Abstract
Based on remarkable success of PTH as an anabolic drug for osteoporosis, case reports of off-label use of teriparatide (1-34 PTH) in patients with complicated fractures and non-unions are emerging. We investigated the mechanisms underlying PTH accelerated fracture repair. Bone marrow cells from 7 days 40cg/kg of teriparatide treated or saline control mice were cultured and Osx and osteoblast phenotypic gene expression assessed by real time RT-PCR in these cells. Fractured animals injected daily with either saline or 40cg/kg of teriparatide for up to 21 days were X-rayed and histological assessment performed, as well as immunohistochemical analyses of the Osx expression in the fracture callus. Osx, Runx2 and osteoblast or chondrocyte phenotypic gene expression was also assessed in fracture calluses. Our data shows that Osx and Runx2 are up-regulated in marrow-derived MSCs isolated from mice systemically treated with teriparatide. Furthermore, these MSCs undergo accelerated osteoblast maturation compared to saline injected controls. Systemic teriparatide treatments also accelerated fracture healing in these mice concomitantly with increased Osx expression in the PTH treated fracture calluses compared to controls. Collectively, these data suggest a mechanism for teriparatide mediated fracture healing possibly via Osx induction in MSCs.
Keywords: Teriparatide, PTH, Osterix/Sp7, Runx2, fractures
Introduction
Osteoporosis affecting more than 20 million Americans accounts for approximately 1.5 million fragility fractures every year [Finkelstein J.S., 2000], including more than 700, 000 vertebral fractures and over 300, 000 hip fractures [Riggs et al., 1995]. The probability of death following a hip fracture in osteoporotic patients is estimated to be 10 to 20% within the first year, and less than 50% of survivors regain their pre-fracture level of mobility and independence [Finkelstein J.S., 2000]. Furthermore, mortality within 90 days of an osteoporotic fracture in individuals who are older than 65 years is substantially higher than predicted; and for a subset of these fractures the risk for early lethality increases by approximately seven fold [Heaney, 2003]. During the past decade, the prevention of osteoporotic fractures has been limited to the use of anti-resorptive drugs [Cranney et al., 2002], which have proven to be insufficient for decreasing the mortality and morbidity in this patient population.
The known anabolic effects of parathyroid hormone (PTH) on bone formation has led to the development of a human recombinant peptide, teriparatide (1-34 human parathyroid hormone), corresponding to the first 34 amino acids of PTH. This drug was initially developed to treat postmenopausal osteoporotic women to enhance bone mineral density [Neer et al., 2001], and cortical thickness and trabecular bone volume [Jiang et al., 2005; Kurland et al., 2000] compared to placebo controls. In addition to its anabolic effects on bone turnover [Dempster et al., 2001; Neer et al., 2001; Rodan et al., 2000] teriparatide used in clinical trials was shown to significantly reduce the risk of vertebral and non-vertebral fractures in osteoporotic women [Black et al., 2003; Neer et al., 2001]. Using various animal models, several groups have shown that intermittent exposure to PTH stimulates osteoblast differentiation and function [Hock et al., 1992; Kulkarni et al., 2007] in vivo. In addition, this intermittent PTH treatment was also shown to enhance fracture callus formation [Nakazawa et al., 2005] and increase mechanical strength in adult rat fractures [Andreassen et al., 1999; Andreassen et al., 2004] in vivo. This PTH mediated anabolic effect was sustained throughout the remodeling phase of fracture healing [Alkhiary et al., 2005].
We hypothesized that PTH may enhance Osx expression in mesenchymal stem cells thereby inducing their differentiation into mature osteoblasts to achieve bony union. Our data show that daily teriparatide exposure induces Osx expression in mouse fracture callus compared to saline controls and promotes the differentiation of bone marrow derived MSCs to the osteoblastic lineage. We further show that intermittent PTH treatment up-regulates Osx in osteoprogenitor cells as well as markers of osteoblast differentiation in vitro.
Materials and Methods
Animals and treatments
7–9 week old C57BL/6 mice (Jackson Labs, Bar Harbor, ME) were used for fracture experiments. Mice were housed at the University of Rochester animal facilities according to state and federal law requirements. The animal studies were conducted in accordance with the principles and procedures approved by the University of Rochester IACUC. Mice were randomly selected to control or teriparatide (a generous gift from Eli Lilly) treated groups. Immediately following fracture experiments as described below, mice received daily subcutaneous teriparatide 40cg/kg or saline as control for 21 days.
Fracture experiments
Groups of 6 mice were anesthetized with ketamine (60 mg/kg) and xylazine (4 mg/kg) in normal saline via intraperitoneal injection. The femur fracture were performed using a 3-point bending system adapted from Bonnarens and Einhorn [Bonnarens et al., 1984] and as we have previously described [Kaback et al., 2008]. Fractures were radiographed by the Faxitron x-ray system (Faxitron, X-ray, Wheeling, IL) under anesthesia on Days 7, 10, 14, and 21 to monitor initial pin fixation as well as ongoing callus formation. Mice were sacrificed 7, 10, 14, and 21 days following fracture by induction of CO2 narcosis followed by cervical dislocation. For each experiment legs from 6 saline control mice and 6 teriparatide treated mice were harvested, and whole calluses (Including vessels, cartilage and bone) isolated for RNA extraction prior to gene expression analyses. Another set of control and treated mice (6 each) were used for histologic study as described below.
