Abstract
The aryl hydrocarbon receptor (AhR) is a ligand-dependent transcription factor that is bound and activated by many toxic ubiquitous environmental contaminants, including the halogenated aromatic hydrocarbon, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). The AhR belongs to a family of proteins that contain basic helix-loop-helix/Per-ARNT-SIM (bHLH/PAS) domains. The circadian clock protein, BMAL1, is also a bHLH-PAS transcription factor and has been shown to interact with the AhR. AhRs are expressed in nearly every mammalian tissue, including the suprachiasmatic nuclei (SCN), and previous studies have suggested that activation of the AhR with dioxins affects rhythmicity in circadian clocks. In this study, the authors tested the hypothesis that activation of the aryl hydrocarbon receptor with the potent dioxin, TCDD, alters the organization of the mammalian circadian system by measuring bioluminescence from tissues explanted from PER2::LUCIFERASE mice. They found that in vitro treatment of explanted tissues with TCDD did not alter the periods, amplitudes, or damping rates of the PER2::LUC rhythms compared with controls. Likewise, in vivo treatment with TCDD had no effect on the phase relationship between central and peripheral oscillators. Together, these data demonstrate that activation of the AhR with TCDD does not directly or systemically alter the mouse circadian system.
Keywords: TCDD; 2,3,7,8-tetrachlorodibenzo-p-dioxin; aryl hydrocarbon receptor; Per-ARNT-SIM (PAS) domain; PER2::LUCIFERASE; mice; suprachiasmatic nucleus
Dioxins are chemical contaminants that are formed as unintentional by-products of industrial processes, including the production of pesticides and herbicides and bleaching of paper products. The halogenated aromatic hydrocarbon, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), is a toxic dioxin that is found ubiquitously in the environment and is concentrated at high levels of the food chain. TCDD evokes adverse effects on multiple organ systems in animals, including tumorigenesis, wasting syndrome, decreased fertility, immunosuppression, and acute lethality (Gao et al. 2000; Knerr and Schrenk 2006; Seefeld et al. 1984; Whysner and Williams 1996). Most of the toxic effects of TCDD are mediated by its binding to the aryl hydrocarbon receptor (AhR), a ligand-activated transcription factor that belongs to the bHLH/PAS (basic-helix-loop-helix/Period [PER]–aryl hydrocarbon receptor nuclear translocator [ARNT]–single minded [SIM]) family of heterdimeric transcription factors. AhR-deficient mice are resistant to the toxic effects of TCDD, including teratogenesis, thymic atrophy, and liver pathology (Fernandez-Salguero et al. 1996; Mimura et al. 1997). Upon binding of TCDD, the AhR translocates from the cytoplasm to the nucleus, where it forms a heterodimer with ARNT. This heterodimer then binds to xenobiotic response elements that are upstream of genes encoding xenobiotic metabolizing enzymes (XMEs), such as Cytochrome P450 1A1 (Cyp1a1).
AhR mRNA is widely expressed in embryonic (Abbott et al. 1995) and adult (Li et al. 1994; Su et al. 2002) mouse tissues, and AhR-deficient mice have physiological deficits in multiple organ systems (Fernandez-Salguero et al. 1995; Fernandez-Salguero et al. 1997; Mimura et al. 1997; Schmidt et al. 1996), suggesting that AhRs have physiological functions that are distinct from their role in the metabolism of xenobiotics. One of AhR’s functions may be a role in circadian rhythms since the bHLH-PAS proteins, BMAL1 and AhR, have been shown to interact in vitro and in vivo (Hogenesch et al. 1997; Tischkau et al. 2011; Xu et al. 2010). AhR mRNA is expressed in the mouse and rat SCN (Mukai et al. 2008; Petersen et al. 2000) and in many peripheral oscillators, including the liver, lung, and thymus, so it is anatomically poised to participate in circadian functions (Li et al. 1994; Okey 2007). Circadian gene expression is altered in the liver of AhR-deficient mice, and treatment of wild-type mice with TCDD affects the phase of their locomotor activity rhythms and alters circadian gene expression in the SCN, ovary, liver, and bone marrow (Garrett and Gasiewicz 2006; Miller et al. 1999; Mukai et al. 2008; Mukai and Tischkau 2007; Tischkau et al. 2011; Xu et al. 2010). Together these data suggest that there is crosstalk between the circadian system and AhR signaling pathways.
MATERIALS AND METHODS
Animals
Male and female heterozygous mPer2Luc mice (Yoo et al. 2004), backcrossed with the C57BL/6J strain for at least 19 generations (age 124 ± 70 [mean ± SD] days for in vitro experiments or 44 ± 5 days at time of in vivo TCDD treatment), were bred and maintained in 12L:12D for all experiments. Mice were group-housed after weaning. All experiments were conducted in accordance with the guidelines and approval of the Institutional Animal Care and Use Committee at Vanderbilt University.
