Abstract
In chronic kidney disease (CKD), once injury from any number of disease processes reaches a threshold, there follows an apparently irreversible course toward decline in kidney function. The tubulointerstitium may play a key role in this common progression pathway. Direct injury, high metabolic demands, or stimuli from various other forms of renal dysfunction activate tubular cells. These, in turn, interact with interstitial tissue elements and inflammatory cells, causing further pathologic changes in the renal parenchyma. The tissue response to these changes thus generates a feed-forward loop of kidney injury and progressive loss of function. This article reviews the mechanisms of this negative cycle mediating CKD.
Keywords: Fibrosis, Inflammation, Hypoxia, Proteinuria
Keywords: Medicine & Public Health, Pediatrics
Introduction
The tubulointerstitium includes the tubules, which comprise about 80% of the kidney volume, and the compartment of the kidney bounded by the vasculature and nephrons. This component of the kidney performs critical functions (Table 1), including the selective reabsorption and excretion of filtered molecules and the production and release of hormones. As such, the tubulointerstitium has a high energy demand and is susceptible to injury in situations of relative nutrient deprivation. It is involved in a multitude of disease processes, including obstructive uropathy, reflux nephropathy, pyelonephritis, metabolic disorders, hereditary diseases, and toxin exposures. Given the importance of the tubulointerstitium and its sensitivity to injury, it is not surprising that many of its physiological functions, when exaggerated, account for clinical manifestations of chronic kidney disease (CKD).
Table 1.
Functions of the tubulointerstitium
| Functions of the tubulointerstitium |
|---|
| Transport functions |
| Electrolyte balance |
| Volume homeostasis |
| Osmolarity |
| Acid–base balance |
| -chloride transport |
| -bicarbonate regeneration |
| -ammoniagenesis |
| Phosphate metabolism |
| Selective protein reabsorption |
| Metabolic function |
| Gluconeogenesis |
| Hormonal function |
| Vitamin D metabolism |
| Erythropoiesis |
| Blood pressure |
| -renin–angiotensin system |
| -endothelins |
| -prostaglandins, prostacyclin |
The contribution of deranged tubulointerstitial function to these manifestations raises a question as to the possible involvement of this compartment in the pathophysiology of progressive loss of kidney tissue as well. In CKD, once kidney damage reaches a threshold, the subsequent progression appears to be largely irreversible and independent of the initial kidney injury. It is theorized that the myriad primary kidney injuries that lead to end-stage kidney disease (ESKD) converge into one or more final common pathway(s) of disease progression. Tubulointerstitial injury and the cascade of events that it elicits may be one of these pathways.
The purpose of this educational review is to consider the mechanisms of progression that have been proposed to involve the tubulointerstitium. We emphasize that a purely negative physiological process is unlikely to exist, since biology has evolved to preserve and improve function. Therefore, in other circumstances the involved mechanisms are beneficial. For example, macrophages may help repair lesions by recruiting cellular elements and producing a provisional extracellular matrix (ECM), but these same actions may mediate inflammation or produce fibrogenic cytokines. This concept of misdirected repair has been considered in a previous issue of Pediatric Nephrology [1].
Model theories of CKD progression
Two models have been proposed for how kidney injury leads to ESKD, with emphasis on the involvement of the glomeruli and the tubulointerstitium, respectively [2]. In the “overload hypothesis,” an initial kidney injury results in a decrease in the number of functioning nephrons. In response, the remaining nephrons manifest compensatory increases in function. This causes further nephron damage and loss, generating a feed-forward cycle that leads to ESKD. Alternatively, the “fibrosis hypothesis” suggests that a variety of initial kidney insults result in tubulointerstitial injury, eliciting further inflammation and damage to the tubulointerstitium that proceeds to ESKD. These pathways are not mutually exclusive, but represent two ways of conceptualizing the progressive nature of CKD.
The scientific literature has implicated the tubulointerstitium in progressive kidney loss for several decades. In 1970, Schainuck and colleagues defined a pathologic correlate for declining glomerular filtration rate (GFR) in patients with various glomerulopathies [3]. These researchers found that GFR was inversely related to the severity of interstitial damage seen in biopsy samples. In contrast, a relationship between GFR and histologic severity of glomerular injury was not found. Further studies showed that increased interstitial volume and fibrosis, a decrease in peritubular capillaries, morphologic changes in tubular epithelial cells, and intensity of interstitial inflammation all correlate with kidney function deterioration [4].
This relationship between tubulointerstitial injury and deteriorating kidney function could have been influenced by both sampling bias and the pattern of glomerular damage [5]. Glomerular histology may be more subject to sample bias in diseases with focal changes. Furthermore, with advancing kidney disease in general, glomerular pathology can be heterogeneous due to the coexistence of hyperfunctioning, enlarged glomeruli and scarred glomeruli in the same biopsy sample [6]. However, the possibility must be considered that tubulointerstitial injury is the major causal event associated with the progressive decline in kidney function in all forms of CKD. To understand this concept further, it is important to consider the mechanisms by which tubulointerstitial injury occurs and to examine the pathologic consequences of the response to that injury.
