Abstract
AIM: To examine cytokeratin-18 (CK-18) and caspase-cleaved CK-18 expression in tumours and correlate with clinicopathological outcomes including tumour regression grade (TRG) response.
METHODS: Formalin-fixed human gastro-oesophageal cancers were constructed into tissue microarrays. The first set consisted of 122 gastric/gastro-oesophageal cancer cases not exposed to neoadjuvant chemotherapy and the second set consisted of 97 gastric/gastro-oesophageal cancer cases exposed to pre-operative platinum-based chemotherapy. Expression of CK-18 and caspase-cleaved CK-18 was investigated using immunohistochemistry.
RESULTS: CK18 was commonly expressed in gastro-oesophageal tumours (92.6%). Fifty-six point seven percent of tumours previously exposed to neoadjuvant chemotherapy were positive for caspase-cleaved CK-18 expression compared to only 24.6% of tumours not previously exposed to neoadjuvant chemotherapy (P = 0.009). In patients who received neoadjuvant chemotherapy, caspase-cleaved cytokeratin-18 expression correlated with favourable TRG response (TRG 1, 2 or 3, P = 0.043).
CONCLUSION: This is the largest study to date of CK-18 and caspase-cleaved CK-18 expression in gastro-oesophageal tumours. We provide the first evidence that caspase-cleaved CK-18 predicts tumour regression with neoadjuvant chemotherapy.
Keywords: Tumour regression grade, Gastro-oesophageal cancers, Chemotherapy, Full length cytokeratin-18, Caspase-cleaved cytokeratin-18
INTRODUCTION
Neoadjuvant platinum-based chemotherapy followed by surgery is the standard of care for patients with gastro-oesophageal adenocarcinoma[1,2]. However, there is an urgent need to develop predictive markers to individualize patient therapy[3]. We have recently shown that tumour regression grade (TRG) is a marker of histopathological response and tumour down-staging in tumours receiving neoadjuvant chemotherapy[4]. TRG was defined as per Mandard’s criteria[5]. TRG1 (complete regression) showed absence of residual cancer and fibrosis extending through the different layers of the oesophageal wall; TRG2 was characterised by the presence of rare residual cancer cells scattered through the fibrosis; TRG3 was characterised by an increase in the number of residual cancer cells but fibrosis predominated; TRG4 showed residual cancer outgrowing fibrosis; and TRG5 was characterised by the absence of regressive changes[5]. In patients who received neoadjuvant chemotherapy (CS group), 46.7% of gastric/gastro-oesophageal junction adenocarcinomas and 45.5% of lower third oesophageal adenocarcinomas had TRG 1, 2 or 3 compared to 13.7% in patients who did not receive neoadjuvant chemotherapy but proceeded to primary surgery. In the CS group, responders (TRG 1, 2 or 3) showed significant tumour down-staging [early ypT-stage disease (P = 0.002)]. In gastric cancers specifically, additional associations were seen with negative nodal disease (P = 0.044) and absence of vascular invasion (P = 0.027)[4]. More recently, we have also demonstrated that favourable tumour regression predicts better clinical outcomes in patients receiving neoadjuvant chemotherapy in gastro-oesophageal adenocarcinomas[6].
The anticancer activity of chemotherapeutic agents is directly related to the induction of apoptosis in tumours. Whilst the apoptotic pathway is complex, the intrinsic mitochondrial pathway is the predominant apoptotic pathway in cancer cells. In the intrinsic pathway, the mitochondrial release of cytochrome c activates caspase-9, which in turn activates caspase-3 and caspase-7[7,8]. Among the several cellular substrates of the capsases, members of the cytokeratin family, including cytokeratin-18 (CK-18), contribute to cellular collapse and apoptosis. Caspase-cleaved CK-18 is a specific marker of epithelial cell death and correlates with apoptosis in gastrointestinal epithelial cancers[9-11].
In the current study we have evaluated full length CK-18 and caspase-cleaved CK-18 protein expression using immunohistochemistry. We show for the first time that caspase-cleaved CK-18 expression in tumours correlates with histopathological tumour regression in early stage gastro-oesophageal adenocarcinomas.
