Abstract
Recognition and management of gastrointestinal and hepatic complications of hematopoietic stem cell transplantation has gained increasing importance as indications and techniques of transplantation have expanded in the last few years. The transplant recipient is at risk for several complications including conditioning chemotherapy related toxicities, infections, bleeding, sinusoidal obstruction syndrome, acute and chronic graft-versus-host disease (GVHD) as well as other long-term problems. The severity and the incidence of many complications have improved in the past several years as the intensity of conditioning regimens has diminished and better supportive care and GVHD prevention strategies have been implemented. Transplant clinicians, however, continue to be challenged with problems arising from human leukocyte antigen-mismatched and unrelated donor transplants, expanding transplant indications and age-limit. This review describes the most commonly seen transplant related complications, focusing on their pathogenesis, differential diagnosis and management.
Keywords: Stem cell transplantation, Graft-versus-host disease, Sinusoidal obstruction syndrome, Complications
INTRODUCTION
The indications of both autologous and allogeneic hematopoietic stem cell transplantation have expanded over the past decade including for malignant and nonmalignant disorders[1,2]. Transplant clinicians routinely encounter gastrointestinal and hepatic disorders and complications before, during and after the transplant. The outcome of transplant is often closely related to how well these complications are managed. This review provides a current overview of these disorders including pathogenesis, clinical diagnosis and management.
EVALUATION OF TRANSPLANT CANDIDATE
Patients referred for hematopoietic stem cell transplantation undergo a detailed evaluation including history and physical examination which encompasses the history of disease requiring transplant as well as pre-existing conditions including dental problems, vaccination, travel, blood transfusion and infectious disease exposure history[3,4]. Imaging studies including computerized tomography (CT) with/without positron emission tomography scan or magnetic resonance imaging (MRI) can be performed to localize and restage the malignancy. Infectious disease markers including human immunodeficiency virus (HIV)-1 and (HIV)-2, human T-cell leukemia virus (HTLV)-1 and (HTLV)-2, hepatitis B virus (HBV) and hepatitis C virus serologies, cytomegalovirus (CMV), Epstein-Barr virus (EBV), herpes simplex virus (HSV)-1 and (HSV)-2, varicella zoster virus (VZV) and rubella titers are checked within 30 d prior to transplant admission. In addition to routine blood count, liver function and coagulation studies are obtained. Donors for allogeneic transplant also undergo the same serologic testing within 30 d of stem cell collection. Donors with viral hepatitis B and hepatitis C pose the risk of disease transmission to the recipient up to 30% and 100% respectively[5,6]. The risk of fatal HBV infection in recipients who become HBsAg positive is about 12%[5] By contrast, HCV transmission during transplant does not usually pose an increased short or mid-term clinical risk to the recipient yet does increase the long-term risk of cirrhosis[7]. Therefore donors should be treated with anti-viral agents; pegylated interferon α (IFN-α), famciclovir or lamivudine for hepatitis B and IFN-α plus ribavirin for hepatitis C before stem cell collection if time permits[8,9]. Of note, IFN-α should be stopped at least 1 wk before stem cell collection to avoid engraftment problems.
Patients with existing nausea, heartburn, dysphagia, abdominal pain, diarrhea, Crohn’s disease or ulcerative colitis should be investigated with endoscopy prior to transplant to rule out mucosal ulcers and infections as the risk of bleeding is increased during the periods of thrombocytopenia.
Abnormal liver enzymes and organomegaly should be investigated with ultrasound, CT or MRI. Liver biopsy is indicated in patients with positive hepatitis B surface antigen or hepatitis C antibody to rule out hepatic fibrosis or cirrhosis which increases the risk of fatal sinusoidal obstruction syndrome (SOS)[10]. Evidence of advanced liver fibrosis is a contraindication to proceed to stem cell transplantation because of excess transplant-related mortality. Pre-existing liver dysfunction can be secondary to viral hepatitis as well as alcoholic or non-alcoholic steatohepatitis, iron overload, fungal infection, chemotherapy-induced cholestatic injury or hepatocyte damage, fibrosis, extramedullary hematopoiesis or prior liver irradiation[11]. Myeloablative conditioning regimens, recent exposure to alkylating agents (especially cyclophosphamide) and exposure to newer drugs, such as imatinib and gemtuzumab ozogamicin, are known associations with increased risk of SOS[12-14].
Hepatitis B infected transplant candidates are at risk for hepatitis flare and fulminant hepatitis can occur in up to 50% of transplant recipients in the absence of antiviral prophylaxis[15,16]. In the presence of isolated HBV core antibody, observation or prophylaxis are both acceptable approaches. If HBV surface antigen is detected, it is usually recommended that prophylaxis with oral nucleoside therapy is initiated prior to transplant and that HBV DNA levels are monitored frequently during the post-transplant period[17]. Even in the absence of cirrhosis, HCV infected patients have an increased risk of SOS especially if pre-transplant aspartate aminotransferase is elevated[18]. There is no effective prophylaxis or treatment of hepatitis C for transplant recipients as pegylated IFN-α is contraindicated due to myelosuppression and has the potential to exacerbate graft-versus-host disease (GVHD). Ribavirin alone can be tried while patient is on immunosuppressive therapy.
Patients with conditions causing transfusion dependency such as myelodysplastic syndrome, leukemia, lymphoma and aplastic anemia should be screened for iron overload as excess iron can impair Kupffer cell function and increase the risk for mold infections[19]. The excess iron can be demonstrated either by quantification of iron in liver biopsy tissue or MRI of the liver. Patients with severe iron overload can benefit from chelation pre-transplant. However the urgent need for the transplant may preclude this option. The relationship between iron overload and transplant-related toxicity has not been well established and in most cases chelation can be postponed after the transplant.
NAUSEA AND VOMITING
Many chemotherapeutic agents, with or without total body irradiation used in the conditioning regimens, have significant emetogenic potential (Table 1). The pathogenesis includes stimulation of the chemotherapy trigger zone in the brainstem which activates the vomiting center by increasing efferent output to target organs in the gastrointestinal tract, resulting in subsequent emesis. Chemotherapy also causes cell damage in the gastrointestinal (GI) tract, resulting in the release of neuroactive agents and vagal stimulation, increasing afferent input to the chemotherapy trigger zone and the vomiting center in the brainstem.
Table 1.
Emetogenic potential of chemotherapeutic agents used in stem cell transplantation
| Chemotherapeutic agent | |
| High (90%) emetogenic risk | Carmustine > 250 mg/m2 |
| Cyclophosphamide > 1500 mg/m2 | |
| Moderate (30%-90%) emetogenic risk | Busulfan |
| Cytarabine > 200 mg/m2 | |
| Melphalan | |
| Low (10%-30%) emetogenic risk | Etoposide |
| Minimal (< 10%) emetogenic risk | Fludarabine |
| Rituximab |
Patients usually experience the chemotherapy related side effects during the early post-transplant (15 d) period before engraftment (Figure 1). Nausea and vomiting in later phases may be due to other potential etiologies including upper GI acute GVHD and infections such as HSV, VZV, CMV, adenovirus, fungus and Helicobacter pylori. Persistent or recurrent nausea not responsive to routine anti-emetic regimens should be investigated further for GVHD with upper GI endoscopy which may show mucosal edema and erythema and biopsy findings consistent with local lymphocytic infiltrates and epithelial apoptosis[20,21]. Specimens should also be studied for bacterial or fungal cultures, HSV and CMV infections as viral infections can be detected in the GI tract without the presence of virus DNA in serum. Other common medical etiologies such as medication intolerance, gastroparesis, intestinal obstruction, intraabdominal infections, neurologic and metabolic causes should also be considered.
