Abstract
Rationale
Identification of biomarkers that establish diagnosis or treatment response is critical to the advancement of research and management of patients with depression.
Objective
Our goal was to identify biomarkers that can potentially assess fluoxetine response and risk to poor treatment outcome.
Methods
We measured behavior, gene expression, and the levels of 36 neurobiochemical analytes across a panel of genetically diverse mouse inbred lines after chronic treatment with water or fluoxetine.
Results
Glyoxylase 1 (GLO1) and guanine nucleotide-binding protein 1 (GNB1) mostly account for baseline anxiety-like and depressive-like behavior, indicating a common biological link between depression and anxiety. Fluoxetine-induced biochemical alterations discriminated positive responders, while baseline neurobiochemical differences differentiated negative responders (p < 0.006). Results show that glial fibrillary acidic protein, S100 beta protein, GLO1, and histone deacetylase 5 contributed most to fluoxetine response. These proteins are linked within a cellular growth/proliferation pathway, suggesting the involvement of cellular genesis in fluoxetine response. Furthermore, a candidate genetic locus that associates with baseline depressive-like behavior contains a gene that encodes for cellular proliferation/adhesion molecule (Cadm1), supporting a genetic basis for the role of neuro/gliogenesis in depression.
Conclusion
We provided a comprehensive analysis of behavioral, neurobiochemical, and transcriptome data across 30 mouse inbred strains that has not been accomplished before. We identified biomarkers that influence fluoxetine response, which, altogether, implicate the importance of cellular genesis in fluoxetine treatment. More broadly, this approach can be used to assess a wide range of drug response phenotypes that are challenging to address in human samples.
Electronic supplementary material
The online version of this article (doi:10.1007/s00213-011-2574-z) contains supplementary material, which is available to authorized users.
Keywords: Depression, Anxiety, Fluoxetine, Genetic, Biochemical, Biomarker, SSRI
Introduction
The social and economic burden of major depressive disorder (MDD) is substantial. Despite the significant cost associated with depression, <30% of patients achieve adequate response to a selective serotonin reuptake inhibitor (SSRI; Trivedi et al. 2006). There are several factors that contribute to poor treatment outcome, including gender, disease onset, and presence of comorbid psychiatric disorders (Kornstein and Schneider 2001). Presently, the therapeutic management of patients who relapse or fail to respond is poorly defined. Partial responders or non-remitters typically undergo a series of antidepressant treatment modifications that are largely based on trial and error, leading to delay in symptom resolution and treatment discontinuation (Olfson et al. 2006; Warden et al. 2007). A set of biomarkers that can prospectively identify patients who will respond to a specific medication can provide an evidence-based rationale for selecting an appropriate course of antidepressant therapy.
Several large studies have been undertaken to identify genetic biomarkers of antidepressant response (Garriock et al. 2010; Ising et al. 2009; Uher et al. 2010). Pharmacogenetic studies of antidepressants in the Sequenced Treatment Alternatives for Depression (STAR*D) trial have led to the identification of genes associated with treatment response (Hu et al. 2007; Kraft et al. 2007; Lekman et al. 2008; McMahon et al. 2006; Paddock 2008), treatment resistance (Perlis et al. 2008), and treatment-emergent suicidal ideation (Laje et al. 2007, 2009; Perlis et al. 2007). In addition, polymorphisms in genes that encode drug-metabolizing enzymes and transporters have been tested for correlation with treatment response (Peters et al. 2008). Although several studies have yielded notable findings, none meet the currently accepted standards in human genetics for replication (Chanock et al. 2007).
The search for antidepressant biochemical markers is complicated by multiple neurobiological abnormalities that have been observed in depression, indicating that it is unlikely for a single biological marker to characterize a multifactorial disease like MDD. Many analytes, including brain-derived neurotrophic factor (BDNF), serotonin transporter, and monoamines, have been linked with depressive symptoms and response to antidepressant therapy (Manji et al. 2001; Nestler et al. 2002; Thase 2007). A few studies have looked at biochemical markers that can be used as diagnostic (Domenici et al. 2010; Jehn et al. 2006) or treatment markers (Ising et al. 2007; Schule et al. 2009) for depression. Other physiologic markers including neurostructural and neurofunctional measures have shown promise as potential markers of antidepressant response (Leuchter et al. 2009, 2010). It is yet to be determined whether these biomarkers can prospectively predict response prior to treatment.
A major limitation in evaluating the relationship between antidepressant response and biochemical alterations in human subjects is sample accessibility, whereby analysis is typically restricted to human serum and postmortem brain samples. The assessment of postmortem brain tissues is limited by the inability to distinguish whether neurobiochemical abnormalities are causal or a consequence of disease or treatment, while serum samples are more accessible but provide a relatively myopic view of the neurobehavioral mechanisms that occur in the brain. Importantly, these limitations can be mitigated through the use of inbred mouse strains, which are easily maintained and manipulated for research purposes. Besides ease in accessibility, mice within the same strain are homogeneous at each genomic locus, which makes the identification of genetic variants of drug response more feasible due to a reduced genomic complexity.
In order to understand how biochemical and genetic differences correlate with treatment response, we measured depressive-like behavior, anxiety-like behavior, gene expression, and the levels of 36 neurobiochemical analytes across a panel of genetically diverse mouse inbred lines after chronic treatment with vehicle or fluoxetine. Neurobiochemical markers were chosen based on their putative molecular function within pathways proposed to underlie depression and anxiety, which include neuronal transmission, HPA axis regulation, and neuroimmune processes. To our knowledge, this is the first study that simultaneously measured and examined the role of 36 putative neurobiochemical markers across 30 mouse inbred strains. In this study, we examined how baseline and treatment-induced biochemical differences affect depressive-like and anxiety-like responses. Our goal was to establish genetic and biochemical biomarkers that can predict fluoxetine response and to propose a molecular pathway that is critical in mediating response to SSRIs. In addition, we examined baseline neurobiological differences that affect depressive-like and anxiety-like behaviors in an effort to identify biomarkers that contribute to risk for poor treatment outcomes.
Materials and methods
Animals
Thirty mouse inbred strains (129S1/SvImJ, A/J, AKR/J, BALB/cJ, BTBRT<t>tf/J, BUB/BnJ, C3H/HeJ, C57BL/6J, C57BLKS/J, C57BR/cdJ, C58/J, CBA/J, CE/J, DBA/2J, FVB/NJ, I/LnJ, LG/J, LP/J, MA/MyJ, MRL/MpJ, NOD/LtJ, NOR/LtJ, NZB/BlNJ, NZW/LacJ, P/J, PL/J, RIIIS/J, SJL/J, SM/J, and SWR/J) aged 5–6 weeks were obtained from The Jackson Laboratory (Bar Harbor, ME). Male mice were housed four per cage in polycarbonate cages on a 12-h light/dark cycle (lights on at 0700 hours) with access to food and water ad libitum. Following 1 week of habituation, mice were randomized to either control or treatment group. Depending on cost, availability, and estimated intra-strain response variability, the number of animals treated per treatment group ranged from 9 to 21 per strain. All procedures were approved by the Institutional Animal Care and Use Committee and followed the guidelines set forth by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Drug treatment
Fluoxetine is a SSRI that has been extensively studied for its effect on depressive behavior (Cipriani et al. 2005; Rossi et al. 2004). Fluoxetine HCl was purchased from Spectrum Chemicals (Gardena, CA). The mean water intake for each strain was determined previously by measuring daily water consumption for 3 weeks. This information, along with the average weight measurements for each strain, was used to determine the amount of fluoxetine required to provide a daily oral dose of 0 or 18 mg/kg per mouse. A chronic fluoxetine regimen of 18 mg/kg for 21 days was selected based on the results of a previous dose–response study which investigated the behavioral effects of fluoxetine administration at 0, 5, 10, 14, or 18 mg/kg for 1, 6, or 21 days (Miller et al. 2008). In each strain, a comparison of the mean daily water intake between vehicle- and fluoxetine-treated animals indicates a minimal effect of fluoxetine on water consumption (Electronic supplementary material (ESM) Online Resource 1). Mice were treated daily with fluoxetine or water throughout the end of the study and were sacrificed 1 week after the open field test between 1300 and 1600 hours.
Tail suspension test
Strains were randomly tested across day, time of testing, and equipment. At any given time, only four to eight animals per strain were tested. Each animal was tested only once in each procedure. After chronic administration of 0 or 18 mg/kg of fluoxetine for 21 days, mice aged 9–10 weeks were tested in a tail suspension apparatus (PHM-300 TST Cubicle, Med Associates, St. Albans, VT) between 1300 and 1600 hours. In this test, the mouse is subjected to a short-term inescapable stress by having its tail suspended. Following failed attempts to escape, the mouse becomes immobile, a response generally considered as behavioral despair, a depressive-like behavior that is proposed to model “hopelessness” (Steru et al. 1985, 1987). Immobility was recorded for 7 min in 60-s blocks using the following parameters: threshold = 3, gain = 8, and resolution = 200 ms. Since all strains were uniformly active for the first minute, percent time spent immobile was calculated for the last 6 min of the test. This procedure has been used previously to determine tail suspension test (TST) responses to antidepressants (Fujishiro et al. 2002; Ukai et al. 1998). Mice that climbed up their tail during testing were excluded from analysis (number of animals excluded = 104 out of 721 mice). Mean depressive-like behavior is reported in ESM Online Resource 2.
Open field test
To minimize potential carryover effects between tests, behavior in the open field was recorded a week following TST. The open field (OF) test was conducted between 1300 and 1600 hours using a 27.3 × 27.3-m open field apparatus (MED-OFA-MS, Med Associates) which was surrounded by infrared detection beams on the x-, y-, and z-axes that automatically recorded the animals’ position and activity over the course of the experiment. Activity in the open field was recorded for 10 min within an environmental chamber that provided white noise (60–64 dB) and low, indirect lighting (ESM Online Resource 2). The OF is commonly used to measure exploratory and locomotive behavior in mice. This test exploits the rodent’s innate aversion to well-lit open spaces. Based on previous observations that showed increased exploration toward illuminated open areas following the administration of anxiolytics (Choleris et al. 2001; Crawley 1985), anxiety-like behavior was measured as percent time spent in the center 25% of the open field. In addition, locomotor activity was recorded by calculating the total distance traveled in the open field. This behavior was measured to assess whether general locomotor activity confounded response in the TST.
Tissue collection
Upon completion of the study, mice were killed by cervical dislocation and decapitation between 0900 and 1300 hours. Trunk blood was quickly collected and allowed to clot on ice. Following centrifugation, serum samples were collected and stored at −20°C for the determination of fluoxetine and norfluoxetine levels using liquid chromatography–tandem mass spectrometry (LC-MS/MS). Micro-dissections of individual regions were performed on serial coronal brain sections that were placed on a cold metal block. The cortex was taken from the same section for each animal and immediately snap-frozen on dry ice and stored at −80°C for gene expression and neurobiochemical analyses. Other brain regions were used for RNA isolation and subsequent gene expression analysis. Given that the neurobiochemical markers we examined were primarily expressed in the brain (Table 1), we measured the levels of these analytes in brain tissue. Tissue size requirements limited brain transcriptome analysis and neurobiochemical quantification to the cortex.
Table 1.