MATERIALS AND METHODS
Patients
We identified patients referred to our centre with resectable gastric, gastro-oesophageal junction (GOJ) and lower third oesophageal adenocarcinomas between January 2001 and May 2008. GOJ tumours were defined as per Siewert’s classification[12]. The Union for International Cancer Control TNM staging system for oesophageal and gastric cancer was used in this study. The study was approved by the Ethics Committee of Nottingham University Hospitals.
Construction of tissue micro-array
Tissue micro-arrays (TMAs) were constructed. In brief, HE-stained slides (5 μm) were used to identify and mark out representative areas of viable tumour tissue. Then 0.6 mm-diameter needle core biopsies from the relevant areas of corresponding paraffin-embedded blocks were placed at defined coordinates in the recipient paraffin array blocks using a tissue microarrayer (Beecher Instruments, Sun Prairie, WI). Array blocks were constructed at a density of 80-150 cores per array. Two broad sets of TMA blocks were constructed. An array set of 97 patient cores to include gastric and gastro-oesophageal tumours that had received neoadjuvant chemotherapy and an array set of 122 cores of patients who had received no neoadjuvant chemotherapy were constructed. These TMA blocks were constructed in triplicate, each containing one sample from a different region of the tumour.
Immunohistochemistry
A standard streptavidin-biotin complex technique was used. In brief, 5 μm TMA sections were deparaffinised with xylene and rehydrated through graded alcohol. Endogenous peroxidase was blocked with 0.3% hydrogen peroxide in methanol for 20 min. Antigen retrieval was carried out by microwave treatment of the slides in sodium citrate buffer (pH 6) for 10 min at 750 W followed by 10 min at 300 W. The slides were rinsed in phosphate buffer saline (PBS) and incubated with Vectastain blocking serum diluted in PBS to block non-specific absorption. The slides were incubated for 30 min with the primary antibody M30 to detect caspase-cleaved CK-18 (Peviva, Bromma, Sweden) at a dilution of 1:75 and primary antibody M6 to detect full length CK-18 (Peviva, Bromma, Sweden) at a dilution of 1:150 at room temperature. After washing with PBS, sections were incubated with secondary antibody (Vectastain) for 30 min followed by avidin-biotin complex for a further 30 min. 3-3’ Diaminobenzidine tetrahydochloride was used as a chromogen. All sections were counterstained with Gill’s haematoxylin, dehydrated and mounted using DPX (a mixture of disterene, plasticizer, and xylene; Sigma).
Evaluation of staining
Evaluation of staining was performed with the observer blinded to the corresponding clinicopathological data. For full length CK-18 expression, cytoplasmic expression in cancer cells was considered positive. For caspase-cleaved CK-18 expression, TMA cores from tumour showing any positively stained apoptotic cells were considered positive. Caspase-cleaved CK-18 positive apoptotic cells were counted in TMA cores and the total number of positive cells from each tumour was taken as the number of positive cells.
Statistical analysis
All statistical analyses were carried out using SPSS package (version 15 for Windows, SPSS, Inc.). Associations between categorical variables were examined using cross-tabulation and the Pearson χ2 test. Kaplan Meier curves were derived to assess disease-specific survival, and the significance of differences in disease-specific survival between groups was calculated using the log-rank test. Patients whose death related to their oesophago-gastric cancer were considered in the disease-specific survival calculations. This was determined by death certification entries. Deaths resulting from non-oesophago-gastric cancer-related causes were censored. Survival rates were calculated from the date of diagnosis until the 13th January 2009, when any remaining survivors were censored and Kaplan Meier curves were plotted. In all cases, P < 0.05 was considered statistically significant.
RESULTS
Patient demographics
There were 2 groups of patients: those who received neoadjuvant chemotherapy (neoadjuvant group) and those who underwent primary surgery only (primary group). There were 97 patients in the neoadjuvant group with a median age of 64 years; 76% (n = 74) were male and 51.5% (n = 50) of cases were T3 tumours. There were 122 cases in the primary group with a median age of 74.5 years; 75.4% (n = 92) were male and 53.2% (n = 65) had T3 tumours. Patients in the primary surgery group did not receive any adjuvant chemotherapy after surgery. In the neoadjuvant group, 78% of patients had received all the planned three cycles of neoadjuvant ECF/ECX chemotherapy (adenocarcinomas) and 96.4% had received all the planned two cycles of neoadjuvant CF chemotherapy (squamous cell carcinomas). Of the patients who received all three cycles of ECF/ECX chemotherapy, 42% went on to receive a further three cycles of ECF/ECX chemotherapy. There was no significant difference between the primary surgery group and the perioperative chemotherapy group (gastric/GOJ) with regards to T stage [T2 (33.6% vs 29.8%), T3 (53.2% vs 51.5%)] and N stage [N0 (26.2% vs 31.9%), > N0 (73.8% vs 68.1%)]. Only adenocarcinomas were included in the immunohistochemical and survival analyses in this study (Table 1).