Figure 1.
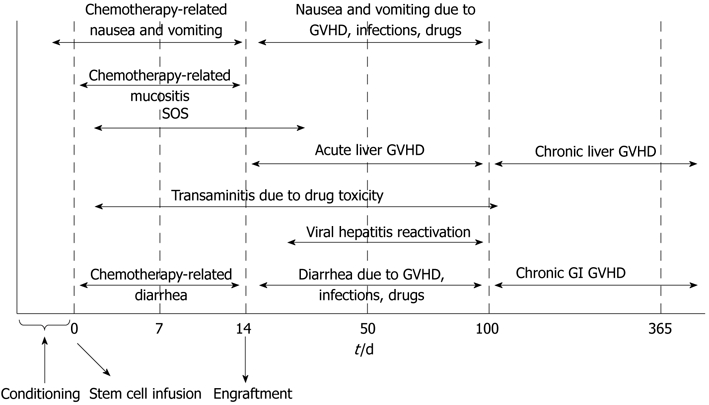
Timeline summary of hepatic and gastrointestinal complications of stem cell transplantation. GVHD: Graft-versus-host disease; SOS: Sinusoidal obstruction syndrome; GI: Gastrointestinal.
Prevention is the key to success in managing nausea and vomiting during the peri-transplant period[22]. Acute emesis prevention (up to 24 h after chemotherapy) can be achieved with a combination of corticosteroids (dexamethasone 10-20 mg iv/po daily or methylprednisolone 40-125 mg iv/po daily) and 5-hydroxytryptamine-3 receptor antagonists (ondansetron 16-24 mg iv/po daily or granisetron 1-2 mg daily). Delayed emesis (up to 5 d after treatment) can usually be prevented with corticosteroids. Aprepitant neurokinin-1 antagonist is an effective agent for this purpose; however it may interact with several post-transplant immunosuppressive agents and therefore is sparingly and cautiously used, especially in the allogeneic stem cell transplant setting.
Treatment options for breakthrough nausea and vomiting include phenothiazines (prochlorperazine, promethazine), metoclopramide, lorazepam, haloperidol, dronabinol and corticosteroids[23].
OROPHARYNGEAL MUCOSITIS AND DYSPHAGIA
Breakdown of mucosal barrier presenting as mucositis is a common complication during the early post-transplant period. It affects up to 80% of transplant recipients, especially with radiation-based myeloablative regimens[24]. Chemotherapeutic agents commonly causing mucositis include busulfan, etoposide, melphalan and methotrexate. Pre-existing periodontal disease and prior radiation to the head and neck area increase the risk of post-transplant complications. Mucositis can result in significant oral pain and dysphagia, decreased oral caloric intake as well as bleeding, infection, upper airway edema and obstruction. Clinically apparent mucositis usually starts 5-10 d after initiation of the conditioning regimen (Figure 1). Initial erythema and atrophy is followed by ulceration and healing phases. It may take up to two weeks for healing of chemotherapy-induced mucositis.
Infectious causes of mucositis include CMV, HSV, VZV, varicella zoster virus, Candida species and bacterial pathogens. The incidence of viral and fungal infections has been significantly lower since the standardization of antiviral and antifungal prophylaxis regimens. Other noninfectious causes of dysphagia should be included in the differential diagnosis such as acid-reflux disease, pill esophagitis and acute and chronic GVHD with esophageal strictures.
Prevention and early treatment is critical to minimize the duration and severity of symptoms. Frequent mouth rinsing with topical agents and oral cryotherapy with ice chips are started with chemotherapy and continued until engraftment. Keratinocyte growth factor (palifermin) has been shown to decrease the incidence of mucositis by 40% in patients receiving autologous stem cell transplant with aggressive total body irradiation (TBI)-based regimens[25,26]. It is administered iv for 3 d before and after cytotoxic therapy. Other supportive measures include saline and bicarbonate rinses, mucosal coating agents (such as aluminum hydroxide), topical anesthetics such as lidocaine rinse and/or narcotic analgesia, topical nystatin for signs of candidiasis and proton-pump inhibitor prophylaxis. Total parenteral nutrition should be considered for patients who are unable to tolerate oral supplementation for more than 7 d.
DIARRHEA
Diarrhea occurs in almost half of patients receiving high-dose chemotherapy conditioning and radiotherapy. It is most commonly associated with toxicity of conditioning regimens within the first 2 wk after transplant (Figure 1). Alkylating agents, busulfan and combination regimens are frequent etiologies and cause diarrhea due to mucosal inflammation. Several other etiologies should be considered in patients having diarrhea in the post-transplant period (Table 2). Acute GVHD is the most common reason for diarrhea after engraftment (> 15 d) in allogeneic transplants[27]; persistent or new diarrhea beyond 3 wk of transplant should be investigated for GVHD. The diarrhea with GVHD can be watery, mucoid and in large volumes; can be accompanied by vomiting, gastrointestinal bleeding and severe abdominal pain[28]. Infectious etiologies account for only 10%-15% of cases, yet diarrhea at any time after transplant should still prompt obtaining stool studies for Clostridium difficile toxin[29,30] as well as bacterial, viral and parasitic cultures if indicated. Abdominal imaging with CT may show bowel wall edema and/or pneumatosis intestinalis which may be associated with either GVHD or CMV infection (Figure 2). If cultures are negative, patients are usually treated with loperamide 4 mg po once followed by 2 mg/24 h as needed up to 24 mg/24 h. If diarrhea persists, strategies include scheduling loperamide every 4-6 h, adding atropine and diphenoxylate or tincture of opium. Octreotide starting at 150 mg iv every 8 h can be considered for protracted cases and can be titrated to response[31]. Other critical measures include maintaining adequate hydration and electrolyte supplementation, treating infections, discontinuation of medications causing diarrhea and assessment of nutritional status. Persistent symptoms despite the above measures and/or new diarrhea presenting after engraftment should be investigated with endoscopy and biopsy.
Table 2.
Differential diagnosis of post-transplant diarrhea
| Conditioning regimen-related | |
| Acute GVHD | |
| Drug toxicity | |
| Antibiotic-related | |
| Opioid withdrawal | |
| Mycophenolate mofetil toxicity | |
| Tacrolimus (thrombotic microangiopathy) | |
| Proton pump inhibitors | |
| Promotility agents | |
| Magnesium salts | |
| Metoclopramide | |
| Infectious | |
| Clostridium difficile | |
| CMV | |
| Rotavirus | |
| Adenovirus | |
| EBV | |
| HSV | |
| Astrovirus | |
| Norovirus | |
| Bacterial infections including ESBL | |
| Fungal infections | |
| Parasitic infections (Cryptosporidium, Microsporidia, Giardia) | |
| Mycobacterial infections | |
| Others | |
| Lactose intolerance | |
| Malabsorption | |
| Pancreatic insufficiency | |
CMV: Cytomegalovirus; EBV: Epstein-Barr virus; HSV: Herpes simplex virus; GVHD: Graft-versus-host disease; ESBL: Extended spectrum β lactamase.
