Abstract
We report the finding of the presence of carbon nanoparticles (CNPs) in different carbohydrate based food caramels, viz. bread, jaggery, sugar caramel, corn flakes and biscuits, where the preparation involves heating of the starting material. The CNPs were amorphous in nature; the particles were spherical having sizes in the range of 4–30 nm, depending upon the source of extraction. The results also indicated that particles formed at higher temperature were smaller than those formed at lower temperature. Excitation tuneable photoluminescence was observed for all the samples with quantum yield (QY) 1.2, 0.55 and 0.63%, for CNPs from bread, jaggery and sugar caramels respectively. The present discovery suggests potential usefulness of CNPs for various biological applications, as the sources of extraction are regular food items, some of which have been consumed by humans for centuries, and thus they can be considered as safe.
The use as well as presence of nanoparticles (NPs) in food is a hotly debated area, owing to their short and long term effects on human health and the environment1,2,3,4. The promise of targeted and/or sustained release of drug, food colourants and flavours, while incorporated with NP, makes the pursuit of understanding of their functioning and fate a worthy exercise5,6,7. Although, substantial development in the engineering of consumable NPs5,6,7 and their effects in vitro and in vivo have taken place8; few biodegradable NPs have entered clinical trials and have been marketed9,10. While NP formulations for topical applications are accepted by majority of population11, the idea of the consumption of these particles, either for curing a disease or for having nutritional or flavouring benefit, creates an alarm for the public. The reason behind this seems to be their potential effect on human health following consumption, which has received little attention; and the lack of awareness, which has raised concerns regarding the safety of nanomaterials in biological and food applications1,2,3,4. A way around this problem, could originate out of direct derivation of nanomaterials from food products, especially from traditional food items. These materials could be considered safe for biological applications when there is no known toxicity and thus may possibly alleviate the misapprehension that all NPs are toxic.
History of nanotechnology is replete with examples of use of nanomaterials dating back to millennia12,13,14. The dye used in colouring hair to black, during the Greco-Roman period, is now known to have been consisted of PbS nanocrystals (NCs)12. Romans exhibited their mastery in technology in the Lycurgus cup by harnessing the optical properties of gold (Au) NPs13. The extraordinary mechanical strength and a sharp cutting edge in Damascus sabre have recently been attributed to the presence of carbon nanotubes (CNTs) and cementite nanowires14. In all the cases mentioned above, while the technology based on nanomaterials were known to different civilizations, the nanoscale nature of their functional constituents have only been revealed recently. The ‘nano’ dimensionality is not only confined to engineered materials or technology; nature also creates NPs or nanostructures which are present as functional components in an organism; either in the form of enzymes which catalyze most of the biological reactions or as ribosomes which act as the sites for protein synthesis. It was the invention of sophisticated microscopic and analytical techniques which has led to the discovery of these nanostructures. In this regard, the idea of searching for nanomaterials within regular food items cannot be inexplicit. This motivated us to search for NPs in food items, which can potentially be used for biological application, where the concern of the origin and toxicity of the nanomaterials can easily be waived off. Herein, we report the presence of carbon nanoparticles (CNPs) in regular carbohydrate based food caramels, such as in bread, jaggery, corn flakes and biscuits. The CNPs have been found to be present in those samples, where the preparation of food mainly involves heating of the starting ingredients in absence of water, leading to formation of caramels. Arguably; this discovery revealed that human consumption of nanomaterials in the form of food caramels has its history possibly from the period when human for the first time started eating bread.