Changes in glomerular function can initiate tubulointerstitial damage
Abnormal glomerular filtration can initiate progression to CKD, inducing a tubulointerstitial response, as depicted in Fig. 1. Here, we will examine the concepts of misdirected filtration, obstruction of filtrate flow, and proteinuria.
Fig. 1.
Glomerular or tubular injury initiates chronic kidney disease (CKD), but the tubulointerstitial response is primarily responsible for progression. (1) Podocyte dysfunction or depletion leads to proteinuria, causing reactive changes in the tubular cells. (2) Podocyte depletion also permits the transudation of glomerular filtrate directly into the periglomerular tubulointerstitium, depositing a number of biologically active molecules in that compartment. (3) As the number of effective nephrons decreases, the remaining nephrons hypertrophy. An increased amount of filtrate per nephron increases metabolic demand on the tubules, which under even normal circumstances have high energy requirements. Thus, further stress on tubule cell homeostasis results in activation of the cells, away from a state of relatively orderly transporter function. ROS Reactive oxygen species
Misdirected filtration
Kriz and colleagues have proposed a mechanism by which filtrate leakage external to the tubular lumen damages the tubulointerstitium [2]. Chronic damage to the glomerulus elicits a consistent pattern of changes characterized by foot process effacement and, eventually, podocyte loss. The resulting areas of denuded glomerular basement membrane can adhere to parietal epithelial cells, forming a bridge between the glomerular and parietal basement membranes. An adhesion, or synechia, thus forms between the affected portion of the glomerular tuft and Bowman’s capsule. At this adhesion, the filtrate is extruded directly into the periglomerular interstitium. In response to plasma proteins, interstitial fibroblasts are recruited to and/or proliferate at the site of the transudate. Inflammation and fibrosis develop around the glomerulus, resulting in local tubular atrophy and subsequent degeneration.
Obstructed flow of filtrate
The local response described in the previous paragraph and/or crescent formation resulting from endocapillary inflammation leading to extracapillary cellular proliferation may obstruct the glomerulotubular junction and the initial segment of the proximal tubule, blocking the outflow of filtrate from Bowman’s capsule [2]. The result is tubular atrophy and degeneration. In a murine model of glomerulonephritis, [7] tubular degeneration was seen only in those tubules associated with glomeruli that had cellular crescents extending into the tubular lumen. Glomeruli with crescent formation not involving the glomerulotubular junction were associated with morphologically normal initial proximal tubule segments.
Proteinuria
Tubulointerstitial injury also can be caused by increased delivery of physiologically active molecules to the tubular lumen. Severity of protein excretion has been consistently associated with a greater likelihood of decline in GFR, and treatments aimed at ameliorating proteinuria slow this decline. Studies of both animal models and human kidney biopsy specimens have shown various pathologic injuries to the tubulointerstitium related to proteinuria, including tubular epithelial cell apoptosis and epithelial-to-mesenchymal transition (EMT) [8]. Increased reabsorption of biologically active molecules increases metabolic demand on the remaining nephrons. A caveat to these observations is that, in some cases, increased nephron damage might cause proteinuria rather than the other way around. Consistent with this view, unremitting proteinuria in cases of treatment-resistant, true minimal change nephropathy has not been associated with progressive kidney disease [8]. Thus, although “severity” of proteinuria usually has been characterized by the amount of protein present, it is likely that a specific characteristic of the proteinuria—such as the nature of the molecules that are present in the filtrate—is essential for it to cause tubulointerstitial damage. Conversely, it is worth noting that, if tubular reabsorptive mechanisms are disturbed, increased urine protein could represent a result, rather than a cause, of tubular damage in progressive CKD.
Activation of tubular cells: a central event in progressive tubulointerstitial disease
Assuming that in many cases proteinuria does cause tubulointerstitial injury, several mechanisms might be implicated based on events observed in acute processes [8, 9]. Protein casts may obstruct the tubular lumen. Tubular cells may be damaged as protein reabsorption is increased in response to filtered load, possibly through overloading and rupture of lysosomes or by an overwhelming energy demand on the tubular cells. However, these mechanisms have not been proven by experimental evidence. Non-protein substances in the urine could be toxic to the cells. Ultimately, the biologically active molecules present in the proteinuric filtrate induce changes in tubular cells from being highly differentiated to a less differentiated state wherein they mediate a variety of pathogenic processes. Thus, instead of serving as a polarized barrier that regulates the transport of molecules into and out of the glomerular filtrate, the activated epithelium releases cytokines, recruits inflammatory cells and fibroblasts, and serves as a source of stimuli of other cellular events that contribute to CKD (Fig. 2).
Fig. 2.