List of neurobiochemical analytes simultaneously measured across multiple mouse inbred strains and their known functions
| Biomarker | Name | Function | GO category |
|---|---|---|---|
| ACTH | Adrenocorticotropin-releasing hormone | Neuroimmune | (GO:0005179), (GO:0007218) |
| APOD | Apolipoprotein D | Neuroimmune | (GO:0006629), (GO:0005215) |
| BAG1 | BCL2-associated athanogene 1 | Apoptosis | (GO:0006916), (GO:0006950) |
| BDNF | Brain-derived neurotrophic factor | Neurogenesis | (GO:0006916) (GO:0048167) |
| c-fos | FBJ osteosarcoma oncogene | Transcription | (GO:0007399), (GO:0045941) |
| CREB | cAMP response element-binding | Signal Transduction | (GO:0007165), (GO:0007202) |
| PPP1R1B | Protein phosphatase 1 subunit 1B | Modulation of phosphatase1 | (GO:0007165), (GO:0004864) |
| DAT | Dopamine transporter | Regulation of dopamine levels | (GO:0005329), (GO:0007268) |
| FKBP51 | FK506-binding protein 51 | Signal transduction | (GO:0005528), (GO:0031072) |
| GAD67 | Glutamic acid decarboxylase-67 | Decarboxylation of glutamate to GABA | (GO:0004351), (GO:0006915) |
| Galanin | Galanin | Neuronal modulation | (GO:0005184), (GO:0006950) |
| GFAP | Glial fibrillary acidic protein | Maintenance of astrocytes | (GO:0005200), (GO:0005882) |
| Ghrelin | Ghrelin | Neurotrophy and appetite regulation | (GO:0006916), (GO:0008343) |
| GLO1 | Glyoxylase1 | Neuroprotection | (GO:0006916), (GO:0006749) |
| GNB1 | Guanine nucleotide-binding protein subunit beta-1 | Signal Transduction | (GO:0006112), (GO:0007200) |
| GSK3β | Glycogen synthase kinase 3 beta | Phosphorylation | (GO:0035255), (GO:0006916) |
| GSR | Glutathione Reductase | Neuroprotection | (GO:0006749), (GO:0016491) |
| HDAC5 | Histone deacetylase 5 | Transcription | (GO:0004407), (GO:0000122) |
| IL-6 | Interleukin6 | Neuroimmune | (GO:0001781), (GO:0006954) |
| KCNJ9 | Potassium inwardly rectifying channel, subfamily J, member 9 | Regulation of K+ levels | (GO:0015467), (GO:0006813) |
| MCH | Melanin concentrating hormone | Sleep and circadian rhythm | (GO:0007218), (GO:0046005) |
| NET | Norepinephrine transporter | Regulation of norepinephrine levels | (GO:0006836), (GO:0008504) |
| NGF | Nerve growth factor | Neuronal growth and signaling | (GO:0008504), (GO:0006954) |
| NMDA1 | Glutamate receptor, subunit 1 | Neurotransmission | (GO:0004972), (GO:0005234) |
| NPY | Neuropeptide Y | Neuropeptide signaling | (GO:0005184), (GO:0001664) |
| NR3C1 | Glucocorticoid receptor | Neuroendocrine | (GO:0004883), (GO:0006351) |
| NSG2 | Neuron-specific family gene member 2 | Dopamine receptor signaling pathway | (GO:0007212), (GO:0050780) |
| P2X7 | Purinergic receptor ligand-gated ion channel 7 | Regulation of Ca2+ | (GO:0010524), (GO:0000187) |
| PAQR8 | Progestin and AdipoQ receptor 8 | Steroid binding | (GO:0004872), (GO:0005496) |
| PTH | Parathyroid hormone | Regulation of Ca2+ | (GO:0031856), (GO:0007186) |
| S100β | S100 beta protein | Glial cell proliferation | (GO:0007417), (GO:0008283) |
| SERT | Serotonin transporter | Regulation of norepinephrine levels | (GO:0008504), (GO:0015222) |
| SGNE | Secretogranin V | Neuroendocrine | (GO:0016486), (GO:0007218) |
| TNF-α | Tumor necrosis factor-alpha | Neuroimmune | (GO:0006955), (GO:0005125) |
| VEGF | Vascular endothelial growth factor | Growth and angiogenesis | (GO:0001525), (GO:0005125) |
Fluoxetine/norfluoxetine quantification
A solution with 10 μL of serum and 150 μL of acetonitrile was spiked with 5 μg/mL of each internal standard (fluoxetine-D6 and norfluoxetine-D6). Following sample filtration with a 0.45-μm filter plate, 50 μL of the filtrate was diluted with 0.1% formic acid and injected into a Sciex 4000 LC-MS/MS (Sciex Inc., Concord, ON) equipped with a Waters YMC Cyano HPLC column (2.0 × 23 mm, Milford, MA). The mobile phase consisted of water/acetonitrile/formic acid (75:25:0.1). A standard curve with concentrations from 20 to 10, 000 ng/mL was used to extrapolate the amounts of fluoxetine and norfluoxetine in each sample (n = 8–19 mice per strain per treatment group). Peak areas were detected for ions with the following mass-to-charge (m/z) ratios: m/z 310 → 44 (fluoxetine), m/z 296 → 134 (norfluoxetine), m/z 316 → 44 (fluoxetine-D6), and m/z 302 → 140 (norfluoxetine-D6). This information was used to quantify serum fluoxetine and norfluoxetine levels (ESM Online Resource 3).
Neurobiochemical analyte quantification
Prior to ELISA experiments, all antibodies were tested for specificity by performing Western blot experiments in pooled brain samples acquired from three mouse inbred strains. Abundance of neurobiochemical markers was measured using a parallelized reverse ELISA methodology (Zeptosens—a division of Bayer (Schweiz) AG, Witterswil, Switzerland). From each strain, cortex tissues were taken from three animals in each treatment group and homogenized in CLB1 buffer (Zeptosens) with zirconium oxide beads for 30 s using QIAgen TissueLyser II (QIAgen, Valencia, CA). Semi-solid brain tissues were mixed gently on a tumbling shaker for 30 min at room temperature. Following centrifugation (2 min at 10,000×g), supernatants were transferred and stored at −80°C until further analysis. The total protein concentrations for each sample were determined using a modified Bradford assay (Coomassie Protein Plus Assay, Pierce Biotechnology, Rockford, IL). All samples were adjusted with CLB1 buffer to obtain a uniform concentration of 2 mg/mL. Immediately after dilution, each sample was spotted twice at concentrations of 0.05, 0.10, 0.15, and 0.2 mg/mL of total protein. Reference signals were obtained from simultaneous spotting of assay buffer and labeled antibodies on the array chip. Following overnight incubation with primary antibodies, arrays were washed and incubated with fluorescence-labeled anti-species secondary antibody for 2.5 h in the dark. Arrays were imaged simultaneously using ZeptoREADER (Zeptosens) at excitation/emission wavelengths of 635/670 nm with exposure times of 0.3, 1, 5, and 10 s.
All images were analyzed using ZeptoVIEW 3.0 (version 3.0.1.17). Background intensities for each individual spot were determined by taking the mean signals of four additional spots equidistant from the sample spot. Net fluorescence intensities (NFI) were calculated by subtracting the background signal from each sample spot signaling. Following NFI determination, each NFI value was normalized to the mean intensity of the reference spots. Using least squares method, eight normalized NFI values obtained from each sample were fitted linearly against tissue lysate protein concentrations. The extrapolated signal intensity that corresponds to the midpoint of the concentration range (0.125 mg/mL) is defined as the reference fluorescence intensity (RFI). Relative abundance for each neurobiochemical marker was obtained by comparing inter-strain RFI values. All neurobiochemical markers measured in this study are listed in Table 1. The mean RFI scores for each analyte are listed in ESM Online Resource 4. Behavioral, neurobiochemical, and drug (fluoxetine and norfluoxetine) concentration data for each animal were deposited at The Jackson Laboratory Phenome Database (http://phenome.jax.org/) under the name Wiltshire 2 and Wiltshire 3.
Statistical analysis
Response to treatment was calculated by taking the ratio of fluoxetine behavioral scores to vehicle behavioral scores. Percent change in immobility was calculated by multiplying the ratio of fluoxetine immobility scores to vehicle immobility scores by 100 and then subtracting the product from 100. We defined positive and negative responders as having at least 20% decreased or increased in immobility scores, respectively. Given that the criterion was defined subjectively, we also investigated the difference in response using more stringent criteria, which were 30% and 40% change in immobility scores. Strain and treatment effects on immobility scores and percent time spent in the center of the OF were obtained using a two-way analysis of variance. Post hoc pairwise comparisons of behavioral scores between control and fluoxetine groups were performed using a Mann–Whitney U test. False discovery rate is controlled at 0.10 using the Benjamini–Hochberg method.
Because both baseline neurobiochemical levels and fluoxetine-induced biochemical alterations can affect the SSRI response, we wanted to identify the relationship between behavior and neurobiochemical alterations at baseline and after fluoxetine treatment. Partial least squares (PLS) regression analysis was used to extract latent vectors that account for most of the covariance between biochemical analytes and behavior in each treatment group. Once the vector was extracted, we determined the biochemical analytes that covary most with behavioral response by assessing the PLS loading score for each analyte onto the vector.
To identify biochemical markers that discriminate positive or negative responders, we used a distance weighted discrimination (DWD) analysis. Unlike traditional multivariate analysis, this approach was intended for the analysis of high dimension, low sample size data sets (Marron et al. 2007; Qiao et al. 2010). The DWD method was used to identify a hyperplane that best distinguishes the positive or the negative response groups. Following identification of a DWD hyperplane, the DWD loading vector (orthonormal to the hyperplane) was calculated, indicating the contribution of each neurobiochemical marker in discriminating negative or positive responders. The Direction Projection Permutation based hypothesis test (DiProPerm) with DWD was used to test for an overall neurobiochemical mean difference in each response group. In this procedure, the neurobiochemical scores were projected onto the DWD vector and the two-sample t statistic was computed. The t statistic obtained from the real data set was compared with the t statistics obtained from 1,000 relabeled group pairs with recomputed DWD vector to determine whether the overall difference in neurobiochemical levels was significant (p < 0.05).
We performed Pearson’s correlation analysis to determine whether neurobiochemical levels correspond with the fluoxetine, norfluoxetine, or mRNA expression levels. Molecular connections between candidate treatment biomarkers were evaluated using Ingenuity Pathway Analysis (IPA) software (Ingenuity Systems, Inc. Redwood, CA). Statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, NC), R Package version 2.13 (http://www.r-project.org/), and MATLAB 7 (The MathWorks, Natwick, MA).
Transcriptome gene expression analysis
Brain regions were homogenized in 500 μL of Trizol using a QIAgen TissueLyser at 30 Hz (QIAgen). The RNA for each animal was isolated by guanidinium thiocyanate–phenol–chloroform extraction and was further purified using the RNAeasy miniprep kit (QIAgen) according to the manufacturer’s directions. For each strain, RNA samples for three animals in each treatment group were pooled and converted to cDNA and biotinylated cRNA (Enzo kit, Affymetrix, Santa Clara, CA). The resulting cRNA was hybridized to Affymetrix GeneChip Mouse Genome 430 2.0 arrays.
Following normalization with the gcRMA algorithm, expression data were analyzed using ArrayAssist software (Stratagene, La Jolla, CA). To identify genetic alterations associated with fluoxetine administration and treatment response, we analyzed the gene expression profiles between the control and fluoxetine-treated mice as well as profiles between negative and positive responders as defined by the tail suspension phenotype. Microarray data were preprocessed to identify genes with expression levels greater than the background levels (intensity, >50). The gene expression profiles for each treatment group (control vs. fluoxetine) were filtered by calculating the fold change difference between probes with minimum and maximum intensity values. In each treatment group, genes that were significantly variable between strains (fold change ≥3 between minimum and maximum values) were analyzed. Alternatively, expression data between response groups (negative vs. positive) were filtered by taking the ratio of fluorescence intensities between fluoxetine and control mice within each strain. Probe sets that were significantly affected by fluoxetine treatment (intensity ratios of fluoxetine to vehicle ≥2 for at least one strain) were selected. Following filtration, genes were prioritized based on their signal-to-noise ratio (SNR), which is the difference in median gene expression values divided by the standard deviation. The p value for each observed SNR was determined from the empirical null distribution of SNRs, which was obtained by permuting the sample labels and recalculating the SNRs 1,000 times (Gould et al. 2006). Genes with a false discovery rate (FDR) of ≤0.05 were considered significant.
