cohort, inferred from the ratio of cone and rod births, changes over time, in parallel with the remaining neurogenic population. Comparable results were obtained using Math5 mutant mice, except that ganglion cells were essentially absent, and late fates were overrepresented within the lineage. We identified Math5-independent RGC precursors in the earliest-born (embryonic day 11) retinal cohort, but these precursors require Math5-expressing cells for differentiation. Math5 thus acts permissively to establish RGC competence within a subset of progenitors, but is not sufficient for fate specification. It does not autonomously promote or suppress the determination of non-RGC fates. These data are consistent with progressive and temporal restriction models for retinal neurogenesis, in which environmental factors influence the final histotypic choice.
Keywords: mouse, genetics, Cre recombinase, BAC transgenic, lineage, expression fate mapping, cell fate determination, retina, optic nerve, atonal, bHLH
INTRODUCTION
The seven major cell types in the vertebrate retina (rod and cone photoreceptors; amacrine, horizontal and bipolar interneurons; Müller glia; and ganglion cells) develop from a common pool of progenitors (Turner and Cepko, 1987; Turner et al., 1990) that are established when the optic vesicles invaginate to form bilayered optic cups (Goldowitz et al., 1996). The inner layer of each optic cup consists of proliferative retinal progenitor cells (RPCs), which are arranged as a pseudostratified epithelium. These RPCs begin to permanently exit mitosis and differentiate around embryonic day 11 (E11) in the mouse. Retinal neurons and glia are fully formed by postnatal day 21 (P21) and are arranged in a highly ordered tri-laminated structure (Rodieck, 1998). The outer nuclear layer (ONL) consists of photoreceptors while the inner nuclear (INL) and ganglion cell (GCL) layers are populated by interneurons, glia and ganglion cells. The mechanism of cell fate determination – how these diverse cell types are generated from an initially homogeneous progenitor population – remains poorly understood.
Birthdating experiments, in which [3H]-thymidine was used to mark the terminal S phase of progenitor cells, have established a characteristic order for the emergence of different retinal cell types during histogenesis (Carter-Dawson and LaVail, 1979; Rapaport et al., 2004; Sidman, 1961; Young, 1985). In all vertebrate species examined, retinal ganglion cells are the first-born neurons (Altshuler et al., 1991). In mammals, these are followed by horizontal cells, cones, amacrines, rods, bipolar cells and Müller glia, in descending birth order. There is considerable overlap in the distribution of birthdates among cell types, particularly for rod photoreceptors, which are born over an extended period (E13-P7 in mice) and are most abundant. Moreover, as a subclass, displaced amacrines, located in the mammalian GCL, are born earlier than amacrines in the INL (LaVail et al., 1991; Reese and Colello, 1992).
Lineage tracing experiments in rodents and frogs show that individual retinal progenitors are multipotent, giving rise to clones with heterogeneous cell type composition and size, and that the histogenic potential of the progenitor pool is gradually restricted over time (Holt et al., 1988; Turner and Cepko, 1987; Turner et al., 1990; Wetts and Fraser, 1988; Wong and Rapaport, 2009). The absence of a strict hierarchical relationship among cell types suggests that fate determination in the retina is a stochastic process (Gomes et al., 2011; Livesey and Cepko, 2001). The observation of discordant two-cell clones in rodent lineage marking studies indicates that at least some cell fate decisions occur during or after the terminal division, and may be subject to environmental influence (Turner and Cepko, 1987). Indeed, multiple extrinsic factors have been shown to alter the ratio of retinal cell types generated from progenitor pools (Altshuler et al., 1991; Ezzeddine et al., 1997; Fuhrmann et al., 1995; Yang, 2004; Young and Cepko, 2004).
Heterochronic mixing experiments, in which early and late retinal cells are co-cultured in unequal ratios, have shown that progenitors have a limited capacity to shift their fate forward or backward in sequence, and suggest that competence is fundamentally a cell-intrinsic property (Belliveau and Cepko, 1999; Rapaport et al., 2001; Reh, 1992; Watanabe and Raff, 1990). Likewise, single-cell dissociation studies have shown that the fates of retinal progenitors, including post-mitotic cells, change over time and are intrinsically programmed (Adler and Hatlee, 1989; Cayouette et al., 2003; Reh and Kljavin, 1989). Thus, it is likely that cell-intrinsic factors, expressed by progenitors in a prescribed temporal order, work in concert with extrinsic factors in the retinal microenvironment to guide cell fate decisions and ensure proper ratios of each cell type.
The basic helix-loop-helix (bHLH) transcription factor Math5 (Atoh7) was identified on the basis of its homology to Drosophila Atonal (Brown et al., 1998), which plays a critical role in the specification of R8 photoreceptors in the eye imaginal disc (Frankfort and Mardon, 2002; Hsiung and Moses, 2002; Jarman, 2000; Sun et al., 2003). The mouse Math5 gene contains a single exon (Prasov et al., 2010) and is specifically expressed by progenitor cells during retinal histogenesis (Brown et al., 1998), similar to frog, chick, and zebrafish orthologs (Kanekar et al., 1997; Liu et al., 2001; Masai, 2000). Math5 mutant mice lack retinal ganglion cells (RGCs) and optic nerves (Brown et al., 2001; Wang et al., 2001) and their circadian rhythms are not photoentrained (Brzezinski et al., 2005; Wee et al., 2002). Retinal vascular development (Brzezinski et al., 2003) and electrophysiology (Brzezinski et al., 2005) are also disrupted in these mice. Finally, the relative abundance of other retinal cell types is altered, through a combination of cell autonomous and non-autonomous effects (Brzezinski et al., 2005; Le et al., 2006). RGC genesis similarly fails in ath5 mutant (lakritz) zebrafish (Kay et al., 2001). In humans, ATOH7 mutations cause optic nerve aplasia (Ghiasvand et al., 2011) and the ATOH7 locus is a major determinant of normal variation in optic disc size, which reflects RGC number (Khor et al., 2011; Macgregor et al., 2010; Ramdas et al., 2010).
Math5 is likely to trigger a regulatory cascade for RGC development. Expression of the POU domain transcription factor Brn3b (Pou4f2) appears to be controlled by Math5 in mice, similar to the orthologous circuit in chick and frog (Hutcheson and Vetter, 2001; Liu et al., 2001; Schneider et al., 2001; Wang et al., 2001). In turn, Brn3b and the homeodomain transcription factor Isl1 form two regulatory nodes that are critical for RGC maturation (Erkman et al., 1996; Gan et al., 1996; Mu et al., 2004; Mu et al., 2008; Pan et al., 2008).
How does Math5 regulate ganglion cell fate determination? In principle, Math5 could act either as an instructive factor, irreversibly directing competent progenitors to differentiate into RGCs, or as a permissive factor, establishing an RGC competence state within a set of multipotent progenitors, only some of which develop into RGCs (Wessells, 1977). The Cre-lox recombination system provides a powerful tool to distinguish these mechanisms, by indelibly marking descendant cells. In a previous lineage analysis, a Math5-Cre knock-in allele was found to mark multiple retinal cell types, suggesting that Math5 acts permissively (Feng et al., 2010; Yang et al., 2003).
In this report, we extend these findings using a Math5>Cre BAC transgene in wild-type and Math5 mutant mice. This approach, coupled with birthdating analysis, has allowed us to quantitatively assess the cell type distribution and unique fate trajectory of the Math5 lineage over time. Our results show Math5 is expressed at equivalent levels in a subset of progenitors that are capable of forming all retinal cell types, with a frequency that decreases according to birth order. Although heavily weighted toward early fates, only 11% of these cells develop into RGCs and only 55% of RGCs descend from Math5+ progenitors. In the absence of Math5 function, lineage-marked cells exhibit a similarly diverse range of fates but do not differentiate as RGCs, suggesting Math5 has both autonomous and non-autonomous roles in RGC development. Using cell cycle markers and nucleoside pulse-chase analysis, we show Math5 expression is confined to progenitors during or after the terminal division, and does not control cell cycle exit. Finally, using retroviral clone analysis of explanted embryonic retinas, we demonstrate that Math5+ cells frequently arise in pairs from symmetric terminal divisions. Our results extend previous observations, but compel different conclusions. We provide new insights into Math5 function, ganglion cell development, and the mechanism of retinal fate determination.
MATERIALS AND METHODS
Quantitative PCR
Eye tissue was collected from 8-12 CD-1 embryos or newborn mice at time-points between E10.5 and P1.5 and homogenized in Trizol reagent (Invitrogen, Carlsbad, CA). Total RNA was purified from pooled homogenates at each time-point. cDNA was synthesized using d(N)6 primer and Superscript II reverse transcriptase (Invitrogen). Quantitative PCR was performed on cDNA using Math5 and Hprt primers (Brown et al., 2001) with the iCycler iQ system (Bio-Rad, Hercules, CA). Seven measurements were made for each cDNA pool. Math5 RNA levels (critical threshold cycles) were normalized to Hprt as described (Livak and Schmittgen, 2001), and are reported relative to the mean P1.5 value.
Math5>Cre BAC transgenic mice
We replaced the Math5 open reading frame on bacterial artificial chromosome (BAC) clone RP23-328P3 with a 2.0 kb nlsCre-actin pA cassette using a two-step recA-mediated recombination protocol in E. coli (Gong et al., 2002; Heintz, 2001). To target the BAC, which contains 110 kb 5′ and 103 kb 3′ genomic DNA flanking the Math5 transcription unit (Prasov et al., 2010), we constructed a plasmid vector with short 5′ (A, 345 bp) and 3′ (B, 378 bp) homology arms flanking Cre-pA. These were amplified by PCR from UTR sequences of the solitary Math5 exon (AF418923) and cloned into the SalI and XhoI sites of p GSU-Cre (Cushman et al., 2000). The resulting A-Cre-B cassette was inserted into the XhoI site of shuttle plasmid pLD53 GFP10 as a 2.4 kb SacI-XhoI fragment and verified by DNA sequencing. Shuttle plasmid pLD53 GFP10 was derived from pLD53.SC1 by partial SpeI digestion and insertion of a XhoI linker in place of the 3.5 kb EGFP fragment. We then targeted RP23-328P3 with the Math5>Cre shuttle vector pLD53 ACreB to obtain ampicillin- and chloramphenicol-resistant cointegrates (Gong et al., 2002). These were resolved by selection on TYE (tryptone-yeast extract) agarose plates with chloramphenicol and 10% (w/v) sucrose. Two recombinant Math5>Cre BAC clones were recovered and verified by PCR and pulsed-field gel electrophoresis (PFGE) Southern analysis.
Purified circular DNA from Math5>Cre BAC clone RP23-328P3-D1-68 was injected into fertilized (SJL/2 C57BL/6J) F2 oocytes by the UM Transgenic Animal Core Facility. Nine transgenic founders were identified by Cre-specific and BAC vector-insert junctional PCRs. Transgene copy number was determined by Southern analysis, using an upstream Math5 genomic probe that hybridizes equally well to 3.5 kb BAC and 6.5 kb mouse chromosomal EcoRI fragments. Transgene integrity was evaluated by Southern analysis following NotI digestion and PFGE. Transgenic offspring were genotyped using PCR primers within the Cre-pA cassette.
Math5>Cre mice (line 872 or 360) were crossed to Z/AP (JAX stock 003919, (Lobe et al., 1999) and R26floxGFP (JAX stock 004077, (Mao et al., 2001) reporter strains, which express membrane-tethered hPLAP (human placental alkaline phosphatase) or cytoplasmic GFP (green fluorescent protein), respectively, from ubiquitously active promoters, upon Cre-mediated excision of floxed upstream stop signals. Tissues from informative double transgenic progeny were collected from E11.5 to 15.5, on P0.5, and at 3-4 weeks of age. To trace lineage in the absence of Math5 function, we crossed Z/AP; Math5 −/− mice (Atoh7tm1Gla, (Brown et al., 2001) to Math5>Cre (line 360); Math5 +/− mice and compared the patterns of hPLAP staining in 3-4 week-old double transgenic mutants and heterozygous controls.
Histology
Embryonic and adult eyes were fixed overnight in 4% paraformaldehyde (PFA) at 4°C, cryoprotected in phosphate-buffered saline (PBS) with 10 to 30% sucrose, frozen in OCT compound (Tissue-Tek, Torrance, CA), and cryosectioned at 5-10 m. For Brn3b (Pou4f2) and cyclin D1 epitopes, fixation was 30 min at room temperature in 2% PFA. For immunodetection, cryosections were blocked for 4 hrs at room temperature in PBTx (0.1 M NaPO4 pH 7.3 0.5% Triton X-100) with 10% normal donkey serum (NDS) and 1% bovine serum albumin (BSA). Sections were incubated overnight at 4 C with primary antisera or biotinylated PNA (peanut agglutinin) lectin diluted in PBTx with 3% NDS and 1% BSA. For fluorescence detection, sections were incubated for 2 hrs at room temperature with appropriate secondary antibodies or streptavidin conjugates (Jackson Immunoresearch, West Grove, PA). Nuclei were identified using 100 ng/mL 4′,6-diamidino-2-phenylindole (DAPI). For chromogenic detection, sections were stained using the avidin-biotin complex method (Vector, Burlingame, CA) with HRP (horeseradish peroxidase)-conjugated streptavidin and diaminobenzidine (Brown et al., 2001).
The primary antibodies were mouse anti-β-galactosidase (βgal, monoclonal 40-1A, 1:500, DSHB, Iowa City, IA); rabbit anti-βgal (1:5000, ICN Cappel, Aurora, OH); rat anti-βgal (1:500, (Saul et al., 2008)); rat anti-BrdU (monoclonal BU1/75, 1:100, Harlan Seralab, Indianapolis, IN); mouse anti-calbindin (monoclonal CB-955, 1:500, Sigma, St. Louis, MO); mouse anti-Cre (monoclonal 7.23, 1:300, Covance, Princeton, NJ); mouse anti-cyclinD1 (sc8396, 1:100, Santa Cruz Biotechology, Santa Cruz, CA); rabbit anti-GFP (1:5000, Upstate, Lake Placid, NY); chicken anti-GFP (1:2000, Abcam, Cambridge, MA); mouse anti-hPLAP (monoclonal 8B6, 1:250, Sigma); mouse anti-Ki67 (monoclonal MM1, 1:25, Novocastra, Newcastle, UK); rabbit anti-mGluR2/3 (1:200, Chemicon); goat anti-Neurod1 (sc1084, 1:50, Santa Cruz); rabbit anti-phosphohistone H3 (1:400, Upstate, Lake Placid, NY); rabbit anti-rhodamine (1:500, Invitrogen). Biotinylated PNA (Vector) was used at 1:250.
For simultaneous detection of BrdU (5-bromo-2-deoxyuridine) and other markers, cryosections were fully stained with primary antibodies and lectins, and fluorescent secondary reagents. Sections were then treated with 2.4 N HCl in PBTx for 60-75 min at room temperature, washed, and immunostained for BrdU. EdU (5-ethynyl-2-deoxyuridine) was detected using an azide-alkyne cycloaddition reaction (Buck et al., 2008) and commercial reagents (Click-iT-647, Invitrogen) after immunostaining. For EdU and BrdU co-labeling, BrdU immunostaining was performed as the final step. For Ki67 immunostaining, sections were unmasked before the blocking step by heating to 95°C for 10 min in 0.01 M citric acid.
For chromogenic detection of hPLAP activity in retina, 5-10 μm cryosections were heat-treated for 30 min in PBS with 2 mM MgCl2 at 70 C and stained with 5-bromo-4-chloro-3-indolyl phosphate (BCIP) and nitroblue tetrazolium (NBT) substrates (Roche, Indianapolis, IN) for 1.5 hrs (Lobe et al., 1999). To detect hPLAP activity in the brain, adult tissues from transgenic animals were immersion-fixed in 4% PFA, 2 mM MgCl2 at 4 C overnight, heat-treated for 45 min in PBS with 2 mM MgCl2 at 70 C, and embedded in 3% agarose. Thick coronal vibratome sections (250 m) were stained for hPLAP activity as floating slices in 24-well plates in AP buffer containing 0.01% Na deoxycholate, 0.02% NP-40, 2 mM levamisole, and BCIP/NBT substrate (Roche), for 5-6 hrs at room temperature. Sections were washed in PBS containing 20 mM EDTA, dehydrated through a graded ethanol series, cleared with BABB (1:2 benzyl alcohol: benzyl benzoate) and mounted in Permount (Fisher Scientific, Pittsburgh, PA). Chromogenic detection of -galactosidase (gal) activity with 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (Xgal) substrate and in situ RNA hybridization were performed as described (Brown et al., 2001).