Activated tubular cells participate in a number of processes that lead to progressive glomerular loss. While it is not clear that the cells directly produce extracellular matrix to yield a scar, they do produce cytokines, support inflammatory responses, and dedifferentiate or die. The result is nephron loss
Response to filtered load of plasma molecules
The roles of different filtered molecules in the pathogenesis of progression are uncertain. In the normal state, the three proteins megalin, cubilin, and amnionless form a receptor complex that efficiently binds and reabsorbs filtered albumin and other proteins in the proximal tubule [10]. Megalin is a large transmembrane protein and a member of the low-density lipoprotein receptor family; its intracellular tail includes phosphorylation, signaling, and protein interaction motifs that may enable it to initiate intracellular signaling cascades [11]. Cubilin colocalizes with megalin on the proximal tubule membrane and is required for albumin binding, whereas megalin is important for internalization of albumin into the cell [12]. Amnionless facilitates the transport of cubilin to the proximal tubule membrane and assists in ligand endocytosis [10]. When tubular protein delivery increases, such as in glomerular damage, the capacity of the receptor complex is likely overwhelmed, resulting in the increased delivery of proteins and other toxins to the more distal tubule [10].
Albumin stimulates tubular cell nuclear factor kappa-B (NF-κB) and signal transducer and activator of transcription (STAT), which in turn upregulate chemokines, including macrophage chemotactic factor 1 (MCP-1) and regulated upon activation, normal T cell expressed, and secreted (RANTES), and lead to inflammatory infiltration and eventual tubular injury [5]. Blocking the action of MCP-1 or chemokine receptor-1 (CCR-1) in animal models of progressive renal injury results in the reduction of tubulointerstitial inflammation and fibrosis [13, 14]. Other mediators that could play a role in disease progression, not necessarily directly attributable to albumin stimulation, include plasminogen activators, matrix metalloproteinases, and their inhibitors [15]. Increased formation of reactive oxygen species (ROS) as a response to albuminuria may further add to tubulointerstitial damage. Reabsorption of albumin in the proximal tubule cell leads to concomitant activation of NADPH oxidase and ROS generation through the activation of Rac1, a GTPase involved in albumin endocytosis [16].
In considering the pathogenesis of tubulointerstitial injury, it is important to bear in mind that albumin is a major intravascular carrier protein, and that some effects attributed to albumin actually may represent the effect of other albumin-bound molecules. For example, iron has been proposed to be a tubular toxin/activator [17] by generating ROS. Additional molecules, bound to albumin or not, may contribute to mechanisms of progression. Several examples are offered here.
Lipids
Lipids or their breakdown products may cause tubular injury and atrophy. In animal models, exposure to albumin-bound free fatty acids resulted in more severe tubulointerstitial damage than exposure to albumin alone, as well as in increased macrophage infiltration and increased expression of markers of epithelial damage and fibrosis [18, 19]. Fatty acids activate peroxisome proliferator-activated receptors (PPAR) in human proximal tubule cells, resulting in cell apoptosis [20]. They also may mediate lipid peroxidation reactions. Oleic acid-bound albumin administration [21] induces ROS production in vitro through a proline-rich tyrosine kinase 2 (Pyk2)-dependent pathway. An acidic environment, as is often present in the tubular lumen in kidney disease, enhances ROS production after exposure to oleic acid-bound albumin.
Complement
Protein overload in the proximal tubule is also associated with complement activation and deposition and with subsequent tubulointerstitial damage [8]. Inhibition of the membrane attack complex, or C5b-9, results in decreased progression of tubulointerstitial damage and less impairment of creatinine clearance in proteinuric rats [22, 23].
Tissue response to tubulointerstitial damage
The activation of the tubular cell represents a dedifferentiation from a highly specialized, polarized transport epithelium to one with a variety of synthetic and other activities. It has been proposed by some investigators that this dedifferentiation represents a return to a primordial phenotype, since the tubular cells were derived during organogenesis from mesenchymal cells condensing around the ureteric bud in a mesenchymal-to-epithelial transition. An important series of questions involves the role of these dedifferentiated tubular cells in progression and the specific origin of the cells that produce excessive ECM [24]. These will be considered further in the next section. Regarding the fate of the dedifferentiated tubular cells, it is likely that such cells manifest a variety of phenotypes that contribute to progression. It has been difficult to detect a transitional form between the tubular epithelial cell and a myofibroblast that produces ECM. However, the dedifferentiated tubular epithelial cell may not involve the synthesis of scar tissue. Increased synthetic activity by these cells creates further metabolic demand. The secreted molecules pathologically activate other cells in the tubulointerstitium. The result is nephron loss, increased filtration of biologically active molecules through overloaded, remnant nephrons, and a cycle of intensification of all the mechanisms of progression that have been activated.
