Abstract
Disorders of the cardiac rhythm are quite prevalent in clinical practice. Though the variability in drug response between individuals has been extensively studied, this information has not been widely used in clinical practice. Rapid advances in the field of pharmacogenomics have provided us with crucial insights on inter-individual genetic variability and its impact on drug metabolism and action. Technologies for faster and cheaper genetic testing and even personal genome sequencing would enable clinicians to optimize prescription based on the genetic makeup of the individual, which would open up new avenues in the area of personalized medicine. We have systematically looked at literature evidence on pharmacogenomics markers for anti-arrhythmic agents from the OpenPGx consortium collection and reason the applicability of genetics in the management of arrhythmia. We also discuss potential issues that need to be resolved before personalized pharmacogenomics becomes a reality in regular clinical practice.
Keywords: Arrhythmia, Pharmacogenomics, Personal genome, Genetic testing, Adverse drug reactions
Arrhythmias or disorders of the cardiac rhythm are not uncommon in clinical settings and one of the major causes of mortality and morbidity. Atrial Fibrillation is supposed to be rare in young healthy individuals unless without underlying cardiac pathology [10], while prevalent in the elderly and affects roughly around 2-5 Million individuals in the United States alone [2]. Ventricular fibrillation has a smaller incidence of close to 0.4 million [3]. Ventricular tachy-arrhythmias contribute significantly to the morbidity and mortality in patients with underlying coronary artery disease. It has been estimated that close to a half of deaths due to coronary artery disease is caused by ventricular arrhythmias [4]. Apart from the genetic and underlying cardiac disease as causes of cardiac rhythm abnormalities, a number of therapeutic agents, including drugs not directly used in the therapy of cardiac rhythm abnormalities have now been implicated to cause significant prolongation of QT interval and a form of ventricular arrhythmia, torsades de pointes, which is potentially fatal [5]. Recent reports also point to cardiac arrhythmias as one of the top causes for drug withdrawal and failure of clinical trials [6].
No major study on the incidence of arrhythmias or adverse drug reactions to anti-arrhythmic drugs throughout India has been performed. The lack of adequate epidemiological data in this important area has been highlighted in recent publications [4]. According to a report of arrhythmia care in India, published in 2002, the prevalence of patients with arrhythmias in the country is around 2 million [7]. Studies have also pointed to the high prevalence of asymptomatic arrhythmias in elderly patients [8]. According to the reports from the National pharmacovigilance programme, several cases of adverse drug reactions to anti-arrhythmic agents have been reported from people across India. Verapamil and Amiodarone have been reported to cause Steven Johnsons syndrome. Atenolol has been similarly reported to have adverse drug events like fatigue, cough and edema in a study conducted in South India [9]. Similar studies have shown Atenolol to be associated with around 4-5% of total adverse drug reactions reported.
Individuals vary widely in their response to therapeutic agents, and a large component of this variability is modulated through the genetic makeup of the individual. Apart from the variability in response, genetic variations are also now known to contribute significantly to Adverse Drug Reactions (ADRs). One of the earliest contributions to understanding of genomics of external agents have stemmed from the observations of the British physician Garrod, who proposed that defects in enzymatic pathways in unusual diseases of metabolism could produce unusual sensitivity to chemical agents. Molecular genetic dissection of congenital conditions in humans has contributed immensely to the overall understanding of the genetics of heart rhythm. The field has now grown by leaps and bounds with the advent of modern tools and techniques, which enables dissecting genetic phenomena at single base-pair resolution. The advent of genomics technologies has paved the way to deciphering the molecular genetic mechanisms of variability in response to therapeutic agents. This variability could be caused by genetic variations, which modulate the pharmacokinetics or pharmacodynamics of the drug. This could involve variations in genes involved in drug transport/metabolism right up to variations in drug-targets and off-targets. The field of understanding genetic variability in the response to drugs has now emerged into a full-fledged branch of biology - pharmacogenomics with the potential to significantly improve disease management. The field has also offered novel clues towards understanding mechanisms and pathways, which involves therapeutic agents.