Images were obtained using a Nikon Eclipse E800 epifluorescence microscope and a SPOT digital camera. Low power images of brain sections were captured using a Zeiss Axioimager Z1 microscope with 5X objective. Confocal images were collected using a Noran OZ Laser Scanning Confocal assembly microscope or Zeiss LSM510 Meta imaging system.
Labeling RGCs by retrograde axonal tracing
To unequivocally identify all RGCs, we performed retrograde axon labeling with a rhodamine dextran tracer (Farah and Easter, 2005; Rachel et al., 2002). Eyes from adult Math5>Cre; R26floxGFP mice were rapidly immersed in artificial cerebral spinal fluid (aCSF) (von Bohlen und Halbach, 1999). The optic nerves were transected within 1 mm of the sclerae and pressed against 4 mm cubes of surgifoam (Ethicon, Somerville, NJ) saturated with 3% l-α-lysophosphatidyl choline (LPC, Sigma) and lysine-fixable tetramethyl rhodamine dextran 3,000 MW powder (Molecular Probes, Eugene, OR). Each eye and surgifoam cube was sealed with 1% agarose and incubated in aerated aCSF for 1 hr at room temperature. The eyes were then incubated overnight in fresh aCSF without surgifoam, fixed in 4% PFA for 4-6 hrs at room temperature, cryoprotected in PBS with 10 to 30% sucrose, and frozen in OCT. For P1 mice, the same retrograde labeling procedure was followed, except that eyes were immersed in Hank’s balanced salt solution containing calcium, magnesium and 1 mM glucose (Gerfen et al., 2001). After labeling, eyes were fixed in 4% PFA for 1 hr. The dissected retinas were post-fixed for 3 hrs, immunostained and flatmounted for imaging.
Math5 cell cycle analysis
Retinas from Math5 +/− and Math5 −/− embryos (carrying the lacZ knock-in allele) were co-labeled for gal, BrdU or EdU (S phase), phosphohistone H3 (M phase (Bradbury, 1992)), cyclinD1 (G1 and early S phases (Yang et al., 2006)), and Ki67 (S, G2, M and late G1 phases, (Key et al., 1993)). To label cells in S phase, pregnant dams were given a single intraperitoneal injection of EdU (6.7 μg/g of body mass) or BrdU (100 μg/g) 30-60 min prior to harvest. To test whether Math5+ progenitors re-enter the cell cycle, lineage-marked Math5>Cre embryos carrying Z/AP or R26floxGFP reporters were similarly pulsed with BrdU or EdU and their retinas co-stained for hPLAP, GFP or Cre and cell cycle markers.
Quantitative lineage analysis
Math5+ descendants were revealed by hPLAP or GFP immunolabeling in 200 adult retinal sections. Cell types were identified by laminar position, characteristic morphology, expression of diagnostic markers, and retrograde axon tracing. Lineage-marked cones were distinguished from rods by co-labeling with anti-hPLAP and PNA lectin (Blanks and Johnson, 1983). Because strong hPLAP staining in cone pedicles obscured horizontal cell bodies, we identified these cells using the R26floxGFP reporter and calbindin immunostaining (Peichl and Gonzalez-Soriano, 1993). Horizontal cells were surveyed in 58 sections from Math5>Cre; R26floxGFP mice (8 eyes). GFP-positive rhodamine-dextran labeled RGCs and DAPI-labeled nuclei (RGCs + displaced amacrines) were counted within the GCL in 33 fields (200X magnification) representing 8 Math5>Cre; R26floxGFP adult eyes. For P1 counts, the fraction of lineage-labeled RGCs was determined in retinal flatmounts from 3 eyes. The fraction of each cell type descending from Math5+ progenitors, and the fraction of Math5+ progenitors giving rise to each cell type, were calculated based on detailed retinal cell counts reported for adult C57BL/6J mice (Jeon et al., 1998). For lineage tracing in the absence of Math5 gene function, labeled cells were counted in 23 fields (200X magnification) representing 6 adult eyes.
Dual reporter concordance
To assess Math5>Cre efficiency and heterogeneity among Math5+ progenitors, we crossed Math5>Cre; Z/AP mice to homozygous R26floxGFP mice. Retinal sections from 3-4 week-old triple transgenic offspring (Math5>Cre; Z/AP; R26floxGFP) were immunostained for GFP and hPLAP. Single- and double-labeled cones, rods, amacrines and GCL neurons were counted in 18 fields (200X magnification) representing 4 eyes. To calculate concordance, we divided the number of double-labeled cells by the total number of labeled cells. Concordance was evaluated statistically using Cohen’s κ test (Cohen, 1960).
Birthdating and window labeling studies
To identify Math5 descendants exiting mitosis before P0, we performed a cumulative BrdU labeling experiment (Miller and Nowakowski, 1988). Pregnant dams carrying Math5>Cre; Z/AP embryos were given a single BrdU injection (100 g/g body mass) on day E10.5 and provided with drinking water containing 500 g/mL BrdU and 1% sucrose (pH 7.0) until birth (Mayer et al., 2000). To maximize labeling efficiency, water bottles were protected from light and replaced daily. Retinal sections from 3-week-old offspring were immunostained for BrdU and hPLAP.
To monitor how the fates of Math5+ progenitors exiting mitosis change during development, we performed birthdating (pulse-labeling) experiments. Pregnant dams carrying Math5>Cre; Z/AP embryos were given a single BrdU injection (as above) on day E14.5, E15.5, E16.5 or E17.5 of development. Eyes from 3-4 week-old mice were stained with BrdU and hPLAP antibodies, and PNA lectin. The total number of cones (PNA+) and the number of hPLAP+ and/or BrdU+ photoreceptors were counted in 14 central retinal fields (200X magnification), corresponding to 3 eyes for each time-point. For birthdating lineage-marked photoreceptors in the absence of Math5 function, we followed the same protocol as above. We immunostained Math5>Cre; R26floxGFP; Math5 −/− retinas for BrdU and GFP, and counted ≥7 fields (200X magnification) from 2-4 eyes for each time-point. The fraction of lineage-marked and birthdated cones was calculated directly from cell counts. The fraction of labeled rods was estimated using a 35.2 rod-to-cone ratio for wild-type mice, based on C57BL/6 data (Jeon et al., 1998), and a 12.1 ratio for Math5 mutants (SEM = 0.8 based on n = 5 animals, 71 fields at 200X magnification).
To determine the contribution of Math5+ cells to the early-born (EB) cohort of neurons, we performed pulse- and window-labeling experiments at the onset of neurogenesis. For pulse-labeling, gravid dams carrying Math5>Cre; R26floxGFP embryos were given a single injection of EdU at day E11, and eyes from the resulting pups were harvested at P1. Whole retinas were stained for GFP and EdU, flatmounted, and imaged as confocal Z-stacks through the ganglion cell layer. The fraction of early-born cells in the Math5 lineage (EdU+ GFP+ / EdU+) was determined from 4 eyes representing 4 mice.
For window labeling (Repka and Adler, 1992), pregnant dams carrying Math5 +/− and −/− embryos were given EdU on day E11, as a single injection or two injections 12 hrs apart. No difference was apparent in the extent of EdU labeling between these schedules. Dams were then given a single injection of BrdU on E12 and provided with BrdU in the drinking water until harvest at E12.5. Early-born cells (EdU+ BrdU−) were counted from 3-4 embryos of each genotype, representing 1-3 litters, and scored for βgal or Brn3b immunoreactivity. Statistical error is reported as the binomial standard deviation. Labeled fractions were compared using Fisher’s exact test (Fisher, 1925).
Retinal explants and clonal analysis
Retinal explant culture and retroviral infections were performed using established methods, which favor RGC survival (Hatakeyama and Kageyama, 2002; Wang et al., 2002). Math5 lacZ/+ retinas were dissected from E12.5 or E13.5 eyes, removing sclerae, pigmented epithelium (RPE) and lens tissue, and were flattened onto 5 mm Nucleopore polycarbonate membranes (0.4 μm pore size, GE Healthcare, Piscataway, NJ). These explants were placed on Transwell inserts (Corning) in 2-cm dishes containing neurobasal media (Invitrogen) with 1X B27 and N2 supplements, glutamine (0.4 mM), BDNF (50 ng/mL, Peprotech, Rocky Hill, NJ), CNTF (10 ng/mL, Peprotech), penicillin (50 U/mL), streptomycin (50 μg/mL), and gentamicin (0.5 μg/mL), and cultured at the gas-media interface at 37 C and 5% CO2.
MIG retroviral stocks (Van Parijs et al., 1999) were generated by transfecting MSCV-IRES-GFP plasmid DNA into the Phoenix ecotropic packaging cell line (Pear, 2001; Swift et al., 2001) and titered on NIH3T3 fibroblasts. Filtered viral preparations (~8×105 colony-forming units/mL) containing polybrene (hexadimethrine bromide, 0.8 μg/mL, Sigma Aldrich, St. Louis, MO) were added directly to the explant surface in one drop (25 μL) to infect mitotic cells. After 2 days in vitro (DIV), half of the media was replaced with fresh media. After 3 DIV, explants were fixed for 30 min in 4% PFA, cryoprotected in 30% sucrose, and frozen in OCT. Thick (30 μm) sections were immunostained for βgal and GFP. For each time-point, the size and composition of clones was determined by 3-dimensional analysis of confocal Z-stacks. Clones were defined as clusters of GFP+ cells directly apposed to each other (within 2-3 μm) and separated by at least 4 cell bodies from any other GFP+ cells. Only clones containing at least two GFP+ cells and one βgal+ cell were scored. Previous studies have shown that the average progenitor cell cycle length is 14-16 hrs at this stage (Alexiades and Cepko, 1996; Sinitsina, 1971), permitting 4-5 divisions during the 72 hr culture period. Accordingly, the largest clones in each set of explants contained 8-16 cells, reflecting a minimum of 3-4 divisions in vitro.
RESULTS
Math5 is transiently expressed by early retinal progenitors during or after their terminal cell cycle
As a first step to determine the mechanism of Math5 action, we defined the timing of Math5 expression during retinal development by quantitative PCR (Fig. 1A). Math5 mRNA increases rapidly at E11, peaks between E12.5 and E14.5, and declines gradually after E14.5. This temporal profile is consistent with RNA in situ hybridization data (Brown et al., 1998) and closely resembles birthdating curves for RGCs (Drager, 1985; Young, 1985). These data suggest Math5 acts transiently during early retinal neurogenesis.
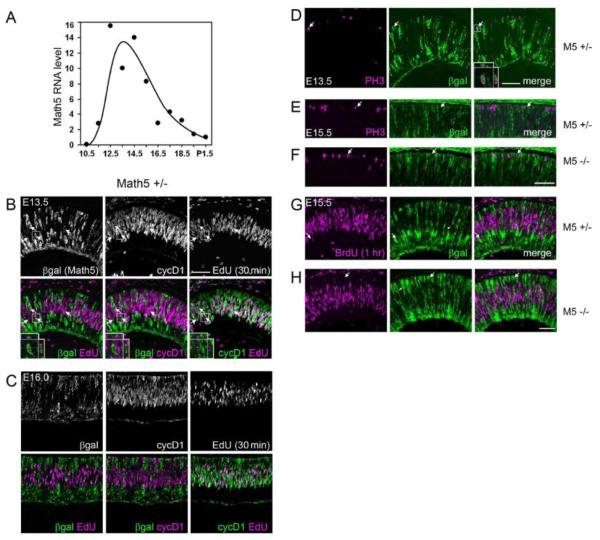
Fig. 1.
Math5 is expressed by early retinal progenitors during or shortly following their terminal cell cycle. (A) Time course of Math5 mRNA expression in developing eyes. Math5 mRNA levels peak at E14, with a profile that resembles RGC birthdating curves (Young 1985; Rapaport et al. 2004). (B-C) Sections from E13.5 (B) or E16.0 (C) Math5 +/− embryos co-stained for βgal (Math5-lacZ allele), EdU (following a 30 min chase), and cyclin D1 (marks G1/early S phase). Upper and lower panels show single- and double-labeled confocal projection images of 10 (B) or 3 (C) 1-m optical slices. Insets show a βgal+ cell in G2 (EdU+ cyclinD1−). At E13.5, some βgal+ EdU+ cells are present (arrows, 18 of 517 βgal+ cells), but none are cyclinD1+ (0 of 517). At E16.0, few or no βgal+ cells are EdU+ or cycD1+. (D-H) Retinal sections from Math5 +/− (D, E, G) and Math5 −/− (E, G) mice co-stained for βgal and cell cycle markers. Math5-lacZ is occasionally co-expressed with M-phase marker PH3 at E13.5 (arrow in D, inset), but does not overlap with PH3 (arrows in E,F) or BrdU (1 hr chase) (arrows in G,H) at E15.5. Therefore, Math5-lacZ expression initiates during terminal G2 phase at E13.5, but after terminal M phase at E15.5 and E16.0 in both Math5 +/− and −/− retinas. M5, Math5; βgal, E. coli β-galactosidase; cycD1, cyclinD1; PH3, phosphohistone H3. Scale bar, 50 m.
The cellular distribution of Math5 mRNA and Math5-lacZ activity across the retinal epithelium (Brown et al., 2001) is consistent with Math5 transcription in actively proliferating and/or postmitotic cells. Both patterns have been reported for different bHLH genes during neurogenesis (Kageyama and Nakanishi, 1997). Indeed, the closely related gene Math1 is expressed in mitotic cells in the developing cerebellum (Helms et al., 2000) and in postmitotic cells in the inner ear (Chen et al., 2002). In frog, zebrafish and chick retinas, orthologous Ath5 genes are expressed in progenitors during their last cell division (Matter-Sadzinski et al., 2001; Perron et al., 1998; Poggi et al., 2005).
To determine the onset of Math5 expression in individual mouse retinal progenitors, we immunostained E13.5, E15.5, and E16.0 eyes from Math5 +/− (lacZ/+) and/or Math5 −/− (lacZ/lacZ) embryos for β-galactosidase (βgal), the cell cycle marker phosphohistone H3 (PH3, M phase), cyclin D1 (cycD1, G1/early S phase) (Yang et al., 2006) or Ki67 (late G1, S and M phase), and the thymidine analog EdU or BrdU (S phase) following a 30-60 minute pulse in vivo. After the EdU pulse, a small fraction of S phase progenitor cells enter G2 and are detected as EdU+ cycD1-. In contrast, cells remaining in S phase are EdU+ cycD1+. After careful 3-dimensional analysis of confocal Z-stack images, we observed a small number of βgal-expressing cells that had incorporated EdU at E13.5 (18 of 517 = 3.5 0.6% SD) for n = 3 sections, Fig. 1B). These βgal+ cells were exclusively cycD1− (0 of 517, upper limit 95% CI = 0.6%), indicating that Math5 is expressed after G1 phase at E13.5. Accordingly, βgal+ PH3+ cells (M phase) were observed at E13.5 (Fig. 1D, (Le et al., 2006)). In contrast, in E15.5 and E16.0 embryos (Fig. 1C,E,G), few or no cells co-expressed βgal and cell cycle markers EdU, BrdU, cyclinD1, or PH3. The dynamics of Math5 expression thus change during development. At early stages (<E14), some progenitors initiate Math5 expression during the last cell cycle, whereas at later stages (>E15), progenitors express Math5 only after terminal mitosis. Similar results were observed in E15.5 Math5 knockout embryos (Fig. 1F,H) (Le et al., 2006), demonstrating that βgal+ mutant cells do not re-enter the cell cycle. In Math5 +/− and −/− mice, βgal+ cells span the entire retinal thickness (arrowheads in Fig. 1G,H), suggesting that radial processes associated with interkinetic nuclear migration may persist transiently, potentially directing the migration of early post-mitotic cells to their final laminar positions (Barnstable et al., 1985; McLoon and Barnes, 1989; Snow and Robson, 1994; Watanabe et al., 1991).