SNR rank scores were used to identify genes that were differentially expressed in either treatment (control vs. fluoxetine) or response (negative vs. positive responders) groups. These genes were then tested for their ability to assign samples in each response or treatment category using a k-nearest neighbors (k-NN) classification with a leave-one-out cross-validation test previously described in Cover and Hart (1967) and Golub et al. (1999). Briefly, the k-NN algorithm assigned a treatment or response label on the unknown sample based on the class assignment most frequently represented among its closest neighbors. Class assignments were determined based on the expression profiles of genes deemed to be informative. The validity of the model was tested using a leave-one-out cross-validation test in which the algorithm withheld a sample, built a class predictor model using the remaining samples, and then predicted the class label of the withheld test sample. Using a distance function, the model determined which members of the training set were closest to the test sample and then weighted each “vote” according to the distance of the unknown test sample from its neighbors, giving the closest neighbor the biggest “vote.” This process was iteratively performed for each sample and the cumulative error rate was calculated (Cover and Hart 1967; Golub et al. 1999). An unsupervised hierarchical clustering analysis was used to evaluate the expression pattern of the informative genes for each treatment and response group. Moreover, we performed eQTL analysis on genes with significant SNR values (FDR ≤ 0.05) as described in Wu et al. (2008) to determine whether changes in gene expression following fluoxetine treatment were under cis-regulation. Gene expression analyses were performed using GenePattern 2.0 (Reich et al. 2006) and R Package version 2.13. Gene expression data were deposited into NCBI Gene Expression Omnibus database (GSE28644).
A logistic regression analysis was used to identify baseline genetic markers that can account for variable fluoxetine response. Probe sets that were variable in the control group (fold change ≥3 between minimum and maximum values) were selected and evaluated for their influence on fluoxetine response. By using these analyses, we can identify baseline genetic markers that may be predictive of SSRI response. Statistical analyses were performed using R Package version 2.13.
QTL mapping
Efficient mixed-model association has been well described elsewhere (Kang et al. 2008) and will be summarized here. Experimental SNP genotypes encompassing over 500,000 polymorphic loci across 72 commonly used laboratory strains were obtained from the Center for Genome Dynamics at http://cgd.jax.org (Szatkiewicz et al. 2008; Yang et al. 2009). Over 190,000 SNPs were informative for the 30 mouse inbred lines, which provided the basis for inferring the genotype. SNPs used in these analyses were fully genotyped, not imputed, and had been specifically selected to discriminate Mus musculus subspecies. This set of SNPs avoids some of the previous biases in genome-wide analyses and enables an accurate ascertainment of population substructure. Associations between genotype and phenotype were calculated by an F statistic corrected for population structure and genetic relatedness. Results were plotted using SpotFire software (TIBCO Palo Alto, CA). Genomic loci that were associated with behavioral or biochemical phenotypes at an FDR ≤ 0.05 were considered significant.
Results
Behavioral measurements
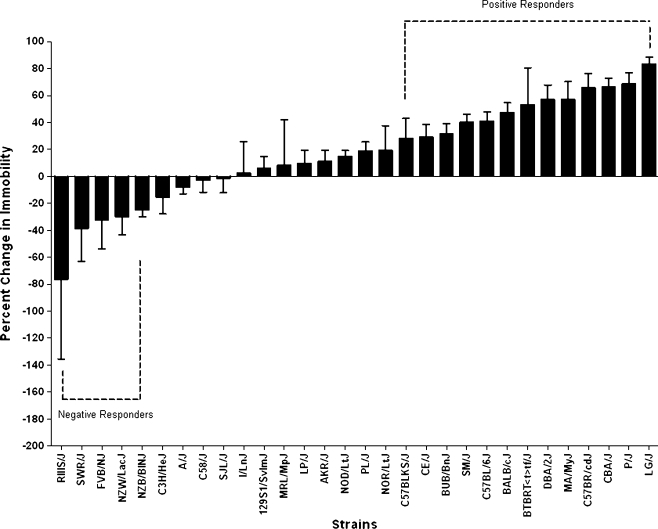
Behavioral responses were measured in the open field test and tail suspension test following chronic treatment with vehicle (water) or fluoxetine. Analysis of immobility scores across the 30 mouse inbred strains revealed significant strain (F = 29.83, p < 0.0001) and treatment (F = 39.14, p < 0.0001) as well as significant strain by treatment effects (F = 3.83, p < 0.0001). Similarly, significant strain (F = 23.16, p < 0.0001), treatment (F = 20.41, p < 0.0001), and strain by treatment effects (F = 1.63, p < 0.02) were observed for percent time spent in the center of the OF. Compared with the control group, an overall reduction in mean immobility was observed in mice treated with fluoxetine (t = 3.253, p < 0.003). However, change in immobility scores (fluoxetine response) was variable between strains. Thirteen strains exhibited a 20% reduction in immobility (positive responders), while five inbred strains exhibited a 20% increase in immobility (negative responders, Fig. 1). Post hoc pairwise comparisons of the behavioral scores between treatment groups revealed a significant difference in immobility scores for BALB/cByJ, BUB/BnJ, C57BL/6J, C57BR/cdJ, CBA/J, MA/MyJ, P/J, PL/J, and SM/J. Significant differences in anxiety-like responses between vehicle- and fluoxetine-treated mice were observed for DBA/2J, MA/MyJ, SJL/J, and SM/J (ESM Online Resource 5).
Fig. 1.
Inter-strain difference in fluoxetine response. Response to treatment was calculated by taking the ratio of fluoxetine behavioral scores to vehicle behavioral scores. Percent change in immobility was calculated by multiplying the ratio of fluoxetine immobility scores to vehicle immobility scores by 100 and then subtracting the product from 100. We defined positive and negative responders as having at least 20% decreased or increased in immobility scores, respectively. Immobility is a measure of “hopelessness” or depressive-like behavior in mice. Strains with positive response to fluoxetine exhibited significant reduction in depressive-like behavior, while negative responders had an increased in immobility
Mean fluoxetine and norfluoxetine levels were variable across the strains; concentration ranges are 470.3–1274.8 ng/mL for fluoxetine and 578.6–1612.2 ng/mL for norfluoxetine. No significant correlation was observed between mean depressive-like behavior and mean serum levels of fluoxetine (r 2 = 0.036) or its metabolite norfluoxetine (r 2 = 0.047). This finding is similar to human studies which did not find a significant association with antidepressant response and serum levels of fluoxetine or norfluoxetine (Amsterdam et al. 1997; Beasley et al. 1990; Norman et al. 1993). With the exception of a few markers, no significant correlation was detected between neurobiochemical levels and drug concentration. Fluoxetine level was minimally correlated with GSK3β (r 2 = 0.213), histone deacetylase 5 (HDAC5, r 2 = 0.131), and vascular endothelial growth factor (VEGF, r 2 = 0.155). A minimal correlation between melanin concentrating hormone (MCH) protein levels and norfluoxetine levels (r 2 = 0.135) was also detected. There was no correlation between mean percent immobility in the TST and total distance traveled in the OF for control (r 2 = 0.013) and treated animals (r 2 = 0.083), indicating that depressive-like behavior was not affected by locomotor activity. We observed a modest correlation between baseline and fluoxetine-induced immobility scores (r 2 = 0.628), which suggests that baseline TST behavior is linked with drug response. Genetic analysis can be performed using inbred strain phenotype data to identify genetic loci associated with behavioral phenotypes (Miller et al. 2010; Williams et al. 2009). Independent QTL mapping analysis for behavioral phenotypes identified significant loci for anxiety, treatment response (data not shown), and baseline behavioral despair. A locus on chromosome 9 (9: 46.7–47.6 Mb) associated with baseline depressive-like behavior (−logP = 6.17, FDR ≤ 0.059), and this was of particular interest because of its co-localization with the genome-wide association analysis for neurobiochemical markers.
Levels of S100β, GSK3β, HDAC5, and GFAP contribute most to treatment response
The levels of 36 neurobiochemical markers proposed to be involved in depression and anxiety were measured in the cortex (Table 1). Dopamine transporter (DAT), interleukin-6 (IL-6), FK506-binding protein 51 (FKBP51), glucocorticoid receptor (NR3C1), and neuron-specific family gene member 2 (NSG2) were excluded from the analysis due to weak intensities, irregular staining, or nonlinear dose–response signals. We examined the relationship between percent change in immobility and neurobiochemical levels. The scatter plots show the strain distribution pattern for each biochemical analyte against depressive-like responses under vehicle (ESM Online Resource 6a) and fluoxetine conditions (ESM Online Resource 6b). In the control group, correlations between protein levels and mRNA expression levels were detected for c-Fos (r 2 = 0.269, 1423100_at), glial fibrillary acidic protein (GFAP; r 2 = 0.222, 1426508_at; r 2 = 0.184, 1426509_at; r 2 = 0.187, 1440142_s_at), glyoxylase 1 (GLO1; r 2 = 0.244, 1424108_at; r 2 = 0.274, 1424109_a_at), and PAQR8 (r 2 = 0.1796, 1431042_at). In the fluoxetine group, correlations between protein levels and mRNA expression levels were detected for GFAP (r 2 = 0.183, 1440142_s_at; r 2 = 0.623, 1426508_at; r 2 = 0.692, 1426509_s_at), guanine nucleotide-binding protein 1 (GNB1; r 2 = 0.144, 1454696_at; r 2 = 0.161, 1417432_at), and NPY (r 2 = 0.154, 1419127_at). The lack of a strong correlation between the neurobiochemical levels and corresponding transcript levels indicates an indirect relationship between mRNA and protein expression.
Partial least squares analysis of the data indicated that the first vector accounted for 56% and 59% of the covariance in neurobiochemical and behavioral differences in the fluoxetine and control groups, respectively (ESM Online Resource 7). Behaviors in the OF and TST accounted for baseline behavior (PLS loading score onto vector, 1; percent time spent in the center OF and immobility TST, 75.1% and 56.4%, respectively). However, the behavioral differences observed after chronic fluoxetine treatment were mainly due to inter-strain responses in the TST (PLS loading score onto vector, 1; percent time spent in the center OF and immobility TST, 17.2% and 72.6%, respectively). Levels of GLO1 and GNB1 account for most of the covariance in anxiety-like and depressive-like behavior in the control group. Higher levels of GLO1 and GNB1 associated with higher baseline anxiety-like and depressive-like behavior. Alternatively, fluoxetine-induced immobility behavior was mostly affected by GFAP, S100 beta protein (S100β), GLO1, and HDAC5 (Fig. 2a). Lower immobility scores following fluoxetine treatment were linked with higher levels of GFAP, S100β, GLO1, and HDAC5.
Fig. 2.
a Neurobiochemical markers that covary with fluoxetine response (top). PLS analysis show that levels of GNB1, GLO1, S100β, GAD67, GFAP, and galanin covary most with response to open field and tail suspension tests (white bars). Levels of GFAP, S100β, GLO1, HDAC5, GAD67, P2X7, and GSK3β covary most with depressive-like response following chronic fluoxetine treatment (black bars). b Neurobiochemical analytes that contribute most to discriminating positive and negative responders (bottom). DWD analysis shows that S100β, GSK3β, HDAC5, and GNB1 discriminate positive responses (black bars) or negative response (white bars). The opposing direction of the S100β and GNB1 vectors indicates that both markers can discriminate negative and positive response groups from each other. Neurobiochemical differences induced by chronic fluoxetine treatment discriminate positive responders, while baseline neurobiological differences discriminate negative responders. Overall, neurobiochemical difference is observed when we defined positive response as a 20% (p < 0.006), 30% (p < 0.014), or a 40% (p < 0.026) reduction in immobility or when we defined negative response as a 20% (p < 0.006), 30% (p < 0.036), or a 40% (p < 0.016) increase in immobility. Data are shown when response is defined as 20% decreased or increased in immobility scores
In order to identify biomarkers of fluoxetine response, we examined which biochemical analytes were most effective in discriminating positive or negative responders. An overall neurobiochemical difference was observed in positive and negative responders. Neurobiochemical alterations following fluoxetine treatment discriminate positive responders, while baseline biological differences distinguish negative responders. This finding was observed irrespective of the stringency in defining positive or negative response, defined as 20–40% reduction or increase in immobility, respectively (Fig. 2b). DWD analysis shows that levels of S100β, GSK3β, HDAC5, GFAP, and GNB1 account for most of the variance in treatment response (Fig. 2b). Baseline levels of S100β, GSK3β, HDAC5, GFAP, and GNB1 can discriminate negative responders. Chronic fluoxetine treatment affects these analytes, and the levels of S100β, GSK3β, HDAC5, GFAP, and GNB1 post-treatment indicate positive response to fluoxetine. The direction of the vectors was the same for GSK3β and HDAC5, but opposite for S100β and GNB1 when discriminating positive or negative responders. Negative responders had lower levels of S100β and higher levels of GNB1, HDAC5, and GSK3β at baseline, while positive responders had lower levels of GNB1 and higher levels of S100β, GSK3β, and HDAC5 after fluoxetine treatment (Fig. 2b). GSK3β and HDAC5 can discriminate negative or positive response, but have poor specificity in identifying negative and positive response groups from each other, while the opposing direction of the S100β and GNB1 vectors indicates that both analytes can discriminate negative and positive response groups from each other. Interestingly, we observed a minimal influence of GFAP on positive response relative to its affect on negative response, which suggests that elevation in GFAP is mainly indicative of negative fluoxetine response.