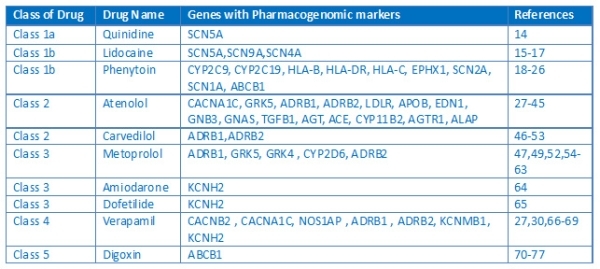
The last couple of decades have seen enormous improvements in the management of cardiac arrhythmias. Due to limited benefits and safety related concerns, very few drugs have been successful and have been commonly used in the treatment of arrhythmias. The field has also seen the emergence of newer classes of drugs which function by normalizing the channel activity rather than blocking them. According to the popular Singh Vaughan Williams classification schema, drugs are placed based on the mechanism of action. The classification scheme has improved over time, presently including the miscellaneous class, which includes drugs which could not fit any of the previous classes. Recent years have seen a number of publications detailing the pharmacogenomics of anti-arrhythmic drugs [10-13]. Though many classes of anti-arrhythmic agents are not particularly used anymore currently in regular clinical practice except in special settings, the wealth of information on pharmacogenomics encompasses the commonly used classes of drugs as well. The Drugs and the genes involved in the pharmacogenomics of anti-arrhythmics and the respective references are summarized in Table 1[14-77].
Table 1.
Summary of drugs, genes involved in the pharmacogenomics of anti-arrhythmic drugs.
Realizing the dream of personalized medicine is not without challenges and focused intervention. The major challenge in understanding the intricacies of genomic variations and deciphering the potential effects on pharmacokinetics and pharmacodynamics is the lack of comprehensive models of drug metabolism and action for many drugs. Understanding and charting drug pathways is the first step towards this dream. A systems level understanding of the drug pathways would enable us to overlay genomic variations and offer smart guesses on drugs that could be involved. The drug pathways for many drugs are complicated, involving multiple and sometimes redundant mechanisms for drug transport, metabolism and targets. Deciphering the pathways is the first step towards understanding how genetic variation could potentially contribute to the changes in functionality of critical components of the drug pathway. In addition, it also provides crucial insights into the molecular mechanisms of drug-drug and drug-environment interactions and how genetic variations could modulate this phenomenon. A comprehensive outline of anti-arrhythmic drugs and their drug pathways are summarized in Figure 1.
Figure 1.
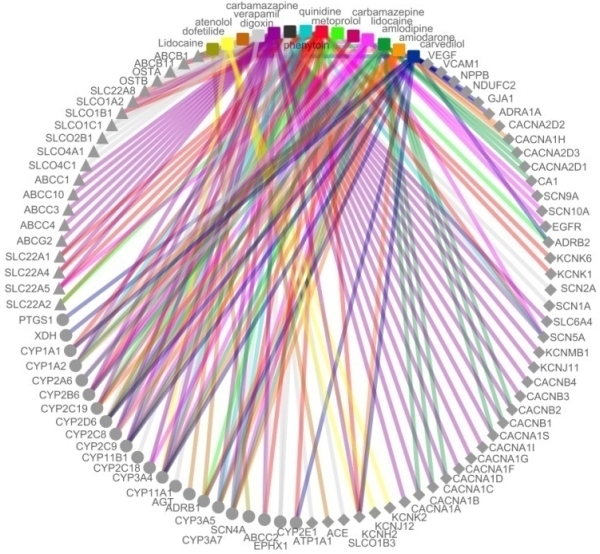
Overview of the drug pathways for anti-arrhythmic drugs. The coloured drugs and edges represent drugs while the metabolizing enzymes are marked as solid circles. The transporters and targets are represented as solid triangles and rhomboids respectively.
Modeling a disease process or pathway is the next critical step in understanding the molecular mechanisms and the genetic architecture of disease processes. Animal models such as rodents and mammals have been used successfully for modeling cardiac arrhythmias. Recently advances include the application of newer model systems for understanding pharmacogenomics principles. Model organisms like zebrafish, which are easy to maintain and study, have been shown to be useful in modeling pharmacological principles and potential mode of action of many therapeutic agents.