Math5>Cre lineage marking system
We designed an expression fate-mapping system to permanently mark lineal descendants of Math5-expressing progenitors and thereby define the range of fates acquired by these cells. The system has two components – transgenic mice expressing Cre recombinase under strict Math5 regulatory control (Math5>Cre) and reporter mice (Z/AP or R26floxGFP) that express a histochemical marker (hPLAP or GFP) wherever Cre excises a loxP-flanked stop signal.
The Math5>Cre recombinant BAC (Fig. 2A,B) includes all likely Math5 regulatory elements (Ghiasvand et al., 2011; Hutcheson et al., 2005). We generated nine Math5>Cre founders, each of which contains 1-5 copies of the BAC transgene (Suppl. Fig. 1A). Five lines were tested using Z/AP reporter mice, which conditionally express hPLAP under control of the ubiquitous CAG promoter (Lobe et al., 1999). Each line gave a similar staining pattern, which is consistent with the spatiotemporal expression of Math5 mRNA (not shown). All subsequent experiments were performed with lines 872 and 360, which contain full-length transgene insertions, as determined by diagnostic PCR, Southern and PFGE analysis (Suppl. Fig. 1B).
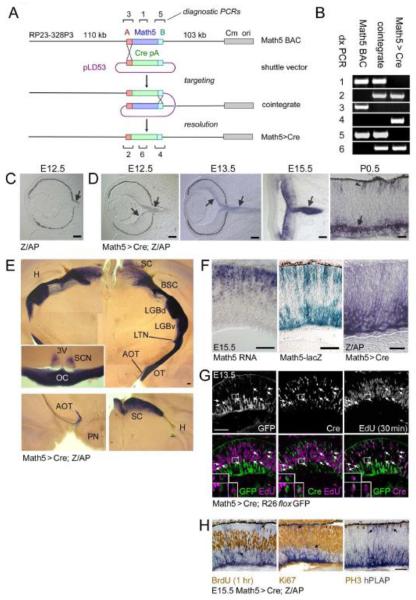
Fig. 2.
Construction and expression of the Math5>Cre transgene. (A) Math5>Cre BACs were generated in E. coli by a two-step homologous recombination procedure. The single-exon Math5 open reading frame was precisely replaced with a nlsCre-pA cassette, using “A” and “B” homology arms derived from 5′ (red box) and 3′ (cyan box) UTRs. The pLD53 shuttle vector contains recA recombinase, positive (amp) and negative (sacB) selection cassettes, and the R6K origin of replication. The pBACe3.6 vector (gray box) contains the chloramphenicol resistance gene (Cm) and P1 origin (ori). (B) Confirmation of recombinant BAC structure using diagnostic (dx) PCRs 1-6 indicated in panel A (assembled from multiple gels). (C-F) Developmental expression pattern. Math5>Cre mice were crossed to mice carrying the Z/AP transgene, which permanently reports Cre activity. Alkaline phosphatase (hPLAP)-positive RGCs (purple, arrows) are first observed at E12.5 in double transgenic embryos (D), while control littermates (C) containing only the Z/AP transgene are negative. hPLAP activity increases from E13.5 to E15.5 as RGCs develop and form the optic nerve. By P0.5, hPLAP activity is abundant in RGCs (arrow) and can be detected in some photoreceptors (arrowhead); however most of the retina is unlabeled. (E) Composite images of 250 μm coronal vibratome sections through the adult thalamus and optic chiasm (inset) show the axonal projections of RGCs derived from Math5+ precursors. Lower panels show sections through the accesory optic system (left) and superior colliculus (right). Labeled RGCs project to all major ganglion cell target sites in the CNS. No significant staining was observed in the cerebral cortex or hippocampus. (F) Kinetics of Math5 expression in the E15.5 retina. Math5 mRNA (in situ hybridization) is expressed in retinal progenitor cells, while the cytoplasmic Math5-lacZ knock-in allele labels progenitors (sclerad) and developing RGCs (vitread), which have recently transcribed Math5 (β-galactosidase activity). hPLAP activity in Math5>Cre; Z/AP mice is localized to developing RGCs on the vitread side of the retinal epithelium. These patterns demonstrate the spatiotemporal progression of Math5 expression, if one considers the perdurance of β-galactosidase, the delay associated with Cre excision, and interkinetic nuclear migration. Math5 is expressed transiently in progenitors that become RGCs. (G-H) Math5>Cre retinas co-stained for lineage tracers and cell cycle markers. Cre is expressed with the same kinetics as Math5, whereas GFP or hPLAP reporters are expressed with a delay. (G) At E13.5, Cre+ EdU+ cells are present (30 min chase, arrows, 33 of 394 Cre+ cells), but no GFP+ EdU+ cells are observed (0 of 309 GFP+ cells). (H) At E15.5, no hPLAP+ cells (arrows) are co-labeled with BrdU (1 hr chase), PH3, or Ki67 (marks late G1 through M phase). Together, these results indicate that cells in the Math5 lineage do not re-enter the cell cycle. pA, polyadenylation signal; SC, superior colliculus; BSC, brachium of the superior colliculus; LGBd, lateral geniculate body, pars dorsalis; LGBv, lateral geniculate body, pars ventralis; LTN, lateral terminal nucleus; AOT, accessory optic tract; OT, optic tract; TV, third ventricle; SCN, suprachiasmatic nucleus; OC, optic chiasm; H, hippocampus; PN, pons. Scale bars, 100 m in C-F; 50 m in G-H.
From the onset of retinal neurogenesis (E11), Math5 mRNA is expressed in cells near the ventricular (sclerad) neuroepithelial surface, where the majority of progenitors undergo mitosis (Brown et al., 1998). In Math5-lacZ knock-in mice, -galactosidase protein is expressed in a similar pattern but perdures (Echelard et al., 1994) in the differentiating descendants of these cells, including RGCs (Brown et al., 2001). In double transgenic Math5>Cre; Z/AP embryos, the alkaline phosphatase (hPLAP) marker first appeared at E12.5 in differentiating RGCs and the developing optic nerve (Fig. 2D), whereas no hPLAP was detected in control embryos carrying Z/AP alone (Fig. 2C). At later developmental stages, some other cell types were labeled with hPLAP (e.g. photoreceptors at P0.5 in Fig. 2D, arrowhead). As expected, hPLAP was only detected in the adult retina and brain, in known Math5 RNA expression domains. In the central nervous system, the hPLAP reporter marks neurons in the auditory hindbrain and cerebellum (Saul et al., 2008) and reveals all known RGC projections (Rodieck, 1998; Simpson, 1984), including those extending to the superior colliculi, lateral geniculate bodies, suprachiasmatic nuclei, and the accessory optic tracts (Fig. 2E).
In the E15.5 retina, a comparison of the spatial and temporal patterns for Math5 mRNA, Math5-lacZ and hPLAP (Fig. 2F) is consistent with a direct role for Math5 in RGC development and highlights the inherent time delay associated with Cre protein synthesis, excisional activation of the Z/AP reporter, and expression of the hPLAP enzyme (Nagy, 2000). Considering the dynamics of retinal interkinetic nuclear migration (Baye and Link, 2008), these results suggest there is a burst of Math5 expression in progenitors exiting the cell cycle. If Math5 is exclusively made during the last division, lineage-marked cells should never re-enter S phase. To test this prediction, we analyzed E13.5 Math5>Cre; R26floxGFP and E15.5 Math5>Cre; Z/AP embryos exposed to EdU or BrdU for 1 hr (Fig. 2G,H). In E13.5 embryos after a 30 min chase, some Cre+ EdU+ cells were present (33 of 394 Cre+ cells = 8.4 0.4% SD for n = 3 sections) and these were restricted to the fresh neurogenic subset (33 of 223 Cre+ GFP-cells = 14.8 1.4% SD). No GFP+ EdU+ cells were observed in the same sections (0 of 309 GFP+ cells, upper limit 95% CI = 0.9%), due to the delay in the Cre-lox system (Fig. 2G). Likewise, in E15.5 embryos, there was no overlap between hPLAP activity and any cell cycle marker (Fig. 2H). Together, these results strongly suggest that Math5 is expressed transiently during or shortly after the terminal cell division. Math5 lineage cells do not re-enter the cell cycle.
Quantitative Math5 lineage analysis
To reveal the fates of Math5+ progenitors, we crossed Math5>Cre mice to Z/AP and R26floxGFP reporter strains and examined mature retinas of 3-4 week old offspring. We observed hPLAP+ cells distributed evenly across the central and peripheral retinas of Math5>Cre; Z/AP mice, but staining was absent in littermates carrying the Z/AP transgene alone (Fig. 3A,B). Because hPLAP protein is membrane-tethered, we could identify most retinal cell types by morphology and laminar position. As expected, RGCs were abundantly labeled. However, we also observed significant staining among rods, cones, horizontal and amacrine cells (Fig. 3A,C,D). The inner plexiform layer (IPL) was intensely labeled due to hPLAP localization in RGC and amacrine dendrites. A thorough survey revealed rare hPLAP+ Müller glia and bipolar cells (Fig. 3C). Importantly, no labeling was observed in retinal cell types that have a separate developmental origin, such as vascular endothelial cells, pericytes, microglia and astrocytes, or in any other parts of the eye, including the anterior chamber and RPE.
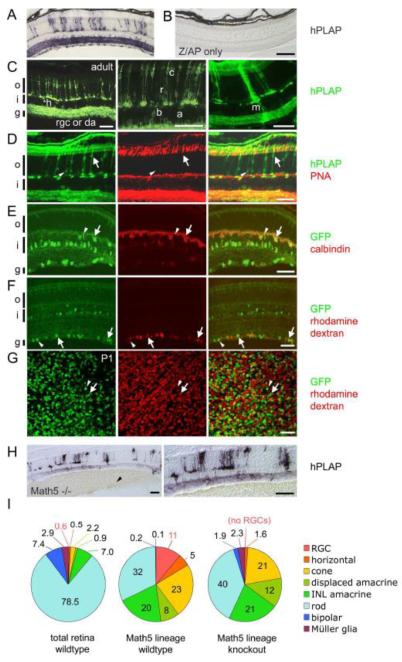
Fig. 3.
Math5+ progenitors contribute differentially to all retinal cell types. Math5>Cre mice were crossed to Z/AP (A-D) or R26floxGFP reporter (E-G) strains. (A) In Math5>Cre; Z/AP mice, hPLAP+ descendants of Math5+ progenitors represent 3% of adult retinal cells (see Table 1) and are present in every cell layer. (B) Z/AP-only control retinas have no hPLAP activity. (C) Math5+ descendants, detected by hPLAP immunostaining, include horizontal (h), ganglion (rgc), displaced amacrine (da), INL amacrine (a), bipolar (b), rod (r), cone (c) and Müller glial (m) cells. (D) Math5+ cone (arrows) and rod (arrowheads) photoreceptors are distinguished by co-labeling with anti-hPLAP and cone-specific PNA lectin. Non-specific labeling of pigment epithelium and choroid reflects mouse IgG crossreactivity. (E-G) In Math5>Cre; R26floxGFP mice, Math5+ horizontal cells (E, arrows) are marked by GFP and calbindin immunoreactivity. The arrowhead shows a solitary Math5+ bipolar cell. (F-G) Math5+ RGCs (arrows) and displaced amacrines (arrowheads) in the GCL are shown in adult retinal sections (F) or P1 retinal flatmounts (G). RGCs are distinguished by retrograde labeling of optic nerve axons with rhodamine dextran. There is no difference in the GFP+ fraction of rhodamine dextran-labeled RGCs between these two ages. (H) The fate of Math5>Cre-expressing progenitors in Math5 −/− mice. hPLAP+ cells are distributed throughout the retina, but RGCs are lacking. Vitreal hemorrhages (arrowhead) are common in Math5−/− mice. (I) The distribution of cell fates in the entire retina (from Jeon et al., 1998), in the Math5 lineage of wild-type mice, and in the Math5 lineage of knockout mice. The Math5 lineage is biased toward early-born cell types (RGC, horizontal, cone), although rods are the most common fate adopted by Math5+ cells. In the Math5 knockout, lineage-derived cells adopt all retinal fates except for RGCs. hPLAP, human placental alkaline phosphatase; o, outer nuclear layer; i, inner nuclear layer; g, ganglion cell layer. Scale bars, 100 m in A-B, H; 50 m in C-G.
To systematically measure the fraction of lineage-marked retinal cells in each class, we co-stained sections for hPLAP or GFP reporters and cell type-specific markers. Equivalent results were obtained using Z/AP and R26floxGFP reporters (see below) and different Math5>Cre lines (data not shown). However, the intensity of expression varied among cell types. Z/AP is strongly expressed in photoreceptors via the CAG promoter, whereas R26floxGFP is weakly expressed by photoreceptors but strongly expressed by other cell types. Consequently, we used Z/AP to count hPLAP+ and hPLAP-cones (arrows in Fig. 3D), and hPLAP+ rods (arrowheads in Fig. 3D) in the outer nuclear layer (ONL), and PNA lectin to distinguish cones from rods (Blanks and Johnson, 1983). We then counted hPLAP+ bipolar cells and Müller glia (Fig. 3C) in the inner nuclear layer (INL) of the same sections. The labeled fraction was calculated for each cell type using reference data for cell populations in the adult mouse retina (Jeon et al., 1998). This fraction ranged from 31% for cones to 1% for rods, and <0.1% for bipolar cells and Müller glia (Table 1).
Table 1. Cell type distribution of Math5 lineage descendants In wild-type Math5>Cre transgenic retinas.
| cells counted |
||||||
|---|---|---|---|---|---|---|
|
Math5 lineage |
total |
Math5 lineage (% of cell type)§ |
cell type† (% of retina) |
Math5 lineage (% of retina) |
cell type‡ (% of Math5 lineage) |
|
| CELL TYPE | (a) | (b) | (a/b) × 100 | (c) × 100 | (a/b)(c) × 100 | (a/b)(c/f) × 100 |
| RGC | 700 | 1,265# | 55§ | 0.6 | 0.3 | 11 |
| cone | 1,515 | 4,914 | 31 | 22 | 0.7 | 23 |
| horizontal | 1,041 | 3,592 | 23 | 0.5 | 0.2 | 5 |
| amacrine | 1,665 | 15,570* | 11 | 7.9 | 0.8 | 29 |
| INL | 1,198 | 13,920* | 9 | 7.0 | 0.6 | 20 |
| displaced | 467 | 1,648# | 28 | 0.9 | 0.2 | 8 |
| rod | 2,085 | 173,000* | 1 | 78.5 | 0.9 | 32 |
| bipolar | 12 | 12,900* | <0.1 | 7.4 | <0.005 | <0.2 |
| Müller glia | 5 | 8,800* | <0.1 | 2.9 | <0.002 | <0.1 |
| TOTAL | 100.0 | 2.9(f) | 100 | |||
RGCs, displaced amacrines, and INL amacrines were counted in 33 fields (200×,8 eyes. 6 mice. R26floxGFP reporter). Horizontal cells were counted in 58 sections (8 eyes,6 mice, R26FLOXGFP reporter). All other cell types were counted in 50 to 70 fields (200X, 16 eyes, 12 mice, Z/AP reporter) Math5+ descendants detected using the Cre lineage system comprise 2.9% of the adult retina (f).
Estimated using cell type ratios reported by Jeon et al. (1998) for adult C57BL/6J mice. The total number of cones counted in surveyed fields was multplied by 35.2 to give the numberof rods, and by 3.32 for bipolar cells and 1.3 for muüller glia The total number of GCL neurons surveyed (RGC and displaced amacrines) was multiplied by 4.78 to estimate the number of inner nuclear layer (INL) amacrines.
RGCs (identified by retrograde axon labeling) represent 43% of GCL neurons (1265/2913 cells). The remaining GCL cells were scored as displaced amacrines.
Among n = 8 eyes, the mean RGC labeling fraction ±SEM was 54 ±2%, with a range between 46 and 63%. The overall labeling fraction for the GCL was 40% (1167/2913 cells), which represents 24% RGCs(700/2913 cells)and 16% displaced amacrines (467/2913 cells).
Calculated from Jeon et al. (1998) and shown in Fig 3I (left pie chart)
These values are shown in Fig 3I (middle pie chart).