In both analyses, the response to fluoxetine was associated with an alteration in candidate neurobiochemical levels (Fig. 2). Results from different methods of analysis (PLS and DWD) show that levels of GFAP, S100β, HDAC5, and GSK3β contribute to differential TST responses after fluoxetine treatment. In order to address whether fluoxetine mediates the levels of candidate treatment markers S100β, GFAP, HDAC5, and GSK3β through a common pathway, we looked for molecular connections between them. Using IPA software, we discovered that all candidate treatment markers are linked either directly or indirectly. S100β, GFAP, HDAC5, and GSK3β connect within a common cellular growth and proliferation network, indicating the importance of cellular genesis in mediating response to chronic fluoxetine treatment (ESM Online Resource 8).
Gene expression profiles for treatment and response groups
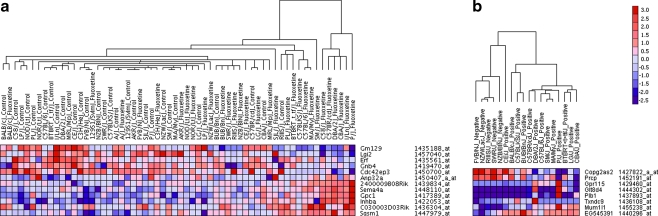
Gene expression data collected from the cortex were preprocessed to eliminate invariable probe sets, reducing the number of probes from 45,101 to 4,818. From genome-wide gene expression analysis, we first identified genes that were strongly correlated with control or fluoxetine treatment by computing the SNR ratio for 4,818 probe sets. We found 203 probe sets that were differentially expressed in either treatment group (FDR ≤ 0.10). Based on the SNR rank scores, 12 genes were significantly up-regulated in the control or fluoxetine group, indicating that these genes are likely to be informative for assigning treatment category (ESM Online Resource 9a). To evaluate whether the expression profiles of the informative genes can be used to predict treatment classifications, we performed the k-NN leave-one-out cross-validation test. The 12 informative genes correctly predicted treatment class for 38 out of the 60 samples (absolute error of 36.6%), indicating that the overall expression patterns of the informative genes do not effectively predict treatment class. Because it is likely that gene expression differences between vehicle- and fluoxetine-treated mice may be confounded by inter-strain difference in response, we then analyzed the gene expression profiles in the negative and positive response groups. There were over 171 genes with variable expression patterns in each response group (FDR ≤ 0.05). Based on the SNR rank scores, eight genes were significantly up-regulated in either positive or negative responders, which make them likely to be genetic predictors of fluoxetine response (ESM Online Resource 9b). Using the k-NN leave-one-out cross-validation algorithm, we tested to see whether responders can be correctly assigned (negative or positive) based on the expression patterns of the eight informative genes. Sixteen out of the 18 samples were correctly assigned to the appropriate response groups, providing an absolute error rate of 11.1%. Results from the regression analysis indicate that several baseline genetic markers affect inter-strain variability in fluoxetine response (ESM Online Resource 10). Using a previously described eQTL analysis (Wu et al. 2008), we found that changes in gene expression for the 171 genes were not likely due to cis-regulation. Gene expression changes as measured by the log ratios of fluorescence intensities (fluoxetine/vehicle) did not significantly associate with polymorphic variants found within 25–500 kb of the putative genes. A heat map was generated following hierarchical clustering analysis wherein the expression patterns of the informative genes were used to cluster each treatment and response group (Fig. 3). Apart from a few specific strain divergences, the gene sets indicate clusters of treatment and response groups. Our results show that gene expression differences were more apparent between the response groups (positive vs. negative) compared with the treatment groups (control vs. fluoxetine), with the gene set for the response groups being able to reasonably predict SSRI response (Fig. 3).
Fig. 3.
a Hierarchical clustering of genes discriminating treatment from control (left). Gene expression patterns of the 12 most informative genes on each treatment group. b Hierarchical clustering of genes discriminating positive and negative response to fluoxetine treatment (right). Gene expression profiles of the eight most informative genes on each response group. Only strains deemed to have a response to treatment are shown. In both figures, color denotes the direction of gene expression changes (red, up-regulated; blue, down-regulated). Intensity illustrates the magnitude of change in gene expression
Quantitative trait loci associated with baseline depressive-like behavior and variable neurobiochemical levels
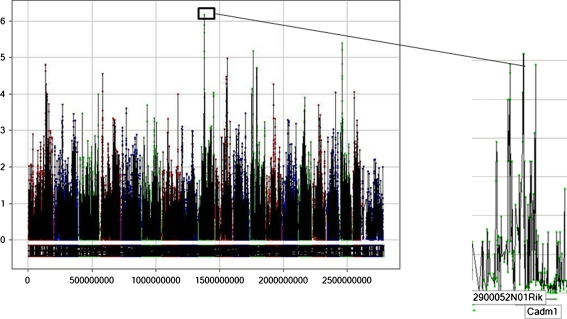
We also investigated whether differences in neurobiochemical analytes, which have been previously linked to anxiety and/or depression, are subject to a common genetic regulatory mechanism. Of particular interest is that the convergence of neurobiochemical and behavioral data revealed a significant loci co-localization on Chr 9 (9: 46.7–47.6 Mb; Fig. 4). The Chr 9 QTL associated with baseline depressive-like behavior as well as baseline levels of cyclic AMP response element-binding protein (CREB, −logP = 4.46, FDR ≤ 0.05) and VEGF (−logP = 5.56, FDR ≤ 0.05). Genes at this locus include Cadm1 and 2900052N01Rik. Cadm1 is involved in the neural cell adhesion processes, facilitating cellular and molecular communication between cells and has been linked with social impairments and anxiety-like behavior in mice (Takayanagi et al. 2010; Zhiling et al. 2008).
Fig. 4.
Genome-wide association plot for depressive-like behavior. Genomic region on Chr. 9 significantly correlates with depressive-like behavior (−logP = 6.17) and baseline levels of VEGF (−logP = 5.56) and CREB (−logP = 4.46). The figure shows baseline behavioral despair QTL. To the right are the putative genes underneath behavioral locus on Chr 9. The y-axis denotes the strength of association between genotype and phenotype (−logP scores), and the x-axis illustrates the cumulative SNP position on the genome
Discussion
Identification of biomarkers that can establish diagnosis, prognosis, and response to treatment is critical to the advancement of research and the management of patients with mood disorder. Biomarkers can be used to objectively assess clinical progression and response to antidepressant therapy. Relative to human studies, the use of mouse inbred strains can be an effective method for investigating biomarkers of drug response due to lower cost, greater tissue accessibility, reduced genomic complexity, and subject availability. However, the use of animal models to parallel subsets of behaviors that typify human disorders is challenging, and many of the symptoms used to establish psychiatric diagnoses in humans cannot be replicated in animals (Nestler and Hyman 2010). Here, we assess limited sets of specific behaviors that are relatively robust and have been previously validated as responsive to antidepressive drug treatment (Cryan et al. 2005; Kulkarni and Dhir 2007) in a panel of genetically diverse inbred mouse strains (Liu and Gershenfeld 2003; Ripoll et al. 2003; Trullas et al. 1989). The TST is primarily used to evaluate the antidepressant activity of novel compounds and does not itself elicit depressive behavior; hence, evaluation of treatment responses following exposure to chronic stress, an animal model of clinical depression that has both face and predictive validity (Nestler and Hyman 2010; Willner 1997, 2005), is needed for an independent validation of treatment biomarkers.
Early studies from the STAR*D trial found a high co-occurrence of anxiety and depression. Over 49% and 42% of patients in the primary care and secondary care setting, respectively exhibited “anxious depressive” symptoms (Fava et al. 2006). Compared with patients with depression alone, patients with comorbid anxiety and depressive disorders are more likely to have prolonged, severe, and recurrent symptoms (Andreescu et al. 2007; Fava et al. 2004) and are less responsive to treatment (Emmanuel et al. 1998; Fava et al. 2008). In this study, we examined how baseline and treatment-induced biochemical differences affect depressive-like and anxiety-like responses. While anxiety-like and depressive-like behavior both contribute to baseline behavioral differences, anxiety-like behavior as measured by percent time spent in the center of the OF was only minimally affected by fluoxetine treatment. This result supports previous findings which have demonstrated that responses in the TST are more sensitive to antidepressant drugs as opposed to other psychotropic agents like anxiolytics, antipsychotics, and stimulants (Cryan et al. 2005). The levels of GLO1 and GNB1 mostly account for anxiety-like and depressive-like behavior in the control group, suggesting that alterations in GLO1 and GNB1 may be common in both depression and anxiety disorders. GLO1 is an antioxidant enzyme that catalyzes the detoxification of methylglyoxal and has been linked with various psychiatric disorders including depression (Fujimoto et al. 2008), panic disorder without agoraphobia (Politi et al. 2006), schizophrenia (Arai et al. 2010), and anxiety-like behavior (Hovatta et al. 2005; Williams et al. 2009). Variable mRNA expression of Glo1 is likely due to the difference in copy number variants. Duplication of a region where Glo1 resides (Chr 17: 30.12–30.65 Mb) was found to be associated with a difference in anxiety-like behavior across a panel of mouse inbred strains (Williams et al. 2009). In agreement with a previous study that has found higher Glo1 mRNA transcripts in more anxious mice (Hovatta et al. 2005), we found that elevation in GLO1 protein corresponds with higher anxiety-like behavior. Elevation in GLO1 protein is also correlated with increased baseline immobility in the TST. This result is discordant with other studies that have found higher expression of GLO1 in less anxious and depressed mice (Ditzen et al. 2006; Kromer et al. 2005). Inconsistent findings may be due to a number of factors including strain, age, and protein quantification methodologies. Therefore, functional and metabolic assessment of GLO1 through measurement of methylglyoxal-mediated glycation is required for clarification (Thornalley 2006). GNB1 belongs to a family of heterotrimeric GTP-binding proteins that integrate signals among receptors and effector proteins. In a study that looked at dominant–subordinate behavior as a rodent model of depression, lower mRNA expression of Gnb1 was associated with dominant behavior (Kroes et al. 2006). In agreement with this finding, we found that increased behavioral despair is associated with higher GNB1 protein. It is interesting to note that mice lacking Gnb1 exhibit reduced cortical thickness, neural tube closure defects, and impaired neural progenitor cell proliferation, suggesting the importance of Gnb1 in neurogenesis (Okae and Iwakura 2010). Treatment with psychostimulants like cocaine and amphetamines up-regulates Gnb1 expression, which indicates that Gnb1 is involved in drug sensitization (Kitanaka et al. 2002). Interestingly, pretreatment with fluoxetine attenuates methamphetamine-induced locomotor sensitization (Takamatsu et al. 2006), suggesting the potential involvement of Gnb1 in fluoxetine response. Our results show that more anxious mice exhibit elevated levels of GNB1 protein. A recent study by Benekareddy et al. (2010) found that early stress induced by maternal separation up-regulates Gnb1 expression, implicating the involvement of Gnb1 in mediating risk to anxiety and mood disorder. Taken together, the association of GLO1 and GNB1 with baseline behavioral despair and anxiety-like behavior offers a novel possibility in which these proteins can be targeted to modulate both disorders and improve treatment outcomes in patients who suffer from comorbid anxious depressive symptoms.