Table 1.
Patient demographics n (%)
| Neoadjuvant chemotherapy group | Primary surgery group | |
| Total number of patients | 97 | 122 |
| Median age (yr) | 64 | 74.5 |
| Sex | ||
| Male | 74 (76) | 92 (75.4) |
| Female | 23 (24) | 30 (24.6) |
| T stage | ||
| T1 | 6 (6.1) | 12 (9.8) |
| T2 | 29 (29.8) | 41 (33.6) |
| T3 | 50 (51.5) | 65 (53.2) |
| T4 | 8 (8.2) | 4 (3.2) |
| TX | 1 (1) | |
| N stage | ||
| N0 | 31 (31.9) | 32 (26.2) |
| ≥ N1 | 66 (68.1) | 90 (73.8) |
| M stage | ||
| M0 | 97 (100) | 122 (100) |
| M1 | - | - |
| Tumour type | ||
| Adenocarcinoma | 83 (85.5) | 122 (100) |
| Squamous cell carcinoma | 12 (12.3) | - |
| Adenosquamous | 2 (2) | - |
| Site of tumour | ||
| Gastric | 20 (20.6) | 122 (100) |
| GOJ | 47 (48.4) | - |
| Lower third of oesophagus | 36 (37.1) | - |
| Surgery | ||
| Total gastrectomy | 22 | 70 |
| Partial gastrectomy | 5 | 39 |
| Oesophagectomy/ Oesophago-gastrectomy | 70 | 13 |
| Survival status | ||
| Alive | 47 (48.4) | 47 (38) |
| Dead | 50 (51.6) | 75 (62) |
GOJ: Gastro-oesophageal junction.
Full length cytokeratin-18 expression
All 122 tumours in the primary surgery group were available for CK-18 analyses. One hundred and thirteen tumours stained positive for CK-18 (92.6%) (Figure 1A) and 9 cores were negative for CK-18 expression. There was no statistically significant correlation between tumour differentiation, T stage, N stage, vascular/perineural invasion, resection margin involvement and full length CK-18 expression in tumours.
Figure 1.
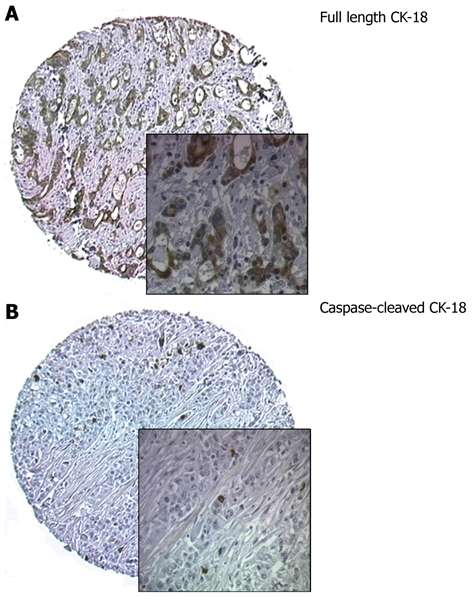
Immunohistochemical staining of full length cytokeratin-18 and caspase-cleaved cytokeratin-18. A: Immunohistochemical staining of full length cytokeratin-18 (CK-18) showing strong cytoplasmic staining; B: Immunohistochemical staining for caspase-cleaved CK-18. Cores from tumour showing positively-stained apoptotic cells. Original magnification × 100; insets × 400.