The major area that would require focused attention in the immediate future is towards standardized efforts to collate pharmacogenomics data and evidence to enable meta-analysis, while at the same time be able to keep pace with the latest avalanche of evidence brought to light by high throughput genomics studies including Genome-wide associations studies (GWAS). Community led approaches like PharmGKB (www.pharmgkb.org) and crowd-sourcing approaches like OpenPGx (www.openpgx.org) are the possible way forward, and both approaches should be organized complementary to each other. Apart from the data, the second focus area is computational tools and resources that can handle the high-throughput datasets. The availability of genome-wide scans as direct-to-consumer services has also provided an immense opportunity and challenge at the same time. With adequate computational tools and resources for interpretation of the data, this has the potential to lower the cost, while at the same time, widen the general acceptability of genetic testing. No healthcare intervention system is complete without adequate education and empowerment of the medical and paramedical professionals and the patients. For the success of widespread acceptability and application of pharmacogenomics testing for cardiac arrhythmias, appropriate focus and emphasis on awareness and healthcare education is essential.
These efforts should be complemented and supplemented by both systematic ways of collecting data and being able to analyze it to unravel emerging phenomena. This would necessitate creation of effective systems for systematic collection and sharing of clinical data, treatment protocols and outcome measures. This includes setting up of registries, which follow standard protocols, metadata and modes of data exchange. This also requires setting up collaborative and shared data resources and analytical approaches. In summary, seamless exchange of ideas, resources and knowhow between research laboratories and clinicians is essential to realize the dream of making pharmacogenomics based personalized medicine a reality.
Acknowledgements
The authors thank Dr. Shantanu Sengupta and Dr Mohd Faruq for reviewing the manuscript. Authors also thank Mr Jatin Talwar for discussions and visualization of data and Dr Yasha Hasija for comments and discussions. The authors acknowledge the OpenPGx (www.openpgx.org) consortium and community for data annotation from literature. This study is funded by CSIR India.
References
- Domanski MJ, et al. The epidemiology of atrial fibrillation. Coronary Artery Disease. 1995;6:95. doi: 10.1097/00019501-199502000-00002. [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- Zheng ZJ, et al. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 104:2158. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- Naik N, et al. Epidemiology of arrhythmias in India: How do we obtain reliable data? Current Science. 2009;97:411. [Google Scholar]
- Nielsen J, et al. Assessing QT interval prolongation and its associated risks with antipsychotics. CNS Drugs. 2011;25:473. doi: 10.2165/11587800-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Crivellente F, et al. The Sooner the Better: utilising biomarkers to eliminate drug candidates with cardiotoxicity in preclinical development. http://www.ddw-online.com/s/summer-2011/p148216/the-sooner-the-better:-utilising-biomarkers-to-eliminate-drug-candidates-with-cardiotoxicity-in-preclinical-development.html [Google Scholar]
- Lokhandwala Y, et al. Arrhythmia care in India-poised for the big leap. Indian Pacing Electrophysiol J. 2002;2:1. [PMC free article] [PubMed] [Google Scholar]
- Singh H, et al. A 24 hour holter study in asymptomatic elderly Indians. JIACM. 2003;4:308. [Google Scholar]