To date, analysis of biochemical alterations has only been performed for a handful of markers in a few inbred strains despite inter-strain differences in response to antidepressants (Crowley et al. 2006; Miller et al. 2008; Ripoll et al. 2003; Vaugeois et al. 1997). In this study, we show that response to fluoxetine treatment mirrors responses seen in human fluoxetine treatment, with a subset of strains exhibiting an “expected response” of reduced immobility as a phenotype of depressive-like behavior. Additionally, a few inbred strains exhibited no significant response, while the remaining strains responded with increased immobility. Similar to previous reports, we observed a strong effect of strain and SSRI treatment on TST responses (Crowley et al. 2006; Ripoll et al. 2003; Trullas et al. 1989). Although the TST immobility scores we measured after chronic fluoxetine treatment were slightly different from what others have reported, our findings are in agreement with a previous study that examined TST responses in multiple inbred strains after fluoxetine treatment (Lucki et al. 2001). Following acute administration of fluoxetine, Lucki et al. (2001) observed reduced immobility responses for BALB/cJ and DBA/2J, but not for C57BL/6J, A/J, and C3H/HeJ. With the exception of the data for C57BL/6J, these results are consistent with our findings.
A low correlation was observed between change in immobility scores (treatment response) and serum fluoxetine and norfluoxetine levels, indicating that, at least in the mouse, pharmacokinetics does not significantly modulate behavior and that other factors like pharmacodynamics and pharmacogenetics may contribute to differential responses to fluoxetine treatment. Of note is that the negative and positive responses to fluoxetine can be distinguished based on neurobiochemical variability. Baseline biological differences discriminate negative responders, while biochemical alterations following fluoxetine treatment delineate positive responders. This result suggests that a positive response to fluoxetine is mostly due to treatment-induced biochemical alterations and that a negative response to fluoxetine is mainly attributed to baseline differences between mouse strains. Therefore, we propose that patients who fail to respond or remit from antidepressant therapy may have baseline biological abnormalities predisposing them to poor therapeutic outcomes.
Overall, we found minimal correlation between mRNA expression and protein levels for a small number of neurobiomolecules. The lack of correlation between protein abundance and mRNA expression levels can be due to variable posttranscriptional and posttranslational mechanisms, different protein synthesis and turnover rates, and high degree of noise in mRNA and protein experiments (Greenbaum et al. 2003). To date, correlation analysis between transcript levels and protein abundance has been performed in a few studies (Anderson and Seilhamer 1997; Chen et al. 2002; Guo et al. 2008; Gygi et al. 1999; Ideker et al. 2001; Pascal et al. 2008; Washburn et al. 2001). In general, these studies found a weak correlation between protein levels and mRNA expression levels, which suggests that protein abundance cannot be sufficiently predicted by mRNA expression data alone. The relationship between transcript levels and its corresponding protein levels are affected by transcription, translation, and regulatory processes within the cell. Given that proteins are more directly linked to the phenotype than mRNA transcripts, protein data are likely to be more useful for characterizing the molecular basis of disease. However, due to the limited ability to comprehensively measure protein levels, global profiling analysis is currently restricted to mRNA expression studies. It is important to note that both transcriptome and protein abundance data provide useful information for disease modeling and pathway analysis. Gene expression and protein abundance data can be used to predict protein–protein interactions (Greenbaum et al. 2003), which can provide insight into the mechanisms underlying the disease.
Results from both DWD and PLS analyses suggest that levels of S100β, GSK3β, HDAC5, and GFAP influence differential TST responses following SSRI treatment (Fig. 2). Chronic fluoxetine treatment results in higher levels of S100β, which triggers the expression of serotonin transporters (SERT) and activates de novo serotonin synthesis (Baudry et al. 2010). Similarly, increased levels of GSK3β have been observed following chronic fluoxetine administration in vivo (Fatemi et al. 2009; Li et al. 2004). Inhibition of GSK3β blocked the fluoxetine-mediated down-regulation of SERT through the overexpression of miR 16 (Baudry et al. 2010), thus linking serotonin regulation with GSK3β phosphorylation (Li et al. 2004). Tsankova et al. (2006) found that treatment with tricyclic antidepressants results in lower mRNA levels of Hdac5 and that overexpression of Hdac5 in the hippocampus blocks the antidepressant effects of imipramine in the chronic social defeat stress paradigm. Consistent with previous studies (Baudry et al. 2010), higher levels of S100β following fluoxetine treatment were observed in positive responders. Interestingly, both negative and positive responders displayed higher levels of GSK3β and HDAC5. GSK3β phosphorylates various molecules typically leading to substrate inhibition, while chromatin remodeling by HDAC5 can prevent the transcription of many downstream genes. Thus, elevation of GSK3β and HDAC5 can affect several different downstream molecular processes, leading to variable treatment outcomes. Additional studies are required to examine the relationship between fluoxetine response and levels of GSK3β and HDAC5. We found minimal correlation between fluoxetine concentrations and GSK3β (r 2 = 0.213) and HDAC5 (r 2 = 0.131) protein levels. This result suggests that in comparison to S100β and GNB1, GSK3β and HDAC5 levels are more sensitive to fluoxetine treatment. The minimum correlation observed suggests that the affects of GSK3β and HDAC5 on fluoxetine response may partially be influenced by fluoxetine levels. Little information is known about the relationship of GFAP with fluoxetine response. In rodents, chronic treatment with fluoxetine results in the elevation of amplifying neural progenitor cells (Encinas et al. 2006), while antidepressant treatment in humans results in the increased numbers of quiescent neural progenitor cells, including nestin and GFAP (Boldrini et al. 2009). Rat astrocytic cells enriched with GFAP exhibit Na+-dependent and fluoxetine-sensitive serotonin uptake, suggesting the involvement of GFAP in mediating serotonin levels (Dave and Kimelberg 1994). The underlying mechanism mediating the influence of GFAP on negative fluoxetine response warrants closer examination, given the low clinical response rates to SSRI treatment (Trivedi et al. 2006).
Although our findings indicate that fluoxetine administration alters the levels of S100β, HDAC5, GSK3β, and GFAP, it is likely that these biochemical changes are not specific to SSRIs. Other psychotropic medications, including clozapine, lithium, valproic acid, and haloperidol, have been shown to alter GFAP (Fatemi et al. 2008) and GSK3β levels (Fatemi et al. 2009) in vivo. Altered levels of S100β are observed in patients with schizophrenia (Gattaz et al. 2000; Wiesmann et al. 1999), which can be normalized by treatment with antipsychotic agents (Ling et al. 2007; Steiner et al. 2010). Treatment with HDAC inhibitors is associated with neuroprotective effects and may be beneficial in the treatment of various psychiatric disorders, including bipolar disorder and schizophrenia (Kazantsev and Thompson 2008; Tsankova et al. 2007). Collectively, these findings suggest that S100β, GSK3β, HDAC5, and GFAP are likely to be involved in a behavioral pathway common to multiple psychiatric disorders, although the downstream mechanisms mediated by each analyte may be specific for each psychiatric disorder. An important avenue for future study is the assessment of these biomarkers in easily accessible samples like plasma or serum, thus facilitating translation of preclinical findings to human studies. Current work in the lab aims to evaluate the relationship between behavioral responses and serum levels of neurobiochemical markers across multiple inbred strains. Additionally, sex differences in the metabolism of fluoxetine (Hodes et al. 2010) necessitate further investigation of these biomarkers in females.
Overall, we observed minimal difference (203 probe sets with variable expression patterns) in gene expression profiles between vehicle- and fluoxetine-treated mice. This result is consistent with what others have found when performing global transcriptome analyses between control and antidepressant treatment groups (Bohm et al. 2006; Lee et al. 2010; Miller et al. 2008; Takahashi et al. 2006). Although other groups have found more genes that were up- or down-regulated following fluoxetine treatment, the filtering criteria we used to preprocess our microarray data are generally more stringent in comparison. We only analyzed genes with a minimum threefold difference in expression between strains and had statistically significant SNR values. In addition, other factors including fluoxetine dosing regimen, microarray methodology, and time of tissue collection can influence gene expression results. When we compared the gene expression profiles between negative and positive responders, we found that we can accurately assign samples as either negative or positive responders (absolute error rate, 11.1%) based on the expression patterns of Copg2S2, Prcp, Gpr115, Gxylt2, Plb1, Txndc9, Mum1/1, and Gm1642, making these genes potential predictors of SSRI response. Although none of these genes have been directly linked to antidepressant response or major depressive disorder, several are involved in molecular processes that may be critical in mediating behavior or neuronal function, such as neuropeptide signaling and lysosomal serine carboxypeptidase activity. For six of the genetic markers of fluoxetine response, there are commercially available ELISA kits and antibodies that can be used to evaluate the relationship between protein levels of these genes and SSRI response. Additionally, we identified baseline genetic markers that affect inter-strain despair responses to fluoxetine treatment, suggesting use as potential prognostic treatment biomarkers. Interestingly, one candidate gene is a poly(A)-binding protein, cytoplasmic 1 (Pabpc1), a gene that was significantly associated with SSRI response in a genome-wide association study which evaluated over 1,800 patients from the STAR*D trial (Shyn et al. 2011). Pabpc1 encodes for a poly(A)-binding protein that binds to the poly(A) tail of mRNA, facilitating ribosome recruitment and translation initiation. Given that the association between Pabpc1 and antidepressant response was observed in both preclinical and human studies, the mechanism underlying this association warrants further investigation.
To identify genetic markers for depressive disorder, we focused on genomic regions that are associated with both behavioral and neurobiochemical phenotypes. We identified a behavioral despair QTL on Chr 9 that correlated with inter-strain neurobiochemical variability. A QTL for depressive-like behavior has not been reported previously at this locus, which contains only two genes—Cadm1 and 2900052N01Rik. Cadm1 encodes for neural cell adhesion molecules that mediate cell-to-cell communication. Furthermore, Cadm1 is involved in synaptic function and neuronal differentiation (Watabe et al. 2003) and has been linked with anxiety-like and social behavior in mice (Takayanagi et al. 2010; Zhiling et al. 2008). A recent study by Takayanagi et al. (2010) showed that mice lacking Cadm1 displayed higher anxiety-like phenotype and impairment in motor coordination and social behavior compared to wild-type mice. However, no significant difference in depressive-like behavior as measured by the TST was observed between groups (Takayanagi et al. 2010). Other behavioral paradigms like chronic social defeat stress and reduced sucrose preference, which assess other features of depressive behavior (e.g., anhedonia; Nestler and Hyman 2010) can be used to further evaluate the role of Cadm1 in depression. Since CADM1 acts as a synaptic adhesive molecule, functional disruption of Cadm1 may lead to impaired neural plasticity and neurogenesis, both of which have been linked with major depressive disorder (Campbell and Macqueen 2004; Schmidt and Duman 2007). Links defining interactions between Cadm1 and the two biochemical markers (CREB and VEGF) that co-localize to the same genetic locus on Chr 9 provide a promising avenue for future study.
Altogether, our results highlight the role of cellular proliferation in mediating SSRI response. Similar to Cadm1, Gpr115, Plb1, Gxylt2, and Prcp are involved in cellular proliferation and/or cellular adhesion (Bjarnadottir et al. 2004; Ganendren et al. 2006; Mallela et al. 2008, 2009; Okajima et al. 2008; Sethi et al. 2010). Furthermore, candidate fluoxetine markers S100β, GSK3β, HDAC5, and GFAP are linked through a common cellular growth and proliferation pathway, suggesting that treatment with fluoxetine stimulates cell growth. Our results indicate that response to fluoxetine is affected by cellular genesis, which is consistent with previous studies that have shown a critical role of neurogenesis in mediating SSRI response (Deltheil et al. 2009; Manev et al. 2001). Regulation of neurogenesis, cellular resilience, neuroplasticity, and cellular death/atrophy is important in the pathogenesis and treatment of depression (Duman 2002; Krishnan and Nestler 2008; Lucassen et al. 2010; Manji et al. 2001, 2003). Neuroimaging and postmortem brain studies show that patients with MDD have impaired neural circuitry and structural abnormalities. Compared with healthy controls, patients with mood disorder display lower number of glial cells, reduced cortical volume and thickness, and decreased neuronal size and density, all of which implicate cellular atrophy/loss (Manji et al. 2001, 2003). Antidepressant therapy increases the levels of neurotrophic factors (Duman et al. 2001; Malberg et al. 2000; Sairanen et al. 2005) that promote neurogenesis and inhibit cell death signaling pathways (Riccio et al. 1999). Hippocampal neurogenesis is critical in facilitating adaptive response to stress (Duman et al. 2001; Malberg et al. 2000), memory development (Mirescu et al. 2004; Schloesser et al. 2009), and learning new experiences (Kempermann 2008); thus, the impairment of neurogenesis may confer risk to depressive behavior. Elucidating the roles of neurogenesis, cellular resilience, neural plasticity, and cell death in the development of depressive behavior requires a system-level exploration of these processes. Whether the interplay between cellular proliferative and survival processes facilitates synaptic remodeling and leads to altered mood remains to be seen.