Caspase-cleaved cytokeratin-18 expression
All tumours were suitable for caspase-cleaved CK-18 expression analyses (Figure 1B). In tumours previously exposed to neoadjuvant chemotherapy (neoadjuvant TMA, n = 97), 56.7% of tumours (55/97) were positive compared to 24.6% (30/122) of tumours not previously exposed to neoadjuvant chemotherapy (primary TMA). This was statistically significant (P = 0.009). The mean total number of caspase-cleaved CK-18 positive cancer cells per tumour was 4.16 in the neoadjuvant group (range: 1-92) compared to 2.7 in the primary surgery group (range: 1-51).
We have previously demonstrated that TRG as assessed using Mandard’s criteria is a useful tool to assess response to neoadjuvant chemotherapy in gastro-oesophageal adenocarcinomas; favourable TRG correlated with tumour down-staging in that study[4]. In the current study we evaluated whether caspase-cleaved CK-18 expression correlated with TRG. We found that 43.6% of tumours that were positive for caspase-cleaved CK18 also had a favourable tumour response (TRG 1-3) compared to 23.8% that were negative for caspase-cleaved CK-18 expression (Table 2). This was statistically significant (P = 0.043). There was no statistically significant correlation between tumour differentiation, T stage, N stage, vascular/perineural invasion, resection margin involvement and caspase-cleaved CK-18 positivity.
Table 2.
Caspase-cleaved cytokeratin-18 and tumour regression in tumours exposed to neoadjuvant chemotherapy n (%)
| Caspase-cleaved CK-18 | TRG1, TRG2, TRG3 | TRG4, TRG5 | Total |
| Negative | 10 (23.8) | 32 (76.2) | 42 (100) |
| Positive | 24 (43.6) | 31 (56.4) | 55 (100) |
| Total | 34 (35.1) | 63 (64.9) | 97 (100) |
CK-18: Cytokeratin-18; TRG: Tumour regression grade.
Clinicopathological correlations were also observed in tumours not exposed to neoadjuvant chemotherapy. Well-differentiated tumours were more likely to be caspase-cleaved CK-18 positive (62.5%) compared to poor- and moderately-differentiated tumours which were only positive in 23.6% and 19% of tumours respectively (P = 0.031). However, no differences between T, N stage, vascular/perineural invasion, resection margin involvement and caspase-cleaved CK-18 positivity were observed. Regarding patients who had not received neoadjuvant chemotherapy, Kaplan Meier plot showed that in patients whose tumours stained positive for caspase-cleaved CK-18, a longer disease-specific survival was observed compared to patients whose tumours were negative (mean 84 mo vs 51 mo, P = 0.003) (Figure 2). However, in patients who had received neoadjuvant chemotherapy, no statistically significant differences were observed (mean 37.3 mo vs 41.6 mo, P = 0.975) (Figure 2).
Figure 2.
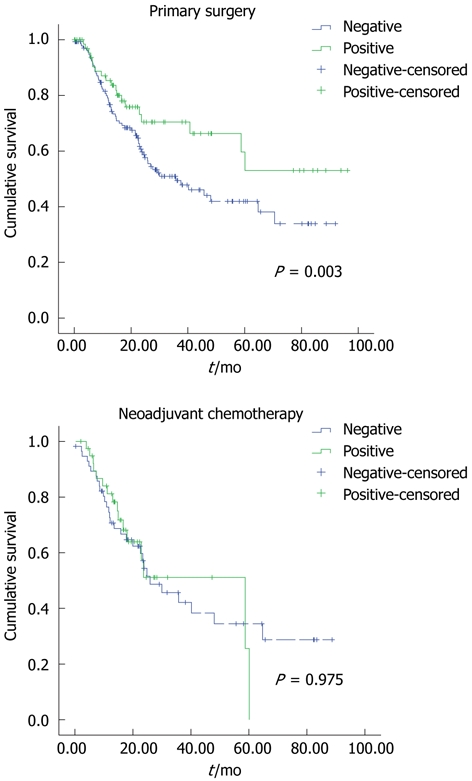
Kaplan Meier curves representing the relationship between caspase-cleaved cytokeratin-18 and disease-specific survival. A: In months from time of diagnosis in patients who received primary surgery only; B: In patients who received neoadjuvant chemotherapy.
Those factors found to be significant in univariate analyses were also included in a multivariate logistic regression analysis to estimate the independent effect of each factor after adjusting for the contributions of other factors. There was no one factor in the cohort of patients studied that showed significance on multivariate analysis (Table 3).
