Abstract
Prior studies have shown that treatment of head and neck squamous cell carcinoma (HNSCC) patients with 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3] reduced intratumoral levels of immune inhibitory CD34+ progenitor cells while increasing levels of mature progeny dendritic cells. This finding was extended to a pilot study to determine whether 1,25(OH)2D3 treatment concurrently increases levels of intratumoral CD4+ and CD8+ T cells, increases intratumoral levels of immune cells expressing the early activation marker CD69, and prolongs time to HNSCC recurrence. The clinical trial comprised 16 patients with newly diagnosed HNSCC being untreated and 16 patients being treated with 1,25(OH)2D3 during the 3-week interval between cancer diagnosis and surgical treatment. Immunologic effects of treatment were monitored by immunohistochemical analyses of surgically removed HNSCC. Clinical effectiveness of 1,25(OH)2D3 treatment in this study was measured by the time to HNSCC recurrence. HNSCC tissues of patients who received treatment with 1,25(OH)2D3 contained increased levels of CD4+ cells and, more significantly, CD8+ T cells. Also prominent was an increase in cells expressing the lymphoid activation marker CD69. Results of this pilot study suggest that patients treated with 1,25(OH)2D3 had a lengthier time to tumor recurrence compared with patients who were not treated before surgery.
Keywords: Head and neck cancer, Head and neck squamous cell carcinoma, Recurrence, T-cell, vitamin D
1. Introduction
Squamous cell carcinoma of the head and neck (head and neck squamous cell carcinoma (HNSCC)) is an aggressive malignancy with a 5-year survival in patients that remains at approximately 50%. Thus the possibility of immunotherapeutic approaches for HNSCC patients has gained interest. Feasibility of immunotherapeutic strategies is supported by studies showing infiltration of CD4+ and CD8+ T cells within HNSCC and a correlation between lymphocyte activity and patient prognosis [1,2]. Immunotherapeutic strategies such as adoptive T-cell transfer, treatment with various immune stimulatory cytokines, or treatment with tumor cell vaccines have suggested therapeutic activity in HNSCC patients [3–7]. Unfortunately, HNSCC patients have profound immune deficiencies that are associated with increased recurrence [1]. CD34+ progenitor cells are among the tumor-induced suppressor cells that contribute to this immune dysfunction [8–10]. CD34+ cells are a part of a spectrum of immature myeloid-lineage cells, the levels of which become prominent in HNSCC patients and which have defects in maturation into dendritic cells able to stimulate T-cell reactivity [11,12]. Our prior in vitro studies had shown that 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3] induced maturation of immune suppressive CD34+ progenitor cells into immune stimulatory dendritic cells [13,14]. Expanding this to a pilot clinical trial with HNSCC patients showed that 1,25(OH)2D3 diminishes intratumoral levels of tumor-induced immune inhibitory CD34+ cells. Concurrent with the decline in intratumoral CD34+ cells was an increase in levels of mature dendritic cells.
CD34+ cells are a part of a spectrum of immature myeloid-lineage cells, the levels of which become prominent in HNSCC patients and which have defects in maturation into dendritic cells able to stimulate T-cell reactivity [11,12]. Our prior in vitro studies had shown that 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3] induces maturation of immune suppressive CD34+ progenitor cells into immune stimulatory dendritic cells [13,14]. Expanding this to a pilot clinical trial with HNSCC patients showed that 1,25(OH)2D3 diminishes intratumoral levels of tumor-induced immune inhibitory CD34+ cells. Concurrent with the decline in intratumoral CD34+ cells was an increase in levels of mature dendritic cells.