In the absence of biological markers, therapeutic outcomes are defined based on the reduction of baseline symptoms, which can lead to inconsistencies and irreproducibility. Genetic and neurobiochemical markers can provide an objective means to measure prognosis and treatment response. We identified genetic and biochemical markers of fluoxetine response that are involved in cellular genesis, highlighting the role of neurogenesis and neuroplasticity in major depressive disorder. Since neurogenesis is not specific to antidepressant treatment, it is critical for future studies to identify how psychotropic medications differentially affect neurogenesis and which downstream neurobiological pathways are affected. Such studies may illuminate differences between closely related psychiatric disorders including depression and anxiety.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 916 kb)
(DOC 56 kb)
Acknowledgments
The authors thank Jim Crowley, Amy Eisener-Dorman, and Patrick Sullivan for their helpful comments. We thank Brandon Young for invaluable assistance in producing the microarray data (Genomics Core, Scripps Florida). This study is funded by a grant from the State of Florida and grants from the National Institute of Mental Health (1R01MH077251 and F32MH084528).
Disclosure
The authors report no conflicts of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Cristina S. Benton, Brooke H. Miller contributed equally to the manuscript.
Contributor Information
Mathew T. Pletcher, Phone: +1-617-6655463, Email: Mathew.Pletcher@pfizer.com
Tim Wiltshire, Phone: +1-919-8435820, Email: timw@unc.edu.
References
- Amsterdam JD, Fawcett J, Quitkin FM, Reimherr FW, Rosenbaum JF, Michelson D, Hornig-Rohan M, Beasley CM. Fluoxetine and norfluoxetine plasma concentrations in major depression: a multicenter study. Am J Psychiatry. 1997;154:963–969. doi: 10.1176/ajp.154.7.963. [DOI] [PubMed] [Google Scholar]
- Anderson L, Seilhamer J. A comparison of selected mRNA and protein abundances in human liver. Electrophoresis. 1997;18:533–537. doi: 10.1002/elps.1150180333. [DOI] [PubMed] [Google Scholar]
- Andreescu C, Lenze EJ, Dew MA, Begley AE, Mulsant BH, Dombrovski AY, Pollock BG, Stack J, Miller MD, Reynolds CF. Effect of comorbid anxiety on treatment response and relapse risk in late-life depression: Controlled study. Br J Psychiatry. 2007;190:344–349. doi: 10.1192/bjp.bp.106.027169. [DOI] [PubMed] [Google Scholar]
- Arai M, Yuzawa H, Nohara I, Ohnishi T, Obata N, Iwayama Y, Haga S, Toyota T, Ujike H, Ichikawa T, Nishida A, Tanaka Y, Furukawa A, Aikawa Y, Kuroda O, Niizato K, Izawa R, Nakamura K, Mori N, Matsuzawa D, Hashimoto K, Iyo M, Sora I, Matsushita M, Okazaki Y, Yoshikawa T, Miyata T, Itokawa M. Enhanced carbonyl stress in a subpopulation of schizophrenia. Arch Gen Psychiatry. 2010;67:589–597. doi: 10.1001/archgenpsychiatry.2010.62. [DOI] [PubMed] [Google Scholar]
- Baudry A, Mouillet-Richard S, Schneider B, Launay JM, Kellermann O. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science. 2010;329:1537–1541. doi: 10.1126/science.1193692. [DOI] [PubMed] [Google Scholar]
- Beasley CM, Jr, Bosomworth JC, Wernicke JF. Fluoxetine: relationships among dose, response, adverse events, and plasma concentrations in the treatment of depression. Psychopharmacol Bull. 1990;26:18–24. [PubMed] [Google Scholar]
- Benekareddy M, Goodfellow NM, Lambe EK, Vaidya VA. Enhanced function of prefrontal serotonin 5-HT(2) receptors in a rat model of psychiatric vulnerability. J Neurosci. 2010;30:12138–12150. doi: 10.1523/JNEUROSCI.3245-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnadottir TK, Fredriksson R, Hoglund PJ, Gloriam DE, Lagerstrom MC, Schioth HB. The human and mouse repertoire of the adhesion family of G-protein-coupled receptors. Genomics. 2004;84:23–33. doi: 10.1016/j.ygeno.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Bohm C, Newrzella D, Herberger S, Schramm N, Eisenhardt G, Schenk V, Sonntag-Buck V, Sorgenfrei O. Effects of antidepressant treatment on gene expression profile in mouse brain: cell type-specific transcription profiling using laser microdissection and microarray analysis. J Neurochem. 2006;97(Suppl 1):44–49. doi: 10.1111/j.1471-4159.2006.03750.x. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John Mann J, Arango V. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009;34:2376–2389. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29:417–426. [PMC free article] [PubMed] [Google Scholar]
- Chanock SJ, Manolio T, Boehnke M, Boerwinkle E, Hunter DJ, Thomas G, Hirschhorn JN, Abecasis G, Altshuler D, Bailey-Wilson JE, Brooks LD, Cardon LR, Daly M, Donnelly P, Fraumeni JF, Jr, Freimer NB, Gerhard DS, Gunter C, Guttmacher AE, Guyer MS, Harris EL, Hoh J, Hoover R, Kong CA, Merikangas KR, Morton CC, Palmer LJ, Phimister EG, Rice JP, Roberts J, Rotimi C, Tucker MA, Vogan KJ, Wacholder S, Wijsman EM, Winn DM, Collins FS. Replicating genotype–phenotype associations. Nature. 2007;447:655–660. doi: 10.1038/447655a. [DOI] [PubMed] [Google Scholar]
- Chen G, Gharib TG, Huang CC, Taylor JM, Misek DE, Kardia SL, Giordano TJ, Iannettoni MD, Orringer MB, Hanash SM, Beer DG. Discordant protein and mRNA expression in lung adenocarcinomas. Mol Cell Proteomics. 2002;1:304–313. doi: 10.1074/mcp.M200008-MCP200. [DOI] [PubMed] [Google Scholar]
- Choleris E, Thomas AW, Kavaliers M, Prato FS. A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev. 2001;25:235–260. doi: 10.1016/S0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Brambilla P, Furukawa T, Geddes J, Gregis M, Hotopf M, Malvini L, Barbui C (2005) Fluoxetine versus other types of pharmacotherapy for depression. Cochrane Database Syst Rev CD004185 [DOI] [PMC free article] [PubMed]
- Cover TM, Hart PE. Nearest neighbor pattern classification. IEEE Trans Inform Theory. 1967;IT-13:21–27. doi: 10.1109/TIT.1967.1053964. [DOI] [Google Scholar]
- Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- Crowley JJ, Brodkin ES, Blendy JA, Berrettini WH, Lucki I. Pharmacogenomic evaluation of the antidepressant citalopram in the mouse tail suspension test. Neuropsychopharmacology. 2006;31:2433–2442. doi: 10.1038/sj.npp.1301065. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Dave V, Kimelberg HK. Na(+)-dependent, fluoxetine-sensitive serotonin uptake by astrocytes tissue-printed from rat cerebral cortex. J Neurosci. 1994;14:4972–4986. doi: 10.1523/JNEUROSCI.14-08-04972.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deltheil T, Tanaka K, Reperant C, Hen R, David DJ, Gardier AM. Synergistic neurochemical and behavioural effects of acute intrahippocampal injection of brain-derived neurotrophic factor and antidepressants in adult mice. Int J Neuropsychopharmacol. 2009;12:905–915. doi: 10.1017/S1461145709000017. [DOI] [PubMed] [Google Scholar]
- Ditzen C, Jastorff AM, Kessler MS, Bunck M, Teplytska L, Erhardt A, Kromer SA, Varadarajulu J, Targosz BS, Sayan-Ayata EF, Holsboer F, Landgraf R, Turck CW. Protein biomarkers in a mouse model of extremes in trait anxiety. Mol Cell Proteomics. 2006;5:1914–1920. doi: 10.1074/mcp.M600088-MCP200. [DOI] [PubMed] [Google Scholar]
- Domenici E, Wille DR, Tozzi F, Prokopenko I, Miller S, McKeown A, Brittain C, Rujescu D, Giegling I, Turck CW, Holsboer F, Bullmore ET, Middleton L, Merlo-Pich E, Alexander RC, Muglia P. Plasma protein biomarkers for depression and schizophrenia by multi analyte profiling of case–control collections. PLoS One. 2010;5:e9166. doi: 10.1371/journal.pone.0009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS. Pathophysiology of depression: the concept of synaptic plasticity. Eur Psychiatry. 2002;17(Suppl 3):306–310. doi: 10.1016/S0924-9338(02)00654-5. [DOI] [PubMed] [Google Scholar]
- Duman RS, Nakagawa S, Malberg J. Regulation of adult neurogenesis by antidepressant treatment. Neuropsychopharmacology. 2001;25:836–844. doi: 10.1016/S0893-133X(01)00358-X. [DOI] [PubMed] [Google Scholar]
- Emmanuel J, Simmonds S, Tyrer P. Systematic review of the outcome of anxiety and depressive disorders. Br J Psychiatry Suppl. 1998;34:35–41. [PubMed] [Google Scholar]
- Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci U S A. 2006;103:8233–8238. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Reutiman TJ, Pandian T, Braun NN, Haug K. Chronic psychotropic drug treatment causes differential expression of connexin 43 and GFAP in frontal cortex of rats. Schizophr Res. 2008;104:127–134. doi: 10.1016/j.schres.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD. Chronic psychotropic drug treatment causes differential expression of Reelin signaling system in frontal cortex of rats. Schizophr Res. 2009;111:138–152. doi: 10.1016/j.schres.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Fava M, Alpert JE, Carmin CN, Wisniewski SR, Trivedi MH, Biggs MM, Shores-Wilson K, Morgan D, Schwartz T, Balasubramani GK, Rush AJ. Clinical correlates and symptom patterns of anxious depression among patients with major depressive disorder in STAR*D. Psychol Med. 2004;34:1299–1308. doi: 10.1017/S0033291704002612. [DOI] [PubMed] [Google Scholar]
- Fava M, Rush AJ, Alpert JE, Carmin CN, Balasubramani GK, Wisniewski SR, Trivedi MH, Biggs MM, Shores-Wilson K. What clinical and symptom features and comorbid disorders characterize outpatients with anxious major depressive disorder: a replication and extension. Can J Psychiatry. 2006;51:823–835. doi: 10.1177/070674370605101304. [DOI] [PubMed] [Google Scholar]
- Fava M, Rush AJ, Alpert JE, Balasubramani GK, Wisniewski SR, Carmin CN, Biggs MM, Zisook S, Leuchter A, Howland R, Warden D, Trivedi MH. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165:342–351. doi: 10.1176/appi.ajp.2007.06111868. [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Uchida S, Watanuki T, Wakabayashi Y, Otsuki K, Matsubara T, Suetsugi M, Funato H, Watanabe Y. Reduced expression of glyoxalase-1 mRNA in mood disorder patients. Neurosci Lett. 2008;438:196–199. doi: 10.1016/j.neulet.2008.04.024. [DOI] [PubMed] [Google Scholar]
- Fujishiro J, Imanishi T, Onozawa K, Tsushima M. Comparison of the anticholinergic effects of the serotonergic antidepressants, paroxetine, fluvoxamine and clomipramine. Eur J Pharmacol. 2002;454:183–188. doi: 10.1016/S0014-2999(02)02557-8. [DOI] [PubMed] [Google Scholar]
- Ganendren R, Carter E, Sorrell T, Widmer F, Wright L. Phospholipase B activity enhances adhesion of Cryptococcus neoformans to a human lung epithelial cell line. Microbes Infect. 2006;8:1006–1015. doi: 10.1016/j.micinf.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Garriock HA, Kraft JB, Shyn SI, Peters EJ, Yokoyama JS, Jenkins GD, Reinalda MS, Slager SL, McGrath PJ, Hamilton SP. A genomewide association study of citalopram response in major depressive disorder. Biol Psychiatry. 2010;67:133–138. doi: 10.1016/j.biopsych.2009.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattaz WF, Lara DR, Elkis H, Portela LV, Goncalves CA, Tort AB, Henna J, Souza DO. Decreased S100-beta protein in schizophrenia: preliminary evidence. Schizophr Res. 2000;43:91–95. doi: 10.1016/S0920-9964(99)00146-2. [DOI] [PubMed] [Google Scholar]