Histological and histomorphometric analyses
Fractured femurs were harvested as described above, fixed in 10% formalin, decalcified in an EDTA solution, embedded in paraffin and cut into 3 micron thick serial sections. These sections were stained in Alcian blue/hematoxylin solution as described previously [Zhang et al., 2002; Kaback et al., 2008]. Histomorphometric analyses were performed using the Osteometrics software (Decatur, GA). Briefly, bone and cartilage formation was quantified on H&E-stained sections by outlining the perimeter of the fracture callus under the 2x objective. Areas of new woven bone and cartilage were then traced. This procedure was repeated until the entire fracture callus has been evaluated. The areas of woven bone, cartilage, and total fracture callus are represented in square millimeters. Data is representative of three separate samples per group and the average total fracture callus size determined in each group. Immunohistochemical staining for Osx was performed using a rabbit polyclonal antibody (a generous gift from AbCam, UK) as previously described [Kaback et al., 2008].
Cell culture
For in vitro experiments, we used primary bone marrow derived cells harvested from tibias and femurs of mice treated daily for 7 days, via intraperitoneal injections, with 40cg/kg with teriparatide or saline control. A 25G5/8 needle (BD biosciences, Franklin Lakes, NJ) was used to flush the marrow with α-MEM (Gibco BRL, Grand Island, NY) containing 20% fetal bovine serum. Marrow was filtered using a swinnex syringe filter (Millipore, Billerica, MA) as previously described [Drissi et al., 1999]. Cells were plated in 6 well plates at the desired density of 2 × 106 cells per well, allowed to adhere for 4 days, and then cultured in complete media containing ascorbic acid (50cg/ml) and beta-glycerophosphate (10mM) for 7, 14 and 28 days prior to either staining or RNA harvesting.
RNA extraction and Real time RT-PCR
For our in vitro experiments, total RNA was isolated using the RNeasy Kit (Qiagen, Valencia, CA) following the manufacturer’s recommendations. Trizol (Invitrogen, Carlsbad, CA) was used to extract RNAs from fracture calluses according to the manufacturer protocol for tissue extraction using a Tissue Lyser (Qiagen, Valencia, CA). For our gene expression experiments, cDNA was synthesized using the advantage Superscript II RT-PCR kit (Invitrogen, Carlsbad, CA), following the manufacturer recommendation. Real time RT-PCR with specific primers for murine (Table 1) were performed using the fluorescent dye SYBR Green I (SYBR Green PCR Master Mix, Applied Biosystems, Foster City, CA) as previously described [Soung et al., 2007].
TABLE 1.
Primers used for real-time RT-PCR experiments
| Genes | Forward primer | Reverse primer | Accession # |
|---|---|---|---|
| Runx2 | CCGGGAATGATGAGAACTA | ACCGTCCACTGTCACTTT | D14636 |
| Osterix | ACTGGCTAGGTGGTGGTCAG | GGTAGGGAGCTGGGTTAAGG | AY803733 |
| Sox9 | AGGAAGCTGGCAGACCAGTA | CGTTCT TCACCGACT TCCTC | AF421878 |
| Type II collagen | ACTGGTAAGTGGGGCAAGAC | CCACACCAAATTCCTGTTCA | BC 052326 |
| Alkaline phosphatase | TGACCTTCTCTCCTCCATCC | CTTCCTGGGAGTCTCATCCT | NM007431 |
| Type I collagen | TGTCCCAACCCCCAAAGAC | CCCTCGACTCCTACATCTTCTGA | NM007742.2 |
| Osteocalcin | TGCTTGTGACGAGCTATCAG | GAGGACAGGGAGGATCAAGT | L24431 |
| β-Actin-F | AGATGTGGATCAGCAAGCAG | GCGCAAGTTAGGTTTTGTCA | NM007393 |
Statistical analysis
Statistical analyses were performed using the student t-test. Mean and standard error (SE) were calculated using GradphPad InStat (Version 4.03, San Diego, CA). The t-test was performed to determine which means were significantly different (p < 0.05).
Results
Teriparatide induces Osx expression in bone marrow derived stem cells while promoting their differentiation into osteoblasts
PTH was previously shown to promote MSC differentiation into mature osteoblasts in vivo [Nishida et al., 1994]. However, the molecular events underlying this PTH anabolic effect on murine bone marrow derived MSCs are still unclear. We harvested bone marrow mesenchymal progenitors from mice previously treated or not with daily teriparatide via intra-peritoneal injections for 7 days. Flushed marrow mesenchymal cells from both groups were then maintained in osteogenic media for up to four weeks. Alkaline phosphatase staining was increased upon systemic teriparatide treatment in these cells in comparison to cells harvested from saline injected controls (data not shown). Using RNA extracted from these cultures, we assessed mRNA levels of Osx and other osteoblast phenotypic genes. Figure 1A shows that Osx expression is significantly and time dependently up-regulated after 14 and 28 days of culture (2.5 and 4 fold respectively) in MSCs from mice treated with teriparatide compared to saline controls. Similarly, Runx2 transcripts were also induced by teriparatide treatment at 14 and 28 days in culture by approximately 2.5 fold (Figure 1B). Figure 1D shows that while teriparatide did not induce alkaline phosphatase mRNA levels at 7 days, it strongly and significantly enhanced its transcript levels at days 14 and 28 by 15 and 8 fold respectively. Finally, transcripts of type I collagen (Figure 1C) and osteocalcin (Figure 1E) were likewise up-regulated by 2 to 3 fold after 14 and 28 days in culture. These results show that systemic teriparatide treatment can induce mesenchymal stem cell differentiation into osteoblasts possibly through induction of Osx and Runx2 expression.