Table 3.
Univariate and multivariate analyses showing predictive factors for disease-specific and overall survival in neoadjuvant and surgery only groups
|
Univariate analysis |
Multivariate analysis | ||
| DSS | OS | OS | |
| Neoadjuvant group | |||
| Caspase-cleaved CK-18 positivity | 0.975 | 0.865 | 1.32 |
| TRG 1-3 vs 4-5 | 0.038 | 0.136 | 0.109 |
| Tumour diff. (well/mod vs poor) | 0.087 | 0.101 | 0.32 |
| T stage (T1, 2 and 3, 4) | 0.07 | 0.09 | 0.101 |
| N stage (N0 and > N1) | 0.106 | 0.124 | 0.23 |
| M stage (M0 and > M0) | 0.23 | 0.14 | 1.72 |
| Vascular invasion | 0.87 | 0.76 | 0.86 |
| Perineural invasion | 1.32 | 1.455 | 1.76 |
| Resection margin involvement | 2.34 | 1.98 | 0.24 |
| Surgery only group | |||
| Caspase-cleaved CK-18 positivity | 0.003 | 0.668 | 0.10 |
| TRG 1-3 vs 4-5 | 0.87 | 0.003 | 0.23 |
| Tumour diff. (well/mod vs poor) | 0.39 | 0.34 | 0.21 |
| T stage (T1, 2 and 3, 4) | 0.04 | 0.08 | 0.19 |
| N stage (N0 and > N1) | 0.16 | 0.10 | 0.43 |
| M stage (M0 and > M0) | 1.27 | 1.64 | 1.99 |
| Vascular invasion | 1.98 | 1.67 | 1.34 |
| Perineural invasion | 1.56 | 1.99 | 2.43 |
| Resection margin involvement | 0.34 | 0.25 | 0.34 |
DSS: Disease-specific survival; OS: Overall survival; CK-18: Cytokeratin-18; TRG: Tumour regression grade; diff.: Differentiation.
DISCUSSION
The ability to predict response to chemotherapy and individualize patient treatment is a high priority in gastro-oesophageal adenocarcinomas. Whilst the role of multimodality therapy in improving patient outcomes is well established, these treatments are toxic and have a considerable impact on patient morbidity. In the United Kingdom, neoadjuvant chemotherapy followed by surgery is routinely offered to patients who are fit with no significant co-morbidities. Accordingly, these patients generally tend to be young and can tolerate toxic chemotherapy. Patients considered not suitable for chemotherapy are usually elderly with significant co-morbidities. Moreover, until 2006 in the United Kingdom, the standard treatment for patients with early stage gastro-oesophageal adenocarcinoma was surgery only and patients were not routinely offered adjuvant chemotherapy. With the publication of results from a large United Kingdom trial of perioperative chemotherapy[1], neoadjuvant chemotherapy was established as a standard treatment option for patients. This is reflected in the differences in mean age between the two groups in our study.
In the current study we have evaluated the potential role of full length CK-18 and caspase-cleaved CK-18 as biomarkers in gastro-oesophageal adenocarcinomas. To evaluate the prognostic significance of full length CK-18 in gastro-oesophageal adenocarcinomas, we first evaluated CK-18 expression in tumours not exposed to neoadjuvant chemotherapy (n = 122). We found that CK-18 was commonly expressed in tumours. This is consistent with a previously reported study[13]. Although Xu et al[13] demonstrated that CK18 mRNA expression correlated with lymph node metastasis and tumour differentiation in gastric cancer, we were unable to demonstrate any positive clinicopathological correlations in our study.