- Golub TR, Slonim DK, Tamayo P, Huard C, Gaasenbeek M, Mesirov JP, Coller H, Loh ML, Downing JR, Caligiuri MA, Bloomfield CD, Lander ES. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science. 1999;286:531–537. doi: 10.1126/science.286.5439.531. [DOI] [PubMed] [Google Scholar]
- Gould J, Getz G, Monti S, Reich M, Mesirov JP. Comparative gene marker selection suite. Bioinformatics. 2006;22:1924–1925. doi: 10.1093/bioinformatics/btl196. [DOI] [PubMed] [Google Scholar]
- Greenbaum D, Colangelo C, Williams K, Gerstein M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 2003;4:117. doi: 10.1186/gb-2003-4-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Xiao P, Lei S, Deng F, Xiao GG, Liu Y, Chen X, Li L, Wu S, Chen Y, Jiang H, Tan L, Xie J, Zhu X, Liang S, Deng H. How is mRNA expression predictive for protein expression? A correlation study on human circulating monocytes. Acta Biochim Biophys Sin (Shanghai) 2008;40:426–436. doi: 10.1111/j.1745-7270.2008.00418.x. [DOI] [PubMed] [Google Scholar]
- Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol. 1999;19:1720–1730. doi: 10.1128/mcb.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, Hill-Smith TE, Suckow RF, Cooper TB, Lucki I. Sex-specific effects of chronic fluoxetine treatment on neuroplasticity and pharmacokinetics in mice. J Pharmacol Exp Ther. 2010;332:266–273. doi: 10.1124/jpet.109.158717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovatta I, Tennant RS, Helton R, Marr RA, Singer O, Redwine JM, Ellison JA, Schadt EE, Verma IM, Lockhart DJ, Barlow C. Glyoxalase 1 and glutathione reductase 1 regulate anxiety in mice. Nature. 2005;438:662–666. doi: 10.1038/nature04250. [DOI] [PubMed] [Google Scholar]
- Hu XZ, Rush AJ, Charney D, Wilson AF, Sorant AJ, Papanicolaou GJ, Fava M, Trivedi MH, Wisniewski SR, Laje G, Paddock S, McMahon FJ, Manji H, Lipsky RH. Association between a functional serotonin transporter promoter polymorphism and citalopram treatment in adult outpatients with major depression. Arch Gen Psychiatry. 2007;64:783–792. doi: 10.1001/archpsyc.64.7.783. [DOI] [PubMed] [Google Scholar]
- Ideker T, Thorsson V, Ranish JA, Christmas R, Buhler J, Eng JK, Bumgarner R, Goodlett DR, Aebersold R, Hood L. Integrated genomic and proteomic analyses of a systematically perturbed metabolic network. Science. 2001;292:929–934. doi: 10.1126/science.292.5518.929. [DOI] [PubMed] [Google Scholar]
- Ising M, Horstmann S, Kloiber S, Lucae S, Binder EB, Kern N, Kunzel HE, Pfennig A, Uhr M, Holsboer F. Combined dexamethasone/corticotropin releasing hormone test predicts treatment response in major depression—a potential biomarker? Biol Psychiatry. 2007;62:47–54. doi: 10.1016/j.biopsych.2006.07.039. [DOI] [PubMed] [Google Scholar]
- Ising M, Lucae S, Binder EB, Bettecken T, Uhr M, Ripke S, Kohli MA, Hennings JM, Horstmann S, Kloiber S, Menke A, Bondy B, Rupprecht R, Domschke K, Baune BT, Arolt V, Rush AJ, Holsboer F, Muller-Myhsok B. A genomewide association study points to multiple loci that predict antidepressant drug treatment outcome in depression. Arch Gen Psychiatry. 2009;66:966–975. doi: 10.1001/archgenpsychiatry.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehn CF, Kuehnhardt D, Bartholomae A, Pfeiffer S, Krebs M, Regierer AC, Schmid P, Possinger K, Flath BC. Biomarkers of depression in cancer patients. Cancer. 2006;107:2723–2729. doi: 10.1002/cncr.22294. [DOI] [PubMed] [Google Scholar]
- Kang HM, Zaitlen NA, Wade CM, Kirby A, Heckerman D, Daly MJ, Eskin E. Efficient control of population structure in model organism association mapping. Genetics. 2008;178:1709–1723. doi: 10.1534/genetics.107.080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazantsev AG, Thompson LM. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat Rev Drug Discov. 2008;7:854–868. doi: 10.1038/nrd2681. [DOI] [PubMed] [Google Scholar]
- Kempermann G. The neurogenic reserve hypothesis: what is adult hippocampal neurogenesis good for? Trends Neurosci. 2008;31:163–169. doi: 10.1016/j.tins.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Kitanaka J, Kitanaka N, Takemura M, Wang XB, Hembree CM, Goodman NL, Uhl GR. Isolation and sequencing of a putative promoter region of the murine G protein beta 1 subunit (GNB1) gene. DNA Seq. 2002;13:39–45. doi: 10.1080/10425170290019883. [DOI] [PubMed] [Google Scholar]
- Kornstein SG, Schneider RK. Clinical features of treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl 16):18–25. [PubMed] [Google Scholar]
- Kraft JB, Peters EJ, Slager SL, Jenkins GD, Reinalda MS, McGrath PJ, Hamilton SP. Analysis of association between the serotonin transporter and antidepressant response in a large clinical sample. Biol Psychiatry. 2007;61:734–742. doi: 10.1016/j.biopsych.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroes RA, Panksepp J, Burgdorf J, Otto NJ, Moskal JR. Modeling depression: social dominance–submission gene expression patterns in rat neocortex. Neuroscience. 2006;137:37–49. doi: 10.1016/j.neuroscience.2005.08.076. [DOI] [PubMed] [Google Scholar]
- Kromer SA, Kessler MS, Milfay D, Birg IN, Bunck M, Czibere L, Panhuysen M, Putz B, Deussing JM, Holsboer F, Landgraf R, Turck CW. Identification of glyoxalase-I as a protein marker in a mouse model of extremes in trait anxiety. J Neurosci. 2005;25:4375–4384. doi: 10.1523/JNEUROSCI.0115-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni SK, Dhir A. Effect of various classes of antidepressants in behavioral paradigms of despair. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1248–1254. doi: 10.1016/j.pnpbp.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Laje G, Paddock S, Manji H, Rush AJ, Wilson AF, Charney D, McMahon FJ. Genetic markers of suicidal ideation emerging during citalopram treatment of major depression. Am J Psychiatry. 2007;164:1530–1538. doi: 10.1176/appi.ajp.2007.06122018. [DOI] [PubMed] [Google Scholar]
- Laje G, Allen AS, Akula N, Manji H, John Rush A, McMahon FJ. Genome-wide association study of suicidal ideation emerging during citalopram treatment of depressed outpatients. Pharmacogenet Genomics. 2009;19:666–674. doi: 10.1097/FPC.0b013e32832e4bcd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Ko E, Kim YE, Min JY, Liu J, Kim Y, Shin M, Hong M, Bae H. Gene expression profile analysis of genes in rat hippocampus from antidepressant treated rats using DNA microarray. BMC Neurosci. 2010;11:152. doi: 10.1186/1471-2202-11-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekman M, Laje G, Charney D, Rush AJ, Wilson AF, Sorant AJ, Lipsky R, Wisniewski SR, Manji H, McMahon FJ, Paddock S. The FKBP5-gene in depression and treatment response—an association study in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Cohort. Biol Psychiatry. 2008;63:1103–1110. doi: 10.1016/j.biopsych.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuchter AF, Cook IA, Marangell LB, Gilmer WS, Burgoyne KS, Howland RH, Trivedi MH, Zisook S, Jain R, McCracken JT, Fava M, Iosifescu D, Greenwald S. Comparative effectiveness of biomarkers and clinical indicators for predicting outcomes of SSRI treatment in major depressive disorder: results of the BRITE-MD Study. Psychiatry Res. 2009;169:124–131. doi: 10.1016/j.psychres.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Leuchter AF, Cook IA, Hamilton SP, Narr KL, Toga A, Hunter AM, Faull K, Whitelegge J, Andrews AM, Loo J, Way B, Nelson SF, Horvath S, Lebowitz BD. Biomarkers to predict antidepressant response. Curr Psychiatry Rep. 2010;12:553–562. doi: 10.1007/s11920-010-0160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhu W, Roh MS, Friedman AB, Rosborough K, Jope RS. In vivo regulation of glycogen synthase kinase-3beta (GSK3beta) by serotonergic activity in mouse brain. Neuropsychopharmacology. 2004;29:1426–1431. doi: 10.1038/sj.npp.1300439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling SH, Tang YL, Jiang F, Wiste A, Guo SS, Weng YZ, Yang TS. Plasma S-100B protein in Chinese patients with schizophrenia: comparison with healthy controls and effect of antipsychotics treatment. J Psychiatr Res. 2007;41:36–42. doi: 10.1016/j.jpsychires.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Liu X, Gershenfeld HK. An exploratory factor analysis of the tail suspension test in 12 inbred strains of mice and an F2 intercross. Brain Res Bull. 2003;60:223–231. doi: 10.1016/S0361-9230(03)00033-9. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Meerlo P, Naylor AS, van Dam AM, Dayer AG, Fuchs E, Oomen CA, Czeh B. Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: implications for depression and antidepressant action. Eur Neuropsychopharmacol. 2010;20:1–17. doi: 10.1016/j.euroneuro.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacol (Berl) 2001;155:315–322. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallela J, Perkins R, Yang J, Pedigo S, Rimoldi JM, Shariat-Madar Z. The functional importance of the N-terminal region of human prolylcarboxypeptidase. Biochem Biophys Res Commun. 2008;374:635–640. doi: 10.1016/j.bbrc.2008.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallela J, Yang J, Shariat-Madar Z. Prolylcarboxypeptidase: a cardioprotective enzyme. Int J Biochem Cell Biol. 2009;41:477–481. doi: 10.1016/j.biocel.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Manev H, Uz T, Smalheiser NR, Manev R. Antidepressants alter cell proliferation in the adult brain in vivo and in neural cultures in vitro. Eur J Pharmacol. 2001;411:67–70. doi: 10.1016/S0014-2999(00)00904-3. [DOI] [PubMed] [Google Scholar]
- Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- Manji HK, Quiroz JA, Sporn J, Payne JL, Denicoff K, Gray A, Zarate CA, Jr, Charney DS. Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression. Biol Psychiatry. 2003;53:707–742. doi: 10.1016/S0006-3223(03)00117-3. [DOI] [PubMed] [Google Scholar]
- Marron JS, Todd MJ, Ahn J. Distance-weighted discrimination. J Am Stat Assoc. 2007;102:1267–1271. doi: 10.1198/016214507000001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon FJ, Buervenich S, Charney D, Lipsky R, Rush AJ, Wilson AF, Sorant AJ, Papanicolaou GJ, Laje G, Fava M, Trivedi MH, Wisniewski SR, Manji H. Variation in the gene encoding the serotonin 2A receptor is associated with outcome of antidepressant treatment. Am J Hum Genet. 2006;78:804–814. doi: 10.1086/503820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BH, Schultz LE, Gulati A, Cameron MD, Pletcher MT. Genetic regulation of behavioral and neuronal responses to fluoxetine. Neuropsychopharmacology. 2008;33:1312–1322. doi: 10.1038/sj.npp.1301497. [DOI] [PubMed] [Google Scholar]