Dendritic cells are highly efficient antigen presenting cells that can generate Th1 cells and cytotoxic T-lymphocytes responses to cancer [15]. Cancers, including HNSCC, are vulnerable to such generated immune effector cell responses [16,17]. However, there has been an accumulation of evidence of defects in the maturation and differentiation of dendritic cells in cancer patients, including those with HNSCC [11,18]. Maturation of dendritic cells is critical in regard to their capacity to stimulate activity of naive T cells, as their ability to form stable adhesions with T cells only develops after maturation [15]. Consistent with this are our past studies showing only low levels of mature dendritic cells within cancers in HNSCC patients and, instead, the presence of dendritic cells expressing markers of immature dendritic cells [19].
Whether decreases in levels of immune inhibitory CD34+ progenitor cells and increases in intratumoral mature dendritic cells previously observed after treatment of HNSCC patients with 1,25(OH)2D3 [19] also translates into increased levels of immune infiltrating cells and, in particular, T cells within the HNSCC had not been previously determined. The present study used immunohistochemical analyses to determine whether the intratumoral content of cells expressing CD69, which is primarily indicative of early stimulated T cells and monocytes, and intratumoral levels of CD4+ and CD8+ T cells were increased in tissues from 1,25(OH)2D3-treated patients compared with untreated patients. Also determined was whether there were any suggestions of clinical responses to the 1,25(OH)2D3 treatment, as measured by the time between surgical treatment and HNSCC recurrence.
2. Subjects and methods
2.1. Study population
Recruitment of patients with a pathologic diagnosis of HNSCC into this 1,25(OH)2D3 (Calcitriol) study was institutional review board approved. Pathologic findings were read before patient enrollment by a pathologist who was not affiliated with this study and was therefore blinded to the treatment arms into which the patients would be enrolled. Patients with newly diagnosed HNSCC who were being scheduled for surgery were eligible for enrollment into this randomized trial with preoperative treatment of 1,25(OH)2D3. Control patients with HNSCC did not receive 1,25(OH)2D3 treatment. Patients were excluded if they had received immunotherapy or radiation treatment in the previous 3 weeks or had concurrent malignancies.
2.2. (OH)2D3 treatment and collection of specimens
Patients were treated orally with 4 μg of 1,25(OH)2D3 for each of 3 sequential days, followed by 4 days of no treatment. This treatment schedule has previously been shown to be associated with minimal toxicity [19,20]. Nevertheless serum calcium and parathyroid hormone levels were measured weekly to monitor possible toxicity. At the conclusion of three cycles of treatment, patients underwent surgical treatment for their HNSCC. Sixteen patients enrolled in this study completed the 1,25(OH)2D3 treatment, and 16 patients enrolled to the control untreated arm underwent surgical excision and were clinically followed up after surgery.
2.3. Immunohistochemistry
Upon surgical collection of HNSCC tissues, the tissues were frozen in optimum cutting temperature (OCT) compound (Miles Laboratories, Elkhart, IN). Tissue blocks were then cryosectioned into 10–nm-thick slices and placed onto slides. Approximately 50 slides were made per frozen block. Slides were stored at −80°C until used for immunohistochemical staining. Cryosectioned HNSCC tissue was used to measure levels of infiltrating immune cells, namely CD4+, CD8+, and CD69+ cells. The procedure for immunostaining for these cells was similar to that previously used [19]. Briefly, the cryosections were fixed onto slides with 100% acetone for 10 minutes and slides were then allowed to dry. The tissue was outlined with a PAP pen. Slides were then washed and incubated in phosphate-buffered saline (PBS) for 10 minutes. Endogenous peroxidase was quenched by incubating the tissues three times for 5 minutes each time in a 0.3% H2O2/PBS solution. Nonspecific mouse antibody was added for 20 minutes to bind to Fc epitopes and to reduce background staining. Next, the primary antibodies and corresponding isotype control antibodies were added to slides for 1 hour at room temperature. Primary antibodies that were used to detect infiltrating cells within HNSCC tissues were against CD4 to detect CD4+ T cells, CD8 to detect CD8+ T cells, and CD69 to detect T cells and monocytes expressing this early activation marker (BD Biosciences, San Jose, CA). Slides were rewashed with buffer for 5 minutes, and then positive staining cells were visualized using the Vecastain ABC immunoperoxidase kit (Vector Labs, Burlingame, CA) with counterstaining using hematoxylin.