Tissue Culture and Luminescence Recording
Within 1.5 h before lights-off, cultures of white adipose tissue (from above the adrenal gland), adrenal, kidney, liver, lung, pituitary, SCN, and thymus were prepared as previously described, except that CellGro (cat. no. 90-013PB plus L-glutamine) recording medium was used (Yamazaki and Takahashi 2005). The recording media that we used contained 16 mg tryptophan per liter. Previous in vitro studies have demonstrated that Cyp1a1 mRNA levels are increased by ultraviolet irradiation in media containing tryptophan, likely through the production of tryptophan photoproducts that that can bind to and activate the AhR (Rannug et al. 1987; Rannug et al. 1995; Wei et al. 1999). One study carefully analyzed Cyp1a1 mRNA expression in vitro in media with an identical tryptophan concentration as our media and found that after 3 h of exposure of the media to room lighting and daylight exposure through the windows of the laboratory, there was an induction of Cyp1a1 mRNA corresponding to 12% of the maximal effect of 155 pM of TCDD (Oberg et al. 2005). Oberg et al. (2005) also found that exposure of the media to light did not alter the potency of TCDD in the media. Although these studies demonstrate that tryptophan photo products in culture media only minimally affect Cyp1a1 expression, we nonetheless took every effort to minimize the production of tryptophan photoproducts in our protocol. Our recording media were stored and heated in the dark and exposed to fluorescent lights in the culture hood for a maximum of 10 min as the media were added to the cultures (the media were never exposed to daylight). Bioluminescence was monitored in real time with the LumiCycle, and photon counts were integrated over 10-min intervals.
In Vitro TCDD Treatment
At the time of culture, vehicle (DMSO) or 1 nM, 10 nM, or 100 nM TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin in DMSO; Cambridge Isotope Laboratories, Inc, Andover, MA) was added to the recording medium before the dish was placed in the LumiCycle. LumiCycle software (Actimetrics, Inc., Evanston, IL) was used to subtract the 24-h moving average from the raw luminescence data and to smooth the data by 0.5-h adjacent averaging. LumiCycle software was used to determine the amplitude (of the first cycle) and damping rate as previously described, where damping rate is the number of days required for the amplitude of the rhythm to decrease to 1/e (≈36.79%) of the starting value (Izumo et al. 2003). Samples with a goodness of fit less than 90% were excluded. Amplitude and damping rate could not be analyzed in liver explants because the goodness of fit was always less than 90% because the amplitudes and periods of the PER2::LUC rhythms changed during the recording (see Supplemental Online Material). In almost every liver explant examined, we observed a spontaneous change in period after 2 to 4 cycles in culture (Suppl. Fig. S1). The first period measured in culture is shown in Figure 1, and the secondary period in liver explants is shown in Supplementary Figure S2. In addition, the amplitude of the second cycle of the PER2::LUC rhythms was often larger than the amplitude of the first cycle (Suppl. Fig. S1). To determine period, the detrended and smoothed data were exported to ClockLab (Actimetrics, Inc.) and a regression line was fit to the acrophase of 3 to 4 cycles. Tissues were collected 7 days later (all tissues collected from 15:00–17:00 local time) for analysis of Cyp1a1 expression.