To evaluate horizontal, ganglion and amacrine cell types, we used the R26floxGFP reporter, which co-localizes with cell type-specific markers in the perinuclear cytoplasm. We identified horizontal cells by calbindin immunostaining (Peichl and Gonzalez-Soriano, 1994) and their position at the outer edge of the INL (Fig. 3E). Twenty-nine percent of horizontals were GFP+ (Table 1). This value is significantly lower than that reported by Yang et al. (2003), but consistent with horizontal cell labeling data of Feng et al. (2010, cf. Suppl. Fig. 3E) obtained using a Math5-Cre knock-in allele. RGCs were distinguished from displaced amacrine cells (Hayden et al., 1980; Perry and Walker, 1980) by retrograde rhodamine-dextran tracing of optic nerve axons. Forty-three percent of neurons in the ganglion cell layer (GCL) were labeled with rhodamine in this experiment (arrows, Fig. 3F), in close accord with previous data (Jeon et al., 1998). All other cells in the GCL were scored as displaced amacrines (arrowheads, Fig. 3F). The frequency of GFP labeling in the adult retina was 55% for ganglion cells, 28% for displaced amacrines, and 9% for INL amacrines (Table 1). To evaluate the Math5 lineage fraction prior to the normal period of RGC culling (Galli-Resta and Ensini, 1996), we performed a similar analysis in early postnatal retinas, limited to the GCL (Fig. 3G). The fraction of GFP+ ganglion cells in P1 retinas (53 ± 1%, n = 3, 948/1777 cells) was similar to that observed in the adult (55 ± 2%, Table 1, Fisher’s exact test P = 0.3).
A clear pattern emerges from these data. First, Math5+ progenitors have the potential to develop into all seven major retinal cell types. Second, the distribution of Math5+ descendents differs from the retina as a whole (Fig. 3I, χ2 test with df = 7, P < 0.0001). Third, the labeling fraction of each cell type (Table 1) decreases according to the birth order (Carter-Dawson and LaVail, 1979; Rapaport et al., 2004; Sidman, 1961; Young, 1985). Early-born cell types – RGCs, cones, horizontal cells and displaced amacrines – frequently descend from Math5+ progenitors, whereas late-born bipolar and Müller glial cells rarely derive from Math5+ progenitors. INL amacrines are born during the middle phase of retinal development, prior to the peak of rod births, and these cell types have an intermediate labeling fraction. We estimate that 3% of adult retinal cells descend from Math5+ progenitors (Table 1). Fourth, only one in nine Math5+ descendants is a ganglion cell (11%). Because RGCs represent ~0.5% of neuroretinal cells in adult mice (Jeon et al., 1998) and Math5 status does not affect RGC survival between P1 and adulthood, Math5 descendants are 50-fold more likely on average to develop as RGCs than the remaining neuroretinal population (approx. 1 in 500). Fifth, 45% of ganglion cells are not marked by the Math5>Cre transgene, suggesting the possibility of a substantial Math5-independent RGC subpopulation. Although the fraction of GCL neurons labeled by Math5>Cre (40%, Table I) approximates the RGC fraction (43%), this value includes both RGC (24%) and displaced amacrine cell types (16%).
The fate of Math5 mutant cells
In mutant mice, the Math5 transcription unit is active, expressing lacZ mRNA, but lineage-marked progenitors are blocked from developing as RGCs. To determine the fates of these cells, we examined retinas from adult Math5 −/− mice carrying Z/AP and Math5>Cre transgenes (Fig. 3H). The extent of hPLAP labeling in the mutant retina was roughly similar to wild-type (Fig. 3A). However, the fate profile within the Math5 lineage was different (χ2 test with df = 7, P < 0.0001). First, RGCs were absent, as expected, decreasing the amount of IPL staining. Second, there was an obvious increase in ‘late-born’ cell types among the hPLAP+ neurons (Suppl. Table 1). For example, rod photoreceptors increase from 32% to 40% of the Math5 lineage. Labeled bipolar cells and Müller glia were visible in most low power fields (200X magnification) of mutant mice, but were difficult to find in wild-type Math5>Cre; Z/AP retinas (Table 1), consistent with results observed by (Feng et al., 2010). This effect is more striking if one considers that the total number of rods, bipolar cells and Müller glia are decreased in Math5 mutants (Brown et al., 2001; Brzezinski et al., 2005). In Math5 mutant mice, the cohort of progenitors expressing Math5>Cre does not differentiate into RGCs, but retains competence to develop into any of the remaining principal cell types.
Math5+ progenitors have equivalent Cre activity
Only a small fraction (11%) of the Math5 lineage develops into RGCs. In principle, the Math5+ population may be heterogeneous, such that one group of progenitors expresses high levels of Math5>Cre and develops as RGCs, while a second group expresses low levels of Math5>Cre and adopts other fates (Fig. 4A). In this model, the low-level multi-lineage Math5>Cre expression could represent ‘priming’ (Hu et al., 1997) of the Math5 gene, or leaky transgene expression, an ‘over-reporting’ artifact that is not biologically meaningful (Dymecki et al., 2002). Alternatively, all Math5+ progenitors may express equivalent levels of Math5>Cre (Fig. 4B), consistent with a permissive role for Math5 in retinal development.
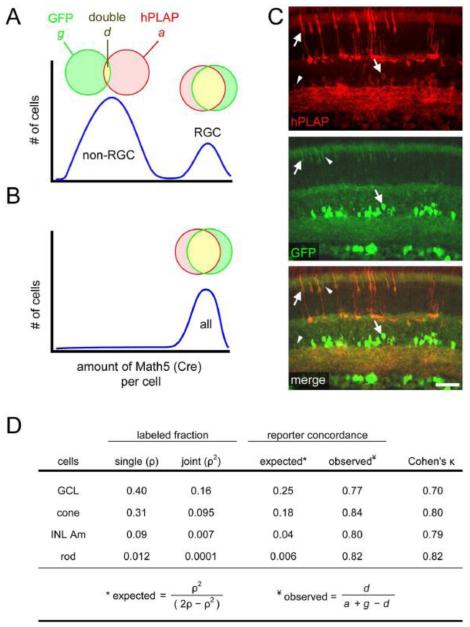
Fig. 4.
All Math5>Cre progenitors express similar levels of Cre, regardless of cell fate. Math5>Cre lineage analysis was performed using Z/AP and R26floxGFP reporters simultaneously, to evaluate the heterogeneity of Math5 expression among progenitors. This analysis assumes that the probability of reporter activation in a given cell is determined by the cumulative amount of Cre recombinase expressed by that cell. (A-B) Two models for Math5 (Cre) expression. (A) Bimodal expression. In this model, Math5+ progenitors giving rise to non-ganglion cell types express Cre weakly (left peak), so reporter activation in these cells is inefficient, and consequently few of their descendants co-express GFP and hPLAP. RGCs in the same retinas express Cre strongly (right peak) and are expected to have high concordance. (B) Uniform expression. In this model, every Math5+ progenitor expresses Cre strongly, so concordance is very high for all cell types (B, right). (C) Retinas of adult Math5>Cre; Z/AP; R26floxGFP mice immunostained for hPLAP and GFP. Double-labeled cells (arrows) greatly outnumber single-labeled cells (arrowheads). (D) The observed concordance between hPLAP and GFP reporters was ~80%, which is significantly greater than expected by chance (Cohen’s κ > 0.7). This value was similar for all cell types, indicating that Math5 is expressed at uniform levels by a subpopulation of progenitor cells, only some of which develop as RGCs. The labeled fractions (ρ) are based on data in Table 1. GCL includes RGCs and displaced amacrines; INL Am, inner nuclear layer amacrine. Scale bar, 50 m.
To test these alternatives, we examined retinas from triple transgenic (Math5>Cre; Z/AP; R26floxGFP) adult mice, using the concordance of hPLAP and GFP labeling in Math5 descendants as an indirect measure of Cre activity (Fig. 4C). In this experiment, we assume that the probability of reporter activation in a particular cell depends on the concentration and stability of intracellular Cre protein, and that neither reporter is saturated at the Cre levels under investigation. Progenitors with strong Cre expression are expected to activate both reporters, while progenitors with weak Cre expression may randomly activate one reporter, Z/AP or R26floxGFP, at a low frequency (Fig. 4A,B). If these events occur independently with equal probability (ρ), then the joint probability of observing both reporters in a single cell (expected concordance) should equal ρ2/(2ρ-ρ2), where ρ2 is the fraction of cells that activate both reporters and 2ρ-ρ2 is the fraction of cells that activate at least one reporter. The observed concordance was uniformly high (~80%) for rods, cones, INL amacrines and GCL neurons, and much greater than expected by chance (Cohen’s κ > 0.7, Fig. 4D, Suppl. Table 2). Thus, the labeling of non-RGC cell types cannot be attributed to differential or leaky Math5>Cre expression.
The fate of the Math5+ progenitor population changes over time
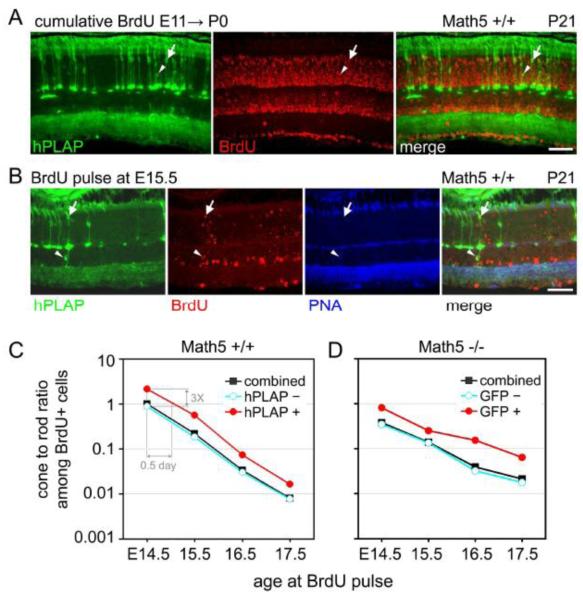
The discovery that some rods, bipolars and Müller glia descend from Math5>Cre progenitors (Table 1) is somewhat surprising because the vast majority of these cell types undergo terminal mitosis (Rapaport et al., 2004; Young, 1985) after the temporal envelope of Math5 mRNA expression (Fig. 1A). To explain these findings, we performed a cumulative labeling experiment, in which Math5>Cre; Z/AP embryos were continuously exposed to BrdU from E10.5 until P0 and analyzed at P21. Retinal progenitors that exit mitosis before P0 should be heavily BrdU-labeled, whereas those that continue to divide after P0 should be weakly labeled. We found that essentially all hPLAP+ cells in the central retina were heavily labeled with BrdU (98.8%), including rods (arrowheads in Fig. 5A), cones (arrows in Fig. 5A), and INL and GCL neurons (Suppl. Table 3). Therefore, Math5+ rods, bipolars and Müller glia are born at the ‘leading edge’ of birthdating curves for these ‘late’ cell types.
Fig. 5.
The fate distribution of Math5+ progenitors changes over time. (A) Cumulative BrdU labeling experiment. Math5>Cre; Z/AP embryos were continuously exposed to BrdU from E10.5 to P0 and their retinas were collected at P21. Nearly all Math5+ descendants (hPLAP+) are heavily labeled with BrdU, indicating that the majority exited mitosis before P0, including lineage-labeled cones (arrows) and rods (arrowheads). There is a distinct gradient of BrdU labeling (birthdates) within the inner and outer nuclear layers, such that cells with nuclei closest to the lens have earlier birthdates (brightest BrdU signal). (B) Pulsed BrdU labeling experiment. Math5>Cre; ZAP embryos were transiently exposed to BrdU at E15.5. Adult retinas were stained with hPLAP and BrdU antibodies and PNA lectin. Math5+ cone (hPLAP+ PNA+ BrdU+, arrow) and bipolar (hPLAP+ BrdU+, arrowhead) cells are indicated. (C) Cone-rod ratio plots for birthdated hPLAP+ (red), hPLAP− (blue) and combined (black) photoreceptor groups. The ratio of cone-to-rod births decreases steadily between E14.5 and E17.5 for hPLAP+ and hPLAP-populations. The curves are parallel, indicating that the fate of Math5+ cells changes over time, similar to other retinal progenitors. However, the cone-to-rod ratio is 3-fold higher for Math5+ progenitors at every time point, suggesting that these cells have a fixed cone vs. rod bias, or are shifted by 0.5 days, compared to other neurogenic cells (hPLAP−) in the same retinal environment. (D) Cone-rod ratio plot for birthdated GFP+ (red), GFP− (blue) and combined (black) photoreceptor groups in Math5 −/−; Math5>Cre; R26floxGFP mice. Scale bar, 50 m.
The fate profile of neurogenic cells emerging from the RPC population is known to change over time, in response to intrinsic factors and environmental signals (Livesey and Cepko, 2001; Rapaport et al., 2004; Young, 1985). This can occur through alterations in the fate bias of individual cells or the composition of the RPC pool (heterogeneity). In principle, the Math5+ cohort may behave similarly. The fate profile of these cells may be intrinsically programmed, or it may vary depending on the time that an individual RPC exits mitosis and initiates Math5 transcription. To test these alternatives, we compared the fates of Math5+ progenitors born on different days. Math5>Cre; Z/AP embryos were exposed to a pulse of BrdU on E14.5, E15.5, E16.5 or E17.5 and their adult retinas were examined by hPLAP, PNA, and BrdU staining (Fig. 5B). A variety of lineage-marked cell types were born on each of these days, including RGCs, rods, cones, amacrines and horizontal cells, as well as rare ‘late’ cell types (arrowhead in Fig. 5B). For quantitative analysis, we focused on photoreceptors, which are relatively numerous and could be directly compared within the ONL. At each time-point, we determined the fraction of hPLAP+ and heavily BrdU+ rods and cones in the central retina (arrows in Fig. 5B, Suppl. Table 4). The fraction of photoreceptors (rods plus cones) that were derived from Math5+ progenitors decreased between E14.5 and E17.5, from 20.6% to 4.3% (Suppl. Fig. 2, Suppl. Table 4), in parallel with the decrease in the total number of Math5+ cells.
The fate of the Math5+ cell population also changed significantly between E14.5 and E17.5, together with the retina as a whole. Math5+ cells born on E14.5 were >2 times as likely to develop into cones as compared to rods (136 vs. 64), whereas those born on E17.5 were >60 times as likely to develop into rods as compared to cones (122 vs. 2, Suppl. Table 4). The fates of progenitors inside and outside the Math5 lineage shifted in parallel, as shown by plots of the cone-to-rod ratio (Fig. 5C), derived from birthdating curves (Suppl. Fig. 2). This shift is primarily determined by the overall decrease in cone births during this interval. Math5+ cells appear to follow the same fate trajectory as other progenitors. However, the ratio curves are displaced by one-half day. In comparison to other neurogenic cells (hPLAP−) exiting mitosis on the same day in the same retinal environment, Math5+ progenitors (hPLAP+) were three times more likely to develop into cones. Surprisingly, similar results were obtained in the absence of Math5 function, in mutant embryos carrying R26floxGFP and Math5>Cre transgenes (Fig. 5D).
These findings support three conclusions. First, the fate profile of Math5+ cells changes over time, similar to that of other retinal progenitors. Second, the fate bias of Math5+ cells extends beyond RGC specification, influencing the choice among alternative fates (e.g. cone vs. rod). Third, the bias among alternative fates is independent of Math5 action, suggesting that upstream or parallel factors are responsible.