- Miller BH, Schultz LE, Gulati A, Su AI, Pletcher MT. Phenotypic characterization of a genetically diverse panel of mice for behavioral despair and anxiety. PLoS One. 2010;5:e14458. doi: 10.1371/journal.pone.0014458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/S0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Norman TR, Gupta RK, Burrows GD, Parker G, Judd FK. Relationship between antidepressant response and plasma concentrations of fluoxetine and norfluoxetine. Int Clin Psychopharmacol. 1993;8:25–29. doi: 10.1097/00004850-199300810-00004. [DOI] [PubMed] [Google Scholar]
- Okae H, Iwakura Y. Neural tube defects and impaired neural progenitor cell proliferation in Gbeta1-deficient mice. Dev Dyn. 2010;239:1089–1101. doi: 10.1002/dvdy.22256. [DOI] [PubMed] [Google Scholar]
- Okajima T, Matsuura A, Matsuda T. Biological functions of glycosyltransferase genes involved in O-fucose glycan synthesis. J Biochem. 2008;144:1–6. doi: 10.1093/jb/mvn016. [DOI] [PubMed] [Google Scholar]
- Olfson M, Marcus SC, Tedeschi M, Wan GJ. Continuity of antidepressant treatment for adults with depression in the United States. Am J Psychiatry. 2006;163:101–108. doi: 10.1176/appi.ajp.163.1.101. [DOI] [PubMed] [Google Scholar]
- Paddock S. Genetic variation in GRIK4 and the implications for antidepressant treatment. Pharmacogenomics. 2008;9:133–135. doi: 10.2217/14622416.9.2.133. [DOI] [PubMed] [Google Scholar]
- Pascal LE, True LD, Campbell DS, Deutsch EW, Risk M, Coleman IM, Eichner LJ, Nelson PS, Liu AY. Correlation of mRNA and protein levels: cell type-specific gene expression of cluster designation antigens in the prostate. BMC Genomics. 2008;9:246. doi: 10.1186/1471-2164-9-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis RH, Purcell S, Fava M, Fagerness J, Rush AJ, Trivedi MH, Smoller JW. Association between treatment-emergent suicidal ideation with citalopram and polymorphisms near cyclic adenosine monophosphate response element binding protein in the STAR*D Study. Arch Gen Psychiatry. 2007;64:689–697. doi: 10.1001/archpsyc.64.6.689. [DOI] [PubMed] [Google Scholar]
- Perlis RH, Moorjani P, Fagerness J, Purcell S, Trivedi MH, Fava M, Rush AJ, Smoller JW. Pharmacogenetic analysis of genes implicated in rodent models of antidepressant response: association of TREK1 and treatment resistance in the STAR(*)D Study. Neuropsychopharmacology. 2008;33:2810–2819. doi: 10.1038/npp.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters EJ, Slager SL, Kraft JB, Jenkins GD, Reinalda MS, McGrath PJ, Hamilton SP. Pharmacokinetic genes do not influence response or tolerance to citalopram in the STAR*D sample. PLoS One. 2008;3:e1872. doi: 10.1371/journal.pone.0001872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politi P, Minoretti P, Falcone C, Martinelli V, Emanuele E. Association analysis of the functional Ala111Glu polymorphism of the glyoxalase I gene in panic disorder. Neurosci Lett. 2006;396:163–166. doi: 10.1016/j.neulet.2005.11.028. [DOI] [PubMed] [Google Scholar]
- Qiao X, Zhang HH, Liu Y, Todd MJ, Marron JS. Weighted distance weighted discrimination and its asymptotic properties. J Am Stat Assoc. 2010;105:401–414. doi: 10.1198/jasa.2010.tm08487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich M, Liefeld T, Gould J, Lerner J, Tamayo P, Mesirov JP. GenePattern 2.0. Nat Genet. 2006;38:500–501. doi: 10.1038/ng0506-500. [DOI] [PubMed] [Google Scholar]
- Riccio A, Ahn S, Davenport CM, Blendy JA, Ginty DD. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science. 1999;286:2358–2361. doi: 10.1126/science.286.5448.2358. [DOI] [PubMed] [Google Scholar]
- Ripoll N, David DJ, Dailly E, Hascoet M, Bourin M. Antidepressant-like effects in various mice strains in the tail suspension test. Behav Brain Res. 2003;143:193–200. doi: 10.1016/S0166-4328(03)00034-2. [DOI] [PubMed] [Google Scholar]
- Rossi A, Barraco A, Donda P. Fluoxetine: a review on evidence based medicine. Ann Gen Hosp Psychiatry. 2004;3:2. doi: 10.1186/1475-2832-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairanen M, Lucas G, Ernfors P, Castren M, Castren E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25:1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloesser RJ, Manji HK, Martinowich K. Suppression of adult neurogenesis leads to an increased hypothalamo-pituitary–adrenal axis response. Neuroreport. 2009;20:553–557. doi: 10.1097/WNR.0b013e3283293e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- Schule C, Baghai TC, Eser D, Hafner S, Born C, Herrmann S, Rupprecht R. The combined dexamethasone/CRH test (DEX/CRH test) and prediction of acute treatment response in major depression. PLoS One. 2009;4:e4324. doi: 10.1371/journal.pone.0004324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi MK, Buettner FF, Krylov VB, Takeuchi H, Nifantiev NE, Haltiwanger RS, Gerardy-Schahn R, Bakker H. Identification of glycosyltransferase 8 family members as xylosyltransferases acting on O-glucosylated notch epidermal growth factor repeats. J Biol Chem. 2010;285:1582–1586. doi: 10.1074/jbc.C109.065409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyn SI, Shi J, Kraft JB, Potash JB, Knowles JA, Weissman MM, Garriock HA, Yokoyama JS, McGrath PJ, Peters EJ, Scheftner WA, Coryell W, Lawson WB, Jancic D, Gejman PV, Sanders AR, Holmans P, Slager SL, Levinson DF, Hamilton SP. Novel loci for major depression identified by genome-wide association study of sequenced treatment alternatives to relieve depression and meta-analysis of three studies. Mol Psychiatry. 2011;16:202–215. doi: 10.1038/mp.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J, Schroeter ML, Schiltz K, Bernstein HG, Muller UJ, Richter-Landsberg C, Muller WE, Walter M, Gos T, Bogerts B, Keilhoff G. Haloperidol and clozapine decrease S100B release from glial cells. Neuroscience. 2010;167:1025–1031. doi: 10.1016/j.neuroscience.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacol (Berl) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Mico JA, Lenegre A, Steru M, Simon P, Porsolt RD. The automated tail suspension test: a computerized device which differentiates psychotropic drugs. Prog Neuropsychopharmacol Biol Psychiatry. 1987;11:659–671. doi: 10.1016/0278-5846(87)90002-9. [DOI] [PubMed] [Google Scholar]
- Szatkiewicz JP, Beane GL, Ding Y, Hutchins L, Pardo-Manuel de Villena F, Churchill GA. An imputed genotype resource for the laboratory mouse. Mamm Genome. 2008;19:199–208. doi: 10.1007/s00335-008-9098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Washiyama K, Kobayashi T, Hayashi S. Gene expression in the brain from fluoxetine-injected mouse using DNA microarray. Ann N Y Acad Sci. 2006;1074:42–51. doi: 10.1196/annals.1369.004. [DOI] [PubMed] [Google Scholar]
- Takamatsu Y, Yamamoto H, Ogai Y, Hagino Y, Markou A, Ikeda K. Fluoxetine as a potential pharmacotherapy for methamphetamine dependence: studies in mice. Ann N Y Acad Sci. 2006;1074:295–302. doi: 10.1196/annals.1369.026. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Fujita E, Yu Z, Yamagata T, Momoi MY, Momoi T, Onaka T. Impairment of social and emotional behaviors in Cadm1-knockout mice. Biochem Biophys Res Commun. 2010;396:703–708. doi: 10.1016/j.bbrc.2010.04.165. [DOI] [PubMed] [Google Scholar]
- Thase ME. Molecules that mediate mood. N Engl J Med. 2007;357:2400–2402. doi: 10.1056/NEJMcibr0706377. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ. Unease on the role of glyoxalase 1 in high-anxiety-related behaviour. Trends Mol Med. 2006;12:195–199. doi: 10.1016/j.molmed.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Trullas R, Jackson B, Skolnick P. Genetic differences in a tail suspension test for evaluating antidepressant activity. Psychopharmacol (Berl) 1989;99:287–288. doi: 10.1007/BF00442824. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- Uher R, Perroud N, Ng MY, Hauser J, Henigsberg N, Maier W, Mors O, Placentino A, Rietschel M, Souery D, Zagar T, Czerski PM, Jerman B, Larsen ER, Schulze TG, Zobel A, Cohen-Woods S, Pirlo K, Butler AW, Muglia P, Barnes MR, Lathrop M, Farmer A, Breen G, Aitchison KJ, Craig I, Lewis CM, McGuffin P. Genome-wide pharmacogenetics of antidepressant response in the GENDEP project. Am J Psychiatry. 2010;167:555–564. doi: 10.1176/appi.ajp.2009.09070932. [DOI] [PubMed] [Google Scholar]
- Ukai M, Maeda H, Nanya Y, Kameyama T, Matsuno K. Beneficial effects of acute and repeated administrations of sigma receptor agonists on behavioral despair in mice exposed to tail suspension. Pharmacol Biochem Behav. 1998;61:247–252. doi: 10.1016/S0091-3057(98)00093-8. [DOI] [PubMed] [Google Scholar]
- Vaugeois JM, Passera G, Zuccaro F, Costentin J. Individual differences in response to imipramine in the mouse tail suspension test. Psychopharmacol (Berl) 1997;134:387–391. doi: 10.1007/s002130050475. [DOI] [PubMed] [Google Scholar]
- Warden D, Trivedi MH, Wisniewski SR, Davis L, Nierenberg AA, Gaynes BN, Zisook S, Hollon SD, Balasubramani GK, Howland R, Fava M, Stewart JW, Rush AJ. Predictors of attrition during initial (citalopram) treatment for depression: a STAR*D report. Am J Psychiatry. 2007;164:1189–1197. doi: 10.1176/appi.ajp.2007.06071225. [DOI] [PubMed] [Google Scholar]
- Washburn MP, Wolters D, Yates JR., 3rd Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotechnol. 2001;19:242–247. doi: 10.1038/85686. [DOI] [PubMed] [Google Scholar]
- Watabe K, Ito A, Koma YI, Kitamura Y. IGSF4: a new intercellular adhesion molecule that is called by three names, TSLC1, SgIGSF and SynCAM, by virtue of its diverse function. Histol Histopathol. 2003;18:1321–1329. doi: 10.14670/HH-18.1321. [DOI] [PubMed] [Google Scholar]
- Wiesmann M, Wandinger KP, Missler U, Eckhoff D, Rothermundt M, Arolt V, Kirchner H. Elevated plasma levels of S-100b protein in schizophrenic patients. Biol Psychiatry. 1999;45:1508–1511. doi: 10.1016/S0006-3223(98)00217-0. [DOI] [PubMed] [Google Scholar]
- Williams RT, Lim JE, Harr B, Wing C, Walters R, Distler MG, Teschke M, Wu C, Wiltshire T, Su AI, Sokoloff G, Tarantino LM, Borevitz JO, Palmer AA. A common and unstable copy number variant is associated with differences in Glo1 expression and anxiety-like behavior. PLoS One. 2009;4:e4649. doi: 10.1371/journal.pone.0004649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacol (Berl) 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Wu C, Delano DL, Mitro N, Su SV, Janes J, McClurg P, Batalov S, Welch GL, Zhang J, Orth AP, Walker JR, Glynne RJ, Cooke MP, Takahashi JS, Shimomura K, Kohsaka A, Bass J, Saez E, Wiltshire T, Su AI. Gene set enrichment in eQTL data identifies novel annotations and pathway regulators. PLoS Genet. 2008;4:e1000070. doi: 10.1371/journal.pgen.1000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Ding Y, Hutchins LN, Szatkiewicz J, Bell TA, Paigen BJ, Graber JH, de Villena FP, Churchill GA. A customized and versatile high-density genotyping array for the mouse. Nat Methods. 2009;6:663–666. doi: 10.1038/nmeth.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhiling Y, Fujita E, Tanabe Y, Yamagata T, Momoi T, Momoi MY. Mutations in the gene encoding CADM1 are associated with autism spectrum disorder. Biochem Biophys Res Commun. 2008;377:926–929. doi: 10.1016/j.bbrc.2008.10.107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 916 kb)
(DOC 56 kb)