Abstract
AIM: To investigate the mucosal morphology in Barrett’s oesophagus by chromo and magnifying endoscopy.
METHODS: A prospective pilot study at a tertiary medical centre was conducted to evaluate the use of acetic acid pulverisation combined with virtual chromoendoscopy using Fujinon intelligent chromoendoscopy (FICE) for semiological characterization of the mucosal morphology in Barrett’s oesophagus and its neoplastic complications. Upper endoscopy using high definition white light, 2% acid acetic pulverisation and FICE with high definition videoendoscopy were performed in 20 patients including 18 patients who presented with aspects of Barrett’s oesophagus at endoscopy examination. Two patients used as controls had normal endoscopy and histological results. Prospectively, videos were watched blind from histological results by three trained FICE technique endoscopists.
RESULTS: The videos of patients with high-grade dysplasia showed an irregular mucosal pattern in 14% using high definition white light endoscopy and in 100% using acid acetic-FICE combined. Videos did not identify irregular vascular patterns using high definition white light endoscopy, while acid acetic-FICE combined visualised one in 86% of cases.
CONCLUSION: Combined acetic acid and FICE is a promising method for screening high-grade dysplasia and early cancer in Barrett’s oesophagus.
Keywords: Acetic acid, Barrett’s metaplasia, Chromoendoscopy, Fujinon intelligent chromoendoscopy
INTRODUCTION
Barrett’s oesophagus is a premalignant lesion of oesophageal adenocarcinoma and endoscopic surveillance has been proposed for the diagnosis of this condition[1,2]. A stepwise four quadrant biopsy protocol is considered the gold standard procedure[3]. Current guidelines advise that biopsies should be obtained from any visible abnormality and that four random quadrant biopsies every 2 cm should be taken to detect inconspicuous dysplasia during endoscopic surveillance[4,5]. In theory, a high sensitivity endoscopic technique for the detection of high-grade dysplasia or early carcinoma is warranted and targeting biopsies and a four quadrant biopsy protocol will be unnecessary to improve Barrett’s oesophagus cancer detection.
Chromoendoscopy with methylene blue, acetic acid, or virtual chromoendoscopy have been proposed to improve the detection of preneoplastic lesions of Barrett’s oesophagus. Although acid acetic pulverisation improves visibility of the mucosal pattern by removing the superficial mucus and enhancing the pit pattern, virtual chromoendoscopy was developed to identify abnormalities from superficial mucosal or vascular patterns, and, therefore, facilitate diagnosis of Barrett’s associated neoplasias[6,7]. Virtual chromoendoscopy using narrow band imaging (NBI) was first studied in this indication and abnormalities of pit and vascular patterns have been described[6]. Improvements in endoscopic material allows functional imaging to be incorporated, which, in turn, permits visualisation of more detail in mucosal and vascular patterns and may complement high-resolution endoscopy to increase the sensitivity of the endoscopic detection of early neoplasia in Barrett’s oesophagus. Kim et al[8] showed that NBI was not reproducible and had a sensitivity of 89% in detecting preneoplastic lesions of Barrett’s oesophagus. Virtual chromoendoscopy using Fujinon intelligent chromoendoscopy (FICE) has been shown to be a useful tool in identifying gastric lesions[9]. Pohl et al[10] failed to show, in a single prospective study, significant differences between FICE and acetic acid combined with conventional chromoendoscopy for the detection of high-grade dysplasia or early cancer in patients with Barrett’s oesophagus. No study has shown any benefit with the combination of acetic acid and FICE. These two techniques could be complementary, since acetic acid enhances visualisation of the pit pattern and FICE allows detection of vascular abnormalities in Barrett’s oesophagus. With the Pohl et al[10] study in mind, we conducted a pilot study to evaluate the combination of 2% acetic acid pulverisation and FICE for semiological characterization of the mucosal morphology in Barrett’s oesophagus.
These two techniques could be complementary, since acetic acid enhances visualisation of the pit pattern[11] and FICE allows detection of vascular abnormalities in Barrett’s oesophagus.