Figure 1. Teriparatide induces bone marrow derived mesenchymal stem cell differentiation into osteoblasts.
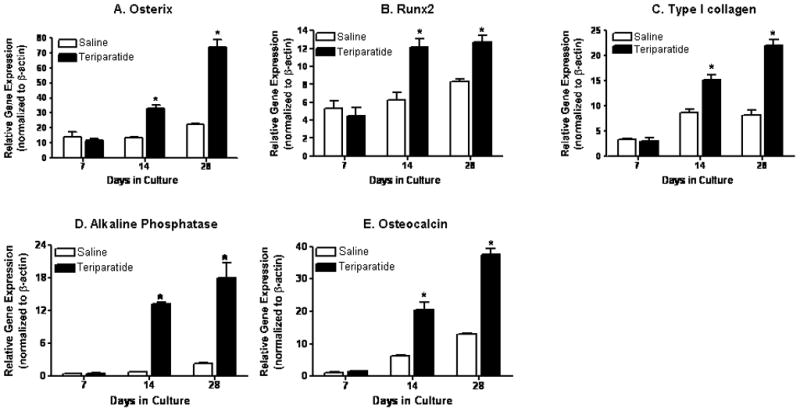
Bone marrow derived progenitor cells were isolated from femurs of mice previously injected daily with teriparatide or saline solution for 7 days. Cells were cultured for 7, 14, and 28 days for total RNA analysis. mRNA levels of Osx (A), Runx2 (B), type I collagen (C), alkaline phosphatase (D), and osteocalcin (E) were measured using real time RT-PCR. Values, which are normalized to β actin, are expressed as means ± SE (n=3). * denotes statistical significance from controls at each time point (p<0.05).
Teriparatide effects on fracture healing in a mouse model
Intermittent 1-34 PTH was previously shown to increase callus size and bone mineral density in rats [Alkhiary et al., 2005; Andreassen et al., 1999]. Here we investigated the effects of human recombinant PTH (teriparatide) on bone repair in a mouse femoral fracture model. We first examined the effects of teriparatide on fracture healing by radiographic and histological analyses. Saline injected animals were also used as controls. Figure 2 shows that while at day 7 post-fracture no differences are radiographically apparent between teriparatide and saline treated controls, the size of the radiolucent soft callus at 10 days post-fracture in the teriparatide treated mice was increased compared to saline controls. By day 14 this difference in callus size persists in the teriparatide treated mice compared to controls as shown by X-ray analysis (Figure 2A). Histological assessments of these fractures (Figure 2B) show that 10 days following teriparatide treatment, enhanced cartilage formation, as evidenced by Alcian blue staining, was observed in these calluses compared to the controls. By day 14 of treatment, increased bone formation was observed in the teriparatide treated mice compared to the saline injected animals. Our histomorphometric measurements of callus sizes in teriparatide treated compared to saline injected controls show that teriparatide induced a significant increase in callus formation at 7, 10, and 14 days post fracture by 35%, 264%, and 75%, respectively (Figure 2C). These results suggest that PTH enhances fracture healing possibly through enhanced differentiation of mesenchymal progenitor cells into mature osteoblasts at the fracture site.
Figure 2. Teriparatide accelerates femur fracture repair.
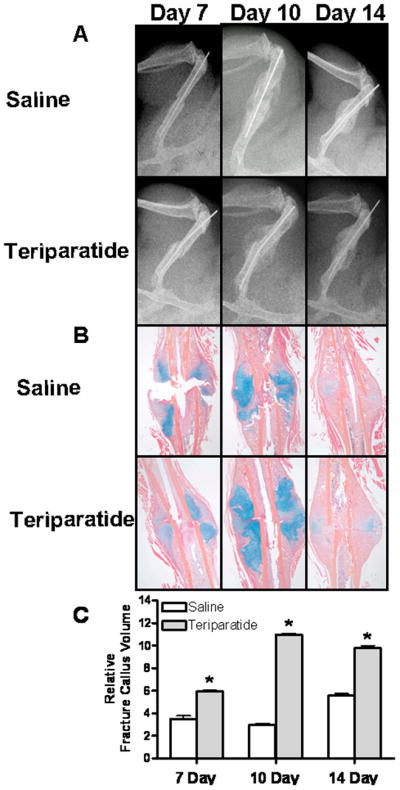
7–9 week old C57BL/6 mice underwent unilateral femur fractures and were subsequently injected with daily teriparatide (lower panels) or saline solution (upper panels) as described in the Methods section. X-rays (A), histology (B), and histomophometric analysis (C) were performed 7, 10, and 14 days post-fracture. Values are expressed as means ± SE (n=3). * denotes statistical significance from controls at each time point (p<0.05).