The origin of the fibrogenic cell
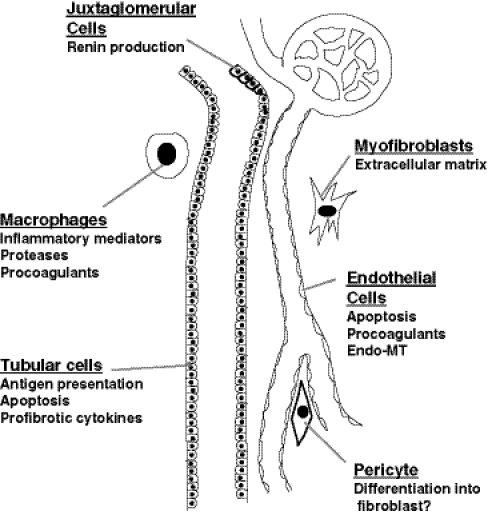
These changes in the tubulointerstitium alter the existing balance between ECM degradation and synthesis. Disruption of this balance affects mechanisms that are activated to heal injuries, resulting in misdirected repair that leads to scar formation [1]. As alluded to in the previous section, a critical question, yet to be resolved, is the origin of the myofibroblast that is actually producing the ECM that becomes scar tissue. While some investigators have suggested that this myofibroblast represents a dedifferentiated tubular cell, referred to as EMT, others have implicated circulating cells, perhaps those derived from the bone marrow as either fibroblast precursors or macrophages, or resident kidney cells with tissue stem-cell potential, such as the vascular pericyte (Fig. 3) [25]. Recent evidence also has suggested a potential role for the transition of endothelial cells to a more mesenchymal phenotype (EndoMT), either causing the loss of peritubular vessels [26] or directly leading to myofibroblasts [27]. It is important to again emphasize that these changes may not be required solely for the generation of scar-producing cells. Alterations in the endothelial or tubular cell phenotype could contribute to the activation of myofibroblasts, or to the recruitment and/or activation of other cells that contribute to progressive disease.
Fig. 3.
Different cell types contribute to progressive nephron loss. Juxtaglomerular cells produce renin, affecting tubuloglomerular feedback to alter glomerular filtration and activating extracellular matrix (ECM) production by multiple cell types. Tubular cells dedifferentiate to recruit inflammatory cells and activated fibroblasts. These myofibroblasts may be derived from vascular pericytes, circulating or resident fibroblasts, or endothelial cells. Macrophages also promote inflammation and tissue damage through protease production. Endothelial cell apoptosis accelerates hypoxic and ischemic changes of the tubular and interstitial cells. The production of soluble mediators by these cells permits them to interact in a way that under ideal circumstances orchestrates a healing response, but in CKD promotes a vicious cycle of misdirected repair
Changes consistent with EMT in the tubulointerstitium have been demonstrated in studies of animal models and human biopsy samples [28]. A primary mediator of this response in the kidney is transforming growth factor-beta (TGF-β), a ubiquitous cytokine that effects changes in gene expression that impact cell growth, differentiation, apoptosis, and ECM deposition. In response to nephron injury of various etiologies, TGF-β expression is upregulated and induces EMT in renal tubule cells [29]. Other potential contributors include connective tissue growth factor (CTGF) [30], which can, like TGF-β, stimulate cellular production of ECM, and plasminogen activator inhibitor (PAI)-1, which can impair the degradation of accumulated ECM [31]. Importantly, these growth factors may be contributed by different types of cell or tissue, so that communication among the tubular and interstitial cells plays a significant part in the pathogenesis of progression.
Inflammation
The dedifferentiated tubular cell produces biologically active molecules, such as those listed above. Cytokines, chemokines, biologically active lipids, and other molecules stimulate the production of more mediators, recruiting inflammatory cells to the tubulointerstitium. Macrophages accumulate and release toxic enzymes, peroxides, cytokines, and pro-coagulants [32]. These may propagate further injury; for example, in murine cell cultures, activated macrophages induce proximal tubule cell apoptosis [33]. In transplant rejection and tubulointerstitial nephritis, the injured tubular epithelial cell can express molecules that mediate antigen presentation, thereby provoking a cycle of inflammatory mediator production, recruitment of inflammatory cells, and further inflammation. Although there are no data on specific antigen presentation in the inflammation of CKD, such an action could potentially play a role here as well.
Hypoxia
Oxygen delivery to the tubulointerstitium relies on diffusion between parallel arterial and venous vessels. This countercurrent multiplier system results in a reduced oxygen tension in the renal medulla of about 10 mm Hg; the renal cortex has a more variable oxygen tension, with an average partial pressure of oxygen of approximately 30 mmHg [34]. This comparatively low oxygen delivery makes the kidney sensitive to changes in systemic oxygenation. In disease processes that result in hypoxia, therefore, renal tissue is prone to injury.
Recent evidence has indicated that patients who have experienced acute kidney injury (AKI) have an increased likelihood of progression to CKD, and patients with mild CKD exhibit a markedly increased incidence of progression to ESKD if they have an episode of AKI. Further, their rate of progression is more rapid [35].
There are several potential explanations for this phenomenon. The circulation to peritubular capillaries is derived from the glomerular capillary effluent. Damage to the glomerular capillary bed thus directly impairs peritubular perfusion and oxygen supply and elicits microvascular dysfunction [36]. Ischemia–reperfusion injury to the kidney stimulates the dedifferentiation of endothelial cells [26]. Significant tubulointerstitial injury has been associated with renal arteriolar and arterial damage and peritubular capillary loss as well as with impaired oxygen diffusion [34]. Blood flow delivered to the nephron may be inadequate for the relatively increased metabolic demand of remaining hyperfunctioning nephrons in CKD. Finally, anemia occurs in CKD due to several causes, including a deficiency of erythropoietin production, and negatively affects the body’s oxygen-carrying capacity.