2.4. Quantification of data and analysis
The number of positive staining cells in each microscopic field was quantitated by individual cell counts. Four random areas of slides were identified and graded independently by four graders. At least two randomly selected slides were scored for each patient sample. The same four graders were used for each of the primary antibodies. The graders were blinded as to the identity of the tissues. Data for the treated and untreated patients were statistically compared using a two-tailed Student’s t-test.
2.5. Clinical outcomes
The possibility of clinical effectiveness of treatment with 1,25(OH)2D3 was determined by measuring the time to recurrence after surgical treatment and comparing results for patients who received 1,25(OH)2D3 treatment or no treatment during the interval between cancer diagnosis and surgery. Results were analyzed using Kaplan–Meier Curves.
3. Results
3.1. Patient population
In all, 16 patients with newly diagnosed HNSCC who were enrolled in this pilot study completed a 3-week course of 1,25(OH)2D3 treatment before surgical treatment, and 16 newly diagnosed HNSCC patients were untreated during the interval between diagnosis and surgical treatment (Table 1). There was no evidence of toxicity resulting from 1,25(OH)2D3 treatment, based on serum calcium and parathyroid hormone measurements. Patient ages ranged from 45 to 92 years. The mean ages of patient groups were similar after randomization into groups receiving 1,25(OH)2D3 treatment preoperatively for 3 weeks (mean 66 years of age) versus those who received no treatment during the same interval (mean 63 years of age). Most of the patients had cancers that originated in the oral cavity. The range of disease stages, based on pathologic evaluation, also didn not differ among patients enrolled in the two arms. For each of the patient groups, seven of 16 patients were female (44%) and nine of 16 were male (56%).
Table 1.
Patient population
| Age | Gender | Primary site | Stage | Margins | Postsurgical treatment |
|---|---|---|---|---|---|
| Untreated | |||||
| 74 | M | Oral cavity | T3N2bM0 | Negative | RT |
| 70 | M | Oral cavity | T4N2cM0 | Negative | Chemo/RT |
| 49 | M | Oral cavity | T4aN2bM0 | Negative | Chemo/RT |
| 59 | M | Sinonasal | T2N0M0 | Positive | RT |
| 80 | M | Floor of mouth | T4aN0M0 | Positive | RT |
| 54 | M | Oral cavity | T3N1M0 | Negative | Chemo/RT |
| 64 | F | Tongue | T3N0M0 | Negative | None |
| 64 | M | Tongue | T2N0M0 | Positive | None |
| 92 | F | Palate | T4aN2bM0 | Positive | RT |