- Arulmani R, et al. Adverse drug reaction monitoring in a secondary care hospital in South India. Br J Clin Pharmacol. 2008;65:210. doi: 10.1111/j.1365-2125.2007.02993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkcom WT, et al. Emerging concepts in the pharmacogenomics of arrhythmias: ion channel trafficking. Expert Rev Cardiovasc Ther. 2010;8:1161. doi: 10.1586/erc.10.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden DM, et al. Arrhythmia pharmacogenomics: methodological considerations. Curr Pharm Des. 2009;15:3734. doi: 10.2174/138161209789649529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy CE, et al. Pharmacogenetics and anti-arrhythmic drug therapy: a theoretical investigation. Am J Physiol Heart Circ Physiol. 2007;292:H66. doi: 10.1152/ajpheart.00312.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbar D, et al. Pharmacogenetics of antiarrhythmic therapy. Expert Opin Pharmacother. 2006;7:1583. doi: 10.1517/14656566.7.12.1583. [DOI] [PubMed] [Google Scholar]
- Shuraih M, et al. A common SCN5A variant alters the responsiveness of human sodium channels to class I antiarrhythmic agents. J Cardiovasc Electrophysiol. 2007;18:434. doi: 10.1111/j.1540-8167.2007.00777.x. [DOI] [PubMed] [Google Scholar]
- Barajas-Martinez HM, et al. Lidocaine-induced Brugada syndrome phenotype linked to a novel double mutation in the cardiac sodium channel. Circ Res. 2008;103:396. doi: 10.1161/CIRCRESAHA.108.172619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets PL, et al. A Nav1.7 channel mutation associated with hereditary erythromelalgia contributes to neuronal hyperexcitability and displays reduced lidocaine sensitivity. J Physiol. 2007;581:1019. doi: 10.1113/jphysiol.2006.127027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z, et al. Two human paramyotonia congenita mutations have opposite effects on lidocaine block of Na+ channels expressed in a mammalian cell line. J Physiol. 1996;496:275. doi: 10.1113/jphysiol.1996.sp021684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavan R, et al. Influence of CYP2C9 and CYP2C19 genetic polymorphisms on phenytoin-induced neurological toxicity in Indian epileptic patients. Eur J Clin Pharmacol. 2010;66:689. doi: 10.1007/s00228-010-0817-2. [DOI] [PubMed] [Google Scholar]
- Hung SI, et al. Common risk allele in aromatic antiepileptic-drug induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Han Chinese. Pharmacogenomics. 2010;11:349. doi: 10.2217/pgs.09.162. [DOI] [PubMed] [Google Scholar]
- Azzato EM, et al. Maternal EPHX1 polymorphisms and risk of phenytoin-induced congenital malformations. Pharmacogenet Genomics. 2010;20:58. doi: 10.1097/FPC.0b013e328334b6a3. [DOI] [PubMed] [Google Scholar]
- Chaudhry AS, et al. CYP2C9*1B promoter polymorphisms, in linkage with CYP2C19*2, affect phenytoin autoinduction of clearance and maintenance dose. J Pharmacol Exp Ther. 2010;332:599. doi: 10.1124/jpet.109.161026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan P, et al. Multidrug resistance in epilepsy and polymorphisms in the voltage-gated sodium channel genes SCN1A, SCN2A, and SCN3A: correlation among phenotype, genotype, and mRNA expression. Pharmacogenet Genomics. 2008;18:989. doi: 10.1097/FPC.0b013e3283117d67. [DOI] [PubMed] [Google Scholar]
- Locharernkul C, et al. Carbamazepine and phenytoin induced Stevens-Johnson syndrome is associated with HLA-B*1502 allele in Thai population. Epilepsia. 2008;49:2087. doi: 10.1111/j.1528-1167.2008.01719.x. [DOI] [PubMed] [Google Scholar]
- Man CB, et al. Association between HLA-B*1502 allele and antiepileptic drug-induced cutaneous reactions in Han Chinese. Epilepsia. 2007;48:1015. doi: 10.1111/j.1528-1167.2007.01022.x. [DOI] [PubMed] [Google Scholar]
- Tate SK, et al. A common polymorphism in the SCN1A gene associates with phenytoin serum levels at maintenance dose. Pharmacogenet Genomics. 2006;16:721. doi: 10.1097/01.fpc.0000230114.41828.73. [DOI] [PubMed] [Google Scholar]