Osterix expression in the fracture calluses of teriparatide treated mice
The effects of teriparatide on fracture healing suggest the recruitment of mesenchymal progenitor cells into the fracture site and their differentiation into mature osteoblasts. In addition, systemic treatment of mice with teriparatide enhanced Osx expression in marrow derived osteogenic cells (shown above in Figure 1). In order to determine the cellular representation of Osx during fracture repair in response to teriparatide, we performed immunohistochemical assays using an Osx specific antibody [Kaback et al., 2008]. Figure 3A shows that Osx protein is expressed in periosteal cells and confined in the immature chondrocyte cell population while it is absent from fibrous tissue and chondrocytes undergoing hypertrophy in the saline injected controls at day 7 post-fracture. After 10 days of treatment, while both the saline controls and the teriparatide injected mice exhibit onset of trabecular bone formation in the fracture calluses, the number of Osx expressing osteoblasts and the volume of de novo trabecular bone is increased in the treated fractures compared to controls. The expression of Osx in the fracture calluses was also evaluated after cartilage remodeling and at the onset of bony union and trabecular bone remodeling at days 14 and 21. Fourteen days following teriparatide treatment, massive bone formation is observed and Osx expression is seen in the osteoblast lining cells as well as in osteocytes embedded in the woven bone. This expression is also observed in the saline injected controls. This same effect is seen after 21 days of treatment with an enhanced representation of trabecular bone compared to day 14. This accelerated trabecular bone formation is also concomitant with the absence of marrow cavities in the calluses of teriparatide treated animals compared to saline controls. Negative controls using secondary antibody alone did not show any reactivity at all time points (data not shown). Together, these results suggest that PTH accelerates fracture healing through enhanced commitment of progenitor cells towards an osteoblastic phenotype possibly through induction of Osx expression.
Figure 3. The effects of teriparatide on Osx protein expression during fracture repair.
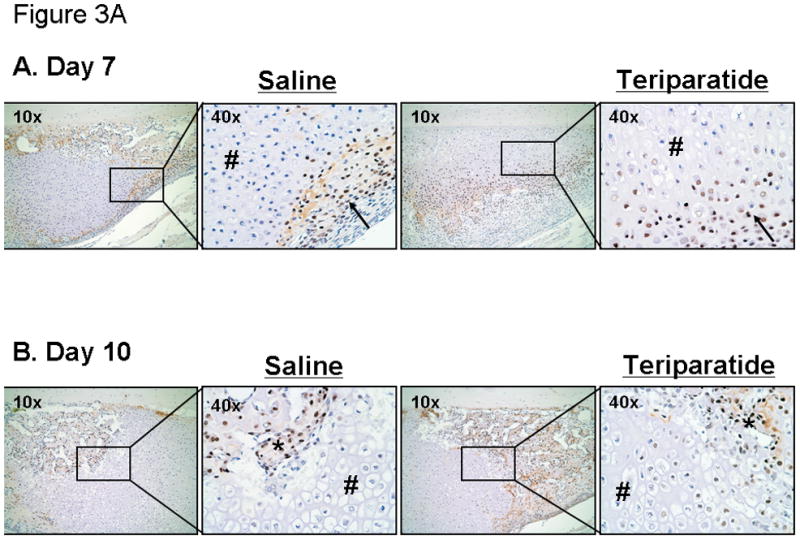
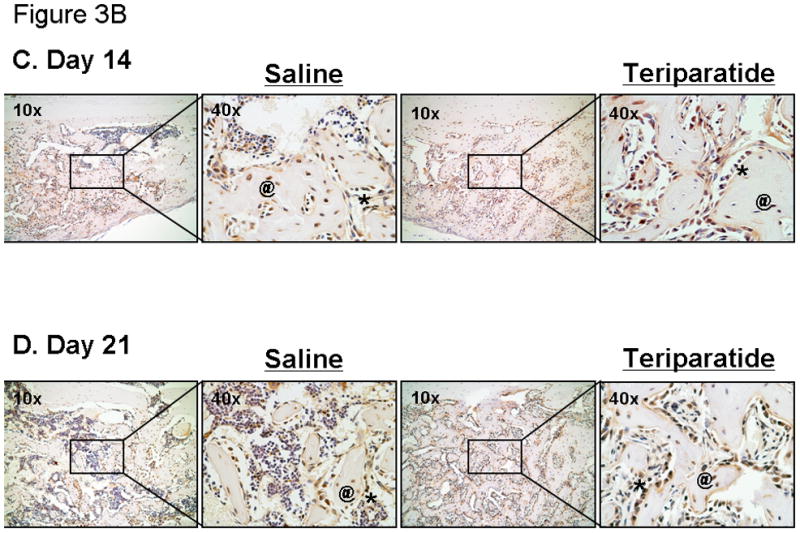
7–9 week old C57BL/6 mice underwent unilateral femur fractures and were subsequently injected daily with teriparatide (right panels; x10 and x40) or saline solution (left panels; x10 and x40) as described in the Methods section. Osx proteins were detected in the fracture callus at day 7, 10, 14, and day 21 post-fracture using immunohistochemical analysis. Of note is the strong immunostaining in the progenitor cells (arrows) as well as osteoblasts (*) and osteocytes (@) seen in newly formed bone. In addition, there is a lack of staining in the hypertrophic chondrocytes (#).