Math5 expression in early-born retinal cells
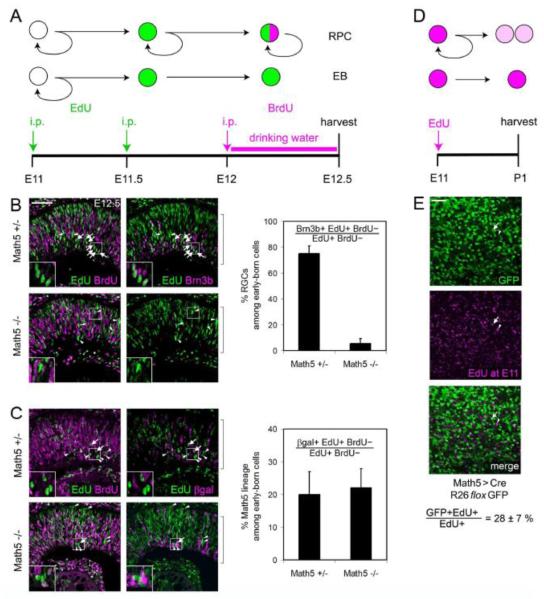
Because Math5 expression is closely correlated with the onset of retinal neurogenesis (~E11.5) (Hufnagel et al., 2010) and is essential for specification of the earliest born cell type, RGCs (Brown et al., 2001; Wang et al., 2001), we expected that most or all early-born retinal cells would express Math5 and adopt RGC fates. To test this hypothesis, we performed a series of window-labeling experiments. Embryos were sequentially exposed to EdU at E11 and BrdU at E12 (Fig. 6A). In this paradigm, cells incorporate EdU if they are in S phase at E11. Because the average cycle length at this stage is less than 24 hrs (Sinitsina, 1971), EdU+ BrdU+ cells scored at E12.5 are interpreted as RPCs that underwent one additional division (and S phase). In contrast, EdU+ BrdU-cells define the early-born (EB) cohort. These cells were in S phase at E11, but exited the cell cycle before E12.5.
Fig. 6.
Math5 marks many of the earliest born cells in the retina. (A-C) Window labeling analysis. (A) Embryos were exposed to pulses of EdU at E11 (onset of neurogenesis) and E11.5, and to continuous BrdU from E12 to E12.5. Progenitors (RPCs) that continue to cycle through E12.5 are EdU+ BrdU+, while cells that have exited mitosis between E11 and E12 are EdU+ BrdU-, and represent the earliest born cohort of retinal neurons. (B-C) Sections through the neural retina (brackets). (B) Most early-born cells in Math5 +/− mice adopt RGC fate (EdU+ BrdU-Brn3b+, arrows). The Brn3b-cells in this cohort are likely to include horizontal cell precursors (arrowheads). Few Brn3b+ RGCs (arrows) are present in Math5 −/− embryos, and the abundance of non-RGC fates increases accordingly (arrowheads). (C) Early-born Math5-lacZ (EdU+ BrdU-βgal+, arrows) and βgal− (arrowheads) cells are shown in Math5 +/− (top) and Math5 −/− (bottom) mice. Only ~20% of the early-born cohort expresses the Math5 transcription unit (βgal+), in both genotypes. (D-E) Birthdating analysis. (D) E11 Math5>Cre; R26floxGFP embryos were exposed to a single EdU injection and analyzed at P1. (E) Flatmounted retinas were stained for EdU and GFP and imaged through the GCL. Strongly EdU+ cells mark the earliest born retinal neurons. Confocal projection image (6-10 μm) shows EdU+ GFP+ (arrow) and EdU+ GFP− (arrowhead) cells. Only 28% of the GCL cells born at E11 are in the Math5+ lineage. i.p., intraperitoneal; EB, early-born. Error bars represent the binomial standard deviation. Scale bar, 50 m.
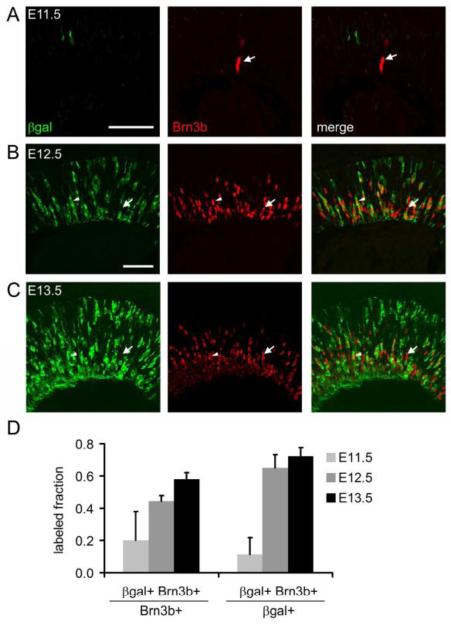
To evaluate RGCs within the EB cohort, we counted the fraction of EdU+ BrdU-cells that were Brn3b+ (RGCs) in Math5 heterozygous and mutant mice (Fig. 6B). In Math5 +/− embryos, 75% of the EB cohort expressed Brn3b, confirming that RGCs are the predominant first-born cell type (Farah and Easter, 2005; Rachel et al., 2002). The abundance of EdU+ BrdU-cells was similar in Math5 −/− and +/− embryos (5.7 vs. 7.1 per 0.001 mm2 field, respectively) and comparable to previous birthdating results (Le et al., 2006). However, in Math5 mutant embryos, only 6% EdU+ BrdU-cells expressed Brn3b. This was expected from the deficiency of RGCs in these mice, and confirms that the loss of RGCs is an early event. We next determined the fraction of EdU+ BrdU-cells that expressed Math5, using the lacZ allele (βgal) as a short-term lineage tracer (Wang et al., 1999). Surprisingly, only 20% of EdU+ BrdU-cells were βgal+, in both Math5 +/− and Math5 −/− mice (Fig. 6C). To independently test this result, we exposed Math5>Cre; R26floxGFP embryos to a single pulse of EdU at E11, harvested their retinas at P1, and determined that 28% of strongly EdU+ cells in the GCL were GFP+ (Fig. 6D,E). As a third test, we evaluated retinas from early Math5-lacZ/+ embryos for coexpression of lacZ and Brn3b. The fraction of gal+ RGCs was relatively low at E11.5, consistent with the EB analysis, but increased from 20% to 60% between E11.5 and E13.5 (Fig. 7). Taken together, the results from these three experiments suggest that Math5 is expressed by a subset of early neurogenic cells, and that only a fraction of Brn3b+ RGCs generated at E11-13 derive from the Math5+ cohort.
Fig. 7.
A subset of Brn3b+ RGCs derives from the Math5 lineage. (A-C) Sections from embryonic Math5-lacZ/+ retinas co-stained for βgal and Brn3b. At E11.5, relatively few Brn3b+ cells are βgal+ (A, arrow). The fraction of Brn3b+ cells expressing Math5-lacZ (arrowheads) increases from E12.5 (B) to E13.5 (C). However, there are many Math5-independent RGCs (arrows) at each age. (D) Histograms showing the fraction of Math5+ cells among Brn3b+ RGCs and the fraction of Brn3b+ RGCs within the Math5+ cohort. Error bars show the standard deviation (n = 3 sections). The total number of cells counted at E11.5, E12.5 and E13.5 was 13, 228 and 667, respectively. Scale bar, 50 μm.
The Math5-independent early-born cells may express other proneural bHLH transcription factors in the Atonal family, such as Neurod1 or Neurog2. At E11.5, Neurod1 was detected in a pattern that partially overlaps Math5-lacZ (Suppl. Fig. 3), consistent with mRNA in situ hybridization data (Hufnagel et al., 2010). A similar overlap has been noted later in development (Kiyama et al., 2011; Le et al., 2006). This may explain the small number of early-born Brn3b+ RGCs present in Math5 −/− mice (Fig. 6B), as Neurod1 can partially substitute for Math5 function in RGC fate specification (Mao et al., 2008).
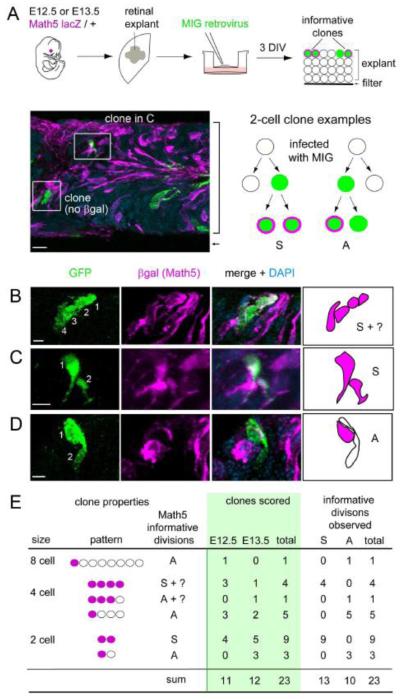
Symmetry of Math5 expression in marked retroviral clones
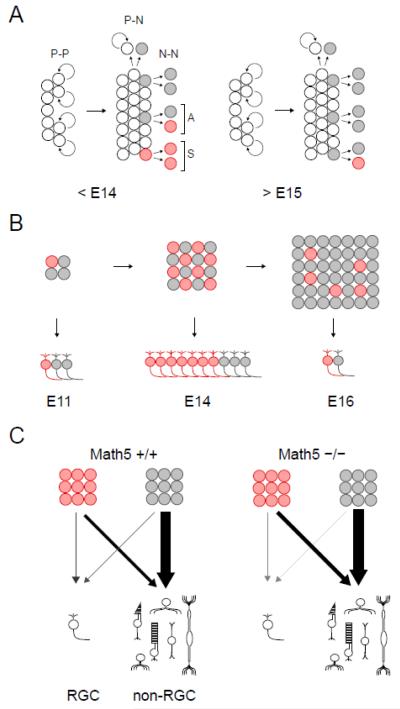
During nervous system development, the mode of progenitor cell divisions changes over time (Gotz and Huttner, 2005; Huttner and Kosodo, 2005; Lu et al., 2000). Prior to neurogenesis, cell divisions predominantly follow the symmetric self-renewing mode (P-P), which expands the progenitor pool. During early neurogenesis, an asymmetric mode is frequently used to generate one mitotic daughter and one differentiating neuron (P-N). During late neurogenesis, most progenitors undergo a symmetric neurogenic mode of division (N-N), in which both daughters permanently exit the cell cycle. The fates adopted by neuronal daughters may also be symmetric (Na-Na) or asymmetric (Na-Nb). In zebrafish, retinal progenitors expressing ath5-GFP undergo terminal neurogenic cell divisions (Poggi et al., 2005). These are symmetric with respect to ath5-GFP expression (NGFP-NGFP), but the daughters may have different cell fates, depending on the retinal environment.
To examine the mode of RPC division giving rise to Math5+ daughter cells in mice, we infected retinal explants from E12.5 or E13.5 Math5-lacZ/+ embryos with MSCV-IRES-GFP (MIG) retrovirus at low density to mark independent GFP+ clones. After culturing explants for 3 days in vitro (DIV), we immunostained 30 μm cryosections for GFP and βgal (Math5-lacZ), and determined the size and composition of clones containing at least one βgal+ cell (Nβgal) (Fig. 8A). These GFP+ clones ranged from 1 to 16 cells. We then focused our analysis on small clones (2-4 cell) containing ≥1 βgal+ cell, as these were most informative for symmetry of βgal+ expression. These clones are likely to represent terminal lineages, given their small size and time in culture. Indeed, all cells in these small clones were postmitotic, as judged by expression of the cell cycle inhibitor p27Kip1 (Dyer and Cepko, 2001) (data not shown). Among 23 clones scored, we observed both symmetric (Nβgal-Nβgal) (Fig. 8B,C) and asymmetric (N-Nβgal, or possibly P-Nβgal) (Fig. 8D) patterns of Math5 expression. Of 23 informative neurogenic divisions, 13 (57%) were symmetric with respect to Math5 expression and 10 were asymmetric (Fig. 8E). The fraction of symmetric divisions did not differ significantly between the E12.5 and E13.5 explant time-points (0.64 vs. 0.50 respectively, Fisher’s exact test P = 0.7). Although few symmetric terminal divisions are expected at this age in the retina as a whole, the high frequency observed among the Math5+ cohort (Nβgal-Nβgal) in this small sample confirms that early progenitors are capable of N-N divisions in mice as in zebrafish (Poggi et al., 2005). Unlike zebrafish, neurogenic divisions can be asymmetric with respect to Math5 expression in mice. These findings confirm that many retinal progenitors express Math5 after terminal M phase.
Fig. 8.
Retrovirally marked clones exhibit symmetric and asymmetric patterns of Math5 expression. (A) E12.5 or E13.5 retinas were explanted from Math5 lacZ/+ embryos, flattened on polycarbonate membranes, infected at low density with a retroviral stock to mark clonal lineages (green), and cultured for 3 days in vitro (DIV). The micrograph shows a cross-section from a representative explant (bracket) co-stained for cytoplasmic βgal (magenta) and GFP (green). The diagram shows hypothetical 2-cell clone with βgal+ cells. Each clone reflects one informative terminal division: a symmetric [S] division which gave rise to two Math5+ daughters (left); or an asymmetric [A] division, which gave rise to one Math5+ and one Math5- daughter (right). (B-D) Confocal Z-stack projections and drawings showing representative clones that are symmetric (B, C) or asymmetric (D) with respect to Math5 expression. (E) Summary of observed clones containing at least one Math5+ cell. Informative divisions have a unique interpretation, and give rise to one [A] or two [S] Math5+ daughters. Both types of divisions were identified. MIG, MSCV-IRES-GFP virus. Scale bars: 10 m in A; 5 m in B-D.
DISCUSSION
Math5>Cre transgene recapitulates endogenous Math5 expression
We believe that the Math5>Cre transgene is expressed in the same pattern as endogenous Math5 mRNA for several reasons. First, the BAC transgenes that we examined are intact and contain >100 kb flanking Math5 genomic DNA on both sides of the Cre cassette, while core regulatory elements for Math5 retinal expression are located within 25 kb of the transcriptional start site (Ghiasvand et al., 2011; Hutcheson et al., 2005). Second, the spatiotemporal pattern of Z/AP activation is congruent with Math5 mRNA and Math5-lacZ expression during retinal development. Third, all lineage-marked adult cells are born during the normal period of Math5 expression, including rare ‘late’ cell types. Fourth, similar results were observed with independent BAC transgenic lines, suggesting that chromosomal position effects are minimal or nonexistent. Apart from the retina and RGC projections, hPLAP staining was only noted in the cerebellum and in bushy cells of the cochlear nucleus, tissues that are known to express Math5 mRNA (Saul et al., 2008). Fifth, the dual reporter concordance experiment provides no evidence for leaky or ectopic Cre expression. Sixth, a similar overall retinal pattern has been observed using a targeted Cre insertion (knock-in allele) in the Math5 locus (Feng et al., 2010; Yang et al., 2003).
Our quantitative analysis significantly extends these previous studies and allows us to reach different conclusions regarding: [1] the size of the Math5-independent cohort of RGCs, [2] the relationship between Math5 expression and cell cycle exit, [3] the role of Math5 in determining non-RGC fates, and [4] the diversity of cell types within the Math5 lineage.
Math5>Cre does not mark all RGCs
Since Math5 is necessary for RGC development, and functions as an intracellular factor, we expected all ganglion cells to be labeled by Cre, as descendants of Math5+ progenitors. However, after carefully excluding displaced amacrine cells, we found that only 55% of RGCs were marked by the Math5>Cre transgene. A similar fraction of RGCs is likely to be labeled by the Math5-Cre knock-in allele (Feng et al., 2010) (cf. Suppl. Fig. 5D), although this finding was not originally appreciated (Yang et al., 2003). These Math5 descendants project to all known target sites for RGCs in the brain (Fig. 2E), suggesting that they represent the ganglion cell population as a whole. There are two possible explanations for the incomplete marking of RGCs: [1] inefficiency of the Cre-lox system, and [2] the existence of a sizeable Math5-independent population of RGCs.
In principle, inefficient reporting may account for a substantial fraction of unlabeled RGCs in the birthdating and lineage tracing experiments. RGCs descending from Math5+ precursors may escape detection for two reasons. First, the absolute level or duration of Cre expression in individual cells may not be sufficient to catalyze robust recombination. The Cre polypeptide must assemble into tetramers for enzymatic activity and has a short half-life in mammalian cells (Nagy, 2000). Generally speaking, Cre transgenes that are continuously active in differentiated cells are expected to be more efficient than those that are made briefly in a progenitor population. Math5 is transiently expressed during retinal development (Fig. 1A) and may be transcribed for only a few hours in individual cells (Fig. 2F) (Fu et al., 2009). Consequently, in the dual concordance experiment, Math5>Cre activated only one reporter in 20% of marked cells (Fig. 4). However, because concordance was relatively high among all cell types (Suppl. Table 3), this effect cannot fully explain the incomplete labeling of RGCs. Second, some cells may epigenetically silence the Math5>Cre transgene or the Math5-lacZ allele, or may be otherwise globally resistant to Cre recombination. Indeed, we have observed rare mice with reduced or elevated RGC labeling (data not shown). In these retinas, the extent of labeling varied coordinately across different cell types, consistent with a clonal epigenetic effect. A similar variation has been noted among Tie1>Cre transgenic mice in the efficiency of endothelial cell labeling (Enge et al., 2002). Nonetheless, among the vast majority of Math5>Cre retinas, there was relatively little variation in the RGC labeling fraction (Table 1). Taken together, Cre inefficiency and epigenetic silencing are unlikely to explain the incomplete labeling of RGCs that we observed.