- Tate SK, et al. Genetic predictors of the maximum doses patients receive during clinical use of the anti-epileptic drugs carbamazepine and phenytoin. Proc Natl Acad Sci U S A. 2005;102:5507. doi: 10.1073/pnas.0407346102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitelshees AL, et al. CACNA1C gene polymorphisms, cardiovascular disease outcomes, and treatment response. Circ Cardiovasc Genet. 2009;2:3628:10 PM 4/8/2012. doi: 10.1161/CIRCGENETICS.109.857839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurnik D, et al. GRK5 Gln41Leu polymorphism is not associated with sensitivity to beta(1)-adrenergic blockade in humans. Pharmacogenomics. 2009;10:1581. doi: 10.2217/pgs.09.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurnik D, et al. Beta-1-adrenoceptor genetic variants and ethnicity independently affect response to beta-blockade. Pharmacogenet Genomics. 2008;18:895. doi: 10.1097/FPC.0b013e328309733f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacanowski MA, et al. Beta-adrenergic receptor gene polymorphisms and beta-blocker treatment outcomes in hypertension. Clin Pharmacol Ther. 2008;84:715. doi: 10.1038/clpt.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaccarino G, et al. Beta2-adrenergic receptor polymorphisms and treatment-induced regression of left ventricular hypertrophy in hypertension. Clin Pharmacol Ther. 2006;80:633. doi: 10.1016/j.clpt.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Liljedahl U, et al. Single nucleotide polymorphisms in the apolipoprotein B and low density lipoprotein receptor genes affect response to antihypertensive treatment. BMC Cardiovasc Disord. 2004;4:16. doi: 10.1186/1471-2261-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson J, et al. Beta1-adrenergic receptor gene polymorphisms and response to beta1-adrenergic receptor blockade in patients with essential hypertension. Clin Cardiol. 2004;27:347. doi: 10.1002/clc.4960270610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallberg P, et al. Gender-specific association between preproendothelin-1 genotype and reduction of systolic blood pressure during antihypertensive treatment--results from the Swedish Irbesartan Left Ventricular Hypertrophy Investigation versus Atenolol (SILVHIA) Clin Cardiol. 2004;27:287. doi: 10.1002/clc.4960270510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filigheddu F, et al. Genetic polymorphisms of the beta-adrenergic system: association with essential hypertension and response to beta-blockade. Pharmacogenomics J. 2004;4:154. doi: 10.1038/sj.tpj.6500247. [DOI] [PubMed] [Google Scholar]
- Hallberg P, et al. Transforming growth factor beta1 genotype and change in left ventricular mass during antihypertensive treatment--results from the Swedish Irbesartan Left Ventricular Hypertrophy Investigation versus Atenolol (SILVHIA) Clin Cardiol. 2004;27:169. doi: 10.1002/clc.4960270315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurland L, et al. Angiotensinogen gene polymorphisms: relationship to blood pressure response to antihypertensive treatment. Results from the Swedish Irbesartan Left Ventricular Hypertrophy Investigation vs Atenolol (SILVHIA) trial. Am J Hypertens. 2004;17:8. doi: 10.1016/j.amjhyper.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Diez J, et al. The A1166C polymorphism of the AT1 receptor gene is associated with collagen type I synthesis and myocardial stiffness in hypertensives. J Hypertens. 2003;21:2085. doi: 10.1097/01.hjh.0000098127.00558.d2. [DOI] [PubMed] [Google Scholar]
- Hallberg P, et al. Adipocyte-derived leucine aminopeptidase genotype and response to antihypertensive therapy. BMC Cardiovasc Disord. 2003;3:11. doi: 10.1186/1471-2261-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofowora GG, et al. A common beta1-adrenergic receptor polymorphism (Arg389Gly) affects blood pressure response to beta-blockade. Clin Pharmacol Ther. 2003;73:366. doi: 10.1016/s0009-9236(02)17734-4. [DOI] [PubMed] [Google Scholar]
- Kurland L, et al. Aldosterone synthase (CYP11B2) -344 C/T polymorphism is related to antihypertensive response: result from the Swedish Irbesartan Left Ventricular Hypertrophy Investigation versus Atenolol (SILVHIA) trial. Am J Hypertens. 2002;15:389. doi: 10.1016/s0895-7061(02)02256-2. [DOI] [PubMed] [Google Scholar]
- Kurland L, et al. Polymorphisms in the angiotensinogen and angiotensin II type 1 receptor gene are related to change in left ventricular mass during antihypertensive treatment: results from the Swedish Irbesartan Left Ventricular Hypertrophy Investigation versus Atenolol (SILVHIA) trial. J Hypertens. 2002;20:657. doi: 10.1097/00004872-200204000-00023. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy KM, et al. The gain-of-function G389R variant of the beta1-adrenoceptor does not influence blood pressure or heart rate response to beta-blockade in hypertensive subjects. Clin Sci (Lond) 2000;99:233. [PubMed] [Google Scholar]