| 51 | M | Floor of mouth | T4N2bM0 | Positive | RT |
| 48 | F | Floor of mouth | T4aN2bM0 | Positive | Chemo/RT |
| 58 | F | Floor of mouth | T4aN2cM0 | Positive | Chemo |
| 50 | M | Floor of mouth | T4aN2cM0 | Positive | RT |
| 48 | F | Tongue | T2N2cM0 | Negative | None |
| 67 | F | Tongue | T2N2cM0 | Negative | Chemo/RT |
| 72 | F | Oral cavity | T2N2bM0 | Negative | None |
| 1,25(OH)2D3 treated | |||||
| 72 | F | Larynx | T3N2cM0 | Positive | Chemo/RT |
| 76 | M | Floor of mouth | T2N0M0 | Positive | RT |
| 45 | M | Floor of mouth | T2N2cM0 | Positive | Chemo/RT |
| 72 | M | Anterior mandible | T4aN1M0 | Negative | RT |
| 74 | M | Tongue/floor of mouth | T4aN2cM0 | Positive | RT |
| 58 | M | Oropharynx | T4N2bM0 | Negative | Chemo/RT |
| 63 | M | Oral cavity | T4aN3M0 | Positive | Chemo/RT |
| 47 | M | Lip | T2N0M0 | Negative | None |
| 47 | M | Tongue | T4N0M0 | Positive | RT |
| 59 | F | Floor of mouth | T3N1M0 | Positive | RT |
| 76 | F | Hard palate | T2N2bM0 | Negative | Chemo/RT |
| 76 | F | Oropharynx | T2N2bM0 | Negative | Chemo/RT |
| 75 | F | Oropharynx | T2N0M0 | Negative | None |
| 73 | F | Tongue | T3N2bM0 | Negative | Chemo/RT |
| 66 | F | Oral cavity | T2N0M0 | Negative | None |
| 73 | M | Floor of mouth | T4N2bM0 | Positive | None |
3.2. Levels of CD4+ and CD8+ T cells in untreated HNSCC patients or those treated with 1,25(OH)2D3 before surgery
Prior studies had shown diminished immune functions and altered T-cell content in patients with HNSCC as well as with other cancer types [21,22]. Furthermore reduced immune content and function in HNSCC patients has been correlated with a poor clinical outcomes [1,23]. Therefore this study aimed to quantitatively assess levels of T-cell subpopulations within HNSCC tissue of newly diagnosed HNSCC patients who were either untreated before surgery or who received treatment with the immune modulator 1,25(OH)2D3, which we had previously shown to diminish levels of immune inhibitory progenitor cell and stimulate differentiation of dendritic cells within the tumor mass [19].
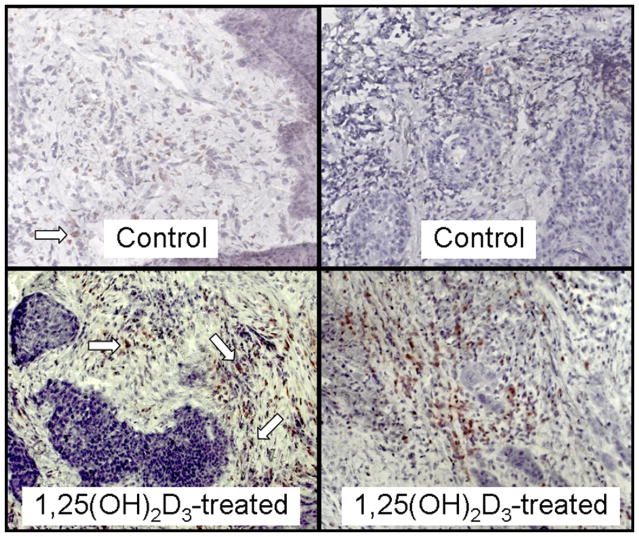
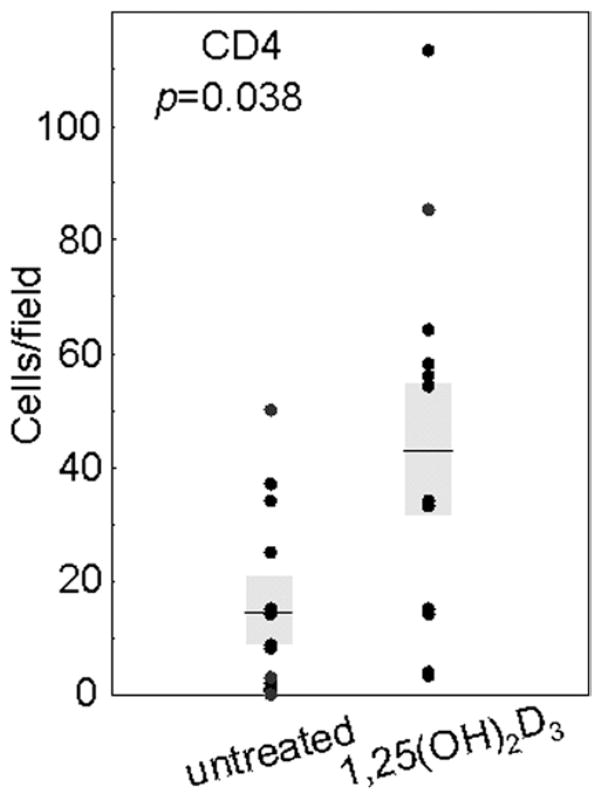
Immunostaining for CD4+ cells showed increases in the numbers of these cells within tissues of patients who received 1,25(OH)2D3 treatment (sample micrographs in Fig. 1). Levels of CD4+ cells within each microscopic field of tissues of untreated patients averaged 14 ± 6. This level was approximately tripled to 44 ± 12 CD4+ cells per field in tissue from patients who had received 3 weeks of 1,25(OH)2D3 treatment (p = 0.038; Fig. 2).