MATERIALS AND METHODS
Ethics
All patients enrolled in this study gave written informed consent. The study was in accordance with the Declaration of Helsinki and the Institutional review board (Centre de protection des personnes d’Ile de France III) approved the study (ref: CPP: AT102).
Patients
The study population consisted of patients with Barrett’s oesophagus, as confirmed by pathological analysis. None of the patients had received previous therapy for Barrett’s oesophagus. The eligibility criteria also included a four-quadrant biopsy protocol, a standardized procedure as described above and a video record of the overall procedure. Patients were required to have at least one dysplastic lesion that could be evaluated. Data were collected retrospectively from a review of the electronic medical records and endoscopy database of our institution from November 2006 to September 2009. Eighteen patients had endoscopic Barrett’s oesophagus (Figure 1). Of these patients, fifteen had specialized intestinal metaplasia, dysplasia or carcinoma and three patients had normal histology results. In addition, two patients with no history of Barrett’s oesophagus or endoscopic lesions, and normal biopsies were included as controls.
Figure 1.
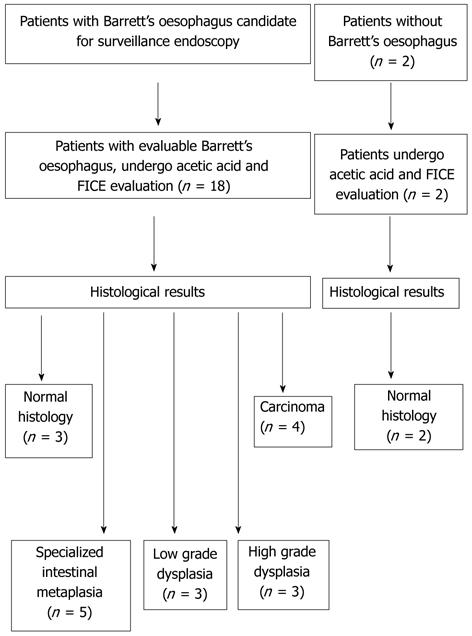
Patient selection for the present study. FICE: Fujinon intelligent chromoendoscopy.
Endoscopy procedure
All explorations were performed using a high definition Fujinon 1.3-million-pixel EG 490 ZW5 gastroscope zoom with optical magnification up to 100 times equipped with a short soft transparent hood by one expert endoscopist and were video recorded. In each case, videos of the oesophagus were taken before biopsies. Endoscopies were carried out following total intravenous anaesthesia of the patient using Propofol. The endoscopic technique was standardized as follows: first the oesophagus was examined with high definition white light endoscopy, followed by 6-10 mL of 2% acetic acid pulverisation, and, finally, FICE was activated. Acetic acid pulverisation was performed with a spray catheter PW-1V-1 (Olympus Optical Co., Ltd., Japan) and the endoscopist gently sucked up excess acetic acid from the oesophageal lumen before inspection. In the case of macroscopic abnormalities in colour or pit-pattern, the zoom was used with a magnification of 10-15. Videos were made with high definition white light and with the combination of acetic acid and FICE. FICE channels 4, 7 and 0 were used following previous studies using FICE in upper endoscopy[9,10]. In the case of macroscopic abnormalities, separate biopsy samples were performed and then systematic biopsies were used in “normal macroscopic areas” of Barrett’s oesophagus using Radial Jaw® Single-Use Biopsy Forceps (Boston Scientific, Fremont, CA, United States) with jumbo capacity.
Histological analysis
All biopsies were evaluated by two pathologists with extensive experience in Barrett’s neoplasia, and reviewed by a third pathologist in cases of dysplasia. Histological results were classified according to the revised Vienna classification[12]. The highest grade of dysplasia obtained from any biopsy sample was used to determine the diagnosis in each patient.