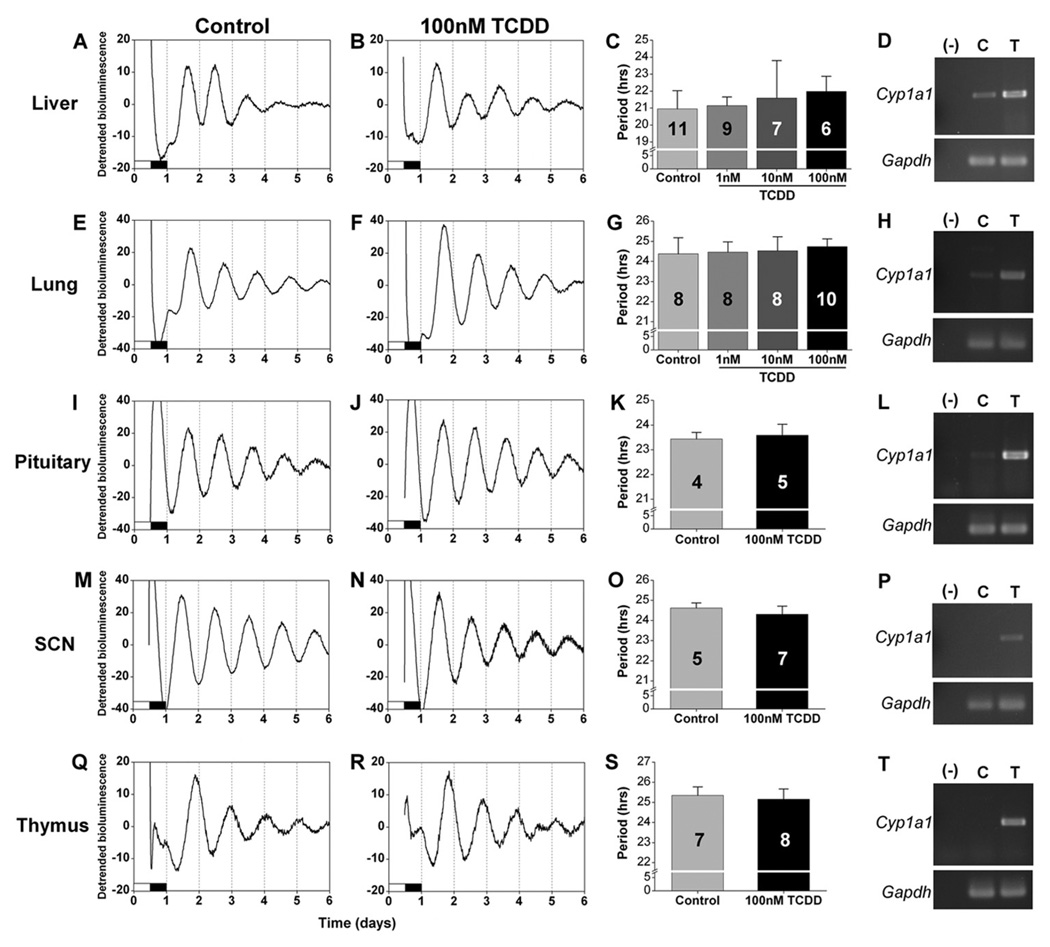
Figure 1.
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) does not directly affect the periods of circadian rhythms in cultured tissues. Representative detrended bioluminescence rhythms in liver (A, B), lung (E, F), pituitary (I, J), SCN (M, N), and thymus (Q, R) explants treated in vitro with DMSO (control, A, E, I, M, Q) or 100 nM TCDD (B, F, J, N, R). The mean period (± SD) of the PER2::LUC rhythm with DMSO (control) or TCDD treatment (1 nM, 10 nM, or 100 nM as indicated) is shown for each tissue (C, G, K, O, S). The first period measured in liver explants is shown (C; secondary period shown in Suppl. Fig. S2). The number of samples for each condition is indicated in the bar graph. Representative examples of the expression of Cyp1a1 and Gapdh in tissues treated in vitro with DMSO (C) or with 100 nM TCDD (T) were assessed by RT-PCR (D, H, L, P, T). Water (−) was added to the PCR reaction as a negative control.
In Vivo TCDD Treatment
At ~6 weeks old, mice were singly-housed and then administered vehicle (DMSO) or 1 µg/kg TCDD dissolved in corn oil by oral gavage. The percentage of DMSO in vehicle- and TCDD-treated mice was 1%. A single 1-µg/kg dose of TCDD administered by oral gavage in corn oil was chosen because this dose and route of administration have been shown to induce Cyp1a1 mRNA expression in liver, alter circadian gene expression, and alter physiological processes such as oxidative stress and immunosuppression (Inouye et al. 2005; Mukai et al. 2008; Narasimhan et al. 1994; Slezak et al. 2000; Tischkau et al. 2011; Xu et al. 2010). Either 4 or 14 days later, tissues were cultured and livers were collected for preparation of RNA and analysis of Cyp1a1 expression. ClockLab was used to measure phase, designated as the value of the first peak of detrended and smoothed PER2::LUC bioluminescence.
RT-PCR Analysis of Cyp1a1 Expression
Total RNA was isolated with the TRIzol reagent (Invitrogen). cDNA was synthesized from total RNA (1 µg RNA for liver for in vivo TCDD administration, 175 ng RNA for liver and lung explants, 350 ng RNA for pituitary explants, 75 ng RNA for thymus explants, and 50 ng RNA for SCN explants treated in vitro with TCDD) using SuperScript II reverse transcriptase (Invitrogen) with random hexanucleotide primers. PCR was performed on the cDNA using specific primers for Cyp1a1 (sense: 5′-AGGGCCACATCCGGGACATCACAG-3′ and antisense: 5′-CAGGCCGGAACTCGTTTGGGTCAC-3′) and glyceraldehyde-3-phosphate dehydrogenase (sense: 5′-ATGGTGAAGGTCGGTGTGAA-3′ and antisense: 5′-GAGTGGAGTCATACTGGAAC-3′). Cyp1a1 and Gapdh expression was assessed in the following numbers of in vitro DMSO- and TCDD-treated samples, respectively: liver: 2, 2; lung: 2, 2; pituitary: 4, 4; SCN: 4, 5; and thymus: 1, 1. For the in vivo treatment experiments, Cyp1a1 and Gapdh expression was assessed in 2 mice administered DMSO, 4 mice administered TCDD and euthanized 4 days later, and 2 mice administered TCDD and euthanized 14 days later.