Alternatively, a subset of RGCs may develop independently of Math5. Detailed analysis of Math5 −/− retinas has revealed a small population of widely dispersed ganglion cells, approximately 4% of wild-type, that survive to adulthood (Lin et al., 2004) and may project to the superior colliculi and laterial geniculate nuclei (Triplett et al., 2011). Moreover, recent data show that a related bHLH factor, Neurod1, can partially substitute for Math5 and allow RGC development (Mao et al., 2008). Indeed, we observed that fewer early-born cells express Math5-lacZ than Brn3b (Fig. 6B,C) and that many Brn3b+ RGCs at E11-E13 do not express Math5-lacZ (Fig. 7). A subset of nascent ganglion cells may develop from Neurod1+ precursors (Suppl. Fig. 3), without Math5. Consistent with this idea, mutant mice lacking both factors mice have even fewer RGCs than Math5 −/− mice (Kiyama et al., 2011).
The fraction of unmarked RGCs (~45%, Table 1) is 10-fold greater than the number of RGCs that survive in Math5 −/− mice (~4%)(Lin et al., 2004). Apart from Cre inefficiency (noted above), there are two possible explanations for this discrepancy. First, RGCs derived from Math5+ progenitors may have a survival advantage during neonatal period (P0-P10) of ganglion cell apoptosis (Young, 1984). However, the deficiency of Math5-independent RGCs in Math5 mutants was clearly evident early in retinal histogenesis, at E12.5 (Fig. 6B,C), well before the neonatal period of RGC culling. In addition, the fraction of Math5+ RGCs in P1 and adult retinas was the same, making this mechanism unlikely. Second, Math5 lineage cells may have a substantial non-autonomous role in RGC fate specification or early differentiation. These cells may represent ‘pioneering’ neurons (Pittman et al., 2008; Raper and Mason, 2010), which promote axon pathfinding and fasciculation within the retina (Erskine and Herrera, 2007; Oster et al., 2004) and survival of Math5-independent RGCs. In the absence of Math5, cells in the inner retina undergo apoptosis during midgestation and surviving RGCs have severe pathfinding defects (Feng et al., 2010; Kiyama et al., 2011; Moshiri et al., 2008; Prasov and Glaser, 2009). Most likely, Math5+ progenitors may favor the formation or survival of other RGCs by para- or juxtacrine signaling. Further work is needed to clarify molecular differences between the Math5+ cohort and other cells in the early retina.
Math5 is made by progenitors exiting the cell cycle
We have determined the precise relationship between onset of Math5 expression and the cell cycle status of retinal progenitors (Fig. 9A). At early stages (<E14), Math5-lacZ was detected in some G2/M phase progenitors but was otherwise present only in non-proliferating cells. Based on the length of G2 phase (~ 2 hrs) (Sinitsina, 1971) and our analysis of retinal cell cycle kinetics in E13.5 Math5>Cre; R26floxGFP embryos, following a 30 min EdU pulse (Fig. 2G), we conclude that at least 15% (and up to 60%) of newly Math5+ cells (Cre+ GFP−) initiate expression before terminal M phase. During later stages (>E15), Math5 was exclusively expressed in post-mitotic cells. Math5 lineage cells did not re-enter the cell cycle at any stage, regardless of the Math5 genotype. This comprehensive analysis reconciles previous disparate observations regarding the timing of Math5 expression (Brown et al., 1998; Le et al., 2006; Yang et al., 2003), including RNA profiling of single retinal cells (Trimarchi et al., 2008). In recent studies, an HA epitope-tagged Math5 allele was expressed with similar kinetics in early E12.5-E14.5 embryos, but was detected in more S, G2, and M phase cells than our Math5-lacZ allele (Feng et al., 2010; Kiyama et al., 2011; Wu et al., 2011). This is comparable to zebrafish, where ath5-GFP expression initiates during terminal S/G2 (Poggi et al., 2005), and is consistent with results obtained in frog and chick (Kay et al., 2001; Matter-Sadzinski et al., 2001; Perron et al., 1998; Poggi et al., 2005).
Fig. 9.
Natural history of the Math5 lineage. (A) The timing of Math5 expression shifts during retinal histogenesis. RPCs (white) shift from a proliferative (P-P) mode of division to stem (N-P) or terminal (N-N) modes, giving rise to neurogenic cells (gray). These express Math5 (red) either during (S, symmetric) or after (A, asymmetric) final mitosis. During early retinal development (<E14), Math5 is frequently expressed during G2 phase of the last cell cycle, generating two Math5+ daughters. During later stages (>E15), Math5 is exclusively expressed by post-mitotic cells. (B) The size of the neurogenic (birthdated) population and proportion of Math5+ cells changes during development. At the onset of neurogenesis (E11), Math5 is expressed by 20-30% of newborn cells. The number of Math5+ cells peaks during midgestation (E14) and rapidly diminishes (E16), while the neurogenic population as a whole continues to expand. The temporal profile for RGC birthdates follows similar kinetics, and reflects Math5+ and Math5 populations. (C) The fate spectrum of Math5 lineage (red) and other neurogenic (gray) cells in wild-type and mutant mice. The thickness and shading of arrows denotes the relative demographic contribution of these cohorts to the mature retina.
The variable timing of Math5 expression was supported by clonal analysis. We observed symmetric Math5-lacZ expression in 13 of 23 informative divisions (56%, NMath5-NMath5) and asymmetric expression in the remaining clones (P/N-NMath5). This frequency is convergent with cell cycle kinetic data discussed above. Together, these findings suggest that early progenitors giving rise to Math5+ cells are heterogeneous in their intrinsic properties and/or responses to the retinal microenvironment. By comparison, zebrafish ath5 is expressed symmetrically in terminal neurogenic divisions (Path5 Nath5-Nath5), but resulting daughters often adopt different fates (Poggi et al., 2005). This difference may be correlated with the accelerated pace of retinal neurogenesis in zebrafish compared to mice. Further studies are needed to determine how the timing of Math5 expression influences the fate choice of daughter cells in mice.
Math5 is unlikely to autonomously regulate the decision to exit the cell cycle for two reasons. First, it is variably expressed during or after the terminal division (Figs. 1B-D, 2G,H). Second, Math5 lineage cells exhibit similar lacZ expression kinetics in mutant and wild-type mice (Fig. 1E-H). Instead, this binary choice must be made upstream or in parallel with Math5 transcription. However, Math5 may affect progenitor cycling indirectly. For example, differentiating RGCs secrete sonic hedgehog (Shh), which acts as a mitogen for RPCs and promotes rod and Müller glial fates (Jensen and Wallace, 1997; Levine et al., 1997; Wang et al., 2005; Yu et al., 2006). Accordingly, Math5 mutants have thinner retinas with fewer rods and glia compared to wild-type mice (Brown et al., 2001), which may reflect a general loss of late-born cells. In zebrafish, ath5 and syu (Shh) mutants exhibit similar defects in retinal cell mitosis, involving a delay in switching polarity of division, from central-peripheral (proliferative) to circumferential (neurogenic) modes (Das et al., 2003). This likewise suggests that nascent RGCs, not the ath5 product per se, affect progenitor cell cycle dynamics.
Math5 establishes an RGC competence state
The expression fate mapping (Fig. 3) and dual concordance (Fig. 4) experiments support six conclusions. First, only a small fraction (3%) of the retina derives from Math5+ progenitors. Second, Math5+ progenitors are multipotent. They retain the potential to generate all seven major retinal cell types. Third, Math5+ progenitors contribute differentially to each cell type. The labeling frequency for a given cell type depends on the histogenic birth order and the temporal expression profile for Math5 (Fig. 9B). Similar overall results were observed in a previous Math5 lineage study (Feng et al., 2010; Yang et al., 2003). However, bipolars and Müller glia were not identified in the wild-type Math5 lineage, presumably because fewer cells were sampled. Fourth, Math5+ progenitors express uniform levels of Math5 and may represent a developmental equivalence group, similar to ato+ progenitors in the fly eye imaginal disc (Dokucu et al., 1996). Fifth, the fates of the Math5+ and Math5-populations change over time with parallel trajectories. The Math5 lineage cells are biased in their selection of non-RGC fates, compared to other neurogenic cells in the same environment, but this difference does not depend on Math5 activity. Sixth, the diversity of retinal cell fates within the Math5 lineage was similar in mutant and wild-type mice, apart from the deficiency of RGCs (Fig. 9C). However, there were modest increases in the labeling fraction of rods, bipolar cells and Müller glia (Fig. 3I). The most parsimonious explanation for these quantitative effects is a difference between wild-type and mutant mice in the fractional distribution of cell types, creating a denominator problem. As noted above, Math5 −/− retinas have significantly fewer late-born cells, presumably due to loss of Shh. Because all Math5 lineage cells retain early birthdates in these retinas (Fig. 6C, Suppl. Fig. 2), this cohort appears expanded and skewed toward late cell fates. Taken together, we conclude Math5 does not directly control the acquisition of multiple retinal cell fates (Feng et al., 2010). Instead, Math5 has an active role in RGC fate specification, as a competence (permissive) factor, and a passive or minor role in the selection of alternative (non-RGC) fates.
Mechanisms of fate determination in the mouse retina
Retinal cell fate choice, differentiation and survival are jointly controlled by intrinsic and extrinsic factors (Livesey and Cepko, 2001). As a nuclear bHLH protein, Math5 is an intrinsic factor. It is necessary but not sufficient for RGC development. During retinogenesis, nine-fold more Math5+ cells are produced than develop into RGCs (Fig. 3, Table 1). These cells have a different fate bias than other neurogenic cells in the same environment (Fig. 5). This property is conferred upstream of Math5. The development of RGCs from Math5+ cells may require the presence of positive cofactors or the absence of inhibitors. Soluble factors and cell-cell signaling are known to negatively regulate RGC genesis, including factors secreted by nascent RGCs (Austin et al., 1995; Belliveau and Cepko, 1999; Waid and McLoon, 1998; Zhang and Yang, 2001), and these may act on Math5+ cells. Together, our data suggest that the Math5+ cohort is influenced by intrinsic and extrinsic factors.
Our finding that Math5+ progenitors born on the same day can give rise to early or late cell types (Fig. 5) is consistent with a progressive restriction model for retinal neurogenesis, in which the progenitor pool is initially multipotent, but gradually loses competence to form early cell types (Pearson and Doe, 2003; Shen et al., 2006). This model is favored by heterochronic co-culture experiments (Reh, 1992; Watanabe and Raff, 1990) and Ascl1 (Mash1) lineage analysis. Mouse Ascl1+ progenitors form all retinal cell types except RGCs (Brzezinski et al., 2011) and may represent the first competence-restricted state. However, our results are also consistent with a temporal restriction model, in which progenitors proceed unidirectionally in time through a relatively fixed series of competence states (Wong and Rapaport, 2009).
The reservoir of neurogenic cells that are competent to form RGCs greatly exceeds the final number. Likewise, the period of RGC competence extends beyond the normal time envelope for RGC births in rat and chick (James et al., 2003; Silva et al., 2003). This excess capacity, which includes Math5+ and Math5-cells, and the fate plasticity of Math5+ cells may serve to enhance the robustness of RGC development and ensure an appropriate histotypic profile in the mammalian retina.
Supplementary Material
Highlights.