- Kurland L, et al. Swedish Irbesartan Left Ventricular Hypertrophy Investigation versus Atenolol (SILVHIA) Trial.: Angiotensin converting enzyme gene polymorphism predicts blood pressure response to angiotensin II receptor type 1 antagonist treatment in hypertensive patients. J Hypertens. 2001;19:1783. doi: 10.1097/00004872-200110000-00012. [DOI] [PubMed] [Google Scholar]
- Dudley C, et al. Prediction of patient responses to antihypertensive drugs using genetic polymorphisms: investigation of renin-angiotensin system genes. J Hypertens. 1996;14:259. doi: 10.1097/00004872-199602000-00016. [DOI] [PubMed] [Google Scholar]
- Dudley C, et al. Role of beta-adrenergic receptor gene polymorphisms in the long-term effects of beta-blockade with carvedilol in patients with chronic heart failure. Cardiovasc Drugs Ther. 2010;24:49. doi: 10.1007/s10557-010-6220-5. [DOI] [PubMed] [Google Scholar]
- Cresci S, et al. Clinical and genetic modifiers of long-term survival in heart failure. J Am Coll Cardiol. 2009;54:432. doi: 10.1016/j.jacc.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troncoso R, et al. Gln(27)->Glubeta(2)-adrenergic receptor polymorphism in heart failure patients: differential clinical and oxidative response to carvedilol. Basic Clin Pharmacol Toxicol. 2009;104:374. doi: 10.1111/j.1742-7843.2008.00370.x. [DOI] [PubMed] [Google Scholar]
- Sehnert AJ, et al. Lack of association between adrenergic receptor genotypes and survival in heart failure patients treated with carvedilol or metoprolol. J Am Coll Cardiol. 2008;52:644. doi: 10.1016/j.jacc.2008.05.022. [DOI] [PubMed] [Google Scholar]
- Chen L, et al. Arg389Gly-beta1-adrenergic receptors determine improvement in left ventricular systolic function in nonischemic cardiomyopathy patients with heart failure after chronic treatment with carvedilol. Pharmacogenet Genomics. 2007;17:941. doi: 10.1097/FPC.0b013e3282ef7354. [DOI] [PubMed] [Google Scholar]
- Mialet Perez J, et al. Beta 1-adrenergic receptor polymorphisms confer differential function and predisposition to heart failure. Nat Med. 2003;9:1300. doi: 10.1038/nm930. [DOI] [PubMed] [Google Scholar]
- Petersen M, et al. Association of beta-adrenergic receptor polymorphisms and mortality in carvedilol-treated chronic heart-failure patients. Br J Clin Pharmacol. 2011;71:556. doi: 10.1111/j.1365-2125.2010.03868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye DM, et al. Beta-adrenoceptor genotype influences the response to carvedilol in patients with congestive heart failure. Pharmacogenetics. 2003;13:79. doi: 10.1097/00008571-200307000-00002. [DOI] [PubMed] [Google Scholar]
- Bhatnagar V, et al. G-protein-coupled receptor kinase 4 polymorphisms and blood pressure response to metoprolol among African Americans: sex-specificity and interactions. Am J Hypertens. 2009;22:332. doi: 10.1038/ajh.2008.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SK, et al. Influence of CYP2D6*10 on the pharmacokinetics of metoprolol in healthy Korean volunteers. J Clin Pharm Ther. 2008;33:567. doi: 10.1111/j.1365-2710.2008.00945.x. [DOI] [PubMed] [Google Scholar]
- Bijl MJ, et al. Genetic variation in the CYP2D6 gene is associated with a lower heart rate and blood pressure in beta-blocker users. Clin Pharmacol Ther. 2009;85:45. doi: 10.1038/clpt.2008.172. [DOI] [PubMed] [Google Scholar]
- Lobmeyer MT, et al. Synergistic polymorphisms of beta1 and alpha2C-adrenergic receptors and the influence on left ventricular ejection fraction response to beta-blocker therapy in heart failure. Pharmacogenet Genomics. 2007;17:277. doi: 10.1097/FPC.0b013e3280105245. [DOI] [PubMed] [Google Scholar]
- Liu J, et al. Beta1-Adrenergic receptor polymorphisms influence the response to metoprolol monotherapy in patients with essential hypertension. Clin Pharmacol Ther. 2006;80:23. doi: 10.1016/j.clpt.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Beitelshees AL, et al. Influence of phenotype and pharmacokinetics on beta-blocker drug target pharmacogenetics. Pharmacogenomics J. 2006;6:174. doi: 10.1038/sj.tpj.6500354. [DOI] [PubMed] [Google Scholar]