Caspase-cleaved CK-18 has recently emerged as a promising marker of apoptosis in gastrointestinal cancers[11]. We therefore evaluated caspase-cleaved CK-18 expression in gastro-oesophageal adenocarcinomas. Fifty-six point seven percent of tumours previously exposed to neoadjuvant chemotherapy were positive for caspase-cleaved CK-18 expression, compared to only 24.6% of tumours not previously exposed to neoadjuvant chemotherapy (P = 0.009). This provides direct evidence that chemotherapy exposure leads to apoptosis-induced increased caspase-cleaved CK-18 expression in gastro-oesophageal tumours. We then demonstrated, for the first time, that the caspase-cleaved CK-18 expression correlated well with favourable tumour regression in patients receiving neoadjuvant chemotherapy (P = 0.043). Factors found to be significant in univariate analyses were not significant in a multivariate logistic regression analysis. This may be because the current study is a small retrospective study and a larger prospective study is required to confirm our observations. However, our study provides evidence that caspase-cleaved CK-18 may be a promising predictive biomarker in gastro-oesophageal adenocarcinomas. Moreover, our study also supports a rational hypothesis for evaluating serial blood caspase-cleaved CK18 secretion, using a recently developed assay[11,14,15], as a promising non-invasive biomarker in operable gastro-oesophageal adenocarcinomas where patients routinely receive three cycles of platinum-based neoadjuvant chemotherapy[1,2]. In a recent study of advanced colorectal cancer, serum levels of caspase-cleaved CK18 were significantly higher in patients who responded to chemotherapy compared to those who did not[11]. Similar results were also reported in advanced colorectal, oesophageal and gastric adenocarcinoma patients receiving palliative chemotherapy[16]. Although these results are promising, whether tumour tissue and serum caspase CK-18 levels are related to each other is not clear. Moreover, whether similar results could be achieved in early stage gastro-oesophageal adenocarcioma patients receiving neoadjuvant chemotherapy is an area of ongoing investigation in our laboratory.
We also made interesting clinicopathological observations in tumours not exposed to chemotherapy. Caspase-cleaved CK18 expression correlated with better differentiation and improved survival in this group. Although the reason for this unexpected finding is not clear, whether host immune factors such as lymphocytic infiltration could contribute to cancer cell death is currently unknown and is an area of ongoing investigation.
In summary, we have conducted a study of full length CK-18 and caspase-cleaved CK-18 expression in gastro-oesophageal cancer. We provide evidence that caspase-cleaved CK-18 is a promising predictive biomarker in patients who receive platinum-based neoadjuvant chemotherapy.
ACKNOWLEDGMENTS
The authors wish to thank Dr. Stewart Martin, Laboratory of Molecular Oncology, University of Nottingham, United Kingdom and Dr. Frank Neumann (BIOAXESS, Malvern, United Kingdom) for helpful discussions.
COMMENTS
Background
Neoadjuvant chemotherapy followed by surgery is a standard treatment option in patients with early stage gastro-oesophageal adenocarcinomas. However, only 40% of patients respond to chemotherapy. There is an urgent need to develop predictive biomarkers.
Research frontiers
Development of a serum biomarker test that can predict response to chemotherapy is highly desirable in gastro-oesophageal adenocarcinomas.
Innovations and breakthroughs
Cytokeratin-18 (CK-18) is commonly expressed in epithelial tumours. Caspase-cleaved CK-18 is expressed in cancer cells undergoing apoptosis following chemotherapy. Caspase-cleaved CK-18 is also secreted by tumour cells and can be evaluated using a blood test. In early stage gastrooesophageal cancers, the authors show that caspase-cleaved CK-18 is prevalent in tumour tissue following chemotherapy and that this correlates with tumour regression. The study suggests that serum testing of caspase-cleaved CK-18 may be feasible to predict response in early stage gastro-oesophageal adenocarcinomas.
Applications
A prospective study of serial serum testing of caspase-cleaved CK-18 elevation and correlation with tumour response to chemotherapy is required to evaluate CK-18 as a promising predictive biomarker in early stage gastro-oesophageal adenocarcinomas.
Terminology
CK-18 is widely expressed in epithelial cancers. Caspase-cleaved CK-18: in epithelial cells undergoing apoptosis, caspase-cleaved CK-18 is expressed and can be detected by immunohistochemistry.
Peer review
This is a study to evaluate whether caspase-cleaved CK-18 can predict response to chemotherapy in gastro-oesophageal adenocarcinomas receiving neoadjuvant chemotherapy. Data presented here support a rational hypothesis to test if secretion of caspase-cleaved CK-18 in blood can be used as a marker of response to chemotherapy in patients.