Math5 bHLH factor confers ganglion cell competence on retinal progenitor cells
Math5 is expressed in neurogenic cells during terminal G2 phase or early G0
Quantitative Math5>Cre lineage analysis and birthdating in wildtype and mutant mice
Identification of Math5-independent ganglion cells
Math5 does not actively determine non-RGC fates
ACKNOWLEDGEMENTS
The authors are grateful to Thom Saunders, Maggie van Keuren and the UM transgenic animal model core for generating the BAC transgenic animals; to Sue Tarlé and Dellaney Rudolph for technical support; to Nadean Brown for in situ hybridization data; to Nathaniel Heintz for BAC targeting plasmids and strains; to Sally Camper for the nlsCre plasmid; and to Sean Morrison for the MIG retroviral construct. The authors thank Chris Edwards, the UM microscopy and image analysis laboratory staff, Rafal Farjo and Mohammad Farah for technical advice. The authors are grateful to Nadean Brown, Chris Chou, David Turner, Matt Wilken, Julia Pollak, Anna La Torre, and Yumi Ueki for valuable discussions and critical reading of the manuscript. This research was funded by National Institutes of Health (NIH) R01 grant EY14259 (TG). JAB and LP were supported by NIH T32 grants EY13934 (JAB, LP), GM07544 (JAB), and GM07863 (LP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adler R, Hatlee M. Plasticity and differentiation of embryonic retinal cells after terminal mitosis. Science. 1989;243:391–3. doi: 10.1126/science.2911751. [DOI] [PubMed] [Google Scholar]
- Alexiades MR, Cepko C. Quantitative analysis of proliferation and cell cycle length during development of the rat retina. Dev Dyn. 1996;205:293–307. doi: 10.1002/(SICI)1097-0177(199603)205:3<293::AID-AJA9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Altshuler DM, Turner DL, Cepko CL. Specification of cell type in the vertebrate retina. In: Lam DMK, Shatz CJ, editors. Cell Lineage and Cell Fate in Visual System Development. MIT Press; Cambridge, MA: 1991. pp. 37–58. [Google Scholar]
- Austin CP, Feldman DE, Ida JA, Jr., Cepko CL. Vertebrate retinal ganglion cells are selected from competent progenitors by the action of Notch. Development. 1995;121:3637–50. doi: 10.1242/dev.121.11.3637. [DOI] [PubMed] [Google Scholar]
- Barnstable CJ, Hofstein R, Akagawa K. A marker of early amacrine cell development in rat retina. Brain Res. 1985;352:286–90. doi: 10.1016/0165-3806(85)90116-6. [DOI] [PubMed] [Google Scholar]
- Baye LM, Link BA. Nuclear migration during retinal development. Brain Res. 2008;1192:29–36. doi: 10.1016/j.brainres.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belliveau MJ, Cepko CL. Extrinsic and intrinsic factors control the genesis of amacrine and cone cells in the rat retina. Development. 1999;126:555–66. doi: 10.1242/dev.126.3.555. [DOI] [PubMed] [Google Scholar]
- Blanks JC, Johnson LV. Selective lectin binding of the developing mouse retina. J Comp Neurol. 1983;221:31–41. doi: 10.1002/cne.902210103. [DOI] [PubMed] [Google Scholar]
- Bradbury EM. Reversible histone modifications and the chromosome cell cycle. Bioessays. 1992;14:9–16. doi: 10.1002/bies.950140103. [DOI] [PubMed] [Google Scholar]
- Brown NL, Kanekar S, Vetter ML, Tucker PK, Gemza DL, Glaser T. Math5 encodes a murine basic helix-loop-helix transcription factor expressed during early stages of retinal neurogenesis. Development. 1998;125:4821–33. doi: 10.1242/dev.125.23.4821. [DOI] [PubMed] [Google Scholar]
- Brown NL, Patel S, Brzezinski J, Glaser T. Math5 is required for retinal ganglion cell and optic nerve formation. Development. 2001;128:2497–508. doi: 10.1242/dev.128.13.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinski JAI, Brown NL, Tanikawa A, Bush RA, Sieving PA, Vitaterna M, Takahashi J, Glaser T. Abnormal circadian behavior and retinal electrophysiology in Math5 mutant mice. Invest Ophthal Vis Sci. 2005;46:2540–51. doi: 10.1167/iovs.04-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinski JA, Schulz SM, Crawford S, Wroblewski E, Brown NL, Glaser T. Math5 null mice have abnormal retinal and persistent hyaloid vasculatures. Dev Biol. 2003;259:394. [Google Scholar]
- Brzezinski JA, Kim EJ, Johnson JE, Reh TA. Ascl1 expression defines a subpopulation of lineage-restricted progenitors in the mammalian retina. Development. 2011;138:3519–31. doi: 10.1242/dev.064006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck SB, Bradford J, Gee KR, Agnew BJ, Clarke ST, Salic A. Detection of S-phase cell cycle progression using 5-ethynyl-2′-deoxyuridine incorporation with click chemistry, an alternative to using 5-bromo-2′-deoxyuridine antibodies. Biotechniques. 2008;44:927–9. doi: 10.2144/000112812. [DOI] [PubMed] [Google Scholar]
- Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. II. Autoradiographic analysis of cell generation using tritiated thymidine. J Comp Neurol. 1979;188:263–72. doi: 10.1002/cne.901880205. [DOI] [PubMed] [Google Scholar]
- Cayouette M, Barres BA, Raff M. Importance of intrinsic mechanisms in cell fate decisions in the developing rat retina. Neuron. 2003;40:897–904. doi: 10.1016/s0896-6273(03)00756-6. [DOI] [PubMed] [Google Scholar]
- Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- Cushman LJ, Burrows HL, Seasholtz AF, Lewandoski M, Muzyczka N, Camper SA. Cre-mediated recombination in the pituitary gland. Genesis. 2000;28:167–74. doi: 10.1002/1526-968x(200011/12)28:3/4<167::aid-gene120>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Das T, Payer B, Cayouette M, Harris WA. In vivo time-lapse imaging of cell divisions during neurogenesis in the developing zebrafish retina. Neuron. 2003;37:597–609. doi: 10.1016/s0896-6273(03)00066-7. [DOI] [PubMed] [Google Scholar]
- Dokucu ME, Zipursky SL, Cagan RL. Atonal, rough and the resolution of proneural clusters in the developing Drosophila retina. Development. 1996;122:4139–47. doi: 10.1242/dev.122.12.4139. [DOI] [PubMed] [Google Scholar]
- Drager UC. Birth dates of retinal ganglion cells giving rise to the crossed and uncrossed optic projections in the mouse. Proc R Soc Lond B Biol Sci. 1985;224:57–77. doi: 10.1098/rspb.1985.0021. [DOI] [PubMed] [Google Scholar]
- Dyer MA, Cepko CL. p27Kip1 and p57Kip2 regulate proliferation in distinct retinal progenitor cell populations. J Neurosci. 2001;21:4259–71. doi: 10.1523/JNEUROSCI.21-12-04259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymecki SM, Rodriguez CI, Awatramani RB. Switching on lineage tracers using site-specific recombination. In: Turksen K, editor. Embryonic Stem Cells: Methods and Protocols. Vol. 185. Humana Press; 2002. pp. 309–34. [DOI] [PubMed] [Google Scholar]
- Echelard Y, Vassileva G, McMahon AP. Cis-acting regulatory sequences governing Wnt-1 expression in the developing mouse CNS. Development. 1994;120:2213–24. doi: 10.1242/dev.120.8.2213. [DOI] [PubMed] [Google Scholar]
- Enge M, Bjarnegard M, Gerhardt H, Gustafsson E, Kalen M, Asker N, Hammes HP, Shani M, Fassler R, Betsholtz C. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. Embo J. 2002;21:4307–16. doi: 10.1093/emboj/cdf418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkman L, McEvilly RJ, Luo L, Ryan AK, Hooshmand F, O’Connell SM, Keithley EM, Rapaport DH, Ryan AF, Rosenfeld MG. Role of transcription factors Brn-3.1 and Brn-3.2 in auditory and visual system development. Nature. 1996;381:603–6. doi: 10.1038/381603a0. [DOI] [PubMed] [Google Scholar]
- Erskine L, Herrera E. The retinal ganglion cell axon’s journey: insights into molecular mechanisms of axon guidance. Dev Biol. 2007;308:1–14. doi: 10.1016/j.ydbio.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Ezzeddine ZD, Yang X, DeChiara T, Yancopoulos G, Cepko CL. Postmitotic cells fated to become rod photoreceptors can be respecified by CNTF treatment of the retina. Development. 1997;124:1055–67. doi: 10.1242/dev.124.5.1055. [DOI] [PubMed] [Google Scholar]
- Farah MH, Easter SS., Jr. Cell birth and death in the mouse retinal ganglion cell layer. J Comp Neurol. 2005;489:120–34. doi: 10.1002/cne.20615. [DOI] [PubMed] [Google Scholar]
- Feng L, Xie ZH, Ding Q, Xie X, Libby RT, Gan L. MATH5 controls the acquisition of multiple retinal cell fates. Mol Brain. 2010;3:36. doi: 10.1186/1756-6606-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA. Statistical Methods for Research Workers. Oliver & Boyd; 1925. [Google Scholar]
- Frankfort BJ, Mardon G. R8 development in the Drosophila eye: a paradigm for neural selection and differentiation. Development. 2002;129:1295–306. doi: 10.1242/dev.129.6.1295. [DOI] [PubMed] [Google Scholar]
- Fu X, Kiyama T, Li R, Russell M, Klein WH, Mu X. Epitope-tagging Math5 and Pou4f2: new tools to study retinal ganglion cell development in the mouse. Dev Dyn. 2009;238:2309–17. doi: 10.1002/dvdy.21974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann S, Kirsch M, Hofmann HD. Ciliary neurotrophic factor promotes chick photoreceptor development in vitro. Development. 1995;121:2695–706. doi: 10.1242/dev.121.8.2695. [DOI] [PubMed] [Google Scholar]
- Galli-Resta L, Ensini M. An intrinsic time limit between genesis and death of individual neurons in the developing retinal ganglion cell layer. J Neurosci. 1996;16:2318–24. doi: 10.1523/JNEUROSCI.16-07-02318.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan L, Xiang M, Zhou L, Wagner DS, Klein WH, Nathans J. POU domain factor Brn-3b is required for the development of a large set of retinal ganglion cells. Proc Natl Acad Sci U S A. 1996;93:3920–5. doi: 10.1073/pnas.93.9.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Holmes A, Sibley D, Skolnick P, Wray S. Current Protocols in Neuroscience. John Wiley & Sons, Inc.; 2001. Common Stock Solutions, Buffers, and Media. [Google Scholar]
- Ghiasvand NM, Rudolph DD, Mashayekhi M, Brzezinski J. A. t., Goldman D, Glaser T. Deletion of a remote enhancer near ATOH7 disrupts retinal neurogenesis, causing NCRNA disease. Nat Neurosci. 2011;14:578–86. doi: 10.1038/nn.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldowitz D, Rice DS, Williams RW. Clonal architecture of the mouse retina. Prog Brain Res. 1996;108:3–15. doi: 10.1016/s0079-6123(08)62528-5. [DOI] [PubMed] [Google Scholar]
- Gomes FL, Zhang G, Carbonell F, Correa JA, Harris WA, Simons BD, Cayouette M. Reconstruction of rat retinal progenitor cell lineages in vitro reveals a surprising degree of stochasticity in cell fate decisions. Development. 2011;138:227–35. doi: 10.1242/dev.059683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Yang XW, Li C, Heintz N. Highly efficient modification of bacterial artificial chromosomes (BACs) using novel shuttle vectors containing the R6Kgamma origin of replication. Genome Res. 2002;12:1992–8. doi: 10.1101/gr.476202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–88. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Hatakeyama J, Kageyama R. Retrovirus-mediated gene transfer to retinal explants. Methods. 2002;28:387–95. doi: 10.1016/s1046-2023(02)00257-8. [DOI] [PubMed] [Google Scholar]
- Hayden SA, Mills JW, Masland RM. Acetylcholine synthesis by displaced amacrine cells. Science. 1980;210:435–7. doi: 10.1126/science.7433984. [DOI] [PubMed] [Google Scholar]
- Heintz N. BAC to the future: the use of bac transgenic mice for neuroscience research. Nat Rev Neurosci. 2001;2:861–70. doi: 10.1038/35104049. [DOI] [PubMed] [Google Scholar]
- Helms AW, Abney AL, Ben-Arie N, Zoghbi HY, Johnson JE. Autoregulation and multiple enhancers control Math1 expression in the developing nervous system. Development. 2000;127:1185–96. doi: 10.1242/dev.127.6.1185. [DOI] [PubMed] [Google Scholar]
- Holt CE, Bertsch TW, Ellis HM, Harris WA. Cellular determination in the Xenopus retina is independent of lineage and birth date. Neuron. 1988;1:15–26. doi: 10.1016/0896-6273(88)90205-x. [DOI] [PubMed] [Google Scholar]
- Hsiung F, Moses K. Retinal development in Drosophila: specifying the first neuron. Hum Mol Genet. 2002;11:1207–14. doi: 10.1093/hmg/11.10.1207. [DOI] [PubMed] [Google Scholar]
- Hu M, Krause D, Greaves M, Sharkis S, Dexter M, Heyworth C, Enver T. Multilineage gene expression precedes commitment in the hemopoietic system. Genes Dev. 1997;11:774–85. doi: 10.1101/gad.11.6.774. [DOI] [PubMed] [Google Scholar]
- Hufnagel RB, Le TT, Riesenberg AL, Brown NL. Neurog2 controls the leading edge of neurogenesis in the mammalian retina. Dev Biol. 2010;340:490–503. doi: 10.1016/j.ydbio.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson DA, Hanson MI, Moore KB, Le TT, Brown NL, Vetter ML. bHLH-dependent and -independent modes of Ath5 gene regulation during retinal development. Development. 2005 doi: 10.1242/dev.01653. in press. [DOI] [PubMed] [Google Scholar]
- Hutcheson DA, Vetter ML. The bHLH factors Xath5 and XNeuroD can upregulate the expression of XBrn3d, a POU-homeodomain transcription factor. Dev Biol. 2001;232:327–38. doi: 10.1006/dbio.2001.0178. [DOI] [PubMed] [Google Scholar]
- Huttner WB, Kosodo Y. Symmetric versus asymmetric cell division during neurogenesis in the developing vertebrate central nervous system. Curr Opin Cell Biol. 2005;17:648–57. doi: 10.1016/j.ceb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- James J, Das AV, Bhattacharya S, Chacko DM, Zhao X, Ahmad I. In vitro generation of early-born neurons from late retinal progenitors. J Neurosci. 2003;23:8193–203. doi: 10.1523/JNEUROSCI.23-23-08193.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarman AP. Developmental genetics: vertebrates and insects see eye to eye. Curr Biol. 2000;10:R857–9. doi: 10.1016/s0960-9822(00)00821-6. [DOI] [PubMed] [Google Scholar]
- Jensen AM, Wallace VA. Expression of Sonic hedgehog and its putative role as a precursor cell mitogen in the developing mouse retina. Development. 1997;124:363–71. doi: 10.1242/dev.124.2.363. [DOI] [PubMed] [Google Scholar]
- Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998;18:8936–46. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama R, Nakanishi S. Helix-loop-helix factors in growth and differentiation of the vertebrate nervous system. Curr Opin Genet Dev. 1997;7:659–65. doi: 10.1016/s0959-437x(97)80014-7. [DOI] [PubMed] [Google Scholar]
- Kanekar S, Perron M, Dorsky R, Harris WA, Jan LY, Jan YN, Vetter ML. Xath5 participates in a network of bHLH genes in the developing Xenopus retina. Neuron. 1997;19:981–94. doi: 10.1016/s0896-6273(00)80391-8. [DOI] [PubMed] [Google Scholar]
- Kay JN, Finger-Baier KC, Roeser T, Staub W, Baier H. Retinal ganglion cell genesis requires lakritz, a Zebrafish atonal Homolog. Neuron. 2001;30:725–36. doi: 10.1016/s0896-6273(01)00312-9. [DOI] [PubMed] [Google Scholar]
- Key G, Becker MH, Baron B, Duchrow M, Schluter C, Flad HD, Gerdes J. New Ki-67-equivalent murine monoclonal antibodies (MIB 1-3) generated against bacterially expressed parts of the Ki-67 cDNA containing three 62 base pair repetitive elements encoding for the Ki-67 epitope. Lab Invest. 1993;68:629–36. [PubMed] [Google Scholar]
- Khor CC, Ramdas WD, Vithana EN, Cornes BK, Sim X, Tay WT, Saw SM, Zheng Y, Lavanya R, Wu R, Wang JJ, Mitchell P, Uitterlinden AG, Rivadeneira F, Teo YY, Chia KS, Seielstad M, Hibberd M, Vingerling JR, Klaver CC, Jansonius NM, Tai ES, Wong TY, van Duijn CM, Aung T. Genome-wide association studies in Asians confirm the involvement of ATOH7 and TGFBR3, and further identify CARD10 as a novel locus influencing optic disc area. Hum Mol Genet. 2011;20:1864–72. doi: 10.1093/hmg/ddr060. [DOI] [PubMed] [Google Scholar]
- Kiyama T, Mao CA, Cho JH, Fu X, Pan P, Mu X, Klein WH. Overlapping spatiotemporal patterns of regulatory gene expression are required for neuronal progenitors to specify retinal ganglion cell fate. Vision Res. 2011;51:251–9. doi: 10.1016/j.visres.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail MM, Faktorovich EG, Hepler JM, Pearson KL, Yasumura D, Matthes MT, Steinberg RH. Basic fibroblast growth factor protects photoreceptors from light-induced degeneration in albino rats. Ann N Y Acad Sci. 1991;638:341–7. doi: 10.1111/j.1749-6632.1991.tb49044.x. [DOI] [PubMed] [Google Scholar]
- Le TT, Wroblewski E, Patel S, Riesenberg AN, Brown NL. Math5 is required for both early retinal neuron differentiation and cell cycle progression. Dev Biol. 2006;295:764–78. doi: 10.1016/j.ydbio.2006.03.055. [DOI] [PubMed] [Google Scholar]