- Terra SG, et al. Beta1-adrenergic receptor polymorphisms and left ventricular remodeling changes in response to beta-blocker therapy. Pharmacogenet Genomics. 2005;15:227. doi: 10.1097/01213011-200504000-00006. [DOI] [PubMed] [Google Scholar]
- Liu J, et al. Gly389Arg polymorphism of beta1-adrenergic receptor is associated with the cardiovascular response to metoprolol. Clin Pharmacol Ther. 2003;74:372. doi: 10.1016/S0009-9236(03)00224-8. [DOI] [PubMed] [Google Scholar]
- White HL, et al. An evaluation of the beta-1 adrenergic receptor Arg389Gly polymorphism in individuals with heart failure: a MERIT-HF sub-study. Eur J Heart Fail. 2003;5:463. doi: 10.1016/s1388-9842(03)00044-8. [DOI] [PubMed] [Google Scholar]
- Johnson JA, et al. Beta 1-adrenergic receptor polymorphisms and antihypertensive response to metoprolol. Clin Pharmacol Ther. 2003;74:44. doi: 10.1016/S0009-9236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- Yang P, et al. Allelic variants in long-QT disease genes in patients with drug-associated torsades de pointes. Circulation. 2002;105:1943. doi: 10.1161/01.cir.0000014448.19052.4c. [DOI] [PubMed] [Google Scholar]
- Sun Z, et al. Role of a KCNH2 polymorphism (R1047 L) in dofetilide-induced Torsades de Pointes. J Mol Cell Cardiol. 2004;37:1031. doi: 10.1016/j.yjmcc.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Niu Y, et al. Genetic variation in the beta2 subunit of the voltage-gated calcium channel and pharmacogenetic association with adverse cardiovascular outcomes in the INternational VErapamil SR-Trandolapril STudy GENEtic Substudy (INVEST-GENES) Circ Cardiovasc Genet. 2010;3:548. doi: 10.1161/CIRCGENETICS.110.957654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noord C, et al. Calcium channel blockers, NOS1AP, and heart-rate-corrected QT prolongation. Pharmacogenet Genomics. 2009;19:260. doi: 10.1097/FPC.0b013e328324e556. [DOI] [PubMed] [Google Scholar]
- Beitelshees AL, et al. KCNMB1 genotype influences response to verapamil SR and adverse outcomes in the INternational VErapamil SR/Trandolapril STudy (INVEST) Pharmacogenet Genomics. 2007;17:719. doi: 10.1097/FPC.0b013e32810f2e3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan JJ, et al. Verapamil blocks HERG channel by the helix residue Y652 and F656 in the S6 transmembrane domain. Acta Pharmacol Sin. 2007;28:959. doi: 10.1111/j.1745-7254.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- Bartnicka L, et al. Effect of ABCB1 (MDR1) 3435C >T and 2677G >A,T polymorphisms and P-glycoprotein inhibitors on salivary digoxin secretion in congestive heart failure patients. Pharmacol Rep. 2007;59:323. [PubMed] [Google Scholar]
- Chowbay B, et al. Meta-analysis of the influence of MDR1 C3435T polymorphism on digoxin pharmacokinetics and MDR1 gene expression. Br J Clin Pharmacol. 2005;60:159. doi: 10.1111/j.1365-2125.2005.02392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita N, et al. Human MDR1 polymorphism: G2677T/A and C3435T have no effect on MDR1 transport activities. Biochem Pharmacol. 2003;65:1843. doi: 10.1016/s0006-2952(03)00178-3. [DOI] [PubMed] [Google Scholar]
- Morita Y, et al. MDR1 genotype-related duodenal absorption rate of digoxin in healthy Japanese subjects. Pharm Res. 2003;20:552. doi: 10.1023/a:1023282312757. [DOI] [PubMed] [Google Scholar]
- Verstuyft C, et al. Digoxin pharmacokinetics and MDR1 genetic polymorphisms. Eur J Clin Pharmacol. 2003;58:809. doi: 10.1007/s00228-003-0567-5. [DOI] [PubMed] [Google Scholar]
- Gerloff T, et al. MDR1 genotypes do not influence the absorption of a single oral dose of 1 mg digoxin in healthy white males. Br J Clin Pharmacol. 2002;54:610. doi: 10.1046/j.1365-2125.2002.01691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim RB, et al. Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin Pharmacol Ther. 2001;70:189. doi: 10.1067/mcp.2001.117412. [DOI] [PubMed] [Google Scholar]
- Hoffmeyer S, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci U S A. 2000;97:3473. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]