Figure 2.
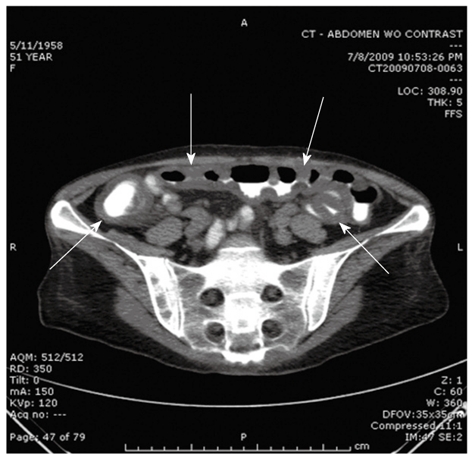
Bowel wall edema in a patient with gastrointestinal graft-versus-host disease.
Visual findings of acute GVHD may include mucosal edema/erythema and ulceration/bleeding. The diagnostic yield is the best when biopsies are obtained either from the stomach and distal colon or from the colon and ileum[32]. Histologic findings include crypt epithelial cell apoptosis and dropout, crypt destruction (Figure 3), pericapillary hemorrhage and variable lymphocytic and eosinophilic infiltration of the epithelium and lamina propria[33]. It is also important to study the biopsy specimens for CMV involvement which is the only common infectious cause of enteritis after transplant that requires biopsy for diagnosis. The negative predictive value of other infectious studies in stools is high and therefore usually does not necessitate endoscopy.
Figure 3.
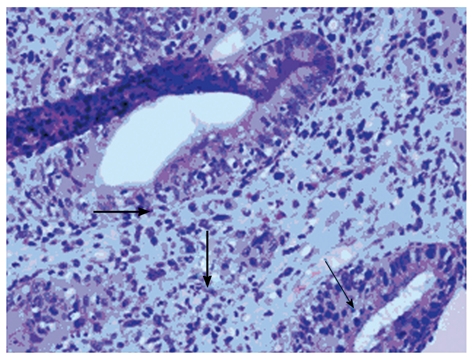
Histologic findings of acute graft-versus-host disease of the colon (hematoxylin and eosin stain, x 400). Thin arrow marks apoptotic bodies; thick arrow marks pericryptal acute inflammation.
The severity of acute GVHD is clinically determined by the amount of diarrhea which helps defining the staging and grading (Tables 3 and 4). In addition to the general management measures described above, patients with high clinical suspicion for ≥ grade II acute GVHD or biopsy proven acute GVHD should be promptly started on high dose steroids[34]; methylprednisolone 0.5-2.0 mg/kg per day iv in addition to their existing GVHD prophylaxis regimens. Half of the patients respond to steroid treatment[35] which can be tapered starting after approximately 1 wk. Patients who do not respond to steroids tend to have a poor prognosis; several second line immunosuppressive agents have been tried with variable success[34]. Other supportive measures consist of addition of oral non-absorbable steroids (budesonide)[36] and cholestyramine as well as dietary adjustments (bowel rest and starting total parenteral nutrition) particularly for moderate to severe diarrhea.
Table 3.
Staging of acute graft-versus-host disease (modified Keystone criteria)
| Stage | Intestinal tract | Liver | Skin |
| 0 | Diarrhea ≤ 500 mL/d | Bilirubin < 2.0 mg/dL | No rash |
| 1 | Diarrhea 501-1000 mL/d or nausea (± vomiting) | Bilirubin 2.0-3.0 mg/dL | Maculopapular rash < 25% of body surface |
| 2 | Diarrhea 1001-1500 mL/d | Bilirubin 3.1–6.0 mg/dL | Maculopapular rash 25%-50% of body surface |
| 3 | Diarrhea > 1501 mL/d | Bilirubin 6.1–15 mg/dL | Generalized erythroderma |
| 4 | Severe abdominal pain +/- ileus | Bilirubin > 15 mg/dL | Generalized erythroderma with blister/bullous formation and desquamation |
Table 4.
Grading of acute graft-versus-host disease (modified Keystone criteria)
| Grade | Gut | Liver | Skin |
| 0 (none) | 0 | 0 | 0 |
| I(mild) | 0 | 0 | 1-2 |
| II (moderate) | 1 | 1 or | 3 or |
| III (severe) | 2-4 | 2-3 or | 0-3 |
| IV (life-threatening) | 4 | 4 or |
GASTROINTESTINAL BLEEDING
The incidence of bleeding has been significantly reduced (1%-2%) as post-transplant care has improved with routine anti-viral, anti-fungal and GVHD prophylaxis, yet it remains one of the major causes of transplant related mortality, particularly among patients with GVHD[37,38]. Common infectious etiologies include CMV, VZV, adenovirus, fungal and clostridial infections. Mucosal necrosis from conditioning therapy, acute and chronic GVHD, peptic ulcer disease, mycophenolate-related ulcerations and gastric antral vascular ectasia (GAVE) are the common known etiologies of noninfectious causes of bleeding. Treatment is mainly focused on supportive care (platelet transfusion and continuous octreotide infusion) as well as implementing prophylaxis for viral infections and GVHD. Treating the underlying cause is important as the benefit of endoscopic methods alone is limited to focal lesions.
LIVER FUNCTION ABNORMALITIES AND JAUNDICE
Severe liver dysfunction after transplant (total serum bilirubin > 4 mg/dL) may be an indicator of poor outcome as there is often no curative treatment[39]. Therefore, efforts are usually geared towards identifying the transplant candidates at risk as well as routine implementation of prophylactic measures such as ursodiol[40] (through 80 d post transplant), viral and fungal prophylaxis and careful selection of conditioning regimens to minimize hepatotoxicity[41]. The incidence of liver-related complications has declined significantly over the past decade with the preventive measures integrated into standard care. Some common etiologies for liver function abnormalities after transplant are summarized in Table 5. Drug toxicity, sepsis, GVHD and SOS are the most common etiologies for liver dysfunction. Calcineurin inhibitors (cyclosporine and less commonly tacrolimus, sirolimus), azole antifungal agents, trimethoprim-sulfamethoxazole, ribavirin, busulfan and bis-chloroethylnitrosourea are commonly associated with cholestasis. A declining incidence of acute GVHD is observed in 20%-25% of allogeneic transplant recipients due to widespread use of prophylactic immunosuppressive drugs, with peak incidence after engraftment (day 15) until the first 100 d of transplant[42]. It typically follows skin and/or GI GVHD and manifests by progressive parallel elevations of serum bilirubin and alkaline phosphatase; serum aminotransferase enzymes are elevated up to 10 times the upper limit of normal (Tables 3 and 4). Hepatitic-variant of GVHD has also been described where serum aminotransferase enzymes are elevated more than 10 times the upper limit of normal and clinical presentation resembles acute viral hepatitis[43]. The diagnosis is usually made by transjugular liver biopsy which typically reveals lymphocytic infiltration of small bile ducts with epithelial cell apoptosis[44]. Routine ursodiol prophylaxis is usually continued through day 80 of allogeneic transplants and reduces the incidence of GVHD[45]. The initial treatment of acute hepatic GVHD is similar to cutaneous and GI GVHD with high dose steroids as described above. However, only 30%-50% of patients respond to initial treatment and half of patients develop chronic GVHD.