- Levine EM, Roelink H, Turner J, Reh TA. Sonic hedgehog promotes rod photoreceptor differentiation in mammalian retinal cells in vitro. J Neurosci. 1997;17:6277–88. doi: 10.1523/JNEUROSCI.17-16-06277.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Wang SW, Masland RH. Retinal ganglion cell type, size, and spacing can be specified independent of homotypic dendritic contacts. Neuron. 2004;43:475–85. doi: 10.1016/j.neuron.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Liu W, Mo Z, Xiang M. The Ath5 proneural genes function upstream of Brn3 POU domain transcription factor genes to promote retinal ganglion cell development. Proc Natl Acad Sci U S A. 2001;98:1649–54. doi: 10.1073/pnas.98.4.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: lessons from the retina. Nat Rev Neurosci. 2001;2:109–18. doi: 10.1038/35053522. [DOI] [PubMed] [Google Scholar]
- Lobe CG, Koop KE, Kreppner W, Lomeli H, Gertsenstein M, Nagy A. Z/AP, a double reporter for cre-mediated recombination. Dev Biol. 1999;208:281–92. doi: 10.1006/dbio.1999.9209. [DOI] [PubMed] [Google Scholar]
- Lu B, Jan L, Jan YN. Control of cell divisions in the nervous system: symmetry and asymmetry. Annu Rev Neurosci. 2000;23:531–56. doi: 10.1146/annurev.neuro.23.1.531. [DOI] [PubMed] [Google Scholar]
- Macgregor S, Hewitt AW, Hysi PG, Ruddle JB, Medland SE, Henders AK, Gordon SD, Andrew T, McEvoy B, Sanfilippo PG, Carbonaro F, Tah V, Li YJ, Bennett SL, Craig JE, Montgomery GW, Tran-Viet KN, Brown NL, Spector TD, Martin NG, Young TL, Hammond CJ, Mackey DA. Genome-wide association identifies ATOH7 as a major gene determining human optic disc size. Hum Mol Genet. 2010;19:2716–24. doi: 10.1093/hmg/ddq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao CA, Wang SW, Pan P, Klein WH. Rewiring the retinal ganglion cell gene regulatory network: Neurod1 promotes retinal ganglion cell fate in the absence of Math5. Development. 2008;135:3379–88. doi: 10.1242/dev.024612. [DOI] [PubMed] [Google Scholar]
- Mao X, Fujiwara Y, Chapdelaine A, Yang H, Orkin SH. Activation of EGFP expression by Cre-mediated excision in a new ROSA26 reporter mouse strain. Blood. 2001;97:324–6. doi: 10.1182/blood.v97.1.324. [DOI] [PubMed] [Google Scholar]
- Masai I. [Mechanisms underlying induction and progression of a neurogenic wave in the zebrafish developing retina] Tanpakushitsu Kakusan Koso. 2000;45:2782–90. [PubMed] [Google Scholar]
- Matter-Sadzinski L, Matter JM, Ong MT, Hernandez J, Ballivet M. Specification of neurotransmitter receptor identity in developing retina: the chick ATH5 promoter integrates the positive and negative effects of several bHLH proteins. Development. 2001;128:217–31. doi: 10.1242/dev.128.2.217. [DOI] [PubMed] [Google Scholar]
- Mayer W, Smith A, Fundele R, Haaf T. Spatial separation of parental genomes in preimplantation mouse embryos. J Cell Biol. 2000;148:629–34. doi: 10.1083/jcb.148.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoon SC, Barnes RB. Early differentiation of retinal ganglion cells: an axonal protein expressed by premigratory and migrating retinal ganglion cells. J Neurosci. 1989;9:1424–32. doi: 10.1523/JNEUROSCI.09-04-01424.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Nowakowski RS. Use of bromodeoxyuridine-immunohistochemistry to examine the proliferation, migration and time of origin of cells in the central nervous system. Brain Res. 1988;457:44–52. doi: 10.1016/0006-8993(88)90055-8. [DOI] [PubMed] [Google Scholar]
- Moshiri A, Gonzalez E, Tagawa K, Maeda H, Wang M, Frishman LJ, Wang SW. Near complete loss of retinal ganglion cells in the math5/brn3b double knockout elicits severe reductions of other cell types during retinal development. Dev Biol. 2008;316:214–27. doi: 10.1016/j.ydbio.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu X, Beremand PD, Zhao S, Pershad R, Sun H, Scarpa A, Liang S, Thomas TL, Klein WH. Discrete gene sets depend on POU domain transcription factor Brn3b/Brn-3.2/POU4f2 for their expression in the mouse embryonic retina. Development. 2004;131:1197–1210. doi: 10.1242/dev.01010. [DOI] [PubMed] [Google Scholar]
- Mu X, Fu X, Beremand PD, Thomas TL, Klein WH. Gene regulation logic in retinal ganglion cell development: Isl1 defines a critical branch distinct from but overlapping with Pou4f2. Proc Natl Acad Sci U S A. 2008;105:6942–7. doi: 10.1073/pnas.0802627105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A. Cre recombinase: the universal reagent for genome tailoring. Genesis. 2000;26:99–109. [PubMed] [Google Scholar]
- Oster SF, Deiner M, Birgbauer E, Sretavan DW. Ganglion cell axon pathfinding in the retina and optic nerve. Semin Cell Dev Biol. 2004;15:125–36. doi: 10.1016/j.semcdb.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Pan L, Deng M, Xie X, Gan L. ISL1 and BRN3B co-regulate the differentiation of murine retinal ganglion cells. Development. 2008;135:1981–90. doi: 10.1242/dev.010751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pear W. Transient transfection methods for preparation of high-titer retroviral supernatants. Curr Protoc Mol Biol. 2001 doi: 10.1002/0471142727.mb0911s36. Chapter 9, Unit9 11. [DOI] [PubMed] [Google Scholar]
- Pearson BJ, Doe CQ. Regulation of neuroblast competence in Drosophila. Nature. 2003;425:624–8. doi: 10.1038/nature01910. [DOI] [PubMed] [Google Scholar]
- Peichl L, Gonzalez-Soriano J. Unexpected presence of neurofilaments in axon-bearing horizontal cells of the mammalian retina. J Neurosci. 1993;13:4091–100. doi: 10.1523/JNEUROSCI.13-09-04091.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peichl L, Gonzalez-Soriano J. Morphological types of horizontal cell in rodent retinae: a comparison of rat, mouse, gerbil, and guinea pig. Vis Neurosci. 1994;11:501–17. doi: 10.1017/s095252380000242x. [DOI] [PubMed] [Google Scholar]
- Perron M, Kanekar S, Vetter ML, Harris WA. The genetic sequence of retinal development in the ciliary margin of the Xenopus eye. Dev Biol. 1998;199:185–200. doi: 10.1006/dbio.1998.8939. [DOI] [PubMed] [Google Scholar]
- Perry VH, Walker M. Amacrine cells, displaced amacrine cells and interplexiform cells in the retina of the rat. Proc R Soc Lond B Biol Sci. 1980;208:415–31. doi: 10.1098/rspb.1980.0060. [DOI] [PubMed] [Google Scholar]
- Pittman AJ, Law MY, Chien CB. Pathfinding in a large vertebrate axon tract: isotypic interactions guide retinotectal axons at multiple choice points. Development. 2008;135:2865–71. doi: 10.1242/dev.025049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggi L, Vitorino M, Masai I, Harris WA. Influences on neural lineage and mode of division in the zebrafish retina in vivo. J Cell Biol. 2005;171:991–9. doi: 10.1083/jcb.200509098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasov L, Brown NL, Glaser T. A critical analysis of Atoh7 (Math5) mRNA splicing in the developing mouse retina. PLoS One. 2010;5:e12315. doi: 10.1371/journal.pone.0012315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasov L, Glaser T. Math5 Confers Multipotency to Fate-Restricted Post-Mitotic Retinal Precursors. ARVO Meeting Abstracts. 2009;50:1310. [Google Scholar]
- Rachel RA, Dolen G, Hayes NL, Lu A, Erskine L, Nowakowski RS, Mason CA. Spatiotemporal features of early neuronogenesis differ in wild-type and albino mouse retina. J Neurosci. 2002;22:4249–63. doi: 10.1523/JNEUROSCI.22-11-04249.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramdas WD, van Koolwijk LM, Ikram MK, Jansonius NM, de Jong PT, Bergen AA, Isaacs A, Amin N, Aulchenko YS, Wolfs RC, Hofman A, Rivadeneira F, Oostra BA, Uitterlinden AG, Hysi P, Hammond CJ, Lemij HG, Vingerling JR, Klaver CC, van Duijn CM. A genome-wide association study of optic disc parameters. PLoS Genet. 2010;6:e1000978. doi: 10.1371/journal.pgen.1000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport DH, Patheal SL, Harris WA. Cellular competence plays a role in photoreceptor differentiation in the developing Xenopus retina. J Neurobiol. 2001;49:129–41. doi: 10.1002/neu.1070. [DOI] [PubMed] [Google Scholar]
- Rapaport DH, Wong LL, Wood ED, Yasumura D, LaVail MM. Timing and topography of cell genesis in the rat retina. J Comp Neurol. 2004;474:304–24. doi: 10.1002/cne.20134. [DOI] [PubMed] [Google Scholar]
- Raper J, Mason C. Cellular strategies of axonal pathfinding. Cold Spring Harb Perspect Biol. 2010;2:a001933. doi: 10.1101/cshperspect.a001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese BE, Colello RJ. Neurogenesis in the retinal ganglion cell layer of the rat. Neuroscience. 1992;46:419–29. doi: 10.1016/0306-4522(92)90062-7. [DOI] [PubMed] [Google Scholar]
- Reh TA. Cellular interactions determine neuronal phenotypes in rodent retinal cultures. J Neurobiol. 1992;23:1067–83. doi: 10.1002/neu.480230811. [DOI] [PubMed] [Google Scholar]
- Reh TA, Kljavin IJ. Age of differentiation determines rat retinal germinal cell phenotype: induction of differentiation by dissociation. J Neurosci. 1989;9:4179–89. doi: 10.1523/JNEUROSCI.09-12-04179.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repka AM, Adler R. Accurate determination of the time of cell birth using a sequential labeling technique with [3H]-thymidine and bromodeoxyuridine (“window labeling”) J Histochem Cytochem. 1992;40:947–53. doi: 10.1177/40.7.1607643. [DOI] [PubMed] [Google Scholar]
- Rodieck RW. The First Steps in Seeing. Sinauer; Sunderland, MA: 1998. [Google Scholar]
- Saul SM, Brzezinski J. A. t., Altschuler RA, Shore SE, Rudolph DD, Kabara LL, Halsey KE, Hufnagel RB, Zhou J, Dolan DF, Glaser T. Math5 expression and function in the central auditory system. Mol Cell Neurosci. 2008;37:153–69. doi: 10.1016/j.mcn.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ML, Turner DL, Vetter ML. Notch signaling can inhibit Xath5 function in the neural plate and developing retina. Mol Cell Neurosci. 2001;18:458–72. doi: 10.1006/mcne.2001.1040. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Dimos JT, Fasano CA, Phoenix TN, Lemischka IR, Ivanova NB, Stifani S, Morrisey EE, Temple S. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nat Neurosci. 2006;9:743–51. doi: 10.1038/nn1694. [DOI] [PubMed] [Google Scholar]
- Sidman RL. Histogenesis of mouse retina studied with thymidine-H3. In: Smelser GK, editor. Structure of the Eye. Academic Press; New York: 1961. pp. 487–506. [Google Scholar]
- Silva AO, Ercole CE, McLoon SC. Regulation of ganglion cell production by Notch signaling during retinal development. J Neurobiol. 2003;54:511–24. doi: 10.1002/neu.10156. [DOI] [PubMed] [Google Scholar]
- Simpson JI. The accessory optic system. Annu Rev Neurosci. 1984;7:13–41. doi: 10.1146/annurev.ne.07.030184.000305. [DOI] [PubMed] [Google Scholar]
- Sinitsina VF. [DNA synthesis and cell population kinetics in embryonal histogenesis of the retina in mice] Arkh Anat Gistol Embriol. 1971;61:58–67. [PubMed] [Google Scholar]
- Snow RL, Robson JA. Ganglion cell neurogenesis, migration and early differentiation in the chick retina. Neuroscience. 1994;58:399–409. doi: 10.1016/0306-4522(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Sun Y, Kanekar SL, Vetter ML, Gorski S, Jan YN, Glaser T, Brown NL. Conserved and divergent functions of Drosophila atonal, amphibian, and mammalian Ath5 genes. Evol Dev. 2003;5:532–41. doi: 10.1046/j.1525-142x.2003.03058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift S, Lorens J, Achacoso P, Nolan GP. Rapid production of retroviruses for efficient gene delivery to mammalian cells using 293T cell-based systems. Curr Protoc Immunol. 2001 doi: 10.1002/0471142735.im1017cs31. Chapter 10, Unit 10 17C. [DOI] [PubMed] [Google Scholar]
- Trimarchi JM, Stadler MB, Cepko CL. Individual retinal progenitor cells display extensive heterogeneity of gene expression. PLoS One. 2008;3:e1588. doi: 10.1371/journal.pone.0001588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett JW, Pfeiffenberger C, Yamada J, Stafford BK, Sweeney NT, Litke AM, Sher A, Koulakov AA, Feldheim DA. Competition is a driving force in topographic mapping. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1102834108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DL, Cepko CL. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987;328:131–6. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- Turner DL, Snyder EY, Cepko CL. Lineage-independent determination of cell type in the embryonic mouse retina. Neuron. 1990;4:833–45. doi: 10.1016/0896-6273(90)90136-4. [DOI] [PubMed] [Google Scholar]
- Van Parijs L, Refaeli Y, Lord JD, Nelson BH, Abbas AK, Baltimore D. Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation-induced cell death. Immunity. 1999;11:281–8. doi: 10.1016/s1074-7613(00)80103-x. [DOI] [PubMed] [Google Scholar]
- von Bohlen und Halbach O. The isolated mammalian brain: an in vivo preparation suitable for pathway tracing. Eur J Neurosci. 1999;11:1096–100. doi: 10.1046/j.1460-9568.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- Waid DK, McLoon SC. Ganglion cells influence the fate of dividing retinal cells in culture. Development. 1998;125:1059–66. doi: 10.1242/dev.125.6.1059. [DOI] [PubMed] [Google Scholar]
- Wang SW, Kim BS, Ding K, Wang H, Sun D, Johnson RL, Klein WH, Gan L. Requirement for math5 in the development of retinal ganglion cells. Genes Dev. 2001;15:24–9. doi: 10.1101/gad.855301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SW, Mu X, Bowers WJ, Klein WH. Retinal ganglion cell differentiation in cultured mouse retinal explants. Methods. 2002;28:448–56. doi: 10.1016/s1046-2023(02)00264-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Dakubo GD, Thurig S, Mazerolle CJ, Wallace VA. Retinal ganglion cell-derived sonic hedgehog locally controls proliferation and the timing of RGC development in the embryonic mouse retina. Development. 2005;132:5103–13. doi: 10.1242/dev.02096. [DOI] [PubMed] [Google Scholar]
- Wang Y, Smallwood PM, Cowan M, Blesh D, Lawler A, Nathans J. Mutually exclusive expression of human red and green visual pigment-reporter transgenes occurs at high frequency in murine cone photoreceptors. Proc Natl Acad Sci U S A. 1999;96:5251–6. doi: 10.1073/pnas.96.9.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Rutishauser U, Silver J. Formation of the retinal ganglion cell and optic fiber layers. J Neurobiol. 1991;22:85–96. doi: 10.1002/neu.480220109. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Raff MC. Rod photoreceptor development in vitro: intrinsic properties of proliferating neuroepithelial cells change as development proceeds in the rat retina. Neuron. 1990;4:461–7. doi: 10.1016/0896-6273(90)90058-n. [DOI] [PubMed] [Google Scholar]
- Wee R, Castrucci AM, Provencio I, Gan L, Van Gelder RN. Loss of photic entrainment and altered free-running circadian rhythms in math5−/− mice. J Neurosci. 2002;22:10427–33. doi: 10.1523/JNEUROSCI.22-23-10427.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells NK. Tissue Interactions and Development. W.A. Benjamin; Menlo Park, CA: 1977. [Google Scholar]
- Wetts R, Fraser SE. Multipotent precursors can give rise to all major cell types of the frog retina. Science. 1988;239:1142–5. doi: 10.1126/science.2449732. [DOI] [PubMed] [Google Scholar]
- Wong LL, Rapaport DH. Defining retinal progenitor cell competence in Xenopus laevis by clonal analysis. Development. 2009;136:1707–15. doi: 10.1242/dev.027607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Sapkota D, Li R, Mu X. Onecut 1 and onecut 2 are potential regulators of mouse retinal development. J Comp Neurol. 2011 doi: 10.1002/cne.22741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Hitomi M, Stacey DW. Variations in cyclin D1 levels through the cell cycle determine the proliferative fate of a cell. Cell Div. 2006;1:32. doi: 10.1186/1747-1028-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XJ. Roles of cell-extrinsic growth factors in vertebrate eye pattern formation and retinogenesis. Semin Cell Dev Biol. 2004;15:91–103. doi: 10.1016/j.semcdb.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Ding K, Pan L, Deng M, Gan L. Math5 determines the competence state of retinal ganglion cell progenitors. Dev Biol. 2003;264:240–54. doi: 10.1016/j.ydbio.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Young RW. Cell death during differentiation of the retina in the mouse. J Comp Neurol. 1984;229:362–73. doi: 10.1002/cne.902290307. [DOI] [PubMed] [Google Scholar]
- Young RW. Cell differentiation in the retina of the mouse. Anat Rec. 1985;212:199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
- Young TL, Cepko CL. A role for ligand-gated ion channels in rod photoreceptor development. Neuron. 2004;41:867–79. doi: 10.1016/s0896-6273(04)00141-2. [DOI] [PubMed] [Google Scholar]
- Yu C, Mazerolle CJ, Thurig S, Wang Y, Pacal M, Bremner R, Wallace VA. Direct and indirect effects of hedgehog pathway activation in the mammalian retina. Mol Cell Neurosci. 2006;32:274–82. doi: 10.1016/j.mcn.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Yang XJ. Regulation of retinal ganglion cell production by Sonic hedgehog. Development. 2001;128:943–57. doi: 10.1242/dev.128.6.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.