Fig. 1.
Representative microscopic images of increased intratumoral levels of CD4+ cells in HNSCC tissues from two sample patients treated with 1,25(OH)2D3 compared with levels in HNSCC tissue of two sample untreated patients. Arrows indicate examples of positive-stained cells. Images are shown at ×400 magnification.
Fig. 2.
Increased levels of CD4+ cells in HNSCC tissue from patients treated with 1,25(OH)2D3. Numbers of immunostained cells per microscopic field were determined in HNSCC tissues from untreated and 1,25(OH)2D3-treated patients. Shown are the averages of the numbers of immunostained cells for at least two separate slides for each patient, each counted independently by four blinded scorers. Shaded areas represent SEM.
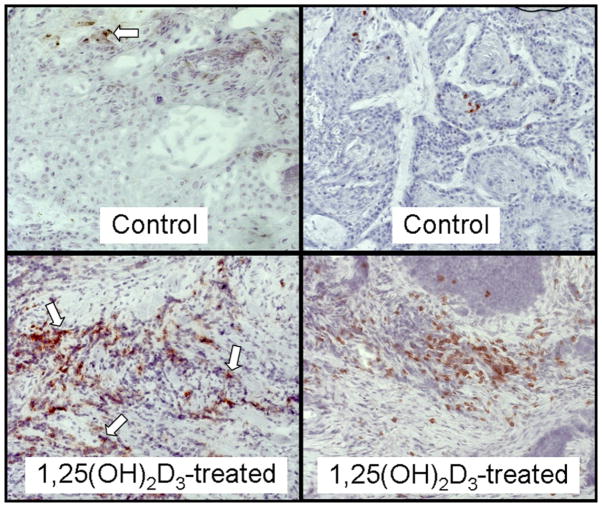
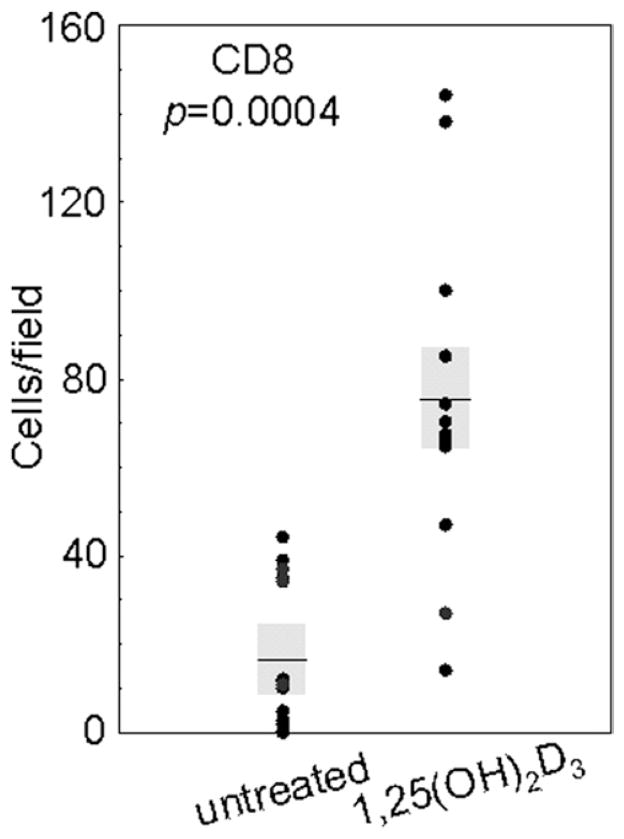
Although HNSCC tissues from patients who received 1,25(OH)2D3 treatment had an increased level of intratumoral CD4+ cells, there was a more highly significant increase in intratumoral CD8+ cells (sample micrographs in Fig. 3). Tissue from untreated patients had an average of 17 ± 6 CD8− staining cells per field. In contrast, tissue from 1,25(OH)2D3-treated patients had an approximate 4.5-fold increase in CD8+ cells, with an average of 75 ± 13 cells per microscopic field (p = 0.0004; Fig. 4). These findings show that a 3-week course of treatment with 1,25(OH)2D3 resulted in an increase in intratumoral infiltration of CD4+ cells, and a more highly significant intratumoral infiltration of CD8+ T cells.