Statistical Analysis
One-way analysis of variance (ANOVA) was used for comparison of more than 2 groups, and independent t tests (2-tailed) were used to compare 2 groups except when variances were not homogeneous, and then the Mann-Whitney rank sum test was used. Significance was ascribed at p < 0.05.
RESULTS
To elucidate the effects of AhR signaling pathways on circadian rhythms, we first investigated the direct effects of TCDD treatment on circadian rhythms in SCN and peripheral tissue explants (Fig. 1). We found that the periods of PER2::LUC expression in liver (Fig. 1A–D), lung (Fig. 1E–H), pituitary (Fig. 1I–L), SCN (Fig. 1M–P), and thymus (Fig. 1Q–T) were not altered by treatment with TCDD compared to vehicle (DMSO)–treated controls (see Suppl. Table S1 for statistical values). To confirm that the AhR was activated by in vitro TCDD treatment of the explanted tissues, we collected the tissues at the end of the experiment (after 7 days in culture) and analyzed the expression of Cyp1a1, which is upregulated by TCDD-dependent AhR activation, by semi-quantitative RT-PCR. We found that in vitro treatment with TCDD markedly increased the expression of Cyp1a1 compared to DMSO-treated controls in all tissues we examined, demonstrating that the AhR was activated in our experiment (Fig. 1D,H,L,P,T). Circadian rhythms of Cyp1a1 expression have been observed in SCN and liver, and expression levels vary by 2- to 3-fold each day in untreated mice (Mukai et al. 2008). Therefore, we sometimes detected Cyp1a1 expression in control samples. In all samples, TCDD treatment increased the expression of Cyp1a1 more than 3-fold compared to controls, demonstrating that we strongly activated AhR signaling above baseline rhythmic levels in all tissues.
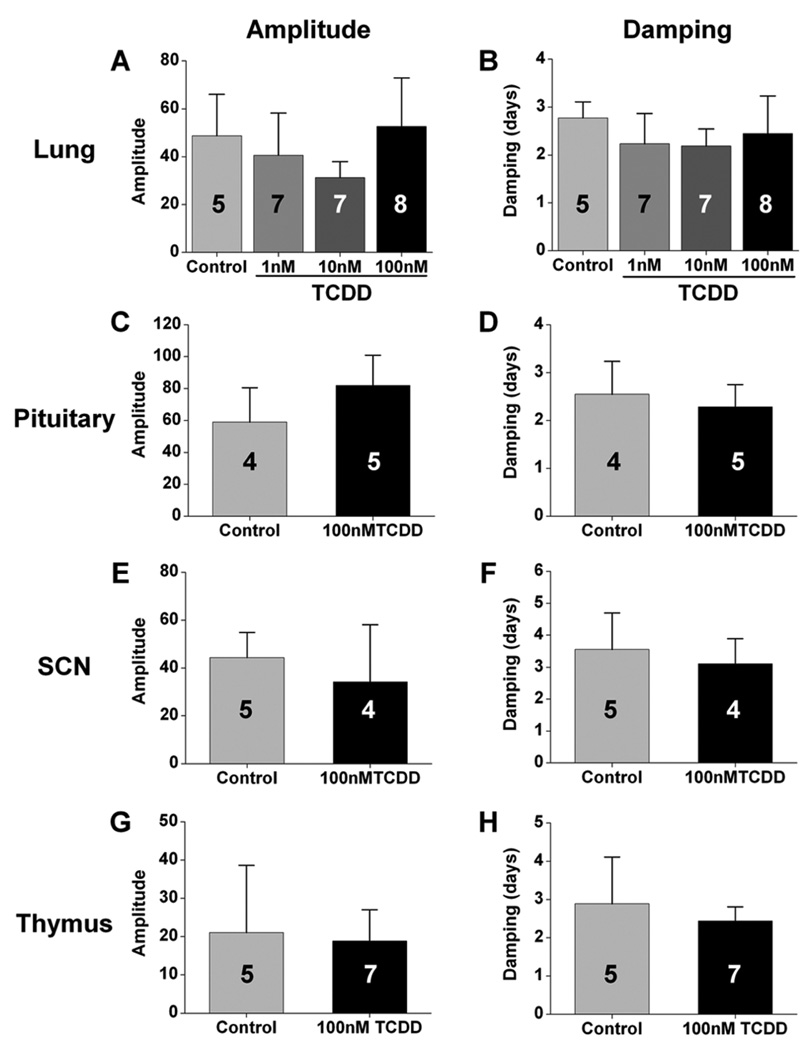
We next analyzed the effect of TCDD treatment on the amplitudes of PER2::LUC rhythms in tissues in vitro. We found that the amplitudes of PER2::LUC rhythms in lung (Fig. 2A), pituitary (Fig. 2C), SCN (Fig. 2E), and thymus (Fig. 2G) were not altered by TCDD treatment compared to vehicle-treated controls (statistical values presented in Suppl. Table S1). Since TCDD or vehicle was added at the time of culture, the effect of TCDD may be reflected in the damping rate of ex vivo rhythms. We found that the damping rates of PER2::LUC rhythms in lung (Fig. 2B), pituitary (Fig. 2D), SCN (Fig. 2F), and thymus (Fig. 2H) did not differ between vehicle- and TCDD-treated tissues (see Suppl. Table S1 for statistical values). Amplitude and damping could not be measured in liver because PER2::LUC rhythms in liver explants have spontaneous changes in period and increases in the amplitude of the second cycle relative to the first cycle, which precludes the use of the curve-fitting method for analysis (see Supplemental Online Material).