Hypoxia itself induces the expression of a number of genes that could contribute to the activation of tubulointerstitial processes leading to CKD. Vascular endothelial cell growth factor (VEGF) and basic fibroblast growth factor (FGF-2) influence the permeability of endothelial and epithelial monolayers, activate cells to migrate and proliferate, and may directly affect fibrogenesis [37]. The activation of these genes is mediated by hypoxia-inducible factor (HIF)-1α, which recently has been implicated in several models of progressive glomerular and tubulointerstitial disease [38, 39]. Through the action of HIF-1α, hypoxia activates fibroblasts and induces EMT, altering ECM metabolism to promote scar accumulation [38]. HIF-1α contributes to TGF-β-stimulated collagen expression even under normoxic conditions [40]. Together, these data strongly support the notion that hypoxia, whether related to AKI or to the changes that occur in CKD, is an important contributor to the progression of CKD.
Renin–angiotensin–aldosterone system
Activation of the renin–angiotensin–aldosterone system (RAAS) in kidney injury elicits tubulointerstitial damage through a variety of mechanisms both related to and beyond hemodynamic changes. RAAS activation exacerbates hypoxia in the tubulointerstitium by constricting the glomerular efferent arterioles, leading to decreased downstream perfusion of the peritubular capillaries [34]. Angiotensin II upregulation alone is associated with endothelial cell damage and increased oxidative stress [34]. Angiotensin II induces FGF, platelet-derived growth factor (PDGF), TGF-β, and PAI-1, all of which may contribute to fibrosis [41]. In addition, aldosterone production leads to the induction of ROS and the production of inflammatory cytokines [42]. In kidney tubule cell cultures, aldosterone administration was observed to cause ROS production and oxidative DNA damage [43]. Beyond its effects on sodium reabsorption, aldosterone has been linked more directly to fibrogenesis [44].
Physiological maintenance of the progression cycle
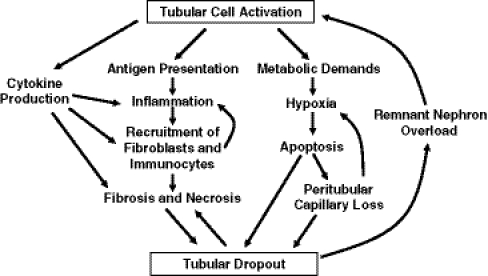
Abnormal glomerular filtration, inflammation, fibrogenesis, and hypoxia, all common pathophysiological consequences of various kidney diseases, contribute to tubulointerstitial injury and activation as described thus far. In turn, tubulointerstitial injury perpetuates a pathway of further kidney injury, likely contributing to the progression of CKD (Fig. 4).
Fig. 4.
The cycle of dysfunction in progressive CKD. Decreased glomerular filtration rate (GFR) or primary tubulointerstitial damage leads to poorly functioning glomeruli and the deleterious activation of tubular cells. These cells communicate with other cells to orchestrate a series of events that trigger mechanisms of interstitial inflammation, fibrosis, and cell death. The result is loss of peritubular capillaries, tubular cell apoptosis and necrosis, and nephron loss. Hypertrophic responses to these events further damage the remnant glomeruli and tubules, propagating the cycle of injury and misdirected repair. RAS Renin–angiotensin–aldosterone system, EMT epithelial-to-mesenchymal transition
Tubulointerstitial injury causes the accumulation of inflammatory cells in the interstitium. Cytokines such as TGF-β inhibit afferent arteriolar vasoconstriction. Extracellular matrix proteins are altered, and there is a decrease in the response of vascular smooth muscle cells to contractile stimuli. Conversely, the activation of hypertensive mechanisms stimulates vasoconstriction and decreased perfusion of the tubulointerstitium [45]. In addition, tubulointerstitial injury itself increases hypoxia and furthers kidney damage. The increased inflammatory cell proliferation and downstream fibrosis that occur in the interstitium increases the distance between tubules and the capillaries that supply oxygen [5]. Moreover, the diffusion of oxygen through the interstitium is limited by inflammation. Tubulointerstitial fibrosis also has been correlated with a loss of peritubular capillaries [5, 34]. A perpetuating pattern of kidney injury is established, whereby hypoxia leads to tubulointerstitial injury and inflammation, which in turn worsens renal hypoxia.
Finally, tubular damage leads to tubular dropout and resultant atubular glomeruli, decreasing the number of functional nephrons. There is a compensatory increase in remnant single-nephron blood flow in response. The remaining nephrons hypertrophy, adapt to increased filtration pressure, and become more vulnerable to disease and pathologic changes. Tubular atrophy also increases fluid delivery to the macula densa and triggers a reduction in GFR via tubuloglomerular feedback. Subsequently, there is exacerbation of glomerulosclerosis, leading to further filtrate leak and proteinuria, again perpetuating tubulointerstitial damage. Eventually, the capacity of the system to respond by autoregulating glomerular blood flow is lost, exacerbating hypoxia/ischemia and decreasing the number of remaining functional nephrons.