Fig. 3.
Representative microscopic images of increased intratumoral levels of CD8+ cells in HNSCC tissues from two sample patients treated with 1,25(OH)2D3 compared with levels in HNSCC tissue of two sample untreated patients. Arrows indicate examples of positive-stained cells. Images are shown at ×400 magnification.
Fig. 4.
Increased levels of CD8+ cells in HNSCC tissue from patients treated with 1,25(OH)2D3. Numbers of immunostained cells per microscopic field were enumerated in HNSCC tissues from untreated and 1,25(OH)2D3-treated patients. Shown are the averages of the numbers of immunostained cells for at least 2 separate slides for each patient, each counted independently by four blinded scorers. Shaded areas represent SEM.
3.3. Increased numbers of intratumoral infiltrating cells expressing the early activation marker CD69
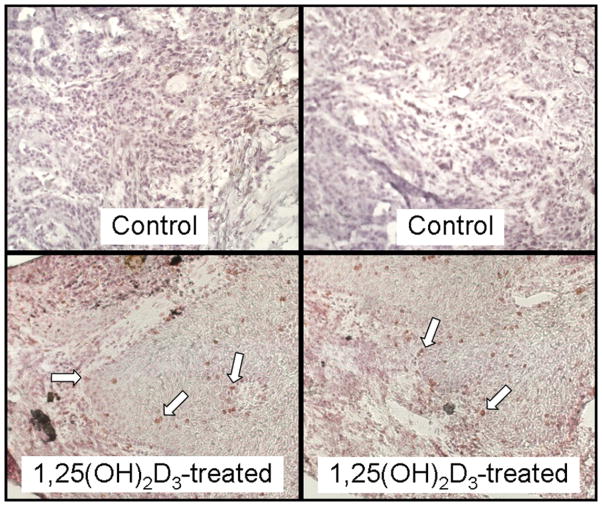
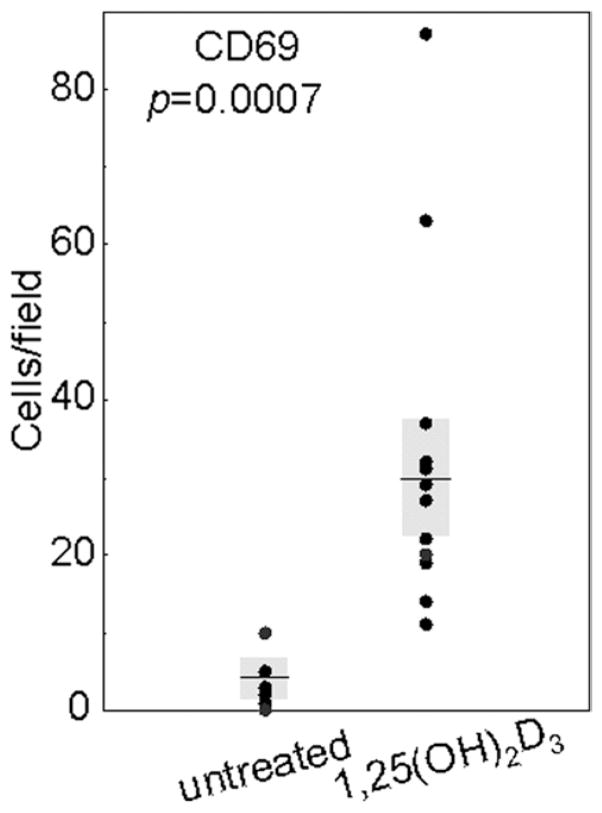
CD69 is an early activation marker that has been shown to be expressed on lymphocytes as well as natural killer cells and monocytes [24–26]. Thus HNSCC tissue from patients who were either untreated or treated with 1,25(OH)2D3 were examined by immunohistochemistry to determine whether treatment with 1,25(OH)2D3 increased levels of immune infiltrating cells that expressed this activation marker (sample micrographs in Fig. 5). CD69+ cells either were not detectable within HNSCC tissues of untreated patients or, occasionally, a few cells could be detected that stained weakly for CD69 (3 ± 2 positive-staining cells/field). In contrast, the presence of cells expressing the activation marker CD69 was approximately 10-fold greater (p = 0.0007; Fig. 6) in tissues from patients who had completed 3 weeks of 1,25(OH)2D3 treatment. Levels of CD69− staining cells in HNSCC of patients treated with 1,25(OH)2D3 averaged 30 ± 7 cells per microscopic field. Although these studies could not further determine the identity of the cells expressing CD69, they did show that, after 1,25(OH)2D3 treatment, there was a prominent increase in infiltrating intratumoral cells with an activated phenotype.
Fig. 5.