Figure 2.
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) does not directly affect the amplitudes and damping rates of circadian rhythms in cultured tissues. The mean (± SD) amplitudes (A, C, E, G) and damping rates (B, D, F, H) of PER2::LUC rhythms in lung (A, B), pituitary (C, D), SCN (E, F), and thymus (G, H) explants did not differ between tissues treated in vitro with DMSO (control) or TCDD (1 nM, 10 nM, or 100 nM as indicated). The number of samples for each condition is indicated in the bar graph.
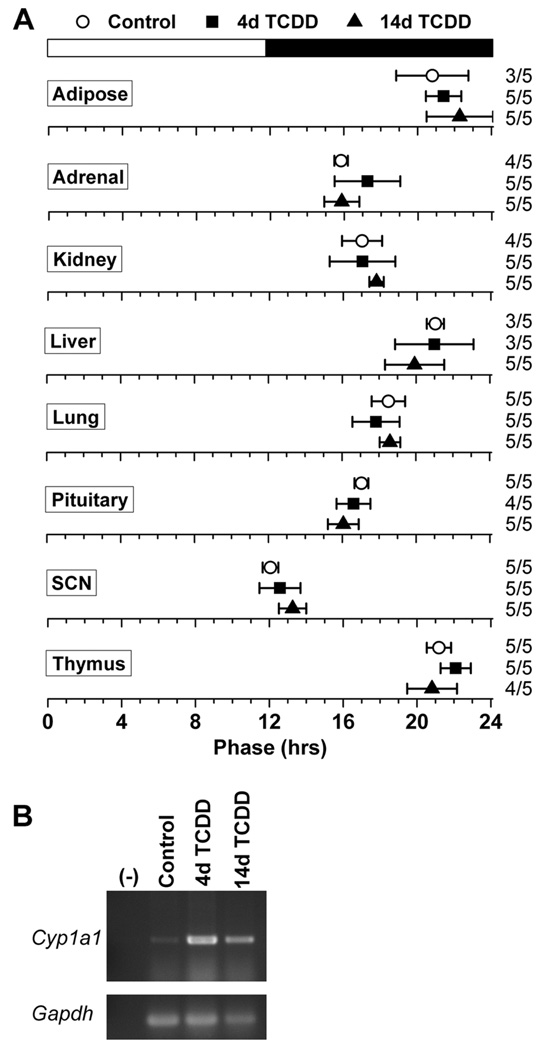
Even though we did not detect a direct effect of AhR activation on circadian rhythms in the SCN and peripheral tissues, it was possible that dioxin could indirectly affect the circadian system. To assess the systemic effect of TCDD on the organization of the circadian system, we administered 1 µg/kg TCDD or vehicle (DMSO) to PER2::LUC mice by oral gavage, cultured tissues either 4 days or 14 days later, and measured the phase of PER2::LUC expression in adipose tissue, adrenals, kidney, liver, lung, pituitary, SCN, and thymus (Fig. 3A). We found that the phases of these tissues were not altered either 4 or 14 days after administration of TCDD. Furthermore, the expression of Cyp1a1 was strongly activated in the liver 4 and 14 days after the single dose of 1 µg/kg TCDD compared to controls, demonstrating that in vivo TCDD treatment induces long-lasting, potent activation of the AhR (Fig. 3B).
Figure 3.