Carbon dots (C-dots), which are CNPs below 10 nm are emerging as viable alternatives to semiconductor quantum dots (Qdots) owing to their important photoluminescent properties and lack of any known cytotoxicity15,16,17. The wavelength-tuneable emission properties have made them promising candidates as new ‘nanolights’17. The optimism has led to increased recent interests in developing methods for their syntheses, involving both top-down and bottom-up approaches17. Incidentally, there are also efforts to understand and tune their optical properties, based on the surface functional groups18,19,20. We have recently observed that caramelization of poly(ethylene glycol) under microwave irradiation constitutes formation of biocompatible C-dots15. This prompted us to analyse the components of different commercial and homemade caramel containing food items for the presence of CNPs. Amazingly, we found that the light to dark brown coloured caramels present in carbohydrate containing foods such as bread, jaggery, corn flakes and biscuit consist of amorphous CNPs, which were similar to those obtained from caramel produced upon heating of commercial sugar. The discovery of the prominent presence of CNPs in regular food items provides simpler and safer sources i.e. daily food items, where the extracted CNPs can be directly used for biological applications. Further, our observations of the hydrophilicity of the surface functional groups of the CNPs, containing carboxylic and alcoholic groups which provide easier alternatives for their conjugation with different therapeutics, could further make them preferred fluorescent candidates for biological applications15,16,17.
Results
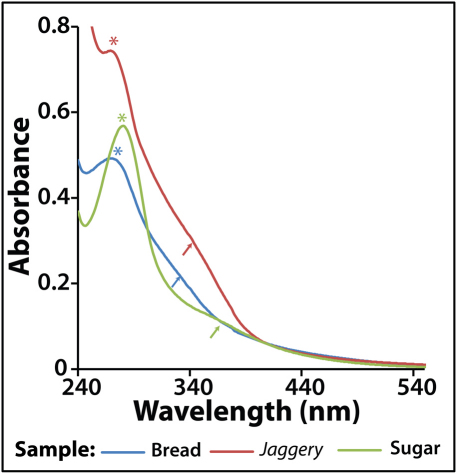
The CNPs isolated from the outer brown part of the bread bun and the caramels obtained from commercially purchased sugar (following caramelization) and jaggery were analysed by UV-vis and fluorescence spectroscopy. The UV-vis spectrum of each of the dispersions consisted of a peak (marked with asterisk) and a shoulder (marked with arrow) between 240 nm and 400 nm and is shown in Figure 1. In addition, there is the presence of a strong background till 540 nm. The peaks and the background extinction are known to occur for CNPs and they are consistent with the literature reports17. The exact assignment of the peaks is still not known and hence the difference among the individual samples could not be explained. CNPs were also extracted from commercially procured biscuits and corn flakes, the spectroscopic data for which are shown in Figure S1 (supplementary information).
Figure 1. UV-vis spectra of CNPs extracted from bread, jaggery and sugar caramel.
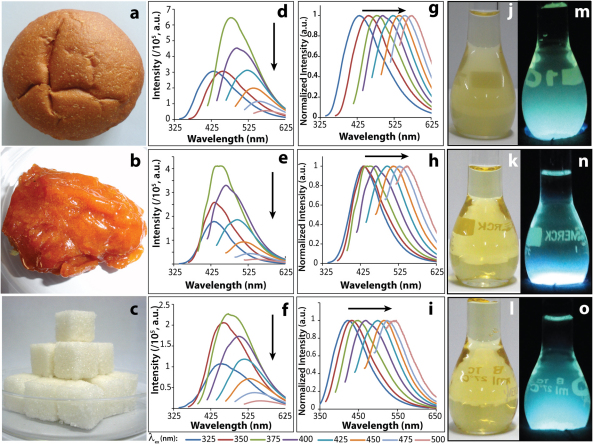
Photographs of the original samples (bread, jaggery and sugar) which served as the preparatory ingredients are shown in Figures 2a, 2b and 2c. The photoluminescence spectra corresponding to the above dispersions (and other samples) are shown in Figures 2d–2i (and Figure S1). The pictures of the dispersions in the presence of white light and UV light are represented in Figures 2j–2o. Under normal white light the dispersions have the characteristic caramel colour, whereas, under UV light (λex = 365 nm) it showed blue luminescence. All of the dispersions exhibited excitation dependent emission spectra as shown in Figures 2g–2i, which were similar to C-dots reported previously17. It was also observed that with increase in the wavelength of excitation from 325 to 375 nm the luminescence intensity increased, the maximum emission intensity being observed for the excitation wavelength of 375 nm, whereas, further increase of the excitation wavelength resulted in the decrease of emission intensity. Additionally, along with decrease in the fluorescence intensity with increasing excitation wavelength the emission maxima showed red-shift, displaying the property of excitation tuneable emission. The excitation dependent emission is an intrinsic property of CNPs, which has been widely reported by several research groups, including us15,17,21,22,23,24,25,26. The quantum yields (QYs) of the CNPs obtained from different food sources were calculated using quinine sulphate as the standard25. At an excitation wavelength of 365 nm, the QYs of the CNPs are summarized in Table 1. The results indicated that these samples had QY typical of C-dots, which is on the order of 1%; with the highest being observed for samples from bread (1.2%) and that from jaggery had the lowest value (0.55%).
Figure 2.
(a), (b), (c) Photographs of commercial bread, jaggery and sugar. (d)–(i) excitation wavelength dependent emission spectra of CNPs from bread, jaggery and sugar caramel. (j)–(l) photographs of dispersions of CNPs from bread, jaggery and sugar caramel observed under white light and (m)–(o) the same under UV light.
Table 1. Quantum yield of CNPs extracted from different food items.
| CNPs extracted from | Size (nm) | QY (%) |
|---|---|---|
| Bread | 27.5 ± 6.1 | 1.2 |
| Jaggery | 20.3 ± 7.5 | 0.55 |
| Sugar caramel | 4.3 ± 1.5 | 0.63 |
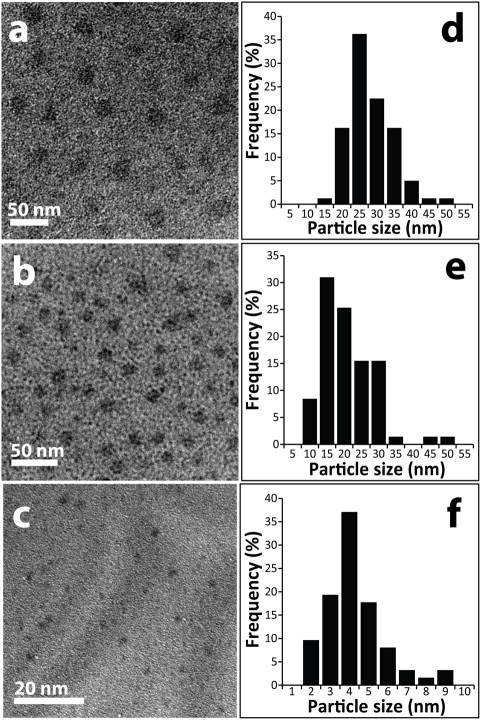
Transmission electron microscopy (TEM) images of the samples obtained from the dispersions of different caramel sources (bread, jaggery and sugar) are represented in Figures 3a–3c, which showed the presence of uniform spherical NPs. The particle distributions calculated from the images are shown in Figures 3d–3f. The average particle sizes as calculated from the TEM images for samples from bread, jaggery and sugar caramel, were determined to be 27.5 ± 6.1, 20.3 ± 7.5, 4.3 ± 1.5 nm respectively. Similarly, samples from corn flakes and biscuits indicated the presence of NPs having sizes of 10.5 ± 2.8 nm and 3.9 ± 1.3 nm respectively (Figure S1, supplementary information). The results clearly indicated that NPs were present in the dispersions extracted from bread, jaggery, caramel of sugar and other materials. While the sample from bread had the highest particle size, the particles from sugar caramel produced at 200°C had the lowest size and the particle sizes of the sample from jaggery were in between the two. In addition, caramels from sugar, produced by heating at 180°C for 10 min, had particles of size 25.8 ± 12.4 nm (Figure S2, supplementary information). Thermogravimetric analysis of sugar indicated decomposition starting at below 200°C with steady decrease in weight till 350°C (Figure S3, supplementary information). Loss of weight signifies the dehydration process of carbohydrate or formation of CO227. Thus, the NPs could possibly be produced at a temperature even lower than 200°C. Samples from bread, jaggery and caramel showed broad X-ray diffraction (XRD) peak at about 2θ = 18° (Figure S4, supplementary information), with no clear signature for crystalline nature of any of the samples. The above results indicated that NPs present in the caramels of bread, jaggery, corn flakes, biscuits and sugar possibly consisted of amorphous carbon.
Figure 3. (a), (b), (c) TEM images of CNPs extracted from bread, jaggery and sugar caramel and (d), (e), (f) corresponding particle size distributions of samples in that order.
Further, 13C NMR (nuclear magnetic resonance) spectra of samples from bread, jaggery and caramel (Figures S5, S6 and S7, supplementary information) revealed the presence of peaks between δ = 60 ppm and δ = 105 ppm. The peaks clearly indicated the presence of carbohydrate unit in the extracted CNPs. There were two additional 13C peaks observed in case of CNPs extracted from bread (δ = 216.951 ppm, δ = 31.778 ppm) and sugar caramel (δ = 215.578 ppm, δ = 30.428 ppm), depicting the presence of keto (-C = O) and methyl groups in the surface functional moieties.
Further, in order to probe the extent of cytotoxicity of the extracted CNPs, we performed XTT based cell viability assay at varying concentrations of CNPs. The plot of percentage viability of cells to that of varying concentration of CNPs (0.05 μg/mL to 2.0 μg/mL) is shown in Figure S11. As is clear from the figure, no cytotoxicity was apparent even at the highest concentration of CNPs (2.0 μg/mL) used. In addition, one way ANOVA showed that the differences in the mean percentage viabilities of cells at different concentrations of CNPs extracted from jaggery (F = 0.652, P = 0.689) and bread (F = 1.152, P = 0.384) were not statistically significant.
Discussion
The UV-visible and fluorescence spectra of the dispersion of CNPs extracted from different food sources displayed features similar to those of C-dots synthesized chemically and thereby suggesting the presence of CNPs in the samples. The fundamental mechanism of photoluminescence of CNPs is still a major question; however, it is thought that the presence of different surface trap sites could be one of the factors for the luminescence.17,22 The origin of fluorescence from the obtained dispersion could only be attributed to the presence of CNPs because the analysis of the starting material for preparation of bread did not show any significant fluorescence (Figure S8, supplementary information). Sugar is known to be a nonfluorescent material, but the caramel prepared upon heating sugar showed the emergence of excitation tuneable luminescence, further confirming the formation of CNPs. Additionally, it was observed that the heating temperature for preparing the caramel had significant effect on the size of NPs formed. Caramels prepared at 180°C and 200°C had the sizes of 25.8 ± 12.4 and 4.3 ± 1.5nm respectively. This indicated that smaller particles were possibly formed at the higher temperature. In other words, the larger particle sizes of NPs obtained from bread and jaggery could be due to their low heating temperatures, whereas, the smaller particles sizes of NPs obtained from sugar caramel, corn flakes and biscuits could be due to higher heating temperatures. It may also be mentioned here that there could be other factors, such as the rate and duration of heating and chemical constituents of the samples, determining the sizes of the produced CNPs. The possibility of the formation of CNPs while preparing and analysing the sample in electron microscopy can be ruled out because when drop cast sample from sugar solution was observed in TEM no such particle formation was detected, even under the exposure of a 200 kV electron beam for several minutes. The images obtained at different time of irradiation, of the sample from sugar solution, in the electron beam of TEM are shown in Figure S9 (supplementary information). It is worth noting that similar extraction process was also used for determining the presence of CNPs, if any, in the interior white part of bread. TEM analysis revealed the presence of inhomogeneous particles (Figure S10a, supplementary information) which could be due to the suboptimal temperature in the inner zone. The fluorescence intensity of this dispersion was significantly low compared to that obtained from the brown part of the bread (Figure S10b, supplementary information). The size of the CNPs produced varied from sample to sample, indicating the possibility of heating temperature as the primary factor determining their sizes. However, it was interesting to observe that for all samples the particles produced were nearly uniform and spherical. To have an idea of the amount of CNPs which can be extracted from a food source we analysed the amount of particles obtained from 1 g each of jaggery and the brown layer of bread. It was observed that about 3 and 2 % w/w of CNPs, in the respective samples were present. The calculation is based on the amount of the starting ingredient taken for the isolation of CNPs and the sample recovered after purification. However, the amount recovered from these materials cannot solely be attributed to CNPs as polymeric layer will always remain surrounding these particles. Isolation of nude CNPs without the polymeric layer has not been possible in the present condition; even then it can give an approximate value about the fraction of particles extracted.
Amorphous nature of NPs present in all the samples is demonstrated by the results of powder XRD data (Figure S4, supplementary information) as no peaks of crystalline origin was detected. The NMR studies revealed that the CNPs were coated with hydrophilic carbohydrate units. No peaks corresponding to the aromatic region was observed, which again supported the luminescence to be originating from the CNPs present in the dispersion.
In summary, our current work revealed the presence of CNPs in carbohydrate based regular food caramels from bread, jaggery, corn flakes and biscuit. The excitation wavelength dependent emission characteristic of the CNPs from food caramels were similar to those generated from sugar; however, the particle sizes varied indicating temperature - dependent formation of CNPs of different sizes. NMR spectroscopy revealed that the CNPs were coated with carbohydrate units. It is interesting to note that for centuries these caramels containing CNPs have been consumed by human beings with no known toxicity and thus it can be considered to have no or minimum risk on human health and may be used as a safe nanomaterial. Our finding of the presence of fluorescent CNPs in food caramels may also help their use in tracking and imaging conjugated biomolecules and drugs in vivo, without being imperilled.
Methods
Preparation and extraction of CNPs
Bread buns were purchased from the local market (Homa Bread, Guwahati, India) and analysed to check the presence of CNPs within it. The top brown layer of bread was carefully excised and 1 g of it was dissolved in 20 mL methanol by sonicating it at 35 kHz in a bath sonicator (Elmasonic TI-H-5 Elma, Germany) for 10 min. Following sonication, the volume of the methanol was reduced to 3 mL in a rotary evaporator before further purification. Jaggery (prepared from sugarcane juice) purchased from the market was heated following a traditional procedure which is as follows. Initially, jaggery (say 50 g) was mixed with water (about 10–15 mL) to make it sufficiently moist. The entire amount was then placed on a hot pan, which was being heated in the medium flame of a commercial gas stove. The mixture was constantly stirred using a kitchen spatula. In about 5 min, when the colour of the mixture turned dark brown, the entire amount was transferred to a pan containing a thin layer of oil and brought to room temperature. The oil layer helped in preventing aggregation of the mass and also in spreading the content over the pan. The jaggery caramel, which was ready for use then, was dissolved in methanol and allowed to stand for a few minutes to remove larger impurities. The sedimented particles from both the samples were removed by filtration. Centrifugation of the supernatant at 5000 rpm was performed further to remove impurities of smaller size. The yellow coloured supernatant thus obtained was further purified by column chromatography (using silica 60–120 mesh) with methanol:dichloromethane (2:3) mixture and finally dialysis (using 1 KDa membrane) was carried out to remove salts and other ions, if any. Similar procedures were followed for the extraction of CNPs from commercially purchased cornflakes and biscuits. Caramel was also prepared in laboratory by heating commercially available sugar. Sugar (Daurala Sugar Works, India) was taken in a glass vial and heated in an oil bath at 200°C for 10 min till the solid turned brown. The brown colored sticky mass was cooled down to room temperature and was then dissolved in methanol, followed by purification using column chromatography. The sample was then concentrated by evaporating the solvent in rotary-evaporator and then it was dissolved in water and finally dialysed before further analysis.
Characterization of CNPs
The extracted CNPs were characterized using TEM (JEOL 2100 UHR-TEM), UV-vis (Perkin Elmer Lambda 25) and fluorescence spectrophotometers (FluoroMax-4, Jobin Yvon). The TEM analysis was performed at an accelerating voltage of 80 kV, unless otherwise mentioned, and the sample was prepared by drop casting 5 μL of the respective sample on a 300 mesh carbon coated copper grids and subsequent air drying before analysis. 13C NMR (100 MHz) of the dispersion was carried out in a Varian 400 MHz FT-NMR using D2O as the solvent. Thermogravimetric analysis (TGA) was performed in Q600 SDT Simultaneous DSC-TGA heat flow analyzer and powder XRD study was done using a Brucker D8 Advanced X-ray diffraction measurement system, with Cu Ka source (λ = 1.54 Å). The quantum yield (QY) was calculated using quinine sulphate in 0.10 M H2SO4 solution as a standard, at an excitation wavelength of 365 nm, and the absorbance was kept below 0.15. The QY of the samples were determined according to equation 1.
Where, QY is the quantum yield, m is the slope of the plot of integrated fluorescence intensity vs absorbance and η is the refractive index of the solvent. For the aqueous solutions ηS/ηR = 1. The subscript R refers to the reference fluorophore i.e. quinine sulphate solution and S for the sample. The values obtained are given in Table S1, supplementary information.
In vitro cytotoxicity assays
HeLa cells were obtained from National Center for Cell Sciences (NCCS), Pune, India and were cultured in Dulbecco's modified Eagle's medium supplemented with penicillin (50 units mL−1), streptomycin (50 mg mL−1), and 10% (v/v) fetal bovine serum. Cells were maintained in 5% CO2 humidified incubator at 37°C. The cells were seeded in a 96 well culture plates at a density of 5000 cells/well and were allowed to grow overnight. The CNPs were then added into the wells in a concentration range of 50 ng/mL to 2 μg/mL and incubated in a humidified incubator for 24 h at 37°C and 5% CO2. XTT (Bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) (Sigma-Aldrich, USA) based cell viability assay was carried out according to the manufacturer's protocol, to determine the percentage of viable cells. The assay is based on the metabolic activity of the cells to reduce the tetrazolium salt XTT to orange coloured compounds of formazan and the intensity of the dye is proportional to the number of metabolic active cells. The percentage cell viability of the untreated cells (control) was taken as 100%. All measurements were collected in triplicate and the values are expressed as mean ± standard error (SE). Statistical analysis for ANOVA was performed using Sigma Plot.
Author Contributions
M.P.S, A.J. and A.C. designed the experiments and analysed the data. M.P.S. and A.J. performed the experiments and A.J. and A.C. prepared the manuscript. A.P. and S.S.G. provided ideas, analyzed the data and helped in the preparation of the manuscript. All authors discussed the results.
Supplementary Material
Supplementary Information
Acknowledgments
This research was supported by the Department of Biotechnology (Nos. No. BT/49/NE/TBP/2010, and BT/01/NE/PS/08), Department of Science and Technology (SR/S1/PC-30/2008 and SR/S5/NM-108/2006). Assistances from CIF, IIT Guwahati, Prof. B. K. Patel, Ms. Dulu Rani Paul and Mr. Subhojit Das are acknowledged. M. P. S. is thankful to CSIR for a fellowship (09/731(0095)/2010-EMR-I).
References
- Sozer N. & Kokini J. L. Nanotechnology and its applications in the food sector. Trends Biotechnol. 27, 82–89 (2009). [DOI] [PubMed] [Google Scholar]
- Sanguansri P. & Augustin M. A. Nanoscale materials development a food industry perspective. Trends Food Sci. Tech. 17, 547–556 (2006). [Google Scholar]
- Duncan T. V. The communication challenges presented by nanofoods. Nat. Nanotechnol. 6, 683-688 (2011). [DOI] [PubMed] [Google Scholar]
- Bass C. Scientific American Home Page, http://www.scientificamerican.com/article.cfm?id=will-nano-particles-present-big-health-problems (accessed Dec 26 2011). [Google Scholar]
- Petros R. A. & DeSimone J. M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 9, 615–627 (2010). [DOI] [PubMed] [Google Scholar]
- Panyam J., & Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 55, 329–347 (2003). [DOI] [PubMed] [Google Scholar]
- Hong M. M., Oh J. M. & Choy J. H. Encapsulation of flavor molecules, 4-hydroxy-3-methoxy benzoic acid, into layered inorganic nanoparticles for controlled release of flavor. J. Nanosci. Nanotechnol. 8, 5018–5021 (2008). [DOI] [PubMed] [Google Scholar]
- Stark W. J. Nanoparticles in biological systems. Angew. Chem. Int. Ed. 50, 1242–1258 (2011). [DOI] [PubMed] [Google Scholar]
- Miele E., Spinelli G. P., Miele E., Tomao F. & Tomao S. Albumin-bound formulation of paclitaxel (Abraxane® ABI-007) in the treatment of breast cancer. Int. J. Nanomedicine. 4, 99–105 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawa R. Nanoparticle-based therapeutics in humans: a survey. Nanotechnology Law and Business 5, 135–155 (2008) [Google Scholar]
- Khundkar R., Malic C. & Burge T. Use of Acticoat™ dressings in burns: what is the evidence? Burns 36, 751–758 (2010). [DOI] [PubMed] [Google Scholar]
- Walter P. et al. Early use of PbS nanotechnology for an ancient hair dyeing formula. Nano Lett. 6, 2215–2219 (2006). [DOI] [PubMed] [Google Scholar]
- Barber D. J. & Frestone I. C. An investigation of the origin of the color of the Lycurgus cup by analytical transmission electron microscopy. Archaeometry 32, 33–45 (1990). [Google Scholar]
- Reibold M., Paufler P., Levin A. A., Kochmann W., Patzke N. & Meyer D. C. Materials: carbon nanotubes in an ancient Damascus sabre. Nature 444, 286 (2006). [DOI] [PubMed] [Google Scholar]
- Jaiswal A., Ghosh S. S. & Chattopadhyay A. One step synthesis of C-dots by microwave mediated caramelization of poly(ethylene glycol). Chem. Commun. 48, 407–409 (2012). [DOI] [PubMed] [Google Scholar]
- Yang S. T. et al. Carbon Dots as Nontoxic and High-Performance Fluorescence Imaging Agents. J. Phys. Chem. C 113, 18110–18114 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S. N. & Baker G. A. Luminescent carbon nanodots: emergent nanolights. Angew. Chem. Int. Ed. 49, 6726–6744 (2010). [DOI] [PubMed] [Google Scholar]
- Yang Z. C. et al. Intrinsically fluorescent carbon dots with tunable emission derived from hydrothermal treatment of glucose in the presence of monopotassium phosphate. Chem. Commun. 47, 11615–11617 (2011). [DOI] [PubMed] [Google Scholar]
- Yang Y. et al. One-step synthesis of amino-functionalized fluorescent carbon nanoparticles by hydrothermal carbonization of chitosan. Chem. Commun. 48, 380–382 (2012). [DOI] [PubMed] [Google Scholar]
- Zheng H., Wang Q., Long Y., Zhang H., Huang X. & Zhu R. Enhancing the luminescence of carbon dots with a reduction pathway. Chem. Commun. 47, 10650–10652 (2011). [DOI] [PubMed] [Google Scholar]
- Zhao Q. L., Zhang Z. L., Huang B. H., Peng J., Zhang M. & Pang D. W. Facile preparation of low cytotoxicity fluorescent carbon nanocrystals by electrooxidation of graphite. Chem. Commun. 5116–5118 (2008). [DOI] [PubMed] [Google Scholar]
- Sun Y-P. et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 128, 7756–7757 (2006). [DOI] [PubMed] [Google Scholar]
- Xu X. et al. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 126, 12736–12737 (2004). [DOI] [PubMed] [Google Scholar]
- Liu H., Ye T. & Mao C. Fluorescent carbon nanoparticles derived from candle soot. Angew. Chem. Int. Ed. 46, 6473–6475 (2007). [DOI] [PubMed] [Google Scholar]
- Zhu H., Wang X., Li Y., Wang Z., Yanga F. & Yang X. Microwave synthesis of fluorescent carbon nanoparticles with electrochemiluminescence properties. Chem. Commun. 34, 5118–5120 (2009). [DOI] [PubMed] [Google Scholar]
- Wang X., Qu K., Xu B., Ren J. & Qu X. Microwave assisted one-step green synthesis of cell-permeable multicolor photoluminescent carbon dots without surface passivation reagents. J. Mater. Chem. 21, 2445–2450 (2011). [Google Scholar]
- Eggleston G., Trask-Morrell B. J. & John R. V. Use of differential scanning calorimetry and thermogravimetric analysis to characterize the thermal degradation of crystalline sucrose and dried sucrose-salt residues. J. Agric. Food Chem. 44, 3319–3325 (1996). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information