Footnotes
Peer reviewer: Steven Hochwald, MD, Surgery, University of Florida, 1600 SW Archer Road, PO Box 100109, Gainesville, FL 32816, United States
S- Editor Gou SX L- Editor Logan S E- Editor Zhang DN
References
- 1.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 2.Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002;359:1727–1733. doi: 10.1016/S0140-6736(02)08651-8. [DOI] [PubMed] [Google Scholar]
- 3.Fareed KR, Kaye P, Soomro IN, Ilyas M, Martin S, Parsons SL, Madhusudan S. Biomarkers of response to therapy in oesophago-gastric cancer. Gut. 2009;58:127–143. doi: 10.1136/gut.2008.155861. [DOI] [PubMed] [Google Scholar]
- 4.Fareed KR, Ilyas M, Kaye PV, Soomro IN, Lobo DN, Parsons SL, Madhusudan S. Tumour regression grade (TRG) analyses in patients with resectable gastro-oesophageal adenocarcinomas treated with platinum-based neoadjuvant chemotherapy. Histopathology. 2009;55:399–406. doi: 10.1111/j.1365-2559.2009.03404.x. [DOI] [PubMed] [Google Scholar]
- 5.Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680–2686. doi: 10.1002/1097-0142(19940601)73:11<2680::aid-cncr2820731105>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 6.Fareed KR, Al-Attar A, Soomro IN, Kaye PV, Patel J, Lobo DN, Parsons SL, Madhusudan S. Tumour regression and ERCC1 nuclear protein expression predict clinical outcome in patients with gastro-oesophageal cancer treated with neoadjuvant chemotherapy. Br J Cancer. 2010;102:1600–1607. doi: 10.1038/sj.bjc.6605686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotter TG. Apoptosis and cancer: the genesis of a research field. Nat Rev Cancer. 2009;9:501–507. doi: 10.1038/nrc2663. [DOI] [PubMed] [Google Scholar]
- 8.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 9.Leers MP, Kölgen W, Björklund V, Bergman T, Tribbick G, Persson B, Björklund P, Ramaekers FC, Björklund B, Nap M, et al. Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. J Pathol. 1999;187:567–572. doi: 10.1002/(SICI)1096-9896(199904)187:5<567::AID-PATH288>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 10.Linder S, Havelka AM, Ueno T, Shoshan MC. Determining tumor apoptosis and necrosis in patient serum using cytokeratin 18 as a biomarker. Cancer Lett. 2004;214:1–9. doi: 10.1016/j.canlet.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 11.Brandt D, Volkmann X, Anstätt M, Länger F, Manns MP, Schulze-Osthoff K, Bantel H. Serum biomarkers of cell death for monitoring therapy response of gastrointestinal carcinomas. Eur J Cancer. 2010;46:1464–1473. doi: 10.1016/j.ejca.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 12.Rüdiger Siewert J, Feith M, Werner M, Stein HJ. Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg. 2000;232:353–361. doi: 10.1097/00000658-200009000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu W, Zhang MW, Huang J, Wang X, Xu SF, Li Y, Wang SJ. Correlation between CK18 gene and gastric carcinoma micrometastasis. World J Gastroenterol. 2005;11:6530–6534. doi: 10.3748/wjg.v11.i41.6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cummings J, Hodgkinson C, Odedra R, Sini P, Heaton SP, Mundt KE, Ward TH, Wilkinson RW, Growcott J, Hughes A, et al. Preclinical evaluation of M30 and M65 ELISAs as biomarkers of drug induced tumor cell death and antitumor activity. Mol Cancer Ther. 2008;7:455–463. doi: 10.1158/1535-7163.MCT-07-2136. [DOI] [PubMed] [Google Scholar]
- 15.Cummings J, Ranson M, Butt F, Moore D, Dive C. Qualification of M30 and M65 ELISAs as surrogate biomarkers of cell death: long term antigen stability in cancer patient plasma. Cancer Chemother Pharmacol. 2007;60:921–924. doi: 10.1007/s00280-007-0437-4. [DOI] [PubMed] [Google Scholar]
- 16.Scott LC, Evans TR, Cassidy J, Harden S, Paul J, Ullah R, O’Brien V, Brown R. Cytokeratin 18 in plasma of patients with gastrointestinal adenocarcinoma as a biomarker of tumour response. Br J Cancer. 2009;101:410–417. doi: 10.1038/sj.bjc.6605175. [DOI] [PMC free article] [PubMed] [Google Scholar]