Evaluation of Barrett’s oesophagus
Retrospectively, videos were watched blind from histological results by three FICE trained endoscopists. For each patient, the most severe lesion was selected for evaluation. Experts noted the characteristics of Barrett’s oesophagus and any other abnormalities with and without combined acetic acid-FICE: macroscopic appearance of the lesion using the Paris classification[12], type of mucosal pattern (regular: ridged/villous, circular, irregular), vascular pattern characteristics (regular, irregular) using the Sharma classification[13], raised lesion, ulcerous lesion, pigmented lesion; and spontaneous bleeding lesion. The last four items were considered on clinical findings.
Statistical analysis
Means and standard deviations were used to summarize continuous variables with an apparently Gaussian distribution, whereas the median and the interquartile range (IQR) were used to summarize variables with a skewed distribution. Kappa statistics with their 95% confidence intervals (CI) were used to test for inter-observer agreement of the 3-step classification system using arbitrary interpretation by Landis and Koch (0, poor agreement; 0.00-0.20, slight agreement; 0.21-0.40, fair agreement; 0.41-0.60, moderate agreement; 0.61-0.80, substantial agreement; 0.80-1.00, almost perfect agreement)[14]. Because Kappa statistics can only be calculated with pair-wise observations, Kappa values were calculated for all pair-wise combinations obtained by the observers.
RESULTS
Patient characteristics
Twenty patients were included. The patients’ characteristics are shown in Table 1. Histological results were normal histology, specialized intestinal metaplasia, low-grade dysplasia, high-grade dysplasia, and carcinoma in 25%, 25%, 15%, 15%, and 20%, respectively. With high definition white light endoscopy, abnormalities in mucosal or vascular pattern were detected in one patient (6%) out of the 18 patients with suspected Barrett’s oesophagus. With combined acetic acid-FICE, seven patients (39%) out of the 18 with suspected Barrett’s oesophagus had a visible irregular mucosal pattern (Table 2). All of these patients had high-grade dysplasia or carcinoma (sensitivity: 100%). With combined acetic acid-FICE, six patients (33%) out of the 18 with suspected Barrett’s oesophagus had a visible irregular vascular pattern. All had high-grade dysplasia or carcinoma (sensitivity: 100%). No patient with metaplasia or low-grade dysplasia had visible irregular mucosal vascular patterns, raised lesions, or spontaneous bleeding (Figure 1).
Table 1.
Clinical characteristics of patients undergoing combined acetic acid and Fujinon intelligent chromoendoscopy endoscopy
| Normal mucosa | SIM or LGD | HGD or carcinoma | All patients | |
| Number of patients (%) | 5 (25) | 8 (40) | 7 (35) | 20 |
| Age (yr) (mean ± SD) | 56.4 ± 21.7 | 65 ± 10.5 | 71 ± 13.8 | 65 ± 15 |
| Sex (men/women) | 5/0 | 7/1 | 7/0 | 19/1 |
| Concomitant therapy PPI (%) | 60 | 100 | 100 | 90 |
| Median Barrett’s oesophagus | 1 (0-1) | 2.3 (2-3.3) | 3.5 (2.5-3.6) | 2 ± 1.3 |
| Length (cm) ± IQR |
SIM: Specialized intestinal metaplasia; LGD: Low-grade dysplasia; HGD: High-grade dysplasia; PPI: Proton-pump inhibitor; IQR: Interquartile range.
Table 2.
Correlation of the predominant mucosal and vascular patterns with histological results during high resolution white light endoscopy or after 2% acetic acid pulverisation and Fujinon intelligent chromoendoscopy 0, 4 and 7
|
High resolution white light |
Acetic acid pulverisation and FICE |
|||||
| Normal histology | SIM or LGD | HGD or carcinoma | Normal histology | SIM or LGD | HGD or carcinoma | |
| Number of patients (%) | 5 | 8 | 7 | 5 | 8 | 7 |
| Regular or not visualized mucosal pattern1 (%) | 100 | 100 | 86 | 100 | 100 | 0 |
| Irregular mucosal pattern (%) | 0 | 0 | 14 | 0 | 0 | 100 |
| Regular or not visualized vascular pattern1 (%) | 100 | 100 | 100 | 100 | 100 | 14 |
| Abnormal blood vessels (%) | 0 | 0 | 14 | 0 | 0 | 86 |
| Raised lesion (%) | 0 | 0 | 14 | 0 | 0 | 71 |
| Pigmented lesion (%) | 0 | 0 | 0 | 0 | 37.5 | 0 |
| Bleeding lesion (%) | 0 | 0 | 14 | 0 | 0 | 57 |
| Ulcerous lesion (%) | 0 | 14 | 14 | 0 | 14 | 14 |
FICE: Fujinon intelligent chromoendoscopy; SIM: Specialized intestinal metaplasia; HGD: High-grade dysplasia; LGD: Indicate Low grade dysplasia.
Endoscopists were not able to exactly classify the mucosal pattern according to Sharma’s classification of mucosal pattern due to lack of visibility, but they judged that the mucosa was regular.
An irregular mucosal pattern was identified in patients with high-grade dysplasia or carcinoma using high definition white light endoscopy or the combination of acetic acid-FICE in 14% and 100%, respectively. An irregular vascular pattern was identified in patients with high-grade dysplasia or carcinoma using high definition white light endoscopy or the combination of acetic acid-FICE in 0% and 86%, respectively (Figures 2 and 3). An irregular mucosal or vascular pattern was not identified in patients without high-grade dysplasia or carcinoma using the combination of acetic acid and FICE (specificity 100%).
Figure 2.
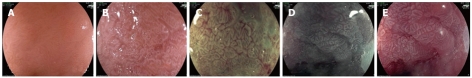
Acetic acid and Fujinon intelligent chromoendoscopy image of the oesophagus. Specialized intestinal metaplasia using high definition white light (A), 2% acetic acid (B), and the combination of acetic acid with Fujinon intelligent chromoendoscopy (FICE) 4 (C), FICE 0 (D) and FICE 7 (E).
Figure 3.
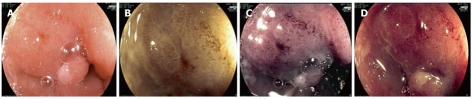
Acetic acid and Fujinon intelligent chromoendoscopy image of an oesophageal carcinoma. Irregular pit pattern and abnormal vascularisation is shown with 2% acetic acid (A), or following the combination of acetic acid and Fujinon intelligent chromoendoscopy (FICE) 4 (B), FICE 0 (C) and FICE 7 (D).
Among the 7 patients with high-grade dysplasia, 4 patients (57%) presented with early carcinoma on biopsy. The sizes of the lesions were 1 cm, 0.5 cm and 0.4 cm. The lesion size was not recorded in one patient. All patients underwent a mucosectomy within 2 mo of the primary endoscopy.
Definitive histological staging was intramucosal carcinoma (pT1m) for 3 of 4 patients following mucosectomy histological analysis, and resection margins were healthy. Only high-grade dysplasia was found in the fourth patient.
Interobserver agreement for mucosal morphology
When the evaluations by the experts were grouped together, the interobserver agreement for mucosal morphology assessed on high definition white light images was substantial on 5 items (mucosal pattern: κ = 0.97, raised lesion: κ = 1.00, pigmented lesion: κ = 1.00, bleeding lesion: κ = 1.00, and ulcerous lesion: κ = 1.00) and moderate on 2 items (vascular pattern: κ = 0.73, abnormal blood vessels: κ = 0.76). There was no difference in interobserver agreement between high definition white light images and combined acetic acid-FICE images, except for 3 evaluations (abnormal blood vessels, vascular pattern and pigmented lesion). In the evaluation of abnormal blood vessels using high definition white light images, the interobserver agreement (κ = 0.76; 95% CI: 0.64-1.00) was lower than that of combined acetic acid-FICE images (κ = 0.91; 95% CI: 0.86-1.00). In the evaluation of vascular pattern using high definition white light images, the interobserver agreement (κ = 0.73; 95% CI: 0.64-0.91) was lower than that of combined acetic acid-FICE images (κ = 0.83; 95% CI: 0.76-0.88). In the evaluation of pigmented lesion using high definition white light images, the interobserver agreement (κ = 1.00; 95% CI: 0.64-1.00) was better than that of combined acetic acid-FICE images (κ = 0.37; 95% CI: 0.28-0.54).
COMMENTS
Background
Barrett’s oesophagus is a pre-neoplastic lesion with an estimated rate of transformation to carcinoma of approximately 0.3%-0.6% each year. Guidelines from the American College of Gastroenterology proposed a surveillance programme to detect high-grade dysplasia or carcinoma in Barrett’s oesophagus. This screening programme recommends performing 4 quadrant biopsies every 2 cm at 1 year in low-grade dysplasia and every 3 mo in high-grade dysplasia.
Research frontiers
Acetic acid pulverisation is a simple and inexpensive method of improving visibility of the pit pattern, but does not allow appreciation of the vascular pattern. Acetic acid instillation increased the detection of cancer compared to white light endoscopy with or without high resolution endoscopy. Whereas acetic acid instillation, indigo carmine chromoendoscopy, narrow-band imaging and chromoendoscopy by Fujinon intelligent chromoendoscopy (FICE) seem to be interesting new techniques, each technique alone seems to be insufficient to warrant abandonment of the Seattle protocol of multiple blind sample biopsies. The authors showed significant differences between FICE and chromoendoscopy with acetic acid for the detection of high-grade dysplasia or early cancer in patients with Barrett’s oesophagus. Both the acetic acid and FICE techniques showed separate per-lesion sensitivity of up to 87% for the detection of high-grade neoplasia and early cancer in patients with Barrett’s oesophagus. The authors conducted a video study to evaluate the combined acetic acid and FICE technique.
Innovations and breakthroughs
Virtual chromoendoscopy has begun to receive greater attention as a potential technique in the diagnosis of Barrett’s oesophagus. In the series, the study highlight the positive effect of the combination of acetic acid and virtual chromoendoscopy. The study confirms the usefulness of FICE technique combined with acetic acid using video reviews. However, the generalization of the results is made difficult by the author’s small population size. Therefore, a larger study is warranted to confirm the author’s results. The data suggest a high sensitivity and specificity with this combination, however, data are lacking on a real-time basis rather than relying on subsequent video reviews. The report, for the first time, on the benefit of the combination of acetic acid and FICE in identifying high-grade dysplasia or early oesophageal neoplasia.
Applications
By using the presented procedure of acetic acid and FICE, this may represent a future strategy for the diagnosis of Barrett’s oesophagus lesions. In the study, taking into account two criteria (irregular mucosal and/or vascular patterns), 100% of patients with high-grade dysplasia or carcinoma were identified by endoscopy, with no errors in the diagnosis of high-grade dysplasia or carcinoma. The study showed that combined acetic acid and FICE had a positive benefit in the identification of high-grade dysplasia or carcinoma. By combining the two techniques of acetic acid and FICE, the results showed improvement in the quality of endoscopic images obtained and visualisation of both mucosal and vascular patterns or irregularities. The endoscopic detection of high-grade dysplasia or carcinoma was enhanced by this combination method as compared to using high definition white light imaging alone. In order to further verify the high sensitivity and specificity findings in this study, future prospective multi-centre studies of patients presenting for endoscopic evaluation of Barrett’s oesophagus with all levels of early neoplasia are required to definitively compare the combination imaging algorithm of acetic acid and FICE to Seattle Protocol random biopsies.
Peer review
This is an interesting study, which adds information to how to tackle detection and surveillance of Barrett’s esophagus.