Table 5.
Differential diagnosis for liver function abnormalities after hematopoietic stem cell transplantation
| First 3 wk post-transplant |
| Drug toxicity |
| Conditioning regimens (cyclophosphamide, total body irradiation, bis-chloroethylnitrosourea, busulfan) |
| Calcineurin inhibitors |
| Azole antifungals |
| SOS |
| Sepsis, candidiasis |
| Ischemic liver disease |
| From 3 wk to 3 mo post-transplant |
| Acute GVHD |
| Drug toxicity |
| SOS |
| Hepatitis (fulminant, acute or chronic): |
| Viral (HBV, HCV, HSV, VZV, adenovirus) reactivation |
| Bacterial or fungal Infection |
| Fungal abscess |
| Gall bladder disease/cholecystitis |
| Hyperalimentation |
| Post-transplant lymphoproliferative disorder (EBV-related) |
| After 3 mo post-transplant |
| Chronic GVHD |
| Iron overload |
| Chronic viral hepatitis |
| Drug toxicity |
| Liver fibrosis or cirrhosis: |
| SOS |
| Viral infections |
| Hemosiderosis |
| Disease recurrence or new malignancy including hepatocellular carcinoma, lymphoproliferative disorder |
| Nodular regenerative hyperplasia |
| Gallbladder disease |
GVHD: Graft-versus-host disease; SOS: Sinusoidal obstruction syndrome; EBV: Epstein-Barr virus; HBV: Hepatitis B virus; HCV: Hepatitis C virus; HSV: Herpes simplex virus; VZV: Varicella zoster virus.
SINUSOIDAL OBSTRUCTION SYNDROME
SOS (AKA veno-occlusive disease or VOD) is a clinical entity characterized by tender hepatomegaly, elevated serum bilirubin levels and weight gain which typically complicates myeloablative hematopoietic stem cell transplantation (HSCT) and was first described in 1979[46]. SOS is a well-recognized conditioning-related toxicity. The incidence is quite variable, ranging from less than 5% to as high as 70% in different reports[47,48].
Endothelial injury appears to be the initiating event triggering the hepatic changes and clinical manifestation of SOS. The prevailing hypothesis centers on damage to the hepatic venular and sinusoidal endothelium as an initial trigger inducing a hypercoagulable state by activation of the coagulation cascade, favoring clot formation over natural anticoagulation[49,50]. As a result, the venular and sinusoidal lumen is reduced due to an edematous concentric subendothelial zone containing fragmented red cells and fibrillar material, inducing partial to complete fibrotic obliteration of the venular lamina.
There are several risk factors for SOS and patients may present with elevated transaminase levels prior to the conditioning regimen due to various pre-existing conditions (Table 6). Previous cumulative exposure to high doses of cytotoxic agents may contribute to these risks including a second HSCT. Infection with hepatitis B is not considered a risk factor alone for SOS unless it is complicated with cirrhosis. The relationship between HCV infection and SOS is somewhat controversial. One report supports the increased risk even in the absence of cirrhosis[18] while another cohort did not confirm the association although there was an increased long term risk of non-relapse mortality for transplant patients who had chronic hepatitis C[51]. Other risk factors include certain conditioning regimens such as cyclophosphamide, busulfan, and/or total body irradiation[52-54].
Table 6.
Risk factors for sinusoidal obstruction syndrome
| Existing liver disease: |
| Chronic viral hepatitis |
| Alcohol related hepatitis |
| Steatohepatitis |
| Cirrhosis, lobular fibrosis |
| Cholestatic disorders |
| Extramedullary hematopoiesis with sinusoidal fibrosis |
| Prior history of: |
| SOS |
| Extensive chemotherapy and stem cell transplantation |
| Hepatic radiation |
| Drugs |
| Recent gemtuzumab ozogamicin use |
| Conditioning agents: |
| High dose TBI (> 14 Gy) |
| Cyclophosphamide metabolite: acrolein |
| Busulfan |
| Melphalan |
| Concomitant use of sirolimus during conditioning |
SOS: Sinusoidal obstruction syndrome; TBI: Total body irradiation.
Cyclophosphamide is a common conditioning agent with the highest incidence of SOS, which becomes a particular concern in regimens combined with TBI and busulfan. The hepatotoxicity is usually dependent on the toxic metabolite acrolein and the exposure to toxic metabolites can be minimized by metabolism-based dosing. The incidence of SOS seems to be higher in transplant recipients who receive TBI doses over 14 Gy[53]. Various fractionated schedules of TBI have been associated with decreased incidence. Increasing the interval between TBI and cytotoxic therapy also may decrease the risk. Busulfan exposure, on the other hand, is not proven to be directly related to SOS although it potentiates the toxicity of cyclophosphamide especially when it is administered after this drug[54]. Oral busulfan has a variable and unpredictable absorption and studies have shown that the risk of SOS increases when the area under the curve for busulfan is greater than 1500 μmol/min. When busulfan is adjusted to normal drug levels by close monitoring, a decreased incidence has been reported[54].
Gemtuzumab ozogamicin may induce sinusoidal injury[55] (15%-40% risk) especially if it is administered preceding a cyclophosphamide-based conditioning regimen.
Most cases are observed within the first 3 wk after transplantation. Usually, an unexplained weight gain is the first symptom. This weight gain, attributable to water and sodium retention by the kidney, appears within 6 d to 8 d following the transplant in 95% of patients. This is often followed by varying degrees of hyperbilirubinemia and elevation in aspartate aminotransferase and alkaline phosphatase levels. Most patients develop ascites and pain in the upper right quadrant, and clinical examination usually reveals a firm and painful hepatomegaly. Platelet refractoriness is a common occurrence[56]. Renal insufficiency in the form of hepatorenal syndrome is also present in 50% of patients developing SOS (mainly patients with severe form) and 25% of them will require hemodialysis. Finally, patients with advanced disease can display severe encephalopathy and/or multiorgan failure.
Doppler ultrasound of the liver usually shows reversal of portal and/or hepatic venous flow in severe cases. Most patients are diagnosed on clinical basis, given the risk of liver biopsy in the setting of coagulopathy and platelet refractoriness. Nevertheless where feasible, the clinical suspicion should be confirmed by transjugular liver biopsy which is the gold standard for diagnosis. Hepatic venous pressure gradients are often measured at the time of biopsy; a gradient greater than 10 mmHg is highly specific for SOS[57] and correlates with worse prognosis.
Given the very high mortality rate in patients with severe SOS, it is critical to implement preventive measures such as ursodeoxycholic acid which has been shown to reduce the incidence of SOS[40]. The efficacy of low-dose heparin has not been confirmed and is usually not part of standard management.