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) does not systemically affect the organization of the circadian system. (A) Phase map for circadian oscillators from PER2::LUC mice administered DMSO (white circles) or 1 µg/kg TCDD (filled symbols) by oral gavage. Tissues were cultured either 4 days (white circles and black squares) or 14 days (black triangles) after treatment. The mean phases (± SD) were determined from the first peak of PER2::LUC expression in vitro and are plotted relative to the time of last lights-on, where 0 h is lights-on and 12 h is lights-off (black and white bar at top). The sample size is shown (number of rhythmic tissues/number of tissues tested). (B) The expression of Cyp1a1 and Gapdh in the liver of mice 4 days after treatment with DMSO (control) or 4 or 14 days after treatment with TCDD was assessed by RT-PCR.
DISCUSSION
Previous studies have described crosstalk between the AhR signaling pathway and the circadian system. TCDD-mediated AhR signaling is altered in the mammary glands and livers of mice that do not have functional PERIOD1 and/or PERIOD2 (Qu et al. 2007, 2009, 2010). Conversely, activation of the AhR signaling pathway has been shown to alter circadian rhythms. For example, TCDD-treated mice have altered circadian gene expression in the SCN, liver, and cultured cells (Garrett and Gasiewicz 2006; Mukai et al. 2008; Xu et al. 2010).
In the current study, we analyzed the effects of TCDD-mediated activation of the AhR signaling pathway on circadian rhythms by measuring the expression of the PER2::LUC fusion protein in real time. We found that direct application of TCDD to tissues in culture did not affect the periods of the PER2::LUC rhythms in the SCN and peripheral tissues. Since AhR interacts with BMAL1, and a function of BMAL1 in cellular clocks is to regulate the amplitudes of rhythms, it was possible that TCDD would affect the amplitudes of circadian rhythms without affecting their periods (Baggs et al. 2009; Hogenesch et al. 1997; Sato et al. 2006; Tischkau et al. 2011; Xu et al. 2010). However, we found that TCDD treatment did not affect the amplitudes and damping rates of PER2::LUC expression in ex vivo tissues.
Although we did not detect a direct effect of TCDD on the periods and amplitudes of circadian rhythms in cultured tissues, it was possible that TCDD could have indirect effects on the circadian system. To determine the systemic effects of TCDD, we measured circadian organization, or the phase relationships between central and peripheral clocks. We found that the phase relationship between circadian clocks was not altered by treatment of mice with TCDD. For our study, we chose to assess circadian organization either 4 or 14 days after TCDD treatment. We chose these time points because our goal was to assess the primary effects of TCDD on the circadian system. If we had assessed circadian organization after a longer duration, we may have measured changes in the circadian system that were secondary to the toxic effects of TCDD.
For both the in vitro and in vivo TCDD treatment studies, we measured increased Cyp1a1 expression, a marker of AhR activation, compared to controls in all of the tissues we examined. A previous study described circadian rhythms of Cyp1a1 expression in animals that had not been exposed to dioxins, consistent with our finding that low levels of Cyp1a1 expression were detected in vehicle-treated tissues (Mukai et al. 2008). Although we cannot rule out the possibility that the cage bedding was contaminated with low levels of dioxins, TCDD treatment markedly increased Cyp1a1 expression in liver, confirming that the AhR signaling pathway was activated in our TCDD-treated mice compared to vehicle controls.
A recent study reported that circadian gene expression in mouse ovary is altered by in vivo TCDD treatment (Tischkau et al. 2011). In the current study, we did not examine the effects of TCDD in reproductive tissues. Future studies could examine the effects of TCDD on real-time circadian gene expression in reproductive tissues.
Together, our data suggest that the TCDD-dependent alterations in circadian gene expression that were described in previous studies may not be translated into effects on the periods, amplitudes, and phases of circadian rhythms. Although TCDD-mediated changes in circadian gene expression may affect other processes related to dioxin toxicity, our study demonstrates that the mammalian circadian system is resistant to TCDD.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by NIH P30 ES000267.
Footnotes
CONFLICT OF INTEREST STATEMENT
The author(s) have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary material for this article is available on the Journal of Biological Rhythms website at http://jbr.sagepub.com/supplemental.