Footnotes
Peer reviewer: Helena Nordenstedt, MD, PhD, Upper Gastrointestinal Research, Department of Molecular Medicine and Surgery, Karolinska Institute, Stockholm 17176, Sweden
S- Editor Sun H L- Editor Webster JR E- Editor Zhang DN
References
- 1.Falk GW. Barrett’s esophagus. Gastroenterology. 2002;122:1569–1591. doi: 10.1053/gast.2002.33427. [DOI] [PubMed] [Google Scholar]
- 2.Sharma P, McQuaid K, Dent J, Fennerty MB, Sampliner R, Spechler S, Cameron A, Corley D, Falk G, Goldblum J, et al. A critical review of the diagnosis and management of Barrett’s esophagus: the AGA Chicago Workshop. Gastroenterology. 2004;127:310–330. doi: 10.1053/j.gastro.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 3.van Sandick JW, van Lanschot JJ, Kuiken BW, Tytgat GN, Offerhaus GJ, Obertop H. Impact of endoscopic biopsy surveillance of Barrett’s oesophagus on pathological stage and clinical outcome of Barrett’s carcinoma. Gut. 1998;43:216–222. doi: 10.1136/gut.43.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirota WK, Zuckerman MJ, Adler DG, Davila RE, Egan J, Leighton JA, Qureshi WA, Rajan E, Fanelli R, Wheeler-Harbaugh J, et al. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63:570–580. doi: 10.1016/j.gie.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788–797. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 6.Curvers WL, van den Broek FJ, Reitsma JB, Dekker E, Bergman JJ. Systematic review of narrow-band imaging for the detection and differentiation of abnormalities in the esophagus and stomach (with video) Gastrointest Endosc. 2009;69:307–317. doi: 10.1016/j.gie.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 7.Pohl J, May A, Rabenstein T, Pech O, Ell C. Computed virtual chromoendoscopy: a new tool for enhancing tissue surface structures. Endoscopy. 2007;39:80–83. doi: 10.1055/s-2006-945045. [DOI] [PubMed] [Google Scholar]
- 8.Kim RE, Singh V, Hall SB, Singh M, Rastogi A, Moloney B, Wani SB, Gaddam S, Sharad CM, Wallace MB, et al. Use of Video-Autofluorescence Imaging (AFI) and Magnification Narrow Band Imaging (Zoom-NBI) in Barrett’s Esophagus: An Inter-Observer Agreement Study. Gastrointest Endosc. 2010;71:S1594 (AB: 1203) (abstract). [Google Scholar]
- 9.Coriat R, Chryssostalis A, Zeitoun JD, Deyra J, Gaudric M, Prat F, Chaussade S. Computed virtual chromoendoscopy system (FICE): a new tool for upper endoscopy? Gastroenterol Clin Biol. 2008;32:363–369. doi: 10.1016/j.gcb.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Pohl J, May A, Rabenstein T, Pech O, Nguyen-Tat M, Fissler-Eckhoff A, Ell C. Comparison of computed virtual chromoendoscopy and conventional chromoendoscopy with acetic acid for detection of neoplasia in Barrett’s esophagus. Endoscopy. 2007;39:594–598. doi: 10.1055/s-2007-966649. [DOI] [PubMed] [Google Scholar]
- 11.Sakai Y, Eto R, Kasanuki J, Kondo F, Kato K, Arai M, Suzuki T, Kobayashi M, Matsumura T, Bekku D, et al. Chromoendoscopy with indigo carmine dye added to acetic acid in the diagnosis of gastric neoplasia: a prospective comparative study. Gastrointest Endosc. 2008;68:635–641. doi: 10.1016/j.gie.2008.03.1065. [DOI] [PubMed] [Google Scholar]
- 12.Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570–578. doi: 10.1055/s-2005-861352. [DOI] [PubMed] [Google Scholar]
- 13.Sharma P, Bansal A, Mathur S, Wani S, Cherian R, McGregor D, Higbee A, Hall S, Weston A. The utility of a novel narrow band imaging endoscopy system in patients with Barrett’s esophagus. Gastrointest Endosc. 2006;64:167–175. doi: 10.1016/j.gie.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 14.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]