Representative microscopic images of increased intratumoral levels of cells expressing the early activation marker CD69 in HNSCC tissues from two sample patients treated with 1,25(OH)2D3 compared with levels in HNSCC tissue of two sample untreated patients. Arrows indicate examples of positive-stained cells. Images are shown at ×400 magnification.
Fig. 6.
Increased levels of cells expressing the early activation marker CD69 in HNSCC tissue from patients treated with 1,25(OH)2D3. Numbers of immunostained cells per microscopic field were determined in HNSCC tissues from untreated and 1,25(OH)2D3-treated patients. Shown are averages of numbers of immunostained cells for at least two separate slides for each patient, each counted independently by four blinded scorers. Shaded areas represent SEM.
3.4. Clinical outcomes for HNSCC patients treated with 1,25(OH)2D3 before surgical treatment
The clinical profile of the population of patients that were recruited into this study is shown in Table 1. Whether treatment of these patients with 1,25(OH)2D3 before surgical cancer treatment might impact on the clinical course of disease was examined. This was accomplished by quantitating possible differences among the patient groups in the time between surgical treatment and recurrence of detectable disease. Although some patients received radiation treatment and/or chemotherapy postoperatively (Table 1), there were no differences in the numbers of control or 1,25(OH)2D3-treated patients who received these added treatments. Therefore the time to postsurgical recurrence of detectable disease was analyzed without stratification for these added treatments.
Shown in Figure 7 is the Kaplan–Meier analysis for the time to HNSCC recurrence for the two patient groups. The HNSCC patient group who received preoperative 1,25(OH)2D3 treatment had a longer time to recurrence than did the group receiving no treatment before surgery (p = 0.048). The median time to recurrence for the control group was 181 days, whereas the median time to recurrence for the patients receiving 1,25(OH)2D3 treatment was 620 days. Although this was a small clinical trial, it nevertheless suggested that presurgical treatment with 1,25(OH)2D3 stimulated intratumoral infiltration of T cells and early activation marker–expressing cells, which coincided with a reduced rate of recurrence of HNSCC disease.
Fig. 7.