REFERENCES
- Abbott BD, Birnbaum LS, Perdew GH. Developmental expression of two members of a new class of transcription factors: I. Expression of aryl hydrocarbon receptor in the C57BL/6N mouse embryo. Dev Dyn. 1995;204:133–143. doi: 10.1002/aja.1002040204. [DOI] [PubMed] [Google Scholar]
- Baggs JE, Price TS, DiTacchio L, Panda S, Fitzgerald GA, Hogenesch JB. Network features of the mammalian circadian clock. PLoS Biol. 2009;7:e52. doi: 10.1371/journal.pbio.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol. 1996;140:173–179. doi: 10.1006/taap.1996.0210. [DOI] [PubMed] [Google Scholar]
- Fernandez-Salguero PM, Ward JM, Sundberg JP, Gonzalez FJ. Lesions of aryl-hydrocarbon receptor-deficient mice. Vet Pathol. 1997;34:605–614. doi: 10.1177/030098589703400609. [DOI] [PubMed] [Google Scholar]
- Gao X, Petroff BK, Rozman KK, Terranova PF. Gonadotropin-releasing hormone (GnRH) partially reverses the inhibitory effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on ovulation in the immature gonadotropin-treated rat. Toxicology. 2000;147:15–22. doi: 10.1016/s0300-483x(00)00161-x. [DOI] [PubMed] [Google Scholar]
- Garrett RW, Gasiewicz TA. The aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin alters the circadian rhythms, quiescence, and expression of clock genes in murine hematopoietic stem and progenitor cells. Mol Pharmacol. 2006;69:2076–2083. doi: 10.1124/mol.105.021006. [DOI] [PubMed] [Google Scholar]
- Hogenesch JB, Chan WK, Jackiw VH, Brown RC, Gu YZ, Pray-Grant M, Perdew GH, Bradfield CA. Characterization of a subset of the basic-helix-loop-helix-PAS superfamily that interacts with components of the dioxin signaling pathway. J Biol Chem. 1997;272:8581–8593. doi: 10.1074/jbc.272.13.8581. [DOI] [PubMed] [Google Scholar]
- Inouye K, Pan X, Imai N, Ito T, Takei T, Tohyama C, Nohara K. T cell–derived IL-5 production is a sensitive target of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Chemosphere. 2005;60:907–913. doi: 10.1016/j.chemosphere.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Izumo M, Johnson CH, Yamazaki S. Circadian gene expression in mammalian fibroblasts revealed by real-time luminescence reporting: temperature compensation and damping. Proc Natl Acad Sci U S A. 2003;100:16089–16094. doi: 10.1073/pnas.2536313100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knerr S, Schrenk D. Carcinogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in experimental models. Mol Nutr Food Res. 2006;50:897–907. doi: 10.1002/mnfr.200600006. [DOI] [PubMed] [Google Scholar]
- Li W, Donat S, Dohr O, Unfried K, Abel J. Ah receptor in different tissues of C57BL/6J and DBA/2J mice: use of competitive polymerase chain reaction to measure Ah-receptor mRNA expression. Arch Biochem Biophys. 1994;315:279–284. doi: 10.1006/abbi.1994.1501. [DOI] [PubMed] [Google Scholar]
- Miller JD, Settachan D, Frame LT, Dickerson R. 2,3,7,8-Tetrachlorodibenzo-p-dioxin phase advances the deer mouse (Peromyscus manuculatus) circadian rhythm by altering expression of clock proteins. Organohalogen Compd. 1999;42:23–28. [Google Scholar]
- Mimura J, Yamashita K, Nakamura K, Morita M, Takagi TN, Nakao K, Ema M, Sogawa K, Yasuda M, Katsuki M, et al. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells. 1997;2:645–654. doi: 10.1046/j.1365-2443.1997.1490345.x. [DOI] [PubMed] [Google Scholar]
- Mukai M, Lin TM, Peterson RE, Cooke PS, Tischkau SA. Behavioral rhythmicity of mice lacking AhR and attenuation of light-induced phase shift by 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Biol Rhythms. 2008;23:200–210. doi: 10.1177/0748730408316022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai M, Tischkau SA. Effects of tryptophan photoproducts in the circadian timing system: searching for a physiological role for aryl hydrocarbon receptor. Toxicol Sci. 2007;95:172–181. doi: 10.1093/toxsci/kfl126. [DOI] [PubMed] [Google Scholar]
- Narasimhan TR, Craig A, Arellano L, Harper N, Howie L, Menache M, Birnbaum L, Safe S. Relative sensitivities of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced Cyp1a-1 and Cyp1a-2 gene expression and immunotoxicity in female B6C3F1 mice. Fundam Appl Toxicol. 1994;23:598–607. doi: 10.1006/faat.1994.1146. [DOI] [PubMed] [Google Scholar]
- Oberg M, Bergander L, Hakansson H, Rannug U, Rannug A. Identification of the tryptophan photoproduct 6-formylindolo[3,2-b]carbazole, in cell culture medium, as a factor that controls the background aryl hydrocarbon receptor activity. Toxicol Sci. 2005;85:935–943. doi: 10.1093/toxsci/kfi154. [DOI] [PubMed] [Google Scholar]