Together, these events create a cycle of injury, cell activation, and misdirected repair that is common to a wide variety of causes of CKD. Further study of these biological responses will enable us to better elucidate the mechanisms of tubulointerstitial damage and determine possible pathways for therapeutic intervention.
Acknowledgments
Supported in part by grants R01 DK049362 and R01 DK075663 from the National Institute of Diabetes, Digestive and Kidney Diseases.
Questions (Answers provided following the reference list)
- Histologic changes shown to correspond to worsening GFR in CKD include all of the following EXCEPT:
- Decreased number of peritubular capillaries
- Severity of glomerular injury
- Intensity of interstitial inflammation
- Increased interstitial volume and fibrosis
- Proteinuria and the presence of other molecules in the filtrate affect tubulointerstitial cells by which of the following mechanisms:
- Proteinuria inhibits activity of the membrane attack complex, or C5b-9
- Albumin, acting through its receptor, megalin, down-regulates NF-κB
- Free fatty acids activate PPAR leading to tubular cell apoptosis
- Generation of ROS is decreased by exposure to iron in the filtrate
- Cells hypothesized to be the source of myofibroblasts responsible for unbalanced production of ECM include all of the following EXCEPT:
- Tubular epithelial cells
- Podocytes
- Bone marrow precursor cells
- Vascular pericytes
- Tissue responses to tubulointerstitial injury include which of the following:
- Renal parenchymal blood flow is increased to maintain unchanged oxygen tension
- Inflammatory cells recruited to the tubulointerstitial milieu are inactivated by high urea concentrations
- Renin is inactivated, resulting in hypoxia and oxidative stress
- EMT is induced in part by the actions of TGF-β, CTGF, and PAI-1
- Tubular injury affects progression of chronic kidney disease by which of the following pathways:
- Hypoxia is worsened by capillary injury and limitation of oxygen diffusion
- Tubular dropout causes a decrease in single-nephron blood flow of remaining glomeruli
- Autoregulation of glomerular blood flow is preserved
- Atrophy of tubules results in increased filtrate delivery to the macula densa, increasing GFR through tubuloglomerular feedback
Footnotes
Answers
1. (B)
2. (C)
3. (B)
4. (D)
5. (A)
References
- 1.Schnaper HW, Hubchak SC, Runyan CE, Browne JA, Finer G, Liu X, Hayashida T. A conceptual framework for the molecular pathogenesis of progressive kidney disease. Pediatr Nephrol. 2010;25:2223–2230. doi: 10.1007/s00467-010-1503-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kriz W, LeHir M. Pathways to nephron loss starting from glomerular diseases—insights from animal models. Kidney Int. 2005;67:404–419. doi: 10.1111/j.1523-1755.2005.67097.x. [DOI] [PubMed] [Google Scholar]
- 3.Schainuck LI, Striker GE, Cutler RE, Benditt EP. Structural-functional correlations in renal diseases. Part II: the correlations. Hum Pathol. 1970;1:631–641. doi: 10.1016/S0046-8177(70)80061-2. [DOI] [PubMed] [Google Scholar]
- 4.Bohle A, Mackensen-Haen S, VonGise, Grund KE, Wehrmann M, Batz C, Bogen Schutz O, Schmitt H, Nagy J, Muller C. The consequences of tubule-interstitial changes for renal function in glomerulopathies. A morphometric and cytological analysis. Pathol Res Pract. 1990;186:135–144. doi: 10.1016/S0344-0338(11)81021-6. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Iturbe B, Garcia Garcia G. The role of tubulointerstitial inflammation in the progression of chronic renal failure. Nephron Clin Pract. 2010;116:c81–c88. doi: 10.1159/000314656. [DOI] [PubMed] [Google Scholar]
- 6.Fogo A, Hawkins EP, Berry PL, Glick AD, Chiang ML, MacDonell RC, Jr, Ichikawa I. Glomerular hypertrophy in minimal change disease predicts subsequent progression to focal glomerular sclerosis. Kidney Int. 1990;38:115–123. doi: 10.1038/ki.1990.175. [DOI] [PubMed] [Google Scholar]
- 7.LeHir M, Besse-Eschmann V. A novel mechanism of nephron loss in a murine model of crescentic glomerulonephritis. Kidney Int. 2003;63:591–599. doi: 10.1046/j.1523-1755.2003.00782.x. [DOI] [PubMed] [Google Scholar]
- 8.Nangaku M. Mechanisms of tubulointerstitial injury in the kidney: final common pathways to end-stage renal failure. Intern Med. 2004;43:9–17. doi: 10.2169/internalmedicine.43.9. [DOI] [PubMed] [Google Scholar]
- 9.Remuzzi G. Nephropathic nature of proteinuria. Curr Opin Nephrol Hypertens. 1999;8:655–633. doi: 10.1097/00041552-199911000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen R, Christensen EI. Proteinuria and events beyond the slit. Pediatr Nephrol. 2010;25:813–822. doi: 10.1007/s00467-009-1381-9. [DOI] [PubMed] [Google Scholar]
- 11.Biemesderfer D. Regulated intramembrane proteolysis of megalin: linking urinary protein and gene regulation in proximal tubule? Kidney Int. 2006;69:1717–1721. doi: 10.1038/sj.ki.5000298. [DOI] [PubMed] [Google Scholar]
- 12.Amsellem S, Gburek J, Hamard G, Nielsen R, Willnow TE, Devuyst O, Nexo E, Verroust PJ, Christensen EI, Kozyraki R. Cubilin is essential for albumin reabsorption in the renal proximal tubule. J Am Soc Nephrol. 2010;21:1859–1867. doi: 10.1681/ASN.2010050492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimizu H, Maruyama S, Yuzawa Y, Kato T, Miki Y, Suzuki S, Sato W, Morita Y, Maruyama H, Egashira K, Matsuo S. Anti-monocyte chemoattractant protein-1 gene therapy attenuates renal injury induced by protein-overload proteinuria. J Am Soc Nephrol. 2003;14:1496–1505. doi: 10.1097/01.ASN.0000069223.98703.8E. [DOI] [PubMed] [Google Scholar]
- 14.Anders H-J, Ninichuk V, Schlondorff D. Progression of kidney disease: blocking leukocyte recruitment with chemokine receptor CCR1 antagonist. Kidney Int. 2006;69:29–32. doi: 10.1038/sj.ki.5000053. [DOI] [PubMed] [Google Scholar]
- 15.Kassiri Z, Oudit GY, Kandalam V, Awad A, Wang X, Ziou X, Maeda N, Herzenberg AM, Scholey JW. Loss of TIMP3 enhances interstitial nephritis and fibrosis. J Am Soc Nephrol. 2009;20:1223–1235. doi: 10.1681/ASN.2008050492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whaley-Connell AT, Morris EM, Rehmer N, Yaghoubian JC, Wei Y, Hayden MR, Habib J, Stump CS, Sowers JR. Albumin activation of NAD(P)H oxidase activity is mediated via Rac1 in proximal tubule cells. Am J Nephrol. 2007;27:15–23. doi: 10.1159/000098432. [DOI] [PubMed] [Google Scholar]
- 17.Cooper MA, Buddington B, Miller NL, Alfrey AC. Urinary iron speciation in nephrotic syndrome. Am J Kidney Dis. 1995;25:314–319. doi: 10.1016/0272-6386(95)90014-4. [DOI] [PubMed] [Google Scholar]
- 18.Kamijo A, Kimura K, Sugaya T, Yamanouchi M, Hase H, Kaneko T, Hirata Y, Goto A, Fujita T, Omata M. Urinary free fatty acids bound to albumin aggravate tubulointerstitial damage. Kidney Int. 2002;62:1628–1637. doi: 10.1046/j.1523-1755.2002.00618.x. [DOI] [PubMed] [Google Scholar]
- 19.Timmeren MM, Bakker SJL, Stegeman CA, Gans RO, Goor H. Addition of oleic acid to delipidated bovine serum albumin aggravates renal damage in experimental protein-overload nephrosis. Nephrol Dial Transplant. 2005;20:2349–2357. doi: 10.1093/ndt/gfh964. [DOI] [PubMed] [Google Scholar]
- 20.Arici M, Chana R, Lewington A, Brown J, Brunskill NJ. Stimulation of proximal tubular cell apoptosis by albumin-bound fatty acids mediated by peroxisome proliferator activated receptor-γ. J Am Soc Nephrol. 2003;14:17–27. doi: 10.1097/01.ASN.0000042167.66685.EA. [DOI] [PubMed] [Google Scholar]
- 21.Souma T, Abe M, Moriguchi T, Takai J, Yanagisawa-Miyazawa N, Shibata E, Akiyama Y, Toyohara T, Suzuki T, Tanemoto M, Abe T, Sato H, Yamamoto M, Ito S. Luminal alkalinization attenuates proteinuria-induced oxidative damage in proximal tubular cells. J Am Soc Nephrol. 2011;22:635–648. doi: 10.1681/ASN.2009111130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nangaku M, Pippin J, Couser W. C6 mediates chronic progression of tubulointerstitial damage in rats with remnant kidneys. J Am Soc Nephrol. 2002;13:928–936. doi: 10.1681/ASN.V134928. [DOI] [PubMed] [Google Scholar]
- 23.He C, Imai M, Song H, Quigg RJ, Tomlinson S. Complement inhibitors targeted to the proximal tubule prevent injury in experimental nephritic syndrome and demonstrate a key role for C5b-9. J Immunol. 2005;174:5750–5757. doi: 10.4049/jimmunol.174.9.5750. [DOI] [PubMed] [Google Scholar]
- 24.Zeisberg M, Duffield JS. Resolved: EMT produces fibroblasts in the kidney. J Am Soc Nephrol. 2010;21:1247–1253. doi: 10.1681/ASN.2010060616. [DOI] [PubMed] [Google Scholar]