Up to 70% of patients with SOS will recover spontaneously and the focus of treatment is supportive care such as maintaining intravascular volume and renal perfusion without causing fluid overload by optimizing sodium restriction and diuretics and transfusions to keep hematocrit levels higher than 40%. The role of albumin or other colloids is unclear but could be considered in patients with severe hypoalbuminemia and large third space fluid accumulations. Low-dose dopamine has been used in patients with renal insufficiency because the mechanism of renal dysfunction appears to be hepatorenal in origin. Avoidance of other hepatotoxic drugs is important in these patients, and infections should be identified and treated promptly. Therapeutic paracentesis can help relieve symptoms in patients with large, tense ascites and may help improve renal function. Also, use of hemodialysis or continuous venous hemofiltration is reported to help with fluid overload in patients with a poor response to diuretics.
There are no effective established treatments for patients with severe SOS characterized by rapidly increasing serum bilirubin and transaminase levels, portal vein thrombosis and multiorgan failure. Several antithrombotic agents have been tested with mixed results. Defibrotide which is an antithrombotic agent without significant systemic effects and with a manageable side effect profile, has been reported to improve signs and symptoms of SOS in 42% of patients[58]. Its mechanism of action is poorly understood. Other tested agents include prostaglandin E1 and tissue plasminogen activator with or without concurrent heparin, intravenous N-acetylcysteine, human antithrombin III concentrate, activated protein C and prednisone. None of these approaches are considered a part of standard management.
LONG-TERM COMPLICATIONS
Chronic GVHD
Long-term survivors of stem cell transplantation are at increased risk of several serious complications related to chronic GVHD which may affect liver, GI tract, skin, mucosal surfaces, lungs, joints, eyes and bone marrow; these patients should be followed regularly. Complications may occur in up to 50% of transplant recipients. Liver GVHD usually manifests with progressive or sudden elevation of alkaline phosphatase and gamma glutamyl transpeptidase. Hyperbilirubinemia is usually a late manifestation that coincides with development of cirrhosis and findings of small bile destruction in biopsy[44]. Viral etiologies should be excluded and liver biopsy should be performed to establish the diagnosis, followed by initiation of immunosuppressive therapy which usually includes steroids with or without a calcineurin inhibitor. An improvement in liver function studies is usually observed within four weeks of treatment and 50%-80% of patients respond to the initial therapy with improvement in histopathologic findings. Addition of ursodeoxycholic acid should also be considered and this is usually well tolerated. The prognosis of patients who do not respond to immunosuppressive regimens is poor and correlates with shortened survival[59].
Chronic GVHD may affect several parts of the GI tract, causing esophageal webs and strictures leading to dysphagia, failure to thrive and chronic aspiration. Early lesions can be reversible with immunosuppression, proton pump inhibitors and dilatation. Patients may also experience chronic intermittent diarrhea, clinically and histologically similar to acute GVHD, which may respond to non-absorbable steroids. Chronic malabsorption is rare and is usually a result of long term inadequate treatment.
Chronic viral hepatitis and cirrhosis
Longstanding viral hepatitis C and B can lead to end-stage liver disease in transplant survivors. Rate of progression to cirrhosis in patients with chronic hepatitis B is comparable to non-transplant patients whereas the incidence of cirrhosis in transplant patients with chronic hepatitis C infection seems to be higher than controls and it can be as high as 24% after 20 years[60]. Otherwise these patients are also at risk of developing hepatocellular carcinoma (HCC) (2%-8% per year) and lymphoproliferative disorders. Patients with chronic hepatitis C should therefore be routinely monitored for viral load and considered for combination therapy with ribavirin and pegylated IFN-α. They should also be screened for HCC with α-feto-protein and ultrasound every 6 mo. Close monitoring is essential for treatment complications such as neutropenia and thrombocytopenia secondary to pegylated IFN-α or exacerbation of coexisting chronic GVHD. Screening and treatment of iron overload may augment the success of anti-viral therapy. Liver transplantation for end-stage liver disease or HCC can be considered, especially from the original stem cell donor[61].
Patients with chronic hepatitis B may have atypical serologic course due to immunosuppression. They may benefit from clearance of antigenemia particularly if the donor has natural HBV immunity. Patients should be monitored for HBV DNA levels and alanine transaminase levels and considered for antiviral treatment at times of tapering of immunosuppressive therapy as well as initiation of new chemotherapy, as they are at risk of flares of hepatitis[62].
Iron overload
The etiology of iron overload in transplant survivors is usually multifactorial, including transfusion dependency and abnormal iron transport by the intestine due to bone marrow dysfunction. It can be an important contributor to chronic liver disease and should be considered in the differential diagnosis[63]. It affects cardiac, endocrine and pancreatic function as well as increasing the risk of opportunistic infections. HFE gene testing should be considered when patients have unexpectedly high levels of iron stores. Clinically significant iron overload usually occurs when serum ferritin exceeds 1000 μg/dL[64]. In the presence of other inflammatory conditions such as chronic GVHD, serum ferritin levels may be falsely elevated. Liver biopsy or noninvasive methods such as liver MRI or FerriScan can be utilized to document the severity of iron overload. Patients with severe iron overload may benefit from mobilization with improved hepatic and cardiac function[65]. If liver iron content is greater than 15 000 μg/g dry weight, both phlebotomy and chelation should be offered. The liver iron content of 7000-15 000 μg/g dry weight should be treated with phlebotomy only and if it is less than 7000 μg/g dry weight, treatment is needed only if there is liver disease[66].
Acute hepatocellular injury
Long term transplant survivors may present with acute elevations of transaminases. The differential diagnosis should include flares of chronic viral hepatitis, hepatitis presentation of chronic GVHD, VZV, HSV infection or drug-induced (antihypertensives, statins, hypoglycemic agents, antibiotics) liver injury.
Malignancies
The risk of new malignancies among transplant survivors increases significantly after 10 years. Patients with chronic hepatitis C have accelerated incidence of HCC[67] and lymphomas[68].
CONCLUSION
Gastrointestinal and hepatic complications count for a significant part of the morbidity during and after hematopoietic stem cell transplant. Recent advances in transplant approaches have changed the outcome and the post-transplant course of many patients[69]. As most transplant survivors are affected by multiple complications, it is imperative that they should receive long-term and systematic follow-up without compromising from individualized care.