- Okey AB. An aryl hydrocarbon receptor odyssey to the shores of toxicology: the Deichmann Lecture, International Congress of Toxicology–XI. Toxicol Sci. 2007;98:5–38. doi: 10.1093/toxsci/kfm096. [DOI] [PubMed] [Google Scholar]
- Petersen SL, Curran MA, Marconi SA, Carpenter CD, Lubbers LS, McAbee MD. Distribution of mRNAs encoding the arylhydrocarbon receptor, arylhydrocarbon receptor nuclear translocator, and arylhydrocarbon receptor nuclear translocator-2 in the rat brain and brainstem. J Comp Neurol. 2000;427:428–439. doi: 10.1002/1096-9861(20001120)427:3<428::aid-cne9>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Qu X, Metz RP, Porter WW, Cassone VM, Earnest DJ. Disruption of clock gene expression alters responses of the aryl hydrocarbon receptor signaling pathway in the mouse mammary gland. Mol Pharmacol. 2007;72:1349–1358. doi: 10.1124/mol.107.039305. [DOI] [PubMed] [Google Scholar]
- Qu X, Metz RP, Porter WW, Cassone VM, Earnest DJ. Disruption of period gene expression alters the inductive effects of dioxin on the AhR signaling pathway in the mouse liver. Toxicol Appl Pharmacol. 2009;234:370–377. doi: 10.1016/j.taap.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Metz RP, Porter WW, Neuendorff N, Earnest BJ, Earnest DJ. The clock genes period 1 and period 2 mediate diurnal rhythms in dioxin-induced Cyp1A1 expression in the mouse mammary gland and liver. Toxicol Lett. 2010;196:28–32. doi: 10.1016/j.toxlet.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rannug A, Rannug U, Rosenkranz HS, Winqvist L, Westerholm R, Agurell E, Grafstrom AK. Certain photooxidized derivatives of tryptophan bind with very high affinity to the Ah receptor and are likely to be endogenous signal substances. J Biol Chem. 1987;262:15422–15427. [PubMed] [Google Scholar]
- Rannug U, Rannug A, Sjoberg U, Li H, Westerholm R, Bergman J. Structure elucidation of two tryptophan-derived, high affinity Ah receptor ligands. Chem Biol. 1995;2:841–845. doi: 10.1016/1074-5521(95)90090-x. [DOI] [PubMed] [Google Scholar]
- Sato TK, Yamada RG, Ukai H, Baggs JE, Miraglia LJ, Kobayashi TJ, Welsh DK, Kay SA, Ueda HR, Hogenesch JB. Feedback repression is required for mammalian circadian clock function. Nat Genet. 2006;38:312–319. doi: 10.1038/ng1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci U S A. 1996;93:6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seefeld MD, Corbett SW, Keesey RE, Peterson RE. Characterization of the wasting syndrome in rats treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 1984;73:311–322. doi: 10.1016/0041-008x(84)90337-5. [DOI] [PubMed] [Google Scholar]
- Slezak BP, Hatch GE, DeVito MJ, Diliberto JJ, Slade R, Crissman K, Hassoun E, Birnbaum LS. Oxidative stress in female B6C3F1 mice following acute and subchronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Toxicol Sci. 2000;54:390–398. doi: 10.1093/toxsci/54.2.390. [DOI] [PubMed] [Google Scholar]
- Su AI, Cooke MP, Ching KA, Hakak Y, Walker JR, Wiltshire T, Orth AP, Vega RG, Sapinoso LM, Moqrich A, et al. Large-scale analysis of the human and mouse transcriptomes. Proc Natl Acad Sci U S A. 2002;99:4465–4470. doi: 10.1073/pnas.012025199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischkau SA, Jaeger CD, Krager SL. Circadian clock disruption in the mouse ovary in response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Lett. 2011;201:116–122. doi: 10.1016/j.toxlet.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei YD, Rannug U, Rannug A. UV-induced CYP1A1 gene expression in human cells is mediated by tryptophan. Chem Biol Interact. 1999;118:127–140. doi: 10.1016/s0009-2797(98)00118-5. [DOI] [PubMed] [Google Scholar]
- Whysner J, Williams GM. 2,3,7,8-Tetrachlorodibenzo-p-dioxin mechanistic data and risk assessment: gene regulation, cytotoxicity, enhanced cell proliferation, and tumor promotion. Pharmacol Ther. 1996;71:193–223. doi: 10.1016/0163-7258(96)00068-x. [DOI] [PubMed] [Google Scholar]
- Xu CX, Krager SL, Liao DF, Tischkau SA. Disruption of CLOCK-BMAL1 transcriptional activity is responsible for aryl hydrocarbon receptor-mediated regulation of Period1 gene. Toxicol Sci. 2010;115:98–108. doi: 10.1093/toxsci/kfq022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Takahashi JS. Real-time luminescence reporting of circadian gene expression in mammals. Methods Enzymol. 2005;393:288–301. doi: 10.1016/S0076-6879(05)93012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.