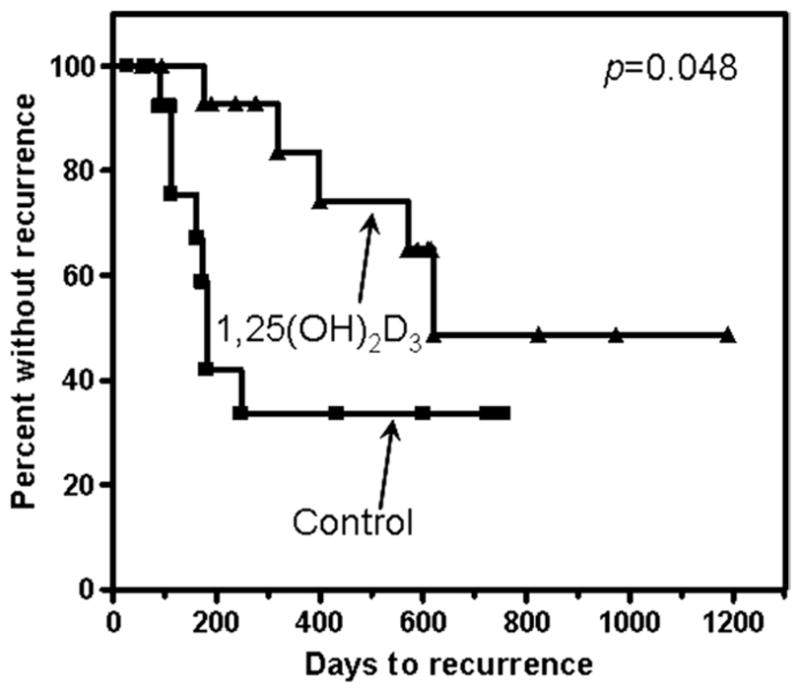
Time to HNSCC recurrence in patient treated with 1,25(OH)2D3. Clinical effectiveness of 1,25(OH)2D3 treatment was analyzed by the number of days between surgical treatment and HNSCC recurrence. Data shown are from Kaplan–Meier analyses of days to recurrence.
4. Discussion
T-cell functional competence is critical for stimulating antitumor immune reactivity. Unfortunately, patients with HNSCC are particularly deficient in their immune competence [1]. There are multiple immune inhibitory mechanisms that are mediated by HNSCC, including their induction of immune suppressor cells that block host immune reactivity [27–29]. Among the immune suppressor cells that are induced to appear in elevated numbers in the peripheral blood and within HNSCC tumor tissue are immature progenitor cells that can be identified by their surface expression of CD34 [29,30]. These CD34+ immune inhibitory cells appear to be defective in their differentiation, but can be driven to differentiate into several different types of mature cells, including immune stimulatory dendritic cells.
Our prior in vitro studies and studies with tumor-bearing animals have shown that, in the presence of the correct cytokine milieu, 1,25(OH)2D3 overcomes the defects in differentiation of CD34+ cells of tumor-bearers. The required cytokines for differentiation toward the dendritic cell lineage include GM-CSF, which HNSCC produce [9,31]. Consequently, several pilot studies were conducted using vitamin D3 analogs to modulate maturation of immune inhibitory progenitor cells. The first study showed that treatment of patients with advanced HNSCC disease with 25-hydroxyvitamin D3 diminished levels of immune inhibitory CD34+ cells in the peripheral blood and concurrently increased immune reactivity of peripheral blood T cells. A subsequent study with newly diagnosed HNSCC patients showed that treatment with 1,25(OH)2D3 reduced intratumoral levels of CD34+ cells and, concurrently, increase levels of mature dendritic cells within the HNSCC tissue.
The above studies using vitamin D3 analogs to modulate D34+ cell differentiation in HNSCC patients prompted further evaluation of whether treatment of newly diagnosed HNSCC patients with 1,25(OH)2D3 between the time of cancer diagnosis and surgical treatment would not only increase the intratumoral levels of mature dendritic cells but also increase infiltrating T-cell levