Abstract
Objective
The aim of the study was to assess the effect on prescription quality and quality of life after intervention with prescription reviews and promotion of patient participation in primary care.
Design
A randomized controlled study with three groups: (A) controls, (B) prescription review sent to physician, and (C) as in B and with a current comprehensive medication record sent to the patient.
Setting
The municipality of Örebro, Sweden (130 000 inhabitants).
Intervention
The study focused on the easiest possible intervention to increase prescription quality and thereby increase quality of life. The intervention should be cost-efficient, focus on colleague-to-colleague advice, and be possible to perform in the primary health care centre without additional resources such as a pharmacist.
Subjects
150 patients recently discharged from hospital. Inclusion criteria were: ≥ 75 years, ≥ five drugs and living in ordinary homes.
Main outcome measures
Quality of life (EQ-5D index, EQ VAS) and quality of prescriptions.
Results
Extreme polypharmacy was common and persistent in all three groups and this was accompanied by an unchanged frequency of drug-risk indicators. There was a low EQ-5D index and EQ VAS in all three groups throughout the study. No statistically significant differences were found anywhere between the groups.
Conclusion
The intervention seems to have had no effect on quality of prescriptions or quality of life. This underlines the major challenge of finding new strategies for improving prescription quality to improve patient outcome measures such as quality of life and reduce the known risks of polypharmacy for the elderly.
Key Words: Frail elderly, inappropriate prescribing, patient participation, polypharmacy, quality of life
Today there are no evidence-based models or smart tools for optimizing available drug treatment.
Prescribing for the elderly is a time-consuming responsibility for physicians in primary care.
A basic colleague-to-colleague intervention with prescription reviews had no effect on quality of prescriptions or quality of life.
New strategies are needed for improving prescription quality to improve patient outcome measures such as quality of life.
Introduction
In the developed world the real challenge for the health care system is the ageing population, accompanied by an increasing burden of chronic diseases and chronic medication [1]. Although modern drugs have made great contributions to health and quality of life (QoL), increasing proportions of negative side effects due to extensive pharmacological treatment are observed. Polypharmacy, defined as ≥ five drugs [2–4] is among the most obvious signs of risks in drug treatment, resulting in increased risks for inappropriate drug use and adverse drug reactions, followed by higher morbidity and hospitalization [5–9].
The Swedish National Board of Health and Welfare (SoS) and Swedish Association of Local Authorities and Regions (SALAR) concur with the WHO recommendations for drug use in the elderly, where the indication is the basic principle, followed by benefits of treatment in relation to harmfulness and inappropriateness [10,11]. The SoS has identified some drug-risk indicators in treatment: drugs not appropriate for use in the elderly. Occurrence of these drugs in the patient's medication list signals increased risks of adverse drug reactions and drug interactions which could affect the quality of drug treatment and the patient's well-being [10]. The most obvious goal for health care is to help people live longer and feel better [12]. As the burden of chronic diseases rises as we live longer, there is a need for focusing on “well-being”, that is QoL, as a main outcome measure [1].
Polypharmacy and/or poor quality of drug treatment are consequently challenges that should be addressed. Drug treatment can be either the facilitator, which gives the opportunities, or the opposite, an intensifier of problems by occurrence of unacceptable side effects possibly leading to decreased QoL.
There are currently no studies that have definitively determined whether various methods designed to reduce drug-related problems in the elderly affect QoL. Most studies in the area focus on prescription reviews done by drug specialists, for example pharmacists [13]. The evidence that this kind of intervention can prevent medication-related adverse events is weak [14,15]. In this study we wanted to investigate whether a more basic kind of intervention and prescription review could be effective. We wanted to conduct a study that focused on the easiest possible intervention to increase prescription quality and thereby increase QoL. The intervention should be cost efficient, focus on colleague to colleague advice and possible to perform in the primary healthcare centre without additional resources such as a pharmacist. The aim of the intervention study was to examine whether prescription reviews sent from a primary care physician to other primary care physicians could affect prescription quality and the patient's QoL, and also whether there were any additive effects by encouraging the patients to question their drug treatment by giving them their medication record.
Material and methods
During the period September 2006 to May 2007, all patients ready for discharge from the University Hospital in Örebro and fulfilling the criteria were eligible for the study. Inclusion criteria were: ≥ 75 years, ≥ five drugs and living in ordinary homes. Exclusion criteria were dementia, abuse, or malignant disease diagnosed before the study start. Moving to a nursing home during the study also resulted in exclusion. The electronic care planning system (Meddix), used throughout the County Council and municipalities, made the surveillance of all discharges complete and all patients had the same opportunity to be included. The study was performed in primary care, since family physicians are responsible for the medical care of the elderly after discharge from hospital. The patients in the study were followed for one year with study end in May 2008.
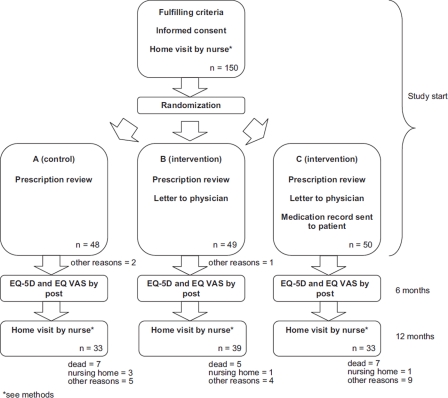
At the time of discharge all patients were registered in the care planning system and a message was sent to the research centre. If the patient was eligible, a letter concerning the study including informed consent was sent to the patient. A research assistant without any connection to the study consecutively randomized the patients to one of the three study groups (Figure 1):
Figure 1.
Study flow chart.
Note: Flow chart of the study and randomization process. Dropouts for other reasons include no answer after three telephone calls, not opening the door at agreed visiting time, and no longer willing to participate.
Group A (control): home visit by study nurse within one month after discharge, QoL survey by post at six months, and second home visit by study nurse at 12 months.
Group B (intervention): as group A and a letter with a prescription review (according to points 1–4 below) sent to the physician/primary health care centre.
Group C (intervention): as group B combined with a current and comprehensive medication record consisting of the patient's written drug regimen and indications sent to the patient to enable participation in his/her drug treatment. This was accompanied by an instruction to utilize the record throughout the health care system, make notes, and discuss their drug treatment with their physicians [11].
During the home visit patients in all three groups were asked about their drug regimen and compliance to capture their “true” medication record. To measure QoL the validated questionnaire EQ-5D was used after approval of the EuroQol group [16,17]. EQ-5D is a generic instrument evaluating function in five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression). The EQ-5D index was used for an overall estimation of QoL [18]. EQ VAS was used for self-rating of current health-related QoL.
The study physician completed a prescription review assessing the following as indicators of prescription quality [10,13,19–21]:
number of drugs; total, on regular basis and on demand;
number of drug-risk indicators (long- and short-acting benzodiazepines, sleeping pills, NSAIDs, digitalis, diuretics, SSRI, PPI, neuroleptics, and drugs with anticholinergic effects);
drug interactions by using a computer program that warns for interactions of C-type (adjustment of dose recommended) and D-type (avoidance of drug recommended) [22];
number of medication errors and/or discrepancies between medication list (prescriptions) and the patient's own regime (drugs noted but not taken, drugs taken but not noted, and wrong dosages).
The prescription reviews were then sent to the primary health care centres to alert the family physicians together with a letter explaining the errors and suggested proceedings.
At study end the comprehensive medication records for the patients in group C were collected by the nurse. All home visits throughout the study were done by the same study nurse who was blinded to the groups. Before study start all primary health care centres and family physicians in the area were informed about the study.
Statistical analyses
There are no data concerning the effect of prescription reviews on QoL and therefore we had to approximate the effect of such an intervention. We estimated that QoL could increase by 20% in the intervention groups. With a power of 80% and a significance level of 5% it was then calculated that a total study population of 150 individuals, with 50 individuals in each arm, should be an appropriate sample size taking into account a dropout rate of 10%. The data were analysed using the SPSS program, version 15.
Results
A total of 150 patients were identified for inclusion in the study. The mean ages in groups A, B, and C were 82.5± 4.9 (mean± SD), 83.4± 5.1 and 83.9± 5.1. The sex distributions were 56% / 44% (female/male), 63% / 37%, and 64% / 36% respectively. No significant differences between the groups were observed in respect of mortality or dropouts (for numbers and reasons see Figure 1).
Table I shows the prescription quality for the patients who completed the study. There were no significant differences when similar comparisons were made with all patients included. Extreme polypharmacy (taking > 10 drugs) was common and persistent in all three groups and this was accompanied by an unchanged frequency of drug-risk indicators (Table I). The frequency of correct medication lists was very low in all three groups (Table I). The frequencies of interactions of types C and D are shown as proportions of patients having them (Table I). The 99 prescription review letters (49 in group B and 50 in group C) sent to physicians/primary care centres, resulted in only eight (three and five respectively) actions.
Table I.
Prescription quality.
| Group A |
Group B |
Group C |
||||||||
| Baseline | 12 months | p-value | Baseline | 12 months | p-value | Baseline | 12 months | p-value | p-value overall | |
| Number of drugs per patient (median) | 8.0 | 9.0 | 0.029 | 10.0 | 11.0 | 0.655 | 10.0 | 10.0 | 0.454 | 0.382 |
| Number of drug-risk indicators per patient (median) | 2.0 | 2.0 | 0.181 | 2.0 | 2.0 | 0.813 | 2.0 | 2.0 | 0.401 | 0.444 |
| Number of medication errors per patient (median) | 5.0 | 2.0 | 0.099 | 3.0 | 2.0 | 0.031 | 3.0 | 3.0 | 0.862 | 0.331 |
| Proportion of correct medication lists (%) | 10.0 | 18.0 | 0.130 | 4.0 | 13.0 | 0.029 | 8.0 | 12.0 | 0.371 | 0.614 |
| Proportion of patients with interactions of C type (%) | 60.6 | 57.8 | 0.135 | 43.9 | 48.7 | 0.327 | 42.4 | 48.5 | 0.705 | 0.788 |
| Proportion of patients with interactions of D type (%) | 3.0 | 3.0 | 0.655 | 2.6 | 7.7 | 0.317 | 21.2 | 6.1 | 0.096 | 0.088 |
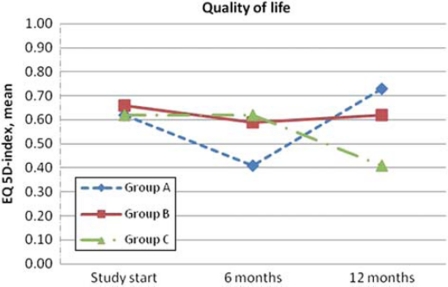
For QoL the EQ-5D results are presented as recommended by the EuroQol group [17]. The dimensions mobility, pain/discomfort, and anxiety/depression show higher percentages with symptoms (Table II). The response frequency for the EQ-5D questionnaires that were sent to the patients at six months was high: 84%, 79% and 80% respectively for each group. The EQ-5D index varied over time, but there were no significant differences in or between the groups (Figure 2). The EQ VAS shows notably low scores for the patients’ own assessment of health-related QoL (Table III). In group C (patient participation), the usage of the medication records was registered when returned to the research centre. From the 33 patients fulfilling the study at 12 months, 21 medication records were returned, but only eight of them had been used. This was accompanied by different messages listing forgetfulness, feeling unaccustomed to participating, and also referring to fear of causing trouble.
Table II.
Frequency distribution (profile) of the EQ-5D descriptive system for comparison.1
| Group A |
Group B |
Group C |
|||||||
| EQ-5D profile | Baseline (n = 47) |
6 months (n = 38) |
12 months (n = 34) |
Baseline (n = 49) |
6 months (n = 37) |
12 months (n = 39) |
Baseline (n = 48) |
6 months (n = 35) |
12 months (n = 33) |
| Mobility: | |||||||||
| No problems (%) | 13 | 19 | 30 | 14 | 17 | 18 | 8 | 18 | 15 |
| Some problems (%) | 79 | 81 | 64 | 78 | 83 | 74 | 83 | 76 | 76 |
| Confined to bed (%) | 8 | 0 | 6 | 8 | 0 | 8 | 8 | 6 | 9 |
| Self-care: | |||||||||
| No problems (%) | 62 | 60 | 76 | 67 | 60 | 71 | 62 | 52 | 66 |
| Some problems (%) | 30 | 34 | 12 | 30 | 35 | 24 | 25 | 39 | 22 |
| Unable to (%) | 8 | 6 | 12 | 4 | 5 | 5 | 13 | 9 | 12 |
| Usual activities: | |||||||||
| No problems (%) | 45 | 47 | 53 | 47 | 38 | 44 | 44 | 24 | 55 |
| Some problems (%) | 30 | 37 | 32 | 31 | 46 | 36 | 29 | 65 | 21 |
| Unable to (%) | 26 | 16 | 15 | 22 | 16 | 20 | 27 | 12 | 24 |
| Pain/discomfort: | |||||||||
| None (%) | 28 | 5 | 23 | 24 | 16 | 18 | 25 | 11 | 24 |
| Moderate (%) | 53 | 63 | 53 | 51 | 54 | 54 | 62 | 74 | 49 |
| Extreme (%) | 19 | 32 | 24 | 25 | 30 | 28 | 13 | 15 | 27 |
| Anxiety/depression: | |||||||||
| None (%) | 36 | 42 | 56 | 51 | 40 | 37 | 56 | 47 | 42 |
| Moderate (%) | 60 | 47 | 38 | 41 | 57 | 55 | 40 | 53 | 54 |
| Extreme (%) | 4 | 11 | 6 | 8 | 3 | 8 | 4 | 0 | 4 |
Note: 1The EQ-5D index varied over time, but there were no statistically significant differences in or between the groups.
Figure 2.
EQ-5D index. Note: Statistical analyses were done within and between the groups using the Friedman test and Kruskal–Wallis test. No significant difference anywhere.
Table III.
Patients’ assessments of their own health-related quality of life, EQ VAS.
| Group A |
Group B |
Group C |
|||||||
| Baseline | 6 months | 12 months | Baseline | 6 months | 12 months | Baseline | 6 months | 12 months | |
| Mean Q VAS score | 50 | 55 | 56 | 51 | 52 | 54 | 51 | 52 | 56 |
| (± SD) | (19) | (19) | (17) | (17) | (19) | (14) | (16) | (20) | (17) |
| Median EQ VAS score | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| (IQR) | (40–60) | (50–72) | (50–68) | (45–60) | (42–60) | (50–60) | (40–60) | (40–70) | (50–64) |
Notes: Statistical analyses were done within and between the groups using the Friedman test and Kruskal–Wallis test. No significant difference anywhere.
Discussion
The aim of the study was to assess the effect in prescription quality and QoL after intervention with prescription reviews and promotion of patient participation via a randomized controlled study. The main results of the study are the persistent low values of QoL, demonstrated by low EQ-5D index and EQ VAS in all three study groups throughout the study. The intervention had no statistically significant effect on QoL or prescription quality. The findings show low interest from the physicians in actions for improving prescription quality to achieve better QoL by reducing risks for this group of vulnerable elderly. The findings also highlight the remaining hierarchic structure in health care where most of the patients still do not dare to discuss their drug treatment.
One reason for the physicians’ unwillingness to change prescriptions according to the prescription review may be the fact that changes require additional work, such as increased monitoring and follow-up and time to consider the suggestions [23]. Another reason is the fact that many prescribers with different specializations are involved in the care of the patient, focusing on their area of specialization and with no one taking the overall responsibility for the patient. All prescribers independently of specialization have the same obligation in the prescribing process [24] but the phenomenon of many caregivers/physicians being involved causes risks and problems when there is no individual caregiver who has an overview of the medication list and where the responsibility is not apparent [25,26].
Part of the intervention was enablement of patient participation in group C. We saw many errors; wrong dosages were taken as well as wrong regimens followed but the patients did not want to cause problems in their relationship with the doctor. They avoided time-consuming questions, although they felt insecure about their medication. The comments here were that they “wanted information and a good relationship”, accompanied by overall trust in the “good” doctors and their judgement on “giving the right treatment”, which is similar to findings in other studies that address patient participation [27]. This reduces discussions concerning the benefits and risks of polypharmacy, since continuity, as well as access and having a “good doctor”, is more important. Empowerment of the patient's involvement in his/her drug treatment is a key issue for the future, and further studies will be needed to evaluate the effects on treatment quality as well as QoL.
The strength of our study is that it was conducted in “care as usual”. The study was completely randomized and there was no external investigators bias since there was only one nurse involved who completed all the home visits throughout the study. A weakness of our study is that our estimation of the effect of the intervention on QoL was too high and therefore the power of the study is low. This means that there could be a small effect on QoL through an intervention like ours but such an eventual small effect is probably of no clinical significance.
Today there are no systematic evidence-based models or smart tools for optimizing the drug treatment available [6]. This study was planned and carried out so that the family physicians involved in the intervention had to perform a minimum of extra work. The physicians’ work was facilitated by the prescription reviews, which showed number of drugs and drug-risk indicators as well as warnings of interactions. Interactions of C and D type are real risk measurements for the patient as well as the health care system, as they signal preventable risks in drug treatment [22]. The results presented here show low responsiveness to the alarm signals. This underlines the major challenge of finding new strategies for improving prescription quality to improve patient outcome measures such as QoL and reduce the known risks of polypharmacy for the elderly.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
This study was supported by grants from Örebro County Council. Special thanks are offered to the study nurse Ewa Löfgren for her sterling work and Susanne Collgård for her excellent work with compilation of the data.
The Regional Ethics Committee of Uppsala University approved the study (Dnr 2006/191).
References
- 1.Hagstrom B, Mattsson B, Wimo A, Gunnarsson RK. More illness and less disease? A 20-year perspective on chronic disease and medication. Scand J Public Health. 2006;34:584–8. doi: 10.1080/14034940600703407. [DOI] [PubMed] [Google Scholar]
- 2.Agostini JV, Han L, Tinetti ME. The relationship between number of medications and weight loss or impaired balance in older adults. J Am Geriatr Soc. 2004;52:1719–23. doi: 10.1111/j.1532-5415.2004.52467.x. [DOI] [PubMed] [Google Scholar]
- 3.Haider SI, Johnell K, Weitoft GR, Thorslund M, Fastbom J. The influence of educational level on polypharmacy and inappropriate drug use: A register-based study of more than 600,000 older people. J Am Geriatr Soc. 2009;57:62–9. doi: 10.1111/j.1532-5415.2008.02040.x. [DOI] [PubMed] [Google Scholar]
- 4.Hovstadius B, Astrand B, Petersson G. Assessment of regional variation in polypharmacy. Pharmacoepidemiol Drug Saf. 2010;19:375–83. doi: 10.1002/pds.1921. [DOI] [PubMed] [Google Scholar]
- 5.Franic DM, Jiang JZ. Potentially inappropriate drug use and health-related quality of life in the elderly. Pharmacotherapy. 2006;26:768–78. doi: 10.1592/phco.26.6.768. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton HJ, Gallagher PF, O'Mahony D. Inappropriate prescribing and adverse drug events in older people. BMC Geriatr. 2009;9:5. doi: 10.1186/1471-2318-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jano E, Aparasu RR. Healthcare outcomes associated with Beers’ criteria: A systematic review. Ann Pharmacother. 2007;41:438–47. doi: 10.1345/aph.1H473. [DOI] [PubMed] [Google Scholar]
- 8.Liu GG, Christensen DB. The continuing challenge of inappropriate prescribing in the elderly: An update of the evidence. J Am Pharm Assoc (Wash) 2002;42:847–57. doi: 10.1331/108658002762063682. [DOI] [PubMed] [Google Scholar]
- 9.O'Mahony D, Gallagher PF. Inappropriate prescribing in the older population: Need for new criteria. Age Ageing. 2008;37:138–41. doi: 10.1093/ageing/afm189. [DOI] [PubMed] [Google Scholar]
- 10.The National Board of Health and Welfare. Indicators for evaluation of quality of drug treatment for elderly. Socialstyrelsen. 2003. pp. 1–74. , editor. Available at: http://www.socialstyrelsen.se/NR/rdonlyres/A65367AB-8F2A-4063-BC79-13103784A838/986/200311020.pdf.
- 11.Swedish Association of Local Authorities and Regions. Stockholm: SKL, Sveriges kommuner och landsting; 2011. Läkemedelsrelaterade problem [Medicines-related probem] [Google Scholar]
- 12.Kaplan RM. The significance of quality of life in health care. Qual Life Res. 2003;12((Suppl 1)):3–16. doi: 10.1023/a:1023547632545. [DOI] [PubMed] [Google Scholar]
- 13.Stockholm: SBU Statens beredning för medicinsk utvärdering; 2009. The Swedish Council on Health Technology Assessment S. How can drug consumption among the elderly be improved. . Report No. 193. [PubMed] [Google Scholar]
- 14.Holland R, Desborough J, Goodyer L, Hall S, Wright D, Loke YK. Does pharmacist-led medication review help to reduce hospital admissions and deaths in older people? A systematic review and meta-analysis. Br J Clin Pharmacol. 2008;65:303–16. doi: 10.1111/j.1365-2125.2007.03071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Royal S, Smeaton L, Avery AJ, Hurwitz B, Sheikh A. Interventions in primary care to reduce medication related adverse events and hospital admissions: Systematic review and meta-analysis. Qual Saf Health Care. 2006;15:23–31. doi: 10.1136/qshc.2004.012153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooks RG, Jendteg S, Lindgren B, Persson U, Bjork S. EuroQol: Health-related quality of life measurement. Results of the Swedish questionnaire exercise. Health Policy. 1991;18:37–48. doi: 10.1016/0168-8510(91)90142-k. [DOI] [PubMed] [Google Scholar]
- 17.The EuroQol Group. Euro-Qol: A new facility for the measurement of health-related quality of life. Health policy. 2009;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 18.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35:1095–108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Fialova D, Onder G. Medication errors in elderly people: Contributing factors and future perspectives. Br J Clin Pharmacol. 2009;67:641–5. doi: 10.1111/j.1365-2125.2009.03419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones BA. Decreasing polypharmacy in clients most at risk. AACN Clin Issues. 1997;8:627–34. doi: 10.1097/00044067-199711000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Maxwell S, Walley T. Teaching safe and effective prescribing in UK medical schools: A core curriculum for tomorrow's doctors. Br J Clin Pharmacol. 2003;55:496–503. doi: 10.1046/j.1365-2125.2003.01878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bjorkman IK, Fastbom J, Schmidt IK, Bernsten CB. Drug–drug interactions in the elderly. Ann Pharmacother. 2002;36:1675–81. doi: 10.1345/aph.1A484. [DOI] [PubMed] [Google Scholar]
- 23.Rytter L, Jakobsen HN, Ronholt F, Hammer AV, Andreasen AH, Nissen A, et al. Comprehensive discharge follow-up in patients’ homes by GPs and district nurses of elderly patients: A randomized controlled trial. Scand J Prim Health Care. 2010;28:146–53. doi: 10.3109/02813431003764466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woodward M, Bird M, Elliot R, Lourens H, Saunders R. Deprescribing: Achieving better health outcomes for older people through reducing medications. J Pharm Pract Res. 2003;33:323–8. [Google Scholar]
- 25.Midlöv P, Bergkvist A, Bondesson A, Eriksson T, Höglund P. Medication errors when transferring elderly patients between primary health care and hospital care. Pharm World Sci. 2005;27:116–20. doi: 10.1007/s11096-004-3705-y. [DOI] [PubMed] [Google Scholar]
- 26.Rahmner PB, Gustafsson LL, Holmstrom I, Rosenqvist U, Tomson G. Whose job is it anyway? Swedish general practitioners’ perception of their responsibility for the patient's drug list. Ann Fam Med. 2010;8:40–6. doi: 10.1370/afm.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moen J. Dissertation. Uppsala University: Uppsala; 2009. Multiple medicine use: Patients’ and general practitioners’ perceptions and patterns of use in relation to age and other patient characteristics. [Google Scholar]