Systemic teriparatide treatment induces Osx and osteoblast phenotypic markers expression in fracture calluses
Intermittent PTH systemic injection was previously shown to increase fracture callus size, bone mineral density, and mechanical strength in rat femur fractures [Alkhiary et al., 2005]. However, the effects of daily teriparatide treatment on gene expression in fracture callus have yet to be defined. To further investigate the molecular targets of PTH in fracture calluses during bone repair, we performed gene expression analyses using RNAs extracted from fracture calluses of teriparatide treated mice and saline controls at 7, 10, 14 and 21 days post-fracture. Figure 4A shows that Osx transcripts are significantly up-regulated (3 fold) in calluses isolated 10 days post-fracture in the teriparatide treated groups compared to saline injected controls. Transcript levels continue to be elevated at 14 days post fracture in the teriparatide treated group (1.5 fold) in comparison to the saline injected animals. However, no difference was observed in Osx expression at 7 and 21 days post-fracture. We also assessed the expression of the bone and cartilage related gene Runx2. Runx2 transcripts are significantly induced at 7 days (2 fold) and 10 days following teriparatide treatment in these fracture calluses (2.5 fold) compared to untreated controls. However, Runx2 transcript levels are similar to saline controls 14 and 21 days post fracture (Figure 4B). Cartilage and bone phenotypic marker gene expression was also evaluated in these calluses. Thus, while teriparatide treatment only induces Sox9 expression in the early time point at 7 days compared to controls (Figure 4C), type II collagen expression is first enhanced at day 7 then inhibited at day 10 upon teriparatide treatment (Figure 4D). As expected, both control and treated levels of the chondrocyte phenotypic genes are reduced 14 and 21 days post-fracture when the cartilage matrix is remodeled in the fracture callus. On the other hand, type I collagen mRNA levels were initially slightly but significantly inhibited at day 7 post-fracture, then up-regulated between 10 and 14 days of teriparatide treatment (2 and 3 fold, respectively), while they were thereafter down-regulated 21 days post-fracture in the teriparatide treated groups compared to controls (by 2 fold) (Figure 4E). The marker for matrix mineralization, osteocalcin was progressively up-regulated by 4 fold following 10 and 14 days of teriparatide treatment compared to saline controls in these fracture calluses. After 21 days of teriparatide exposure, osteocalcin levels were enhanced 2 fold compared to saline treated animals (Figure 4F). Together, these results indicate that daily systemic teriparatide therapy post-fracture is not only able to up-regulate Osx transcripts in fracture callus but also induce mesenchymal stem cell commitment towards osteogenesis in the fracture site.
Figure 4. Teriparatide increases Osx transcript expression in mouse callus during fracture healing.
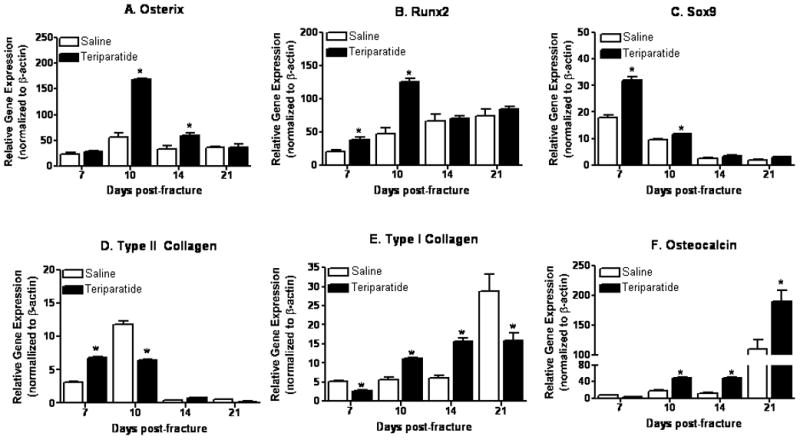
7–9 week old C57BL/6 mice underwent unilateral femur fractures and received daily injection of teriparatide or saline solution for 7 to 21 days. Fracture calluses at various healing time points (7, 10, 14, and 21 days post fracture) were collected and total RNA extracted. mRNA levels of Osx (A), Runx2 (B), Sox9 (C), type II collagen (D), type 1 collagen (E), and osteocalcin (F) were measured using real time RT-PCR. Values are expressed as means ± SE (n=3), normalized to β-actin. * denotes statistical significance from saline controls at each time point (p<0.05).
Discussion
Although the main function of PTH remains to regulate bone metabolism and maintain calcium homeostasis [Arnaud et al., 1970], several studies have investigated its anabolic effects in osteoporotic patients [Bilezikian et al., 2005; Black et al., 2003; Neer et al., 2001]. As fragility fractures continue to pose a unique dilemma to clinicians in terms of understanding fracture biology as well as establishing practical pharmacological agents that will reduce the relative risk of future fractures, the anabolic effects of PTH were also investigated during bone repair both in clinical trials and using animal models [Alkhiary et al., 2005; Jiang et al., 2005; Neer et al., 2001]. Previously, a fracture prevention trial established the role of teriparatide as an anabolic agent capable of reducing the risk of fragility fractures in postmenopausal women [Black et al., 2003; Neer et al., 2001]. However, a possible role for teriparatide in enhancing bony union of post-traumatic fractures should be postulated. Here we report the effects of teriparatide on bone repair in a mouse fracture model. We examined the cellular and molecular pathways underlying PTH enhancement of fracture healing. We believe that one possible mechanism by which PTH mediates fracture repair is through up-regulation of the zinc finger transcription factor Osx in mesenchymal stem cells, which in turn stimulates their recruitment and differentiation into mature osteoblasts.