Footnotes
Peer reviewers: Silvana Zanlungo, Professor, Departamento de Gastroenterología, Pontificia Universidad Católica de Chile, Marcoleta 367, Casilla 114-D, Santiago, Chile; Naoaki Sakata, MD, PhD, Division of Hepato-Biliary Pancreatic Surgery, Tohoku University Graduate School of Medicine, 1-1 Seiryo-machi, Aoba-ku, Sendai, Miyagi 980-8574, Japan
S- Editor Tian L L- Editor O’Neill M E- Editor Zhang DN
References
- 1.Gratwohl A, Baldomero H, Aljurf M, Pasquini MC, Bouzas LF, Yoshimi A, Szer J, Lipton J, Schwendener A, Gratwohl M, et al. Hematopoietic stem cell transplantation: a global perspective. JAMA. 2010;303:1617–1624. doi: 10.1001/jama.2010.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burt RK, Loh Y, Pearce W, Beohar N, Barr WG, Craig R, Wen Y, Rapp JA, Kessler J. Clinical applications of blood-derived and marrow-derived stem cells for nonmalignant diseases. JAMA. 2008;299:925–936. doi: 10.1001/jama.299.8.925. [DOI] [PubMed] [Google Scholar]
- 3.FACT-JACIE International Standards for Cellular Therapy Product Collection, Processing and Administration. 4th ed. Foundation for the Accreditation of Hematopoietic Cell Therapy. Omaha: Donor Evaluation and Management; 2008. pp. 98–105. [Google Scholar]
- 4.Confer DL, Miller JP, Chell JW. Bone marrow and peripheral blood cell donors and donor registries. 4th ed. Thomas’ Hematopoeitic Cell Transplantation. Oxford: Blackwell Publishing; 2009. pp. 544–554. [Google Scholar]
- 5.Arai S, Lee LA, Vogelsang GB. A systematic approach to hepatic complications in hematopoietic stem cell transplantation. J Hematother Stem Cell Res. 2002;11:215–229. doi: 10.1089/152581602753658420. [DOI] [PubMed] [Google Scholar]
- 6.Shuhart MC, Myerson D, Childs BH, Fingeroth JD, Perry JJ, Snyder DS, Spurgeon CL, Bevan CA, McDonald GB. Marrow transplantation from hepatitis C virus seropositive donors: transmission rate and clinical course. Blood. 1994;84:3229–3235. [PubMed] [Google Scholar]
- 7.Peffault de Latour R, Lévy V, Asselah T, Marcellin P, Scieux C, Adès L, Traineau R, Devergie A, Ribaud P, Espérou H, et al. Long-term outcome of hepatitis C infection after bone marrow transplantation. Blood. 2004;103:1618–1624. doi: 10.1182/blood-2003-06-2145. [DOI] [PubMed] [Google Scholar]
- 8.Hui CK, Lie A, Au WY, Ma SY, Leung YH, Zhang HY, Sun J, Cheung WW, Chim CS, Kwong YL, et al. Effectiveness of prophylactic Anti-HBV therapy in allogeneic hematopoietic stem cell transplantation with HBsAg positive donors. Am J Transplant. 2005;5:1437–1445. doi: 10.1111/j.1600-6143.2005.00887.x. [DOI] [PubMed] [Google Scholar]
- 9.Vance EA, Soiffer RJ, McDonald GB, Myerson D, Fingeroth J, Ritz J. Prevention of transmission of hepatitis C virus in bone marrow transplantation by treating the donor with alpha-interferon. Transplantation. 1996;62:1358–1360. doi: 10.1097/00007890-199611150-00032. [DOI] [PubMed] [Google Scholar]
- 10.Strasser SI, McDonald GB. Hepatitis viruses and hematopoietic cell transplantation: A guide to patient and donor management. Blood. 1999;93:1127–1136. [PubMed] [Google Scholar]
- 11.El-Sayed MH, El-Haddad A, Fahmy OA, Salama II, Mahmoud HK. Liver disease is a major cause of mortality following allogeneic bone-marrow transplantation. Eur J Gastroenterol Hepatol. 2004;16:1347–1354. doi: 10.1097/00042737-200412000-00019. [DOI] [PubMed] [Google Scholar]
- 12.McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M, Hardin BJ, Shulman HM, Clift RA. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118:255–267. doi: 10.7326/0003-4819-118-4-199302150-00003. [DOI] [PubMed] [Google Scholar]
- 13.Ayoub WS, Geller SA, Tran T, Martin P, Vierling JM, Poordad FF. Imatinib (Gleevec)-induced hepatotoxicity. J Clin Gastroenterol. 2005;39:75–77. [PubMed] [Google Scholar]
- 14.Wadleigh M, Richardson PG, Zahrieh D, Lee SJ, Cutler C, Ho V, Alyea EP, Antin JH, Stone RM, Soiffer RJ, et al. Prior gemtuzumab ozogamicin exposure significantly increases the risk of veno-occlusive disease in patients who undergo myeloablative allogeneic stem cell transplantation. Blood. 2003;102:1578–1582. doi: 10.1182/blood-2003-01-0255. [DOI] [PubMed] [Google Scholar]
- 15.Hammond SP, Borchelt AM, Ukomadu C, Ho VT, Baden LR, Marty FM. Hepatitis B virus reactivation following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:1049–1059. doi: 10.1016/j.bbmt.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Lalazar G, Rund D, Shouval D. Screening, prevention and treatment of viral hepatitis B reactivation in patients with haematological malignancies. Br J Haematol. 2007;136:699–712. doi: 10.1111/j.1365-2141.2006.06465.x. [DOI] [PubMed] [Google Scholar]
- 17.Liang R. How I treat and monitor viral hepatitis B infection in patients receiving intensive immunosuppressive therapies or undergoing hematopoietic stem cell transplantation. Blood. 2009;113:3147–3153. doi: 10.1182/blood-2008-10-163493. [DOI] [PubMed] [Google Scholar]
- 18.Strasser SI, Myerson D, Spurgeon CL, Sullivan KM, Storer B, Schoch HG, Kim S, Flowers ME, McDonald GB. Hepatitis C virus infection and bone marrow transplantation: a cohort study with 10-year follow-up. Hepatology. 1999;29:1893–1899. doi: 10.1002/hep.510290609. [DOI] [PubMed] [Google Scholar]
- 19.Kontoyiannis DP, Chamilos G, Lewis RE, Giralt S, Cortes J, Raad II, Manning JT, Han X. Increased bone marrow iron stores is an independent risk factor for invasive aspergillosis in patients with high-risk hematologic malignancies and recipients of allogeneic hematopoietic stem cell transplantation. Cancer. 2007;110:1303–1306. doi: 10.1002/cncr.22909. [DOI] [PubMed] [Google Scholar]
- 20.Weisdorf DJ, Snover DC, Haake R, Miller WJ, McGlave PB, Blazar B, Ramsay NK, Kersey JH, Filipovich A. Acute upper gastrointestinal graft-versus-host disease: clinical significance and response to immunosuppressive therapy. Blood. 1990;76:624–629. [PubMed] [Google Scholar]
- 21.Roy J, Snover D, Weisdorf S, Mulvahill A, Filipovich A, Weisdorf D. Simultaneous upper and lower endoscopic biopsy in the diagnosis of intestinal graft-versus-host disease. Transplantation. 1991;51:642–646. doi: 10.1097/00007890-199103000-00019. [DOI] [PubMed] [Google Scholar]
- 22.Trigg ME, Inverso DM. Nausea and vomiting with high-dose chemotherapy and stem cell rescue therapy: a review of antiemetic regimens. Bone Marrow Transplant. 2008;42:501–506. doi: 10.1038/bmt.2008.257. [DOI] [PubMed] [Google Scholar]
- 23.Ettinger DM, Armstrong D, Barbour S, Berger MJ, Bierman PJ, Bradbury B, Ellis G, Kirkegaard S, Kloth DD, Kris MG, Lim D, Noonan K, Rugo HS, Siler D, Sorscher SM, Stucky-Marshall L, Torado B, Urba S. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Antiemesis Version 1.2010. Available from: http//www.nccn.org. [DOI] [PubMed] [Google Scholar]
- 24.Vera-Llonch M, Oster G, Ford CM, Lu J, Sonis S. Oral mucositis and outcomes of allogeneic hematopoietic stem-cell transplantation in patients with hematologic malignancies. Support Care Cancer. 2007;15:491–496. doi: 10.1007/s00520-006-0176-9. [DOI] [PubMed] [Google Scholar]
- 25.Epstein JB, Schubert MM. Managing pain in mucositis. Semin Oncol Nurs. 2004;20:30–37. doi: 10.1053/j.soncn.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Langner S, Staber P, Schub N, Gramatzki M, Grothe W, Behre G, Rabitsch W, Urban C, Linkesch W, Neumeister P. Palifermin reduces incidence and severity of oral mucositis in allogeneic stem-cell transplant recipients. Bone Marrow Transplant. 2008;42:275–279. doi: 10.1038/bmt.2008.157. [DOI] [PubMed] [Google Scholar]
- 27.Cox GJ, Matsui SM, Lo RS, Hinds M, Bowden RA, Hackman RC, Meyer WG, Mori M, Tarr PI, Oshiro LS. Etiology and outcome of diarrhea after marrow transplantation: a prospective study. Gastroenterology. 1994;107:1398–1407. doi: 10.1016/0016-5085(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 28.Ferrara JL, Deeg HJ. Graft-versus-host disease. N Engl J Med. 1991;324:667–674. doi: 10.1056/NEJM199103073241005. [DOI] [PubMed] [Google Scholar]
- 29.Arango JI, Restrepo A, Schneider DL, Callander NS, Ochoa-Bayona JL, Restrepo MI, Bradshaw P, Patterson J, Freytes CO. Incidence of Clostridium difficile-associated diarrhea before and after autologous peripheral blood stem cell transplantation for lymphoma and multiple myeloma. Bone Marrow Transplant. 2006;37:517–521. doi: 10.1038/sj.bmt.1705269. [DOI] [PubMed] [Google Scholar]
- 30.Tomblyn M, Gordon L, Singhal S, Tallman M, Williams S, Winter J, Mehta J. Rarity of toxigenic Clostridium difficile infections after hematopoietic stem cell transplantation: implications for symptomatic management of diarrhea. Bone Marrow Transplant. 2002;30:517–519. doi: 10.1038/sj.bmt.1703703. [DOI] [PubMed] [Google Scholar]
- 31.Ippoliti C, Champlin R, Bugazia N, Przepiorka D, Neumann J, Giralt S, Khouri I, Gajewski J. Use of octreotide in the symptomatic management of diarrhea induced by graft-versus-host disease in patients with hematologic malignancies. J Clin Oncol. 1997;15:3350–3354. doi: 10.1200/JCO.1997.15.11.3350. [DOI] [PubMed] [Google Scholar]
- 32.Thompson B, Salzman D, Steinhauer J, Lazenby AJ, Wilcox CM. Prospective endoscopic evaluation for gastrointestinal graft-versus-host disease: determination of the best diagnostic approach. Bone Marrow Transplant. 2006;38:371–376. doi: 10.1038/sj.bmt.1705453. [DOI] [PubMed] [Google Scholar]
- 33.Ponec RJ, Hackman RC, McDonald GB. Endoscopic and histologic diagnosis of intestinal graft-versus-host disease after marrow transplantation. Gastrointest Endosc. 1999;49:612–621. doi: 10.1016/s0016-5107(99)70390-1. [DOI] [PubMed] [Google Scholar]
- 34.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacMillan ML, Weisdorf DJ, Wagner JE, DeFor TE, Burns LJ, Ramsay NK, Davies SM, Blazar BR. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8:387–394. doi: 10.1053/bbmt.2002.v8.pm12171485. [DOI] [PubMed] [Google Scholar]
- 36.Hockenbery DM, Cruickshank S, Rodell TC, Gooley T, Schuening F, Rowley S, David D, Brunvand M, Berryman B, Abhyankar S, et al. A randomized, placebo-controlled trial of oral beclomethasone dipropionate as a prednisone-sparing therapy for gastrointestinal graft-versus-host disease. Blood. 2007;109:4557–4563. doi: 10.1182/blood-2006-05-021139. [DOI] [PubMed] [Google Scholar]
- 37.Nevo S, Swan V, Enger C, Wojno KJ, Bitton R, Shabooti M, Fuller AK, Jones RJ, Braine HG, Vogelsang GB. Acute bleeding after bone marrow transplantation (BMT)- incidence and effect on survival. A quantitative analysis in 1,402 patients. Blood. 1998;91:1469–1477. [PubMed] [Google Scholar]
- 38.Schwartz JM, Wolford JL, Thornquist MD, Hockenbery DM, Murakami CS, Drennan F, Hinds M, Strasser SI, Lopez-Cubero SO, Brar HS, et al. Severe gastrointestinal bleeding after hematopoietic cell transplantation, 1987-1997: incidence, causes, and outcome. Am J Gastroenterol. 2001;96:385–393. doi: 10.1111/j.1572-0241.2001.03549.x. [DOI] [PubMed] [Google Scholar]
- 39.Gooley TA, Rajvanshi P, Schoch HG, McDonald GB. Serum bilirubin levels and mortality after myeloablative allogeneic hematopoietic cell transplantation. Hepatology. 2005;41:345–352. doi: 10.1002/hep.20529. [DOI] [PubMed] [Google Scholar]
- 40.Tay J, Tinmouth A, Fergusson D, Huebsch L, Allan DS. Systematic review of controlled clinical trials on the use of ursodeoxycholic acid for the prevention of hepatic veno-occlusive disease in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:206–217. doi: 10.1016/j.bbmt.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 41.McDonald GB. Hepatobiliary complications of hematopoietic cell transplantation, 40 years on. Hepatology. 2010;51:1450–1460. doi: 10.1002/hep.23533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cutler C, Li S, Ho VT, Koreth J, Alyea E, Soiffer RJ, Antin JH. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2007;109:3108–3114. doi: 10.1182/blood-2006-09-046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akpek G, Boitnott JK, Lee LA, Hallick JP, Torbenson M, Jacobsohn DA, Arai S, Anders V, Vogelsang GB. Hepatitic variant of graft-versus-host disease after donor lymphocyte infusion. Blood. 2002;100:3903–3907. doi: 10.1182/blood-2002-03-0857. [DOI] [PubMed] [Google Scholar]
- 44.Shulman HM, Sharma P, Amos D, Fenster LF, McDonald GB. A coded histologic study of hepatic graft-versus-host disease after human bone marrow transplantation. Hepatology. 1988;8:463–470. doi: 10.1002/hep.1840080305. [DOI] [PubMed] [Google Scholar]
- 45.Ruutu T, Eriksson B, Remes K, Juvonen E, Volin L, Remberger M, Parkkali T, Hägglund H, Ringdén O. Ursodeoxycholic acid for the prevention of hepatic complications in allogeneic stem cell transplantation. Blood. 2002;100:1977–1983. doi: 10.1182/blood-2001-12-0159. [DOI] [PubMed] [Google Scholar]
- 46.Jacobs P, Miller JL, Uys CJ, Dietrich BE. Fatal veno-occlusive disease of the liver after chemotherapy, whole-body irradiation and bone marrow transplantation for refractory acute leukaemia. S Afr Med J. 1979;55:5–10. [PubMed] [Google Scholar]