- 25.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basile DP, Friedrich JL, Spahic J, Knipe N, Mang H, Leonard EC, Changizi-Ashtiyani S, Bacallao RL, Molitoris BA, Sutton TA. Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. Am J Physiol Renal Physiol. 2011;300:F721–F733. doi: 10.1152/ajprenal.00546.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19:2282–2287. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1–12. doi: 10.1097/01.ASN.0000106015.29070.E7. [DOI] [PubMed] [Google Scholar]
- 29.García-Sánchez O, López-Hernández FJ, López-Novoa JM. An integrative view on the role of TGF-β in the progressive tubular deletion associated with chronic kidney disease. Kidney Int. 2010;77:950–955. doi: 10.1038/ki.2010.88. [DOI] [PubMed] [Google Scholar]
- 30.Phanish MK, Winn SK, Dockrell ME. Connective tissue growth factor-(CTGF, CCN2)—a marker, mediator, and therapeutic target for renal fibrosis. Nephron Exp Nephrol. 2010;114:e83–e92. doi: 10.1159/000262316. [DOI] [PubMed] [Google Scholar]
- 31.Eddy AA, Fogo AB. Plasminogen activator inhibitor-1 in chronic kidney disease: evidence and mechanisms of action. J Am Soc Nephrol. 2006;17:2999–3012. doi: 10.1681/ASN.2006050503. [DOI] [PubMed] [Google Scholar]
- 32.Ricardo SD, Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest. 2008;118:3522–3530. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lange-Sperandio B, Fulda S, Vandewalle A, Chevalier RL. Macrophages induce apoptosis in proximal tubule cells. Pediatr Nephrol. 2003;18:335–341. doi: 10.1007/s00467-003-1116-2. [DOI] [PubMed] [Google Scholar]
- 34.Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17:17–75. doi: 10.1681/ASN.2005070757. [DOI] [PubMed] [Google Scholar]
- 35.Okusa MD, Chertow GM, Portilla D. The nexus of acute kidney injury, chronic kidney disease, and World Kidney Day 2009. Clin J Am Soc Nephrol. 2009;4:520–522. doi: 10.2215/CJN.06711208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fine LG, Norman JT. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int. 2008;74:867–872. doi: 10.1038/ki.2008.350. [DOI] [PubMed] [Google Scholar]
- 37.Xu H, Zeng L, Peng H, Chen S, Jones J, Chew TL, Sadeghi MM, Kanwar YS, Danesh FR. HMG-CoA reductase inhibitor simvastatin mitigates VEGF-induced “inside-out” signaling to extracellular matrix by preventing RhoA activation. Am J Physiol Renal Physiol. 2006;291:F995–F1004. doi: 10.1152/ajprenal.00092.2006. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, Tang L, Zhu Q, Yi F, Zhang F, Li PL, Li N. Hypoxia-inducible factor-1α contributes to the profibrotic action of angiotensin II in renal medullary interstitial cells. Kidney Int. 2011;79:300–310. doi: 10.1038/ki.2010.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haase VH. The sweet side of HIF. Kidney Int. 2010;78:10–13. doi: 10.1038/ki.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Basu RK, Hubchak S, Hayashida T, Runyan CE, Schumacker PT, Schnaper HW. Interdependence of HIF-1α and TGF-β/Smad3 signaling in normoxic and hypoxic renal epithelial cell collagen expression. Am J Physiol Renal Physiol. 2011;300:F898–F905. doi: 10.1152/ajprenal.00335.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fogo AB. Mechanisms of progression of chronic kidney disease. Pediatr Nephrol. 2007;22:2011–2022. doi: 10.1007/s00467-007-0524-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown NJ. Aldosterone and end-organ damage. Curr Opin Nephrol Hypertens. 2005;14:235–241. doi: 10.1097/01.mnh.0000165889.60254.98. [DOI] [PubMed] [Google Scholar]
- 43.Queisser N, Oteiza PI, Stopper H, Oli RG, Schupp N. Aldosterone induces oxidative stress, oxidative DNA damage and NF-κB-activation in kidney tubule cells. Mol Carcinog. 2011;50:123–135. doi: 10.1002/mc.20710. [DOI] [PubMed] [Google Scholar]
- 44.Briet M, Schiffrin EL. Aldosterone: effects on the kidney and cardiovascular system. Nat Rev Nephrol. 2010;6:261–273. doi: 10.1038/nrneph.2010.30. [DOI] [PubMed] [Google Scholar]
- 45.Neuhofer W, Pittrow D. Role of endothelin and endothelin receptor antagonists in renal disease. Eur J Clin Invest Suppl. 2006;3:78–88. doi: 10.1111/j.1365-2362.2006.01689.x. [DOI] [PubMed] [Google Scholar]