Previous studies have described antagonistic effects of PTH on bone formation upon its mode of administration in vitro and in vivo [Alkhiary et al., 2005; Andreassen et al., 1999; Andreassen et al., 2004; Black et al., 2003; Jiang et al., 2005; Nakazawa et al., 2005; Neer et al., 2001; Thomas, 2006]. While continuous exposure to PTH leads to massive bone loss in vivo through increased osteoclast mediated bone resorption following enhanced osteoclast density and activity [Hadjidakis et al., 2006; Potts, 2005], Ishizuya and colleagues demonstrated the ability of PTH treatment administered under an intermittent regimen to induce bone formation through enhanced alkaline phosphatase activity, calcium content and the number of mineralized bone nodules deposited by rat calvaria-derived osteoprogenitor cells in vitro [Ishizuya et al., 1997]. Here we show that intermittent exposure of osteoprogenitor cells to PTH enhances Osx expression and promotes osteoblast differentiation.
The cellular events required for proper fracture healing of long bones through endochondral ossification are well documented [Ferguson et al., 1999; Gerstenfeld et al., 2003; Vortkamp et al., 1998]. Systemic and growth factors are produced in response to skeletal injury leading to the recruitment of progenitor cells from both the bone marrow as well as the periosteum to the site of injury [Hiltunen et al., 1993]. Several investigations reported the requirement for factors including the TGF-β superfamily, the PDGF, FGF, Ihh, prostaglandins, Wnt and VEGF during fracture repair [Gerstenfeld et al., 2003] yet very limited information is available about the mechanisms of action of their downstream targets in fracture calluses. Therefore, we addressed the molecular aspects of mesenchymal stem cell recruitment and differentiation that mediate fracture healing in response to PTH using an animal mouse model. We found that teriparatide increases cartilage and bone formation in the fracture callus at 7 and 10 days post-fracture.
Our assessment of gene expression in the fracture calluses in response to teriparatide shows that Osx expression was up-regulated in the fracture callus following treatment compared to saline injected controls. These results clearly suggest a correlation between PTH enhanced fracture repair and the known Osx mediated commitment of MSCs to the osteoblastic phenotype. We found that while markers of chondrocyte maturation are decreased as the fracture calluses ossify, osteoblast differentiation markers be induced. However, the inductive effects of teriparatide on Sox9 and type II collagen gene expression 7 days post-facture suggest a positive effect on chondrogenesis. Although, chondrocytes exhibit receptors for PTHrP, the effects of PTH on chondrogenesis are not known. These effects may also be indirect. Finally, although the effects of teriparatide on type I collagen gene expression were inductive in the fracture callus between 10 and 14 days post-fracture, PTH treatment inhibited type I collagen expression three weeks following treatment. This could be attributed to enhanced osteoblast maturation in the fracture callus as evidenced by the significant increase in osteocalcin expression at day 21. However, at day 7 post-fracture, teriparatide also inhibited type I collagen expression which in combination with its effects on Sox9 and type II collagen further suggests an effect of PTH on chondrogenesis during fracture healing.
In addition to its role in mediating bi-potent mesenchymal progenitor cell commitment toward osteogenesis [Nakashima et al., 2002], we have recently reported that Osx not only mediates MSC differentiation into mature osteoblasts but also strongly inhibits chondrocyte maturation [Kaback et al., 2008]. Our present study demonstrates that systemic treatment of these mice with teriparatide not only enhanced mesenchymal progenitor cell differentiation into mature osteoblasts, but also increased Osx expression in these marrow derived cells isolated from teriparatide treated mice in vivo. This increase in Osx expression along with osteoblastic phenotypic markers was concomitant with Runx2 expression. Our results demonstrate that systemic teriparatide treatment was able to induce Runx2 levels in the callus in the early time points following fracture healing. It is however noteworthy that Runx2 levels were not induced after day 10 when cartilage is replaced with bone in the callus, while Osx levels were still upregulated. While this shift is Osx versus Runx2 expression during callus formation and remodeling may be in support of the dogma of Osx being downstream of Runx2, it is not clear why PTH did not continue to induce Runx2 expression when cartilage is replaced with bone.
Previous evidence attributed the anabolic effects of PTH on bone formation to the induction of Runx2 [Krishnan et al., 2003]. The anabolic effects of PTH on bone formation were previously attributed to the increased expression of Runx2 through the PKA pathway [Krishnan et al., 2003]. The genetic evidence provided by the loss of Osx or Runx2 function suggested that Osx may be downstream of Runx2. We have previously shown that Osx may be a direct downstream target of Runx2 in various cell lines [Nishio et al., 2006]. However, emerging evidence in recent studies also advocate a Runx2 independent activation of Osx gene expression [Lee et al., 2003; Nakashima et al., 2003; Tu et al., 2006; van der et al., 2005]. It is possible that while PTH targets both Runx2 and Osx, its effects may be directly mediated through Osx and independently from Runx2. Further studies will delineate the precise role of Osx in mesenchymal stem cell recruitment to the fracture site in response to PTH treatment. Together, our findings bring novel insight into the cellular and molecular pathways underlying PTH mediated fracture repair thereby supporting its use not only as an anabolic agent for postmenopausal/osteoporotic bone loss but also for skeletal tissue repair and bone regeneration.
Supplementary Material
Acknowledgments
Contract grant sponsor: National Institutes of Health (NIH) RO1 grant No. AR052674-01 to HD; Contract grant sponsor: NIH CORT P50 grant No. AR05404 to RNR.
The authors would like to thank the National Institutes of Health (NIH RO1 grant No. AR052674-01 to HD and NIH CORT P50 grant No. AR05404 to RNR) for financial support and Krista Scorsone for her technical assistance.
References
- Alkhiary YM, Gerstenfeld LC, Krall E, Westmore M, Sato M, Mitlak BH, Einhorn TA. Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1-34) J Bone Joint Surg Am. 2005;87:731–741. doi: 10.2106/JBJS.D.02115. [DOI] [PubMed] [Google Scholar]
- Andreassen TT, Ejersted C, Oxlund H. Intermittent parathyroid hormone (1-34) treatment increases callus formation and mechanical strength of healing rat fractures. J Bone Miner Res. 1999;14:960–968. doi: 10.1359/jbmr.1999.14.6.960. [DOI] [PubMed] [Google Scholar]
- Andreassen TT, Willick GE, Morley P, Whitfield JF. Treatment with parathyroid hormone hPTH(1-34), hPTH(1-31), and monocyclic hPTH(1-31) enhances fracture strength and callus amount after withdrawal fracture strength and callus mechanical quality continue to increase. Calcif Tissue Int. 2004;74:351–356. doi: 10.1007/s00223-003-0093-6. [DOI] [PubMed] [Google Scholar]
- Arnaud CD, Tsao HS, Oldham SB. Native human parathyroid hormone: an immunochemical investigation. Proc Natl Acad Sci U S A. 1970;67:415–422. doi: 10.1073/pnas.67.1.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilezikian JP, Rubin MR, Finkelstein JS. Parathyroid hormone as an anabolic therapy for women and men. J Endocrinol Invest. 2005;28:41–49. [PubMed] [Google Scholar]
- Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, Garnero P, Bouxsein ML, Bilezikian JP, Rosen CJ. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349:1207–1215. doi: 10.1056/NEJMoa031975. [DOI] [PubMed] [Google Scholar]
- Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. J Orthop Res. 1984;2:97–101. doi: 10.1002/jor.1100020115. [DOI] [PubMed] [Google Scholar]
- Cranney A, Tugwell P, Zytaruk N, Robinson V, Weaver B, Adachi J, Wells G, Shea B, Guyatt G. Meta-analyses of therapies for postmenopausal osteoporosis. IV. Meta-analysis of raloxifene for the prevention and treatment of postmenopausal osteoporosis. Endocr Rev. 2002;23:524–528. doi: 10.1210/er.2001-4002. [DOI] [PubMed] [Google Scholar]
- Dempster DW, Cosman F, Kurland ES, Zhou H, Nieves J, Woelfert L, Shane E, Plavetic K, Muller R, Bilezikian J, Lindsay R. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res. 2001;16:1846–1853. doi: 10.1359/jbmr.2001.16.10.1846. [DOI] [PubMed] [Google Scholar]
- Drissi H, Hushka D, Aslam F, Nguyen Q, Buffone E, Koff A, van Wijnen A, Lian JB, Stein JL, Stein GS. The cell cycle regulator p27kip1 contributes to growth and differentiation of osteoblasts. Cancer Res. 1999;59:3705–3711. [PubMed] [Google Scholar]
- Ferguson C, Alpern E, Miclau T, Helms JA. Does adult fracture repair recapitulate embryonic skeletal formation? Mech Dev. 1999;87:57–66. doi: 10.1016/s0925-4773(99)00142-2. [DOI] [PubMed] [Google Scholar]
- Finkelstein JS. Cecil textbook of medicine. 2000. [Google Scholar]
- Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873–884. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- Hadjidakis DJ, Androulakis II. Bone remodeling. Ann N Y Acad Sci. 2006;1092:385–396. doi: 10.1196/annals.1365.035. [DOI] [PubMed] [Google Scholar]
- Heaney RP. Advances in therapy for osteoporosis. Clin Med Res. 2003;1:93–99. doi: 10.3121/cmr.1.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltunen A, Vuorio E, Aro HT. A standardized experimental fracture in the mouse tibia. J Orthop Res. 1993;11:305–312. doi: 10.1002/jor.1100110219. [DOI] [PubMed] [Google Scholar]
- Hock JM, Gera I. Effects of continuous and intermittent administration and inhibition of resorption on the anabolic response of bone to parathyroid hormone. J Bone Miner Res. 1992;7:65–72. doi: 10.1002/jbmr.5650070110. [DOI] [PubMed] [Google Scholar]
- Ishizuya T, Yokose S, Hori M, Noda T, Suda T, Yoshiki S, Yamaguchi A. Parathyroid hormone exerts disparate effects on osteoblast differentiation depending on exposure time in rat osteoblastic cells. J Clin Invest. 1997;99:2961–2970. doi: 10.1172/JCI119491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Zhao J, Liao EY, Dai RC, Wu XP, Genant HK. Application of micro-CT assessment of 3-D bone microstructure in preclinical and clinical studies. J Bone Miner Metab. 2005;23(Suppl):122–131. doi: 10.1007/BF03026336. [DOI] [PubMed] [Google Scholar]