- 47.Hogan WJ, Maris M, Storer B, Sandmaier BM, Maloney DG, Schoch HG, Woolfrey AE, Shulman HM, Storb R, McDonald GB. Hepatic injury after nonmyeloablative conditioning followed by allogeneic hematopoietic cell transplantation: a study of 193 patients. Blood. 2004;103:78–84. doi: 10.1182/blood-2003-04-1311. [DOI] [PubMed] [Google Scholar]
- 48.Carreras E, Bertz H, Arcese W, Vernant JP, Tomás JF, Hagglund H, Bandini G, Esperou H, Russell J, de la Rubia J, et al. Incidence and outcome of hepatic veno-occlusive disease after blood or marrow transplantation: a prospective cohort study of the European Group for Blood and Marrow Transplantation. European Group for Blood and Marrow Transplantation Chronic Leukemia Working Party. Blood. 1998;92:3599–3604. [PubMed] [Google Scholar]
- 49.Bearman SI. The syndrome of hepatic veno-occlusive disease after marrow transplantation. Blood. 1995;85:3005–3020. [PubMed] [Google Scholar]
- 50.Faioni EM, Krachmalnicoff A, Bearman SI, Federici AB, Decarli A, Gianni AM, McDonald GB, Mannucci PM. Naturally occurring anticoagulants and bone marrow transplantation: plasma protein C predicts the development of venocclusive disease of the liver. Blood. 1993;81:3458–3462. [PubMed] [Google Scholar]
- 51.Frickhofen N, Wiesneth M, Jainta C, Hertenstein B, Heymer B, Bianchi L, Dienes HP, Koerner K, Bunjes D, Arnold R. Hepatitis C virus infection is a risk factor for liver failure from veno-occlusive disease after bone marrow transplantation. Blood. 1994;83:1998–2004. [PubMed] [Google Scholar]
- 52.McDonald GB, Slattery JT, Bouvier ME, Ren S, Batchelder AL, Kalhorn TF, Schoch HG, Anasetti C, Gooley T. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood. 2003;101:2043–2048. doi: 10.1182/blood-2002-06-1860. [DOI] [PubMed] [Google Scholar]
- 53.McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M, Hardin BJ, Shulman HM, Clift RA. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118:255–267. doi: 10.7326/0003-4819-118-4-199302150-00003. [DOI] [PubMed] [Google Scholar]
- 54.McCune JS, Batchelder A, Deeg HJ, Gooley T, Cole S, Phillips B, Schoch HG, McDonald GB. Cyclophosphamide following targeted oral busulfan as conditioning for hematopoietic cell transplantation: pharmacokinetics, liver toxicity, and mortality. Biol Blood Marrow Transplant. 2007;13:853–862. doi: 10.1016/j.bbmt.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 55.McKoy JM, Angelotta C, Bennett CL, Tallman MS, Wadleigh M, Evens AM, Kuzel TM, Trifilio SM, Raisch DW, Kell J, et al. Gemtuzumab ozogamicin-associated sinusoidal obstructive syndrome (SOS): an overview from the research on adverse drug events and reports (RADAR) project. Leuk Res. 2007;31:599–604. doi: 10.1016/j.leukres.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 56.Rio B, Andreu G, Nicod A, Arrago JP, Dutrillaux F, Samama M, Zittoun R. Thrombocytopenia in venocclusive disease after bone marrow transplantation or chemotherapy. Blood. 1986;67:1773–1776. [PubMed] [Google Scholar]
- 57.Shulman HM, Gooley T, Dudley MD, Kofler T, Feldman R, Dwyer D, McDonald GB. Utility of transvenous liver biopsies and wedged hepatic venous pressure measurements in sixty marrow transplant recipients. Transplantation. 1995;59:1015–1022. doi: 10.1097/00007890-199504150-00017. [DOI] [PubMed] [Google Scholar]
- 58.Richardson PG, Elias AD, Krishnan A, Wheeler C, Nath R, Hoppensteadt D, Kinchla NM, Neuberg D, Waller EK, Antin JH, et al. Treatment of severe veno-occlusive disease with defibrotide: compassionate use results in response without significant toxicity in a high-risk population. Blood. 1998;92:737–744. [PubMed] [Google Scholar]
- 59.Vogelsang GB, Pavletic SZ. Chronic Graft Versus Host Disease: Interdisciplinary Management. 1st ed. New York: Cambridge University Press; 2009. [Google Scholar]
- 60.Peffault de Latour R, Lévy V, Asselah T, Marcellin P, Scieux C, Adès L, Traineau R, Devergie A, Ribaud P, Espérou H, et al. Long-term outcome of hepatitis C infection after bone marrow transplantation. Blood. 2004;103:1618–1624. doi: 10.1182/blood-2003-06-2145. [DOI] [PubMed] [Google Scholar]
- 61.Shimizu T, Kasahara M, Tanaka K. Living-donor liver transplantation for chronic hepatic graft-versus-host disease. N Engl J Med. 2006;354:1536–1537. doi: 10.1056/NEJMc052628. [DOI] [PubMed] [Google Scholar]
- 62.Martin BA, Rowe JM, Kouides PA, DiPersio JF. Hepatitis B reactivation following allogeneic bone marrow transplantation: case report and review of the literature. Bone Marrow Transplant. 1995;15:145–148. [PubMed] [Google Scholar]
- 63.Majhail NS, DeFor T, Lazarus HM, Burns LJ. High prevalence of iron overload in adult allogeneic hematopoietic cell transplant survivors. Biol Blood Marrow Transplant. 2008;14:790–794. doi: 10.1016/j.bbmt.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 64.Jensen PD, Jensen FT, Christensen T, Nielsen JL, Ellegaard J. Relationship between hepatocellular injury and transfusional iron overload prior to and during iron chelation with desferrioxamine: a study in adult patients with acquired anemias. Blood. 2003;101:91–96. doi: 10.1182/blood-2002-06-1704. [DOI] [PubMed] [Google Scholar]
- 65.Muretto P, Angelucci E, Lucarelli G. Reversibility of cirrhosis in patients cured of thalassemia by bone marrow transplantation. Ann Intern Med. 2002;136:667–672. doi: 10.7326/0003-4819-136-9-200205070-00009. [DOI] [PubMed] [Google Scholar]
- 66.Angelucci E, Muretto P, Lucarelli G, Ripalti M, Baronciani D, Erer B, Galimberti M, Giardini C, Gaziev D, Polchi P. Phlebotomy to reduce iron overload in patients cured of thalassemia by bone marrow transplantation. Italian Cooperative Group for Phlebotomy Treatment of Transplanted Thalassemia Patients. Blood. 1997;90:994–998. [PubMed] [Google Scholar]
- 67.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 68.Nieters A, Kallinowski B, Brennan P, Ott M, Maynadié M, Benavente Y, Foretova L, Cocco PL, Staines A, Vornanen M, et al. Hepatitis C and risk of lymphoma: results of the European multicenter case-control study EPILYMPH. Gastroenterology. 2006;131:1879–1886. doi: 10.1053/j.gastro.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 69.Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, Martin PJ, Sandmaier BM, Marr KA, Appelbaum FR, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]


