Abstract
Multidrug resistance caused by the overexpression of ABC drug transporters is a major obstacle in clinical cancer chemotherapy. For several years, it appeared that direct inhibition of ABC transporters would be the cheapest and most efficient way to combat this problem. Unfortunately, progress in finding a potent, selective inhibitor to modulate ABC transporters and restore drug sensitivity in multidrug-resistant cancer cells has been slow and challenging. Candidate drugs should ideally be selective, potent and relatively non-toxic. Many researchers in recent years have turned their attention to utilizing natural products as the building blocks for the development of the next generation of inhibitors, especially after the disappointing results obtained from inhibitors of the first three generations at the clinical trial stage. The first step is to discover natural substances (distinct from the first three generation inhibitors) that are potent, selective and relatively non-toxic in order to be used clinically. Here, we present a brief overview of the prospect of using natural products to modulate the function of ABC drug transporters clinically and their impact on human physiology and pharmacology.
Keywords: ATP-binding cassette transporters, Bioavailability, Chemotherapy, Modulators, Multidrug resistance, Natural products
INTRODUCTION
The impact of ATP-binding cassette transporters on cancer chemotherapy
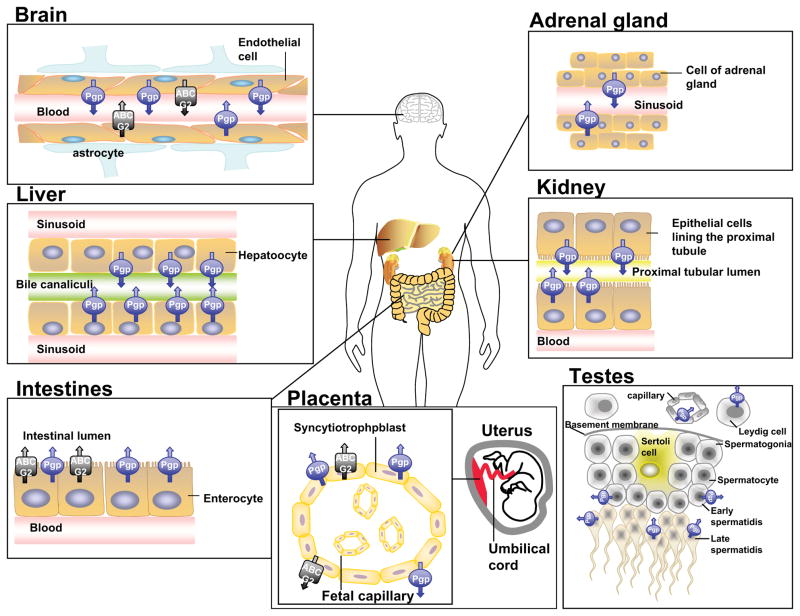
Despite more than two decades of research on the subject, multidrug resistance (MDR) remains one of the major obstacles to successful cancer chemotherapy. This phenomenon occurs when cancer cells spontaneously become insensitive to drugs that are structurally unrelated [1]. A leading cause of MDR in cancer is the overexpression of ATP-Binding Cassette (ABC) transporters that utilize energy derived from ATP hydrolysis to actively transport anticancer drugs across biological membranes, preventing drugs from reaching their targets within a cancer cell [2]. The ABC transporters belong to a superfamily of proteins that are classified into seven subfamilies (ABCA-ABCG) based on their sequence homology and domain organization [3]. To date, sixteen members have been identified to be associated with known human diseases. However, only three major ABC drug transporters, including P-glycoprotein (Pgp; ABCB1), multidrug resistance protein 1 (MRP1; ABCC1) and ABCG2 (BCRP; MXR), are believed to seriously affect cancer chemotherapy [2, 4]. The knock-out mouse models lacking these ABC transporters have shown that they have a major impact on the pharmacological behavior of many anticancer drugs (reviewed in [5, 6]). Collectively, they are capable of transporting the majority of anticancer drugs that are administered in the clinic, including, but not limited to, vinca alkaloids, anthracyclines, mitoxantrone, paclitaxel, etoposide and tyrosine kinase inhibitors such as gleevec or nilotinib [7–9]. Furthermore, in addition to causing MDR in cancer cells, the physiological functions and the localization of these transporters in human tissues also greatly affects the overall adsorption, distribution, metabolism, elimination and toxicity of almost all classes of drugs [10] (Figure 1). To summarize, the presence of high or over-expression of several ABC drug transporters in mRNA and/or protein level can have a significant impact on overall cancer chemotherapy and lead to the development of MDR and treatment failure [11–15]. Consequently, inhibiting the function or expression of these transporters should restore drug sensitivity in some MDR cancer cells and lead to a substantial improvement in the effectiveness of the anticancer drugs in cancer patients.
Figure 1.
Tissue localization of P-glycoprotein and ABCG2 in human organs. These transporters are present on apical or luminal surface of epithelial or endothelial cells.
Development of inhibitors of ABC drug transporters
Many inventive strategies and ideas have recently been proposed and evaluated in an attempt to overcome MDR in cancer patients [16]. Currently, inhibiting the function (or the expression) of respective ABC drug transporters with potent and low toxicity inhibitors (or modulators) is still considered by many researchers to be the easiest and most direct way to restore drug sensitivity in MDR cancer cells. The fundamental concept underlying the use of an inhibitor (or chemosensitizer) in MDR cancer chemotherapy is to directly block drug efflux mediated by ABC transporters (such as Pgp), to improve drug penetration and distribution, and to elevate drug accumulation in MDR cancer cells, eventually to restore drug sensitivity [2, 17–19]. Substantial efforts have been carried out to develop potent modulators of ABC drug transporters for the past two decades, and some of the major discoveries have been summarized in a recent review [8]. Unfortunately, there is still a lack of irrefutable evidence or clinical trial data to demonstrate that this approach can indisputably improve bioavailability or delivery, or can restore drug sensitivity in MDR cancer patients [2]. The difficulty in finding an ideal inhibitor is often associated with specificity, potency and intrinsic toxicity. Adverse interactions of modulators with drugs administered in parallel or nonspecific side effects are also extremely problematic. To make the matter even worse, there are substantial overlapping substrate specificities among the major ABC drug transporters, which are expressed in vital organs (e.g. brain, testes, liver, kidney and intestines) to protect the body from the xenobiotics. There may be some toxicity in these organs associated with the inhibition of these transporters. Furthermore, the variability in expression levels and polymorphisms of ABC transporters among individuals makes clinical trials related to MDR cancer exceptionally challenging [20]. On the other hand, these above mentioned factors are also the reasons why the roles of ABC drug transporters are crucial to advances in personalized medicine [21].
Inhibitors of ABC transporters can be classified into four major categories. The discovery of First Generation Inhibitors involved the screening of drugs or chemicals with known biological activities (such as channel blockers, immunosuppressants and even cardiovascular drugs), to find compounds that also inhibit the function of ABC transporters [8, 22]. In contrast, the Second and Third Generation Inhibitors were specifically designed and synthesized based on structural information from the First Generation Inhibitors [8]. The reasons behind the failure of using inhibitors from the first three generations to restore drug sensitivity in cancer clinical trials are quite clear. Since most of those inhibitors were not designed to select ABC drug transporters as their primary targets, it is not surprising that they affected other biological targets (unspecific and unfavorable interactions with other targets or drugs). Also, higher concentrations of these drugs are required to reach effective inhibitory effect, thus causing undesirable toxicity (e.g. verapamil). In addition, First Generation Inhibitors themselves are often transported substrates of ABC transporters. Mutations in the ABC transporters of interest, the presence of other drug efflux or uptake transporters or channels, and problems with solubility, penetration and distribution, also diminish the effectiveness of these inhibitors [8, 23]. In spite of the lack of effectiveness, it is still crucial to understand the interactions between some of the First Generation Inhibitors and ABC drug transporters, as many are chemotherapeutic drugs used on a daily basis. For instance, the tyrosine kinase inhibitors (TKIs) nilotinib and lapatinib inhibit ABCG2-mediated efflux by competing with the binding of anticancer agents at the ABCG2 substrate binding site. Furthermore, the upregulation of ABCG2 can result in increased efflux of TKIs (gefitinib and vandetanib) after long-term exposure to TKIs [24]. Consequently, their own drug efficacy is also affected by ABCG2-mediated transport [25, 26].
MODULATORS ORIGINATING FROM NATURAL SOURCES: THE FOURTH GENERATION INHIBITORS
Inhibitors or modulators originating from natural sources are sometimes referred to as “Fourth Generation Inhibitors”. In fact, compounds from natural products provide one of the most diverse and novel chemical scaffolds suitable for the development of new inhibitors. It is thus not surprising that many researchers recognize the value of screening for new natural product modulators, because they have the potential to be more successful than many of the modulators already developed. There is a great diversity of materials that can be utilized, as biologically active components are now extracted from plants, fungi and even marine organisms, then purified and characterized. Most importantly, natural extracts are usually low in toxicity and are well tolerated in the human body. For that reason, research groups are actively screening for candidates from natural sources that have a strong modulation effect on the function (or the expression) of disease-linked ABC transporters. Once a lead compound is identified, quantitative structure-activity relationship (QSAR) studies and an optimization process can then be carried out [27, 28]. For instance, the fungal toxin fumitremorgin C (FTC) is an effective ABCG2 inhibitor discovered from natural sources, but has unfavorable neurotoxicity [29]. FTC was therefore optimized into the more potent, specific and less-toxic analog Ko143, which is more appropriate for clinical trials [30]. As a large number of natural product modulators have been discovered over the years, this review will focus on two of the most popular natural products, curcumin and flavonoids, as examples of natural products that modulate ABC drug transporters. A list of key natural product modulators discovered for Pgp, MRP1 and ABCG2 is summarized in Table 1.
Table 1.
List of key natural products that modulate function of P-glycoprotein, multidrug resistant protein 1 and ABCG2
| Name | Targeted ABC transporter | Source | Reference |
|---|---|---|---|
| 3′-4′-7-Trimethoxyflavone | ABCG2 | Plant | [97] |
| 5-Bromotetrandrine | Pgp | Plant | [98] |
| 6-Prenylchrysin | ABCG2 | Plant | [99] |
| Abietane diterpene | Pgp | Plant | [100] |
| Acacetin | ABCG2 | Plant | [101] |
| Agosterol A and derivatives | Pgp | Marine organisms | [102] |
| Alisol B 23-acetate | Pgp | Plant | [103] |
| Amooranin | Pgp | Plant | [104] |
| Asarum heteropoides var. mandshuricum | Pgp | Plant | [105] |
| Baicalein and derivatives | Pgp | Plant | [106] |
| Biochanin A | Pgp | Plant | [77] |
| ABCG2 | Plant | [68] | |
| Bitter melon extract | Pgp | Plant | [107] |
| Cantharidin | Pgp | Insect | [108] |
| Cannabinoids | MRP1 | Plant | [109] |
| Pgp | Plant | [110, 111] | |
| ABCG2 | Plant | [112] | |
| Catechins | Pgp | Plant | [113] |
| Cepharanthine | Pgp | Plant | [114] |
| MRP1 | Plant | [115] | |
| Chrysin | ABCG2 | Plant | [68] |
| Coumarins | Pgp | Plant | [116] |
| Curcumin | Pgp | Plant | [31, 117] |
| MRP1 | Plant | [38] | |
| ABCG2 | Plant | [39] | |
| Cycloartanes | Pgp | Plant | [118] |
| Daizein | ABCG2 | Plant | [119] |
| Deoxyschizandrin | Pgp | Plant | [120] |
| Eudesmin | Pgp | Plant | [121] |
| Eupatin | ABCG2 | Plant | [122] |
| Euphocharacins A-L | Pgp | Plant | [123] |
| Fumitremorgin C | ABCG2 | Fungus | [29, 124] |
| Genistein | ABCG2 | Plant | [101] |
| Ginkgo biloba extract | Pgp | Plant | [125, 126] |
| MRP1 | Plant | [125] | |
| Ginsenoside Rg | Pgp | Plant | [127] |
| Ginsenosides | ABCG2 | Plant | [128] |
| Grapefruit juice extracts | Pgp | Plant | [129] |
| Hapalosin | Pgp | Plant | [130] |
| Harmine | ABCG2 | Plant | [131] |
| Hesperetin | ABCG2 | Plant | [119] |
| Hypericin and hyperforin | Pgp | Plant | [132] |
| Isoquinoline alkaloid, isotetrandrine | Pgp | Plant | [133] |
| Jatrophanes | Pgp | Plant | [134] |
| Kaempferol | ABCG2 | Plant | [101] |
| Kaempferia parviflora extracts | Pgp | Plant | [135] |
| MRP1 | Plant | [136] | |
| Kavalactones | Pgp | Plant | [137] |
| Kendarimide A | Pgp | Marine organisms | [138] |
| Morin | Pgp | Plant | [77] |
| Myricetin | MRP1 | Plant | [139] |
| Naringenin | ABCG2 | Plant | [101] |
| Ningalin B and derivatives | Pgp | Plant | [140, 141] |
| Opiates | Pgp | Plant | [142] |
| Phloretin | Pgp | Plant | [77] |
| Piperine | Pgp | Plant | [143] |
| Plumbagin | ABCG2 | Plant | [144] |
| Polyoxygenated steroids | Pgp | Marine organisms | [145] |
| Protopanaxatriol ginsenosides | Pgp | Plant | [146] |
| Pyranocoumarins | Pgp | Plant | [147] |
| Quercetin | ABCG2 | Plant | [119] |
| Pgp | Plant | [148, 149] | |
| MRP1 | Plant | [67, 69] | |
| Resveratrol | ABCG2 | Plant | [119] |
| Rotenoids | ABCG2 | Plant | [150] |
| Schisandrol A | Pgp | Plant | [151] |
| Sesquiterpenes | Pgp | Plant | [152] |
| Silymarin | Pgp | Plant | [77] |
| ABCG2 | Plant | [119] | |
| Sinensetin | Pgp | Plant | [153] |
| Stemona curtisii root extract | Pgp | Plant | [154] |
| MRP1 | Plant | [154] | |
| Stilbenoids | ABCG2 | Plant | [155] |
| Taxane derivatives | Pgp | Plant | [156, 157] |
| Tectochrysin | ABCG2 | Plant | [99] |
| Terpenoids | ABCG2 | Plant | [158] |
| Pgp | Plant | [159] | |
| Tetrahydrocurcumin | ABCG2 | plant | [37] |
| Tetrandine | Pgp | Plant | [160] |
| Tryprostatin A | Pgp | Fungus | [161] |
| Tryptanthrin | Pgp | Fungus | [162] |
| Vitamin E TPGS | Pgp | Plant | [163] |
Curcumin
Curcumin is a common term used for a mixture of curcuminoids that are purified from the Indian spice turmeric powder, mainly comprised of curcumin (curcumin I), demethoxycurcumin (curcumin II) and bisdemothoxycurcumin (curcumin III) [31]. Curcuminoids are known to have many biological activities, including anti-inflammatory [32, 33], anti-cancer [34], and anti-viral properties [35, 36]. In addition, both curcumin and its major metabolite tertrahydrocurcumin were found to restore drug sensitivity in cancer cells overexpressing the MDR-linked ABC transporters Pgp [31, 37], MRP1 [37, 38] and ABCG2 [37, 39] by directly inhibiting their functions. More recently, curcumin was found to be active against MDR tumors in mice as well [40]. Considering its inhibitory effect on multiple ABC drug transporters and its many beneficiary biological properties, it is not surprising that curcumin has become one of the most exciting natural product modulators in recent years.
Poor bioavailability is the one major problem with using curcumin clinically. The levels of curcumin in plasma and tissues remain low after oral consumption, reported to be in the range of nanomolar and picomolar, respectively [41]. Curcumin is lipophilic and very insoluble in nature, and is also rapidly metabolized in the intestine and excreted in the urine, which means that high doses of curcumin must be consumed for it to be biologically relevant and effective [42]. For instance, in one study, the level of curcumin was only barely detectable in human plasma even after a dose of curcumin as high as 12.0 g [43]. Therefore, several approaches have been investigated to improve the delivery of curcumin in our system [44], including the use of liposomal curcumin [45]; curcumin nanoparticles [46, 47], curcumin phospholipid complex or structural analogues of curcumin [48], or the use of a combination of curcumin and piperine. Piperine has been shown to block the metabolism of curcumin by P450 A3 and by other hepatic and intestinal pathways involved in glucuronidation of this compound [49]. In addition, it was observed that piperine increased the bioavailability of orally given curcumin in both rats and humans with no adverse effect [49]. Thus, it should be useful to test whether the combination of curcumin and piperine improves the bioavailability of coadministered anticancer drugs in cancer patients.
More importantly, both Phase I and II clinical studies with curcumin have been carried out and showed some encouraging results. Despite its poor bioavailability, Phase I studies showed that curcumin is well-tolerated [50] and provided significant improvement in 20–30% of patients with advanced colorectal cancer when treated with 360–500 mg of curcumin [41], with minimal drug-curcumin interactions [51]. Similarly, Phase II studies showed treatment with curcumin clinically can improve the outcome in patients with rheumatoid arthritis [52], chronic anterior uveitis [53], inflammatory bowel disease [54], or psoriasis [55] or in patients with advanced pancreatic cancer [56]. These clinical studies suggest that it would be worthwhile to test curcumin as an adjuvant along with traditional chemotherapy drugs to overcome MDR in cancer patients.
Flavonoids
Among all natural product modulators, the most well-known and well-studied are the flavonoids, which include flavonols, flavones, isoflavones, flavanols, flavanolols, flavanones and chalcones [57]. Typically, each person per day consumes a substantial amount of flavonoids from fruits, vegetables, food supplements, tea and wine. They are known to have many prominent health benefits [58, 59], as well as antitumor [60–62], antimitotic [61], and antiviral [61, 63] properties. They are also able to inhibit kinases [64, 65], and are active in radical scavenging and metal ion chelating [66]. Moreover, it is important to know that many flavonoids are excellent modulators of major ABC drug transporters [67–70]. Collectively, they can change the overall pharmacokinetics, including drug absorption, penetration and elimination by modulating the functions of ABC transporters [71, 72]. The result of such interaction is not at all restricted to inhibition; some flavonoids can stimulate (rather than inhibit) the function of ABC transporters, depending on the substrate of interest [73]. Also, one has to take into account that flavonoids are metabolized into flavonoid conjugates (glucuronide, sulfate or methoxylated) after ingestion, and unlikely to have the exact biological activities or inhibitory effects on ABC transporters as the unmodified forms [74].
In terms of MDR, flavonoids have been studied and characterized extensively by numerous research groups to determine their ability to inhibit Pgp-, MRP1- and ABCG2-mediated efflux and restore drug sensitivity in MDR cancer cells [67–70] (Table 1). A detailed analysis of the structure and inhibitory activity relationships of flavonoids can be found in a review by Boumendjel et al. [75]. Interest in this field developed when one of the earliest reports revealed the potential impact of active components from fruit extracts to affect the outcome of a clinical treatment using Pgp drug substrates [76]. More recently, in vitro and in vivo experiments using cell lines overexpressing ABC drug transporters and knockout animals have been designed for more detailed studies [70]. In vitro biochemical and pharmacological studies have revealed that in most cases, flavonoids modulate ABC drug transporters by competitively binding to the substrate-binding sites of transporters, thus hindering their functions [67, 69, 75, 77]. On the other hand, it appears that some flavonoids also affect ATP binding or hydrolysis at the nucleotide binding domains [75, 78], or alter the surface expression level of ABC transporters [79].
A CLINICAL PERSPECTIVE
Natural products and their derivatives have been investigated clinically for their ability to prevent, inhibit and reverse the progression of cancer. As indicated by surveys, approximately 80% of cancer patients use natural products in combination with classic anti-cancer drugs [80]. This suggests that many cancer patients are quite interested in utilizing natural products either as nutritional supplements or as complementary or alternative medicines. They expect these natural products to significantly reduce the side effects and toxicities caused by anti-cancer drugs, to increase the immune response and enhance the effectiveness of chemoprevention. Some believe they will actually stop or reverse cancer progression. In terms of overcoming ABC transporter-mediated MDR in cancer, many non-toxic natural products or metabolites that inhibit the function of ABC transporters are preferred over the use of First or Second Generation Inhibitors in clinical applications, because the inhibitors from the first two generations can induce undesirable drug-drug interactions or inhibition of physiological functions [72]. Therefore, many researchers are now interested in testing non-toxic natural products to overcome ABC transporter-mediated MDR in clinical settings.
As mentioned earlier, flavonoids are some of the most extensively studied natural products when it comes to their ability to modulate ABC drug transporters [81]. Flavonoids and other natural products usually elevate the level of intracellular concentration of anticancer drugs by directly inhibiting the function of ABC drug transporters that are overexpressed in MDR cancer cells. However, in addition to direct inhibition, many natural products overcome ABC transporter-mediated MDR by altering the bioavailability of various therapeutic drugs upon oral uptake [72]. For instance, Pgp and ABCG2 are both expressed at the apical or luminal membrane of enterocytes in the intestine, which play a role in the elimination of substrate-drugs and food components from the inside to the outside (lumen) of the cells. Although the elimination of substrates is a physiological role of ABC transporters expressed in the intestine, this function limits the absorption of substrate-drugs and food components. A good example is the co-administration of the dietary supplement biochanin A (Table 1) and the anticancer drug paclitaxel. In one report, biochanin A increased the oral bioavailability and peak plasma concentration of the Pgp substrate paclitaxel by 3.7- and 2.0-fold in a rat model [82]. Similarly, other flavonoids such as quercetin and flavone also increased the bioavailability of paclitaxel in male SD rats [83, 84]. Therefore, flavonoids are excellent candidates for modulating ABC drug transporters, as well as reducing the elimination of the co-administered anticancer drugs in in vivo systems. The role of flavonoids as ABC transport modulators affecting the bioavailability of drugs and food components has been summarized in recent reports [72, 81]. These studies demonstrate the pharmacological advantages of using natural products to enhance the uptake of co-administered anticancer drugs by cancer cells.
On the other hand, the use of natural product modulators may also increase the risk of unforeseen toxicities mediated by co-administered anticancer drugs, especially in the case when drugs with a narrow therapeutic index are used [85]. Excessive inhibition of ABC transporters by natural products may result in elevation in anticancer drug toxicity throughout the body, leading to unexpected side effects. In the same way, since both Pgp and ABCG2 are expressed at vital organ barriers such as those protecting the brain, the testes, and the fetus (Figure 1), inhibition of these ABC transporters may lead to undesirable toxicity associated with these organs.
Genetic polymorphisms in drug metabolizing enzymes and ABC transporters may also alter the activity of the anti-cancer drugs, affect the bioavailability and disposition of anticancer drugs, and result in lower therapeutic efficacy or greater toxicity [86]. For example, the natural product biochanin A, mentioned earlier, alters the bioavailability of paclitaxel, a drug substrate of both Pgp and CYP3A. This elevation in systemic paclitaxel exposure is attributed to the synergistic inhibition of both Pgp and CYP3A by biochanin A in the intestine [82]. Moreover, some of the SNPs in the MDR1 (ABCB1) gene appear to be associated with altered transporter function and expression, thereby affecting the metabolism and disposition of drugs [87, 88]. It is essential to know what concentrations of natural products are realistically able to inhibit or chemosensitize MDR cancer cells effectively. For instance, flavonoids in foods are often present as B-glycosides of aglycones and methoxylated forms. After ingestion, flavonoids are metabolized into glucuronide and sulfate and methoxylated conjugates. However, the main metabolites, such as glucuronides and sulfate conjugates may not even interact with Pgp [81]. For that reason, in vivo studies must be carried out in order to identify the active component(s), inhibitory concentrations, and the quantity of natural products needed to be ingested in order to achieve beneficial effects.
In principle, non-toxic natural products can be used as potent candidates to overcome ABC transporter-mediated MDR in cancer, to improve the oral bioavailability of anticancer drugs and provide significant health benefit to cancer patients. However, the altered bioavailability of natural products may result in unexpected side effects of anticancer drugs (or other co-administered drugs). Furthermore, genetic polymorphisms, the variability in metabolic enzymes and the expression of ABC transporters make it difficult to predict the effect of a natural product on the pharmacokinetics of the co-administered anticancer drugs clinically. In other words, when both the inhibitor (a natural product) and the drug substrate (an anticancer drug) of an ABC transporter are administered together, one should assess the potential risk resulting from altered bioavailability. Further in vivo studies and clinical data are required to elucidate the mechanisms of herb-drug and food-drug interactions mediated by ABC transporters, and to understand the pharmacokinetic profile and potential usage of natural products as chemosensitizers in MDR cancer chemotherapy.
CONCLUSIONS AND PERSPECTIVE
Multidrug resistance in cancer caused by the overexpression of ABC drug transporters is a major obstacle in modern chemotherapy. Even though many innovative approaches are available to us, finding a selective, low toxicity inhibitor/ modulator of ABC drug transporters still appears to be the most likely way to resolve this problem. However, finding such a modulator is a complex challenge due to the properties of these modulators themselves, as well as the important physiological and pharmacological roles of these ABC transporters. As a result of some unfavorable clinical outcomes from the first three generations of inhibitors, we are now at a stage of searching for alternative and novel scaffolds from natural sources. The progress of discovering Fourth Generation/ Natural Product Inhibitors is still in the early stages of exploring various extracts/ active components ranging from plants and fungi to marine organisms (Table 1). In addition to those listed in Table 1, many more herbal extracts or traditional Chinese medicines also have great potential to be developed into potent chemosensitizers, since they appear to be biologically active. For instance, traditional herbal medicines are known to have a therapeutic effect on rheumatoid arthritis [89], diabetic nephropathy [90, 91], liver fibrosis and cirrhosis [92–94], heart disease [95, 96] and many other medical conditions. Further effort must be invested in discovering more active compounds from these natural sources.
In summary, natural sources provide us with a multitude of choices ranging from yeast and plants to marine organisms, but the right approach is needed in order to be successful. In our opinion, systematic high-throughput screening of traditional Chinese and South Asian medicines or herbal extract libraries should be the first step in the discovery of non-toxic, potent and selective inhibitors. QSAR studies and manipulations utilizing combinatorial chemistry must then follow if we are to see any success in using modulators to overcome ABC drug transporter-associated multidrug resistance in clinical settings. In addition, we also need to consider the use of natural product modulators as adjuvants to traditional chemotherapy drugs to improve cancer patient treatment.
Acknowledgments
We thank George Leiman for editorial assistance in preparation of the manuscript. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Abbreviations
- ABC
ATP-binding cassette
- FTC
Fumitremorgin C
- MDR
multidrug resistance
- MRP
multidrug-resistance protein
- Pgp
P-glycoprotein
Footnotes
Conflict of Interest: All authors disclose that they do not have any affiliation with any organization with a financial interest, direct or indirect, in the subject matter or materials discussed in the manuscript that may affect the conduct or reporting of the work submitted under the heading.
References
- 1.Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 2.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5(3):219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 3.Dean M, Annilo T. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu Rev Genomics Hum Genet. 2005;6:123–142. doi: 10.1146/annurev.genom.6.080604.162122. [DOI] [PubMed] [Google Scholar]
- 4.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2(1):48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 5.Calcagno AM, Kim IW, Wu CP, Shukla S, Ambudkar SV. ABC drug transporters as molecular targets for the prevention of multidrug resistance and drug-drug interactions. Curr Drug Deliv. 2007;4(4):324–333. doi: 10.2174/156720107782151241. [DOI] [PubMed] [Google Scholar]
- 6.Lagas JS, Vlaming ML, Schinkel AH. Pharmacokinetic assessment of multiple ATP-binding cassette transporters: the power of combination knockout mice. Mol Interv. 2009;9(3):136–145. doi: 10.1124/mi.9.3.7. [DOI] [PubMed] [Google Scholar]
- 7.Sarkadi B, Homolya L, Szakacs G, Varadi A. Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol Rev. 2006;86(4):1179–1236. doi: 10.1152/physrev.00037.2005. [DOI] [PubMed] [Google Scholar]
- 8.Shukla S, Wu CP, Ambudkar SV. Development of inhibitors of ATP-binding cassette drug transporters: present status and challenges. Expert Opin Drug Metab Toxicol. 2008;4(2):205–223. doi: 10.1517/17425255.4.2.205. [DOI] [PubMed] [Google Scholar]
- 9.Loe DW, Deeley RG, Cole SP. Biology of the multidrug resistance-associated protein, MRP. Eur J Cancer. 1996;32A(6):945–957. doi: 10.1016/0959-8049(96)00046-9. [DOI] [PubMed] [Google Scholar]
- 10.Szakacs G, Varadi A, Ozvegy-Laczka C, Sarkadi B. The role of ABC transporters in drug absorption, distribution, metabolism, excretion and toxicity (ADME-Tox) Drug Discov Today. 2008;13(9–10):379–393. doi: 10.1016/j.drudis.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Chan HS, Haddad G, Thorner PS, DeBoer G, Lin YP, Ondrusek N, Yeger H, Ling V. P-glycoprotein expression as a predictor of the outcome of therapy for neuroblastoma. N Engl J Med. 1991;325(23):1608–1614. doi: 10.1056/NEJM199112053252304. [DOI] [PubMed] [Google Scholar]
- 12.Penson RT, Oliva E, Skates SJ, Glyptis T, Fuller AF, Jr, Goodman A, Seiden MV. Expression of multidrug resistance-1 protein inversely correlates with paclitaxel response and survival in ovarian cancer patients: a study in serial samples. Gynecol Oncol. 2004;93(1):98–106. doi: 10.1016/j.ygyno.2003.11.053. [DOI] [PubMed] [Google Scholar]
- 13.Steinbach D, Sell W, Voigt A, Hermann J, Zintl F, Sauerbrey A. BCRP gene expression is associated with a poor response to remission induction therapy in childhood acute myeloid leukemia. Leukemia. 2002;16(8):1443–1447. doi: 10.1038/sj.leu.2402541. [DOI] [PubMed] [Google Scholar]
- 14.van den Heuvel-Eibrink MM, Wiemer EA, Prins A, Meijerink JP, Vossebeld PJ, van der Holt B, Pieters R, Sonneveld P. Increased expression of the breast cancer resistance protein (BCRP) in relapsed or refractory acute myeloid leukemia (AML) Leukemia. 2002;16(5):833–839. doi: 10.1038/sj.leu.2402496. [DOI] [PubMed] [Google Scholar]
- 15.Hipfner DR, Deeley RG, Cole SP. Structural, mechanistic and clinical aspects of MRP1. Biochim Biophys Acta. 1999;1461(2):359–376. doi: 10.1016/s0005-2736(99)00168-6. [DOI] [PubMed] [Google Scholar]
- 16.Wu CP, Calcagno AM, Ambudkar SV. Reversal of ABC drug transporter-mediated multidrug resistance in cancer cells: evaluation of current strategies. Curr Mol Pharmacol. 2008;1(2):93–105. doi: 10.2174/1874467210801020093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker J, Martin C, Callaghan R. Inhibition of P-glycoprotein function by XR9576 in a solid tumour model can restore anticancer drug efficacy. Eur J Cancer. 2004;40(4):594–605. doi: 10.1016/j.ejca.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 18.Pusztai L, Wagner P, Ibrahim N, Rivera E, Theriault R, Booser D, Symmans FW, Wong F, Blumenschein G, Fleming DR, Rouzier R, Boniface G, Hortobagyi GN. Phase II study of tariquidar, a selective P-glycoprotein inhibitor, in patients with chemotherapy-resistant, advanced breast carcinoma. Cancer. 2005;104(4):682–691. doi: 10.1002/cncr.21227. [DOI] [PubMed] [Google Scholar]
- 19.Jekerle V, Wang JH, Scollard DA, Reilly RM, Wiese M, Piquette-Miller M. 99mTc-Sestamibi, a sensitive probe for in vivo imaging of P-glycoprotein inhibition by modulators and mdr1 antisense oligodeoxynucleotides. Mol Imaging Biol. 2006;8(6):333–339. doi: 10.1007/s11307-006-0057-0. [DOI] [PubMed] [Google Scholar]
- 20.Polgar O, Bates SE. ABC transporters in the balance: is there a role in multidrug resistance? Biochem Soc Trans. 2005;33(Pt 1):241–245. doi: 10.1042/BST0330241. [DOI] [PubMed] [Google Scholar]
- 21.Zhou SF, Di YM, Chan E, Du YM, Chow VD, Xue CC, Lai X, Wang JC, Li CG, Tian M, Duan W. Clinical pharmacogenetics and potential application in personalized medicine. Curr Drug Metab. 2008;9(8):738–784. doi: 10.2174/138920008786049302. [DOI] [PubMed] [Google Scholar]
- 22.Ford JM. Experimental reversal of P-glycoprotein-mediated multidrug resistance by pharmacological chemosensitisers. Eur J Cancer. 1996;32A(6):991–1001. doi: 10.1016/0959-8049(96)00047-0. [DOI] [PubMed] [Google Scholar]
- 23.Leonard GD, Polgar O, Bates SE. ABC transporters and inhibitors: new targets, new agents. Curr Opin Investig Drugs. 2002;3(11):1652–1659. [PubMed] [Google Scholar]
- 24.Azzariti A, Porcelli L, Simone GM, Quatrale AE, Colabufo NA, Berardi F, Perrone R, Zucchetti M, D’Incalci M, Xu JM, Paradiso A. Tyrosine kinase inhibitors and multidrug resistance proteins: interactions and biological consequences. Cancer Chemother Pharmacol. 2009;65(2):335–346. doi: 10.1007/s00280-009-1039-0. [DOI] [PubMed] [Google Scholar]
- 25.Shukla S, Sauna ZE, Ambudkar SV. Evidence for the interaction of imatinib at the transport-substrate site(s) of the multidrug-resistance-linked ABC drug transporters ABCB1 (P-glycoprotein) and ABCG2. Leukemia. 2008;22(2):445–447. doi: 10.1038/sj.leu.2404897. [DOI] [PubMed] [Google Scholar]
- 26.Dai CL, Tiwari AK, Wu CP, Su XD, Wang SR, Liu DG, Ashby CR, Jr, Huang Y, Robey RW, Liang YJ, Chen LM, Shi CJ, Ambudkar SV, Chen ZS, Fu LW. Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2. Cancer Res. 2008;68(19):7905–7914. doi: 10.1158/0008-5472.CAN-08-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stouch TR, Gudmundsson O. Progress in understanding the structure-activity relationships of P-glycoprotein. Adv Drug Deliv Rev. 2002;54(3):315–328. doi: 10.1016/s0169-409x(02)00006-6. [DOI] [PubMed] [Google Scholar]
- 28.Raub TJ. P-glycoprotein recognition of substrates and circumvention through rational drug design. Mol Pharm. 2006;3(1):3–25. doi: 10.1021/mp0500871. [DOI] [PubMed] [Google Scholar]
- 29.Rabindran SK, Ross DD, Doyle LA, Yang W, Greenberger LM. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res. 2000;60(1):47–50. [PubMed] [Google Scholar]
- 30.Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, Schellens JH, Koomen GJ, Schinkel AH. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther. 2002;1(6):417–425. [PubMed] [Google Scholar]
- 31.Chearwae W, Anuchapreeda S, Nandigama K, Ambudkar SV, Limtrakul P. Biochemical mechanism of modulation of human P-glycoprotein (ABCB1) by curcumin I, II, and III purified from Turmeric powder. Biochem Pharmacol. 2004;68(10):2043–2052. doi: 10.1016/j.bcp.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Bisht K, Wagner KH, Bulmer AC. Curcumin, resveratrol and flavonoids as anti-inflammatory, cyto- and DNA-protective dietary compounds. Toxicology. 2010;278(1):88–100. doi: 10.1016/j.tox.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14(2):141–153. [PubMed] [Google Scholar]
- 34.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23(1A):363–398. [PubMed] [Google Scholar]
- 35.De Clercq E. Current lead natural products for the chemotherapy of human immunodeficiency virus (HIV) infection. Med Res Rev. 2000;20(5):323–349. doi: 10.1002/1098-1128(200009)20:5<323::aid-med1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 36.Shishodia S, Singh T, Chaturvedi MM. Modulation of transcription factors by curcumin. Adv Exp Med Biol. 2007;595:127–148. doi: 10.1007/978-0-387-46401-5_4. [DOI] [PubMed] [Google Scholar]
- 37.Limtrakul P, Chearwae W, Shukla S, Phisalphong C, Ambudkar SV. Modulation of function of three ABC drug transporters, P-glycoprotein (ABCB1), mitoxantrone resistance protein (ABCG2) and multidrug resistance protein 1 (ABCC1) by tetrahydrocurcumin, a major metabolite of curcumin. Mol Cell Biochem. 2007;296(1–2):85–95. doi: 10.1007/s11010-006-9302-8. [DOI] [PubMed] [Google Scholar]
- 38.Chearwae W, Wu CP, Chu HY, Lee TR, Ambudkar SV, Limtrakul P. Curcuminoids purified from turmeric powder modulate the function of human multidrug resistance protein 1 (ABCC1) Cancer Chemother Pharmacol. 2006;57(3):376–388. doi: 10.1007/s00280-005-0052-1. [DOI] [PubMed] [Google Scholar]
- 39.Chearwae W, Shukla S, Limtrakul P, Ambudkar SV. Modulation of the function of the multidrug resistance-linked ATP-binding cassette transporter ABCG2 by the cancer chemopreventive agent curcumin. Mol Cancer Ther. 2006;5(8):1995–2006. doi: 10.1158/1535-7163.MCT-06-0087. [DOI] [PubMed] [Google Scholar]
- 40.Shukla S, Zaher H, Hartz A, Bauer B, Ware JA, Ambudkar SV. Curcumin inhibits the activity of ABCG2/BCRP1, a multidrug resistance-linked ABC drug transporter in mice. Pharm Res. 2009;26(2):480–487. doi: 10.1007/s11095-008-9735-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer. 2005;41(13):1955–1968. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 42.Singh S. From exotic spice to modern drug? Cell. 2007;130(5):765–768. doi: 10.1016/j.cell.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 43.Lao CD, Ruffin MTt, Normolle D, Heath DD, Murray SI, Bailey JM, Boggs ME, Crowell J, Rock CL, Brenner DE. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 45.Li L, Braiteh FS, Kurzrock R. Liposome-encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer. 2005;104(6):1322–1331. doi: 10.1002/cncr.21300. [DOI] [PubMed] [Google Scholar]
- 46.Bisht S, Feldmann G, Soni S, Ravi R, Karikar C, Maitra A, Maitra A. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J Nanobiotechnology. 2007;5:3. doi: 10.1186/1477-3155-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaikh J, Ankola DD, Beniwal V, Singh D, Kumar MN. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur J Pharm Sci. 2009;37(3–4):223–230. doi: 10.1016/j.ejps.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 48.Mosley CA, Liotta DC, Snyder JP. Highly active anticancer curcumin analogues. Adv Exp Med Biol. 2007;595:77–103. doi: 10.1007/978-0-387-46401-5_2. [DOI] [PubMed] [Google Scholar]
- 49.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64(4):353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 50.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21(4B):2895–2900. [PubMed] [Google Scholar]
- 51.Grant KL, Schneider CD. Turmeric. Am J Health Syst Pharm. 2000;57(12):1121–1122. [PubMed] [Google Scholar]
- 52.Deodhar SD, Sethi R, Srimal RC. Preliminary study on antirheumatic activity of curcumin (diferuloyl methane) Indian J Med Res. 1980;71:632–634. [PubMed] [Google Scholar]
- 53.Lal B, Kapoor AK, Asthana OP, Agrawal PK, Prasad R, Kumar P, Srimal RC. Efficacy of curcumin in the management of chronic anterior uveitis. Phytother Res. 1999;13(4):318–322. doi: 10.1002/(SICI)1099-1573(199906)13:4<318::AID-PTR445>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 54.Hsu CH, Cheng AL. Clinical studies with curcumin. Adv Exp Med Biol. 2007;595:471–480. doi: 10.1007/978-0-387-46401-5_21. [DOI] [PubMed] [Google Scholar]
- 55.Heng MC, Song MK, Harker J, Heng MK. Drug-induced suppression of phosphorylase kinase activity correlates with resolution of psoriasis as assessed by clinical, histological and immunohistochemical parameters. Br J Dermatol. 2000;143(5):937–949. doi: 10.1046/j.1365-2133.2000.03767.x. [DOI] [PubMed] [Google Scholar]
- 56.Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V, Kurzrock R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14(14):4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 57.Raj NK, Sripal RM, Chaluvadi MR, Krishna DR. Bioflavonoids classification, pharmacological, biochemical effects and therapeutic potential. Indian Journal of Pharmacology. 2001;33(1):2–16. [Google Scholar]
- 58.Patil BS, Jayaprakasha GK, Chidambara Murthy KN, Vikram A. Bioactive compounds: historical perspectives, opportunities, and challenges. J Agric Food Chem. 2009;57(18):8142–8160. doi: 10.1021/jf9000132. [DOI] [PubMed] [Google Scholar]
- 59.Crozier A, Jaganath IB, Clifford MN. Dietary phenolics: chemistry, bioavailability and effects on health. Nat Prod Rep. 2009;26(8):1001–1043. doi: 10.1039/b802662a. [DOI] [PubMed] [Google Scholar]
- 60.Cushman M, Nagarathnam D. Cytotoxicities of some flavonoid analogues. J Nat Prod. 1991;54(6):1656–1660. doi: 10.1021/np50078a027. [DOI] [PubMed] [Google Scholar]
- 61.Wang HK, Xia Y, Yang ZY, Natschke SL, Lee KH. Recent advances in the discovery and development of flavonoids and their analogues as antitumor and anti-HIV agents. Adv Exp Med Biol. 1998;439:191–225. doi: 10.1007/978-1-4615-5335-9_15. [DOI] [PubMed] [Google Scholar]
- 62.Choi SU, Ryu SY, Yoon SK, Jung NP, Park SH, Kim KH, Choi EJ, Lee CO. Effects of flavonoids on the growth and cell cycle of cancer cells. Anticancer Res. 1999;19(6B):5229–5233. [PubMed] [Google Scholar]
- 63.Selway JW. Antiviral activity of flavones and flavans. Prog Clin Biol Res. 1986;213:521–536. [PubMed] [Google Scholar]
- 64.Ferriola PC, Cody V, Middleton E., Jr Progress in understanding the structure-activity relationships of P-glycoprotein. Biochem Pharmacol. 1989;38(10):1617–1624. doi: 10.1016/0006-2952(89)90309-2. [DOI] [PubMed] [Google Scholar]
- 65.Cushman M, Zhu H, Geahlen RL, Kraker AJ. Synthesis and biochemical evaluation of a series of aminoflavones as potential inhibitors of protein-tyrosine kinases p56lck, EGFr, and p60v-src. J Med Chem. 1994;37(20):3353–3362. doi: 10.1021/jm00046a020. [DOI] [PubMed] [Google Scholar]
- 66.Cotelle N, Bernier JL, Henichart JP, Catteau JP, Gaydou E, Wallet JC. Scavenger and antioxidant properties of ten synthetic flavones. Free Radic Biol Med. 1992;13(3):211–219. doi: 10.1016/0891-5849(92)90017-b. [DOI] [PubMed] [Google Scholar]
- 67.Leslie EM, Mao Q, Oleschuk CJ, Deeley RG, Cole SP. Modulation of multidrug resistance protein 1 (MRP1/ABCC1) transport and atpase activities by interaction with dietary flavonoids. Mol Pharmacol. 2001;59(5):1171–1180. doi: 10.1124/mol.59.5.1171. [DOI] [PubMed] [Google Scholar]
- 68.Zhang S, Yang X, Morris ME. Flavonoids are inhibitors of breast cancer resistance protein (ABCG2)-mediated transport. Mol Pharmacol. 2004;65(5):1208–1216. doi: 10.1124/mol.65.5.1208. [DOI] [PubMed] [Google Scholar]
- 69.Wu CP, Calcagno AM, Hladky SB, Ambudkar SV, Barrand MA. Modulatory effects of plant phenols on human multidrug-resistance proteins 1, 4 and 5 (ABCC1, 4 and 5) FEBS J. 2005;272(18):4725–4740. doi: 10.1111/j.1742-4658.2005.04888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morris ME, Zhang S. Flavonoid-drug interactions: effects of flavonoids on ABC transporters. Life Sci. 2006;78(18):2116–2130. doi: 10.1016/j.lfs.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 71.Cermak R, Wolffram S. The potential of flavonoids to influence drug metabolism and pharmacokinetics by local gastrointestinal mechanisms. Curr Drug Metab. 2006;7(7):729–744. doi: 10.2174/138920006778520570. [DOI] [PubMed] [Google Scholar]
- 72.Brand W, Schutte ME, Williamson G, van Zanden JJ, Cnubben NH, Groten JP, van Bladeren PJ, Rietjens IM. Flavonoid-mediated inhibition of intestinal ABC transporters may affect the oral bioavailability of drugs, food-borne toxic compounds and bioactive ingredients. Biomed Pharmacother. 2006;60(9):508–519. doi: 10.1016/j.biopha.2006.07.081. [DOI] [PubMed] [Google Scholar]
- 73.Piao Y, Shin SC, Choi JS. Effects of oral kaempferol on the pharmacokinetics of tamoxifen and one of its metabolites, 4-hydroxytamoxifen, after oral administration of tamoxifen to rats. Biopharm Drug Dispos. 2008;29(4):245–249. doi: 10.1002/bdd.593. [DOI] [PubMed] [Google Scholar]
- 74.Forester SC, Waterhouse AL. Metabolites are key to understanding health effects of wine polyphenolics. J Nutr. 2009;139(9):1824S–1831S. doi: 10.3945/jn.109.107664. [DOI] [PubMed] [Google Scholar]
- 75.Boumendjel A, Di Pietro A, Dumontet C, Barron D. Recent advances in the discovery of flavonoids and analogs with high-affinity binding to P-glycoprotein responsible for cancer cell multidrug resistance. Med Res Rev. 2002;22(5):512–529. doi: 10.1002/med.10015. [DOI] [PubMed] [Google Scholar]
- 76.Bailey DG, Spence JD, Munoz C, Arnold JM. Interaction of citrus juices with felodipine and nifedipine. Lancet. 1991;337(8736):268–269. doi: 10.1016/0140-6736(91)90872-m. [DOI] [PubMed] [Google Scholar]
- 77.Zhang S, Morris ME. Effects of the flavonoids biochanin A, morin, phloretin, and silymarin on P-glycoprotein-mediated transport. J Pharmacol Exp Ther. 2003;304(3):1258–1267. doi: 10.1124/jpet.102.044412. [DOI] [PubMed] [Google Scholar]
- 78.Conseil G, Baubichon-Cortay H, Dayan G, Jault JM, Barron D, Di Pietro A. Flavonoids: a class of modulators with bifunctional interactions at vicinal ATPand steroid-binding sites on mouse P-glycoprotein. Proc Natl Acad Sci U S A. 1998;95(17):9831–9836. doi: 10.1073/pnas.95.17.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou S, Lim LY, Chowbay B. Herbal modulation of P-glycoprotein. Drug Metab Rev. 2004;36(1):57–104. doi: 10.1081/dmr-120028427. [DOI] [PubMed] [Google Scholar]
- 80.Dennis T, Fanous M, Mousa S. Natural products for chemopreventive and adjunctive therapy in oncologic disease. Nutr Cancer. 2009;61(5):587–597. doi: 10.1080/01635580902825530. [DOI] [PubMed] [Google Scholar]
- 81.Bansal T, Jaggi M, Khar RK, Talegaonkar S. Emerging significance of flavonoids as P-glycoprotein inhibitors in cancer chemotherapy. J Pharm Pharm Sci. 2009;12(1):46–78. doi: 10.18433/j3rc77. [DOI] [PubMed] [Google Scholar]
- 82.Peng SX, Ritchie DM, Cousineau M, Danser E, Dewire R, Floden J. Altered oral bioavailability and pharmacokinetics of P-glycoprotein substrates by coadministration of biochanin A. J Pharm Sci. 2006;95(9):1984–1993. doi: 10.1002/jps.20664. [DOI] [PubMed] [Google Scholar]
- 83.Choi JS, Jo BW, Kim YC. Enhanced paclitaxel bioavailability after oral administration of paclitaxel or prodrug to rats pretreated with quercetin. Eur J Pharm Biopharm. 2004;57(2):313–318. doi: 10.1016/j.ejpb.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 84.Choi JS, Choi HK, Shin SC. Enhanced bioavailability of paclitaxel after oral coadministration with flavone in rats. Int J Pharm. 2004;275(1–2):165–170. doi: 10.1016/j.ijpharm.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 85.Marchetti S, Mazzanti R, Beijnen JH, Schellens JH. Concise review: Clinical relevance of drug drug and herb drug interactions mediated by the ABC transporter ABCB1 (MDR1, P-glycoprotein) Oncologist. 2007;12(8):927–941. doi: 10.1634/theoncologist.12-8-927. [DOI] [PubMed] [Google Scholar]
- 86.Ekhart C, Rodenhuis S, Smits PH, Beijnen JH, Huitema AD. An overview of the relations between polymorphisms in drug metabolising enzymes and drug transporters and survival after cancer drug treatment. Cancer Treat Rev. 2009;35(1):18–31. doi: 10.1016/j.ctrv.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 87.Wu L, Xu X, Shen J, Xie H, Yu S, Liang T, Wang W, Shen Y, Zhang M, Zheng S. MDR1 gene polymorphisms and risk of recurrence in patients with hepatocellular carcinoma after liver transplantation. J Surg Oncol. 2007;96(1):62–68. doi: 10.1002/jso.20774. [DOI] [PubMed] [Google Scholar]
- 88.Jamroziak K, Mlynarski W, Balcerczak E, Mistygacz M, Trelinska J, Mirowski M, Bodalski J, Robak T. Functional C3435T polymorphism of MDR1 gene: an impact on genetic susceptibility and clinical outcome of childhood acute lymphoblastic leukemia. Eur J Haematol. 2004;72(5):314–321. doi: 10.1111/j.1600-0609.2004.00228.x. [DOI] [PubMed] [Google Scholar]
- 89.Wang LR, Ishiguro N, Yamada E, Nishida Y, Sato K, Iwata H. The effect of da-fang-feng-tang on treatment of type II collagen-induced arthritis in DBA/1 mice. Am J Chin Med. 1999;27(2):205–215. doi: 10.1142/S0192415X99000240. [DOI] [PubMed] [Google Scholar]
- 90.Goto H, Shimada Y, Tanikawa K, Sato S, Hikiami H, Sekiya N, Terasawa K. Clinical evaluation of the effect of daio (rhei rhizoma) on the progression of diabetic nephropathy with overt proteinuria. Am J Chin Med. 2003;31(2):267–275. doi: 10.1142/S0192415X03000850. [DOI] [PubMed] [Google Scholar]
- 91.Yokozawa T, Satoh A, Nakagawa T, Yamabe N. Attenuating effects of wen-pi-tang treatment in rats with diabetic nephropathy. Am J Chin Med. 2006;34(2):307–321. doi: 10.1142/S0192415X06003850. [DOI] [PubMed] [Google Scholar]
- 92.Li CX, Li L, Lou J, Yang WX, Lei TW, Li YH, Liu J, Cheng ML, Huang LH. The protective effects of traditional Chinese medicine prescription, han-dan-gan-le, on CCl4-induced liver fibrosis in rats. Am J Chin Med. 1998;26(3–4):325–332. doi: 10.1142/S0192415X98000361. [DOI] [PubMed] [Google Scholar]
- 93.Schuppan D, Jia JD, Brinkhaus B, Hahn EG. Herbal products for liver diseases: a therapeutic challenge for the new millennium. Hepatology. 1999;30(4):1099–1104. doi: 10.1002/hep.510300437. [DOI] [PubMed] [Google Scholar]
- 94.Stickel F, Schuppan D. Herbal medicine in the treatment of liver diseases. Dig Liver Dis. 2007;39(4):293–304. doi: 10.1016/j.dld.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 95.Kuo C. Successful treatment of complete left bundle branch block complicating acute viral myocarditis employing Chinese herbs. Am J Chin Med. 1986;14(3–4):124–130. doi: 10.1142/S0192415X8600020X. [DOI] [PubMed] [Google Scholar]
- 96.Chen XJ, Bian ZP, Lu S, Xu JD, Gu CR, Yang D, Zhang JN. Cardiac protective effect of Astragalus on viral myocarditis mice: comparison with Perindopril. Am J Chin Med. 2006;34(3):493–502. doi: 10.1142/S0192415X06004028. [DOI] [PubMed] [Google Scholar]
- 97.Katayama K, Masuyama K, Yoshioka S, Hasegawa H, Mitsuhashi J, Sugimoto Y. Flavonoids inhibit breast cancer resistance protein-mediated drug resistance: transporter specificity and structure-activity relationship. Cancer Chemother Pharmacol. 2007;60(6):789–797. doi: 10.1007/s00280-007-0426-7. [DOI] [PubMed] [Google Scholar]
- 98.Jin J, Wang FP, Wei H, Liu G. Reversal of multidrug resistance of cancer through inhibition of P-glycoprotein by 5-bromotetrandrine. Cancer Chemother Pharmacol. 2005;55(2):179–188. doi: 10.1007/s00280-004-0868-0. [DOI] [PubMed] [Google Scholar]
- 99.Ahmed-Belkacem A, Pozza A, Munoz-Martinez F, Bates SE, Castanys S, Gamarro F, Di Pietro A, Perez-Victoria JM. Flavonoid structure-activity studies identify 6-prenylchrysin and tectochrysin as potent and specific inhibitors of breast cancer resistance protein ABCG2. Cancer Res. 2005;65(11):4852–4860. doi: 10.1158/0008-5472.CAN-04-1817. [DOI] [PubMed] [Google Scholar]
- 100.Madureira AM, Molnar A, Abreu PM, Molnar J, Ferreira MJ. A new sesquiterpene-coumarin ether and a new abietane diterpene and their effects as inhibitors of P-glycoprotein. Planta Med. 2004;70(9):828–833. doi: 10.1055/s-2004-827231. [DOI] [PubMed] [Google Scholar]
- 101.Imai Y, Tsukahara S, Asada S, Sugimoto Y. Phytoestrogens/flavonoids reverse breast cancer resistance protein/ABCG2-mediated multidrug resistance. Cancer Res. 2004;64(12):4346–4352. doi: 10.1158/0008-5472.CAN-04-0078. [DOI] [PubMed] [Google Scholar]
- 102.Mitsuo M, Noguchi T, Nakajima Y, Aoki S, Ren XQ, Sumizawa T, Haraguchi M, Kobayashi M, Baba M, Nagata Y, Akiyama S, Furukawa T. Binding site(s) on P-glycoprotein for a newly synthesized photoaffinity analog of agosterol A. Oncol Res. 2003;14(1):39–48. doi: 10.3727/000000003108748595. [DOI] [PubMed] [Google Scholar]
- 103.Wang C, Zhang JX, Shen XL, Wan CK, Tse AK, Fong WF. Reversal of P-glycoprotein-mediated multidrug resistance by Alisol B 23-acetate. Biochem Pharmacol. 2004;68(5):843–855. doi: 10.1016/j.bcp.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 104.Ramachandran C, Rabi T, Fonseca HB, Melnick SJ, Escalon EA. Novel plant triterpenoid drug amooranin overcomes multidrug resistance in human leukemia and colon carcinoma cell lines. Int J Cancer. 2003;105(6):784–789. doi: 10.1002/ijc.11180. [DOI] [PubMed] [Google Scholar]
- 105.Engi H, Hohmann J, Gang G, Pusztai R, Redei D, Kovacs O, Schelz Z, Molnar J. Chemoprevention and inhibition of P-glycoprotein in cancer cells by Chinese medicinal herbs. Phytother Res. 2008;22(12):1671–1676. doi: 10.1002/ptr.2554. [DOI] [PubMed] [Google Scholar]
- 106.Lee Y, Yeo H, Liu SH, Jiang Z, Savizky RM, Austin DJ, Cheng YC. Increased anti-P-glycoprotein activity of baicalein by alkylation on the A ring. J Med Chem. 2004;47(22):5555–5566. doi: 10.1021/jm049949c. [DOI] [PubMed] [Google Scholar]
- 107.Limtrakul P, Khantamat O, Pintha K. Inhibition of P-glycoprotein activity and reversal of cancer multidrug resistance by Momordica charantia extract. Cancer Chemother Pharmacol. 2004;54(6):525–530. doi: 10.1007/s00280-004-0848-4. [DOI] [PubMed] [Google Scholar]
- 108.Zheng LH, Bao YL, Wu Y, Yu CL, Meng X, Li YX. Cantharidin reverses multidrug resistance of human hepatoma HepG2/ADM cells via down-regulation of P-glycoprotein expression. Cancer Lett. 2008;272(1):102–109. doi: 10.1016/j.canlet.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 109.Holland ML, Allen JD, Arnold JC. Interaction of plant cannabinoids with the multidrug transporter ABCC1 (MRP1) Eur J Pharmacol. 2008;591(1–3):128–131. doi: 10.1016/j.ejphar.2008.06.079. [DOI] [PubMed] [Google Scholar]
- 110.Zhu HJ, Wang JS, Markowitz JS, Donovan JL, Gibson BB, Gefroh HA, Devane CL. Characterization of P-glycoprotein inhibition by major cannabinoids from marijuana. J Pharmacol Exp Ther. 2006;317(2):850–857. doi: 10.1124/jpet.105.098541. [DOI] [PubMed] [Google Scholar]
- 111.Holland ML, Panetta JA, Hoskins JM, Bebawy M, Roufogalis BD, Allen JD, Arnold JC. The effects of cannabinoids on P-glycoprotein transport and expression in multidrug resistant cells. Biochem Pharmacol. 2006;71(8):1146–1154. doi: 10.1016/j.bcp.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 112.Holland ML, Lau DT, Allen JD, Arnold JC. The multidrug transporter ABCG2 (BCRP) is inhibited by plant-derived cannabinoids. Br J Pharmacol. 2007;152(5):815–824. doi: 10.1038/sj.bjp.0707467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kitagawa S, Nabekura T, Kamiyama S. Inhibition of P-glycoprotein function by tea catechins in KB-C2 cells. J Pharm Pharmacol. 2004;56(8):1001–1005. doi: 10.1211/0022357044003. [DOI] [PubMed] [Google Scholar]
- 114.Koizumi S, Konishi M, Ichihara T, Wada H, Matsukawa H, Goi K, Mizutani S. Flow cytometric functional analysis of multidrug resistance by Fluo-3: a comparison with rhodamine-123. Eur J Cancer. 1995;31A(10):1682–1688. doi: 10.1016/0959-8049(95)00288-t. [DOI] [PubMed] [Google Scholar]
- 115.Abe T, Koike K, Ohga T, Kubo T, Wada M, Kohno K, Mori T, Hidaka K, Kuwano M. Chemosensitisation of spontaneous multidrug resistance by a 1,4-dihydropyridine analogue and verapamil in human glioma cell lines overexpressing MRP or MDR1. Br J Cancer. 1995;72(2):418–423. doi: 10.1038/bjc.1995.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Raad I, Terreux R, Richomme P, Matera EL, Dumontet C, Raynaud J, Guilet D. Structure-activity relationship of natural and synthetic coumarins inhibiting the multidrug transporter P-glycoprotein. Bioorg Med Chem. 2006;14(20):6979–6987. doi: 10.1016/j.bmc.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 117.Anuchapreeda S, Leechanachai P, Smith MM, Ambudkar SV, Limtrakul PN. Modulation of P-glycoprotein expression and function by curcumin in multidrug-resistant human KB cells. Biochem Pharmacol. 2002;64(4):573–582. doi: 10.1016/s0006-2952(02)01224-8. [DOI] [PubMed] [Google Scholar]
- 118.Madureira AM, Spengler G, Molnar A, Varga A, Molnar J, Abreu PM, Ferreira MJ. Effect of cycloartanes on reversal of multidrug resistance and apoptosis induction on mouse lymphoma cells. Anticancer Res. 2004;24(2B):859–864. [PubMed] [Google Scholar]
- 119.Cooray HC, Janvilisri T, van Veen HW, Hladky SB, Barrand MA. Interaction of the breast cancer resistance protein with plant polyphenols. Biochem Biophys Res Commun. 2004;317(1):269–275. doi: 10.1016/j.bbrc.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 120.Yoo HH, Lee M, Lee MW, Lim SY, Shin J, Kim DH. Effects of Schisandra lignans on P-glycoprotein-mediated drug efflux in human intestinal Caco-2. Planta Med. 2007;73(5):444–450. doi: 10.1055/s-2007-967178. [DOI] [PubMed] [Google Scholar]
- 121.Lim S, Grassi J, Akhmedjanova V, Debiton E, Balansard G, Beliveau R, Barthomeuf C. Reversal of P-glycoprotein-mediated drug efflux by eudesmin from Haplophyllum perforatum and cytotoxicity pattern versus diphyllin, podophyllotoxin and etoposide. Planta Med. 2007;73(15):1563–1567. doi: 10.1055/s-2007-993754. [DOI] [PubMed] [Google Scholar]
- 122.Henrich CJ, Bokesch HR, Dean M, Bates SE, Robey RW, Goncharova EI, Wilson JA, McMahon JB. A high-throughput cell-based assay for inhibitors of ABCG2 activity. J Biomol Screen. 2006;11(2):176–183. doi: 10.1177/1087057105284576. [DOI] [PubMed] [Google Scholar]
- 123.Corea G, Fattorusso E, Lanzotti V, Motti R, Simon PN, Dumontet C, Di Pietro A. Structure-activity relationships for euphocharacins A-L, a new series of jatrophane diterpenes, as inhibitors of cancer cell P-glycoprotein. Planta Med. 2004;70(7):657–665. doi: 10.1055/s-2004-827191. [DOI] [PubMed] [Google Scholar]
- 124.Robey RW, Medina-Perez WY, Nishiyama K, Lahusen T, Miyake K, Litman T, Senderowicz AM, Ross DD, Bates SE. Overexpression of the ATP-binding cassette half-transporter, ABCG2 (Mxr/BCrp/ABCP1), in flavopiridol-resistant human breast cancer cells. Clin Cancer Res. 2001;7(1):145–152. [PubMed] [Google Scholar]
- 125.Nabekura T, Yamaki T, Ueno K, Kitagawa S. Inhibition of P-glycoprotein and multidrug resistance protein 1 by dietary phytochemicals. Cancer Chemother Pharmacol. 2008;62(5):867–873. doi: 10.1007/s00280-007-0676-4. [DOI] [PubMed] [Google Scholar]
- 126.Fan L, Tao GY, Wang G, Chen Y, Zhang W, He YJ, Li Q, Lei HP, Jiang F, Hu DL, Huang YF, Zhou HH. Effects of Ginkgo biloba extract ingestion on the pharmacokinetics of talinolol in healthy Chinese volunteers. Ann Pharmacother. 2009;43(5):944–949. doi: 10.1345/aph.1L656. [DOI] [PubMed] [Google Scholar]
- 127.Kim SW, Kwon HY, Chi DW, Shim JH, Park JD, Lee YH, Pyo S, Rhee DK. Reversal of P-glycoprotein-mediated multidrug resistance by ginsenoside Rg(3) Biochem Pharmacol. 2003;65(1):75–82. doi: 10.1016/s0006-2952(02)01446-6. [DOI] [PubMed] [Google Scholar]
- 128.Jin J, Shahi S, Kang HK, van Veen HW, Fan TP. Metabolites of ginsenosides as novel BCRP inhibitors. Biochem Biophys Res Commun. 2006;345(4):1308–1314. doi: 10.1016/j.bbrc.2006.04.152. [DOI] [PubMed] [Google Scholar]
- 129.de Castro WV, Mertens-Talcott S, Derendorf H, Butterweck V. Grapefruit juice-drug interactions: Grapefruit juice and its components inhibit P-glycoprotein (ABCB1) mediated transport of talinolol in Caco-2 cells. J Pharm Sci. 2007;96(10):2808–2817. doi: 10.1002/jps.20975. [DOI] [PubMed] [Google Scholar]
- 130.Palomo C, Oiarbide M, Garcia JM, Gonzalez A, Pazos R, Odriozola JM, Banuelos P, Tello M, Linden A. A practical total synthesis of hapalosin, a 12-membered cyclic depsipeptide with multidrug resistance-reversing activity, by employing improved segment coupling and macrolactonization. J Org Chem. 2004;69(12):4126–4134. doi: 10.1021/jo0497499. [DOI] [PubMed] [Google Scholar]
- 131.Ma Y, Wink M. The beta-carboline alkaloid harmine inhibits BCRP and can reverse resistance to the anticancer drugs mitoxantrone and camptothecin in breast cancer cells. Phytother Res. 2010;24(1):146–149. doi: 10.1002/ptr.2860. [DOI] [PubMed] [Google Scholar]
- 132.Wang EJ, Barecki-Roach M, Johnson WW. Quantitative characterization of direct P-glycoprotein inhibition by St John’s wort constituents hypericin and hyperforin. J Pharm Pharmacol. 2004;56(1):123–128. doi: 10.1211/0022357022395. [DOI] [PubMed] [Google Scholar]
- 133.Wang TX, Yang XH. Reversal effect of isotetrandrine, an isoquinoline alkaloid extracted from Caulis Mahoniae, on P-glycoprotein-mediated doxorubicin-resistance in human breast cancer (MCF-7/DOX) cells. Yao Xue Xue Bao. 2008;43(5):461–466. [PubMed] [Google Scholar]
- 134.Hohmann J, Redei D, Forgo P, Molnar J, Dombi G, Zorig T. Jatrophane diterpenoids from Euphorbia mongolica as modulators of the multidrug resistance of L5128 mouse lymphoma cells. J Nat Prod. 2003;66(7):976–979. doi: 10.1021/np030036f. [DOI] [PubMed] [Google Scholar]
- 135.Patanasethanont D, Nagai J, Yumoto R, Murakami T, Sutthanut K, Sripanidkulchai BO, Yenjai C, Takano M. Effects of Kaempferia parviflora extracts and their flavone constituents on P-glycoprotein function. J Pharm Sci. 2007;96(1):223–233. doi: 10.1002/jps.20769. [DOI] [PubMed] [Google Scholar]
- 136.Patanasethanont D, Nagai J, Matsuura C, Fukui K, Sutthanut K, Sripanidkulchai BO, Yumoto R, Takano M. Modulation of function of multidrug resistance associated-proteins by Kaempferia parviflora extracts and their components. Eur J Pharmacol. 2007;566(1–3):67–74. doi: 10.1016/j.ejphar.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 137.Weiss J, Sauer A, Frank A, Unger M. Extracts and kavalactones of Piper methysticum G. Forst (kava-kava) inhibit P-glycoprotein in vitro. Drug Metab Dispos. 2005;33(11):1580–1583. doi: 10.1124/dmd.105.005892. [DOI] [PubMed] [Google Scholar]
- 138.Aoki S, Cao LW, Matsui K, Rachmat R, Akiyama S, Kobayashi M. Kendarimide A, a novel peptide reversing P-glycoprotein-mediated multidrug resistance in tumor cells, from a marine sponge of Haliclona sp. Tetrahedron. 2004;60(33):7053–7059. [Google Scholar]
- 139.van Zanden JJ, de Mul A, Wortelboer HM, Usta M, van Bladeren PJ, Rietjens IM, Cnubben NH. Reversal of in vitro cellular MRP1 and MRP2 mediated vincristine resistance by the flavonoid myricetin. Biochem Pharmacol. 2005;69(11):1657–1665. doi: 10.1016/j.bcp.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 140.Soenen DR, Hwang I, Hedrick MP, Boger DL. Multidrug resistance reversal activity of key ningalin analogues. Bioorg Med Chem Lett. 2003;13(10):1777–1781. doi: 10.1016/s0960-894x(03)00294-4. [DOI] [PubMed] [Google Scholar]
- 141.Tao H, Hwang I, Boger DL. Multidrug resistance reversal activity of permethyl ningalin B amide derivatives. Bioorg Med Chem Lett. 2004;14(24):5979–5981. doi: 10.1016/j.bmcl.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 142.Hemauer SJ, Patrikeeva SL, Nanovskaya TN, Hankins GD, Ahmed MS. Opiates inhibit paclitaxel uptake by P-glycoprotein in preparations of human placental inside-out vesicles. Biochem Pharmacol. 2009;78(9):1272–1278. doi: 10.1016/j.bcp.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Han Y, Chin Tan TM, Lim LY. In vitro and in vivo evaluation of the effects of piperine on P-gp function and expression. Toxicol Appl Pharmacol. 2008;230(3):283–289. doi: 10.1016/j.taap.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 144.Shukla S, Wu CP, Nandigama K, Ambudkar SV. The naphthoquinones, vitamin K3 and its structural analogue plumbagin, are substrates of the multidrug resistance linked ATP binding cassette drug transporter ABCG2. Mol Cancer Ther. 2007;6(12 Pt 1):3279–3286. doi: 10.1158/1535-7163.MCT-07-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tanaka J, Trianto A, Musman M, Issa HH, Ohtani II, Ichiba T, Higa T, Yoshida WY, Scheuer PJ. New polyoxygenated steroids exhibiting reversal of multidrug resistance from the gorgonian Isis hippuris. Tetrahedron. 2002;58(32):6259–6266. [Google Scholar]
- 146.Choi CH, Kang G, Min YD. Reversal of P-glycoprotein-mediated multidrug resistance by protopanaxatriol ginsenosides from Korean red ginseng. Planta Med. 2003;69(3):235–240. doi: 10.1055/s-2003-38483. [DOI] [PubMed] [Google Scholar]
- 147.Wu JY, Fong WF, Zhang JX, Leung CH, Kwong HL, Yang MS, Li D, Cheung HY. Reversal of multidrug resistance in cancer cells by pyranocoumarins isolated from Radix Peucedani. Eur J Pharmacol. 2003;473(1):9–17. doi: 10.1016/s0014-2999(03)01946-0. [DOI] [PubMed] [Google Scholar]
- 148.Scambia G, Ranelletti FO, Panici PB, De Vincenzo R, Bonanno G, Ferrandina G, Piantelli M, Bussa S, Rumi C, Cianfriglia M, Mancuso S. Quercetin potentiates the effect of adriamycin in a multidrug-resistant MCF-7 human breast-cancer cell line: P-glycoprotein as a possible target. Cancer Chemother Pharmacol. 1994;34(6):459–464. doi: 10.1007/BF00685655. [DOI] [PubMed] [Google Scholar]
- 149.Limtrakul P, Khantamat O, Pintha K. Inhibition of P-glycoprotein function and expression by kaempferol and quercetin. J Chemother. 2005;17(1):86–95. doi: 10.1179/joc.2005.17.1.86. [DOI] [PubMed] [Google Scholar]
- 150.Ahmed-Belkacem A, Macalou S, Borrelli F, Capasso R, Fattorusso E, Taglialatela-Scafati O, Di Pietro A. Nonprenylated rotenoids, a new class of potent breast cancer resistance protein inhibitors. J Med Chem. 2007;50(8):1933–1938. doi: 10.1021/jm061450q. [DOI] [PubMed] [Google Scholar]
- 151.Fong WF, Wan CK, Zhu GY, Chattopadhyay A, Dey S, Zhao Z, Shen XL. Schisandrol A from Schisandra chinensis reverses P-glycoprotein-mediated multidrug resistance by affecting Pgp-substrate complexes. Planta Med. 2007;73(3):212–220. doi: 10.1055/s-2007-967120. [DOI] [PubMed] [Google Scholar]
- 152.Munoz-Martinez F, Lu P, Cortes-Selva F, Perez-Victoria JM, Jimenez IA, Ravelo AG, Sharom FJ, Gamarro F, Castanys S. Celastraceae sesquiterpenes as a new class of modulators that bind specifically to human P-glycoprotein and reverse cellular multidrug resistance. Cancer Res. 2004;64(19):7130–7138. doi: 10.1158/0008-5472.CAN-04-1005. [DOI] [PubMed] [Google Scholar]
- 153.Choi CH, Sun KH, An CS, Yoo JC, Hahm KS, Lee IH, Sohng JK, Kim YC. Reversal of P-glycoprotein-mediated multidrug resistance by 5,6,7,3′,4′-pentamethoxyflavone (Sinensetin) Biochem Biophys Res Commun. 2002;295(4):832–840. doi: 10.1016/s0006-291x(02)00755-6. [DOI] [PubMed] [Google Scholar]
- 154.Limtrakul P, Siwanon S, Yodkeeree S, Duangrat C. Effect of Stemona curtisii root extract on P-glycoprotein and MRP-1 function in multidrug-resistant cancer cells. Phytomedicine. 2007;14(6):381–389. doi: 10.1016/j.phymed.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 155.Morita H, Koyama K, Sugimoto Y, Kobayashi J. Antimitotic activity and reversal of breast cancer resistance protein-mediated drug resistance by stilbenoids from Bletilla striata. Bioorg Med Chem Lett. 2005;15(4):1051–1054. doi: 10.1016/j.bmcl.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 156.Brooks TA, Kennedy DR, Gruol DJ, Ojima I, Baer MR, Bernacki RJ. Structure-activity analysis of taxane-based broad-spectrum multidrug resistance modulators. Anticancer Res. 2004;24(2A):409–415. [PubMed] [Google Scholar]
- 157.Zhao X, Gu J, Yin D, Chen X. Synthesis and biological evaluation of taxinine analogues as orally active multidrug resistance reversal agents in cancer. Bioorg Med Chem Lett. 2004;14(18):4767–4770. doi: 10.1016/j.bmcl.2004.06.089. [DOI] [PubMed] [Google Scholar]
- 158.Yoshida N, Takada T, Yamamura Y, Adachi I, Suzuki H, Kawakami J. Inhibitory effects of terpenoids on multidrug resistance-associated protein 2- and breast cancer resistance protein-mediated transport. Drug Metab Dispos. 2008;36(7):1206–1211. doi: 10.1124/dmd.107.019513. [DOI] [PubMed] [Google Scholar]
- 159.Yoshida N, Koizumi M, Adachi I, Kawakami J. Inhibition of P-glycoprotein-mediated transport by terpenoids contained in herbal medicines and natural products. Food Chem Toxicol. 2006;44(12):2033–2039. doi: 10.1016/j.fct.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 160.Fu L, Liang Y, Deng L, Ding Y, Chen L, Ye Y, Yang X, Pan Q. Characterization of tetrandrine, a potent inhibitor of P-glycoprotein-mediated multidrug resistance. Cancer Chemother Pharmacol. 2004;53(4):349–356. doi: 10.1007/s00280-003-0742-5. [DOI] [PubMed] [Google Scholar]
- 161.Woehlecke H, Osada H, Herrmann A, Lage H. Reversal of breast cancer resistance protein-mediated drug resistance by tryprostatin A. Int J Cancer. 2003;107(5):721–728. doi: 10.1002/ijc.11444. [DOI] [PubMed] [Google Scholar]
- 162.Yu ST, Chen TM, Tseng SY, Chen YH. Tryptanthrin inhibits MDR1 and reverses doxorubicin resistance in breast cancer cells. Biochem Biophys Res Commun. 2007;358(1):79–84. doi: 10.1016/j.bbrc.2007.04.107. [DOI] [PubMed] [Google Scholar]
- 163.Collnot EM, Baldes C, Wempe MF, Kappl R, Huttermann J, Hyatt JA, Edgar KJ, Schaefer UF, Lehr CM. Mechanism of inhibition of P-glycoprotein mediated efflux by vitamin E TPGS: influence on ATPase activity and membrane fluidity. Mol Pharm. 2007;4(3):465–474. doi: 10.1021/mp060121r. [DOI] [PubMed] [Google Scholar]