- Kaback LA, Soung dY, Naik A, Smith N, Schwarz EM, O'Keefe RJ, Drissi H. Osterix/Sp7 regulates mesenchymal stem cell mediated endochondral ossification. J Cell Physiol. 2008;214:173–182. doi: 10.1002/jcp.21176. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Moore TL, Ma YL, Helvering LM, Frolik CA, Valasek KM, Ducy P, Geiser AG. Parathyroid hormone bone anabolic action requires Cbfa1/Runx2-dependent signaling. Mol Endocrinol. 2003;17:423–435. doi: 10.1210/me.2002-0225. [DOI] [PubMed] [Google Scholar]
- Kulkarni NH, Wei T, Kumar A, Dow ER, Stewart TR, Shou J, N'cho M, Sterchi DL, Gitter BD, Higgs RE, Halladay DL, Engler TA, Martin TJ, Bryant HU, Ma YL, Onyia JE. Changes in Osteoblast, Chondrocyte, and Adipocyte Lineages Mediate the Bone Anabolic Actions of PTH and Small Molecule GSK-3 Inhibitor. J Cell Biochem. 2007;102:1504–1518. doi: 10.1002/jcb.21374. [DOI] [PubMed] [Google Scholar]
- Kurland ES, Cosman F, McMahon DJ, Rosen CJ, Lindsay R, Bilezikian JP. Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab. 2000;85:3069–3076. doi: 10.1210/jcem.85.9.6818. [DOI] [PubMed] [Google Scholar]
- Lee MH, Kwon TG, Park HS, Wozney JM, Ryoo HM. BMP-2-induced Osterix expression is mediated by Dlx5 but is independent of Runx2. Biochem Biophys Res Commun. 2003;309:689–694. doi: 10.1016/j.bbrc.2003.08.058. [DOI] [PubMed] [Google Scholar]
- Nakashima K, de Crombrugghe B. Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet. 2003;19:458–466. doi: 10.1016/S0168-9525(03)00176-8. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Nakajima A, Shiomi K, Moriya H, Einhorn TA, Yamazaki M. Effects of low-dose, intermittent treatment with recombinant human parathyroid hormone (1-34) on chondrogenesis in a model of experimental fracture healing. Bone. 2005;37:711–719. doi: 10.1016/j.bone.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- Nishida S, Yamaguchi A, Tanizawa T, Endo N, Mashiba T, Uchiyama Y, Suda T, Yoshiki S, Takahashi HE. Increased bone formation by intermittent parathyroid hormone administration is due to the stimulation of proliferation and differentiation of osteoprogenitor cells in bone marrow. Bone. 1994;15:717–723. doi: 10.1016/8756-3282(94)90322-0. [DOI] [PubMed] [Google Scholar]
- Nishio Y, Dong Y, Paris M, O'Keefe RJ, Schwarz EM, Drissi H. Runx2-mediated regulation of the zinc finger Osterix/Sp7 gene. Gene. 2006;372:62–70. doi: 10.1016/j.gene.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Potts JT. Parathyroid hormone: past and present. J Endocrinol. 2005;187:311–325. doi: 10.1677/joe.1.06057. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Melton LJ., III The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone. 1995;17:505S–511S. doi: 10.1016/8756-3282(95)00258-4. [DOI] [PubMed] [Google Scholar]
- Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- Soung dY, Dong Y, Wang Y, Zuscik MJ, Schwarz EM, O'Keefe RJ, Drissi H. Runx3/AML2/Cbfa3 regulates early and late chondrocyte differentiation. J Bone Miner Res. 2007;22:1260–1270. doi: 10.1359/jbmr.070502. [DOI] [PubMed] [Google Scholar]
- Thomas T. Intermittent parathyroid hormone therapy to increase bone formation. Joint Bone Spine. 2006;73:262–269. doi: 10.1016/j.jbspin.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Tu Q, Valverde P, Chen J. Osterix enhances proliferation and osteogenic potential of bone marrow stromal cells. Biochem Biophys Res Commun. 2006;341:1257–1265. doi: 10.1016/j.bbrc.2006.01.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der HG, Farih-Sips H, Lowik CW, Karperien M. Multiple mechanisms are involved in inhibition of osteoblast differentiation by PTHrP and PTH in KS483 Cells. J Bone Miner Res. 2005;20:2233–2244. doi: 10.1359/JBMR.050821. [DOI] [PubMed] [Google Scholar]
- Vortkamp A, Pathi S, Peretti GM, Caruso EM, Zaleske DJ, Tabin CJ. Recapitulation of signals regulating embryonic bone formation during postnatal growth and in fracture repair. Mech Dev. 1998;71:65–76. doi: 10.1016/s0925-4773(97)00203-7. [DOI] [PubMed] [Google Scholar]
- Zhang X, Schwarz EM, Young DA, Puzas JE, Rosier RN, O'Keefe RJ. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin Invest. 2002;109:1405–1415. doi: 10.1172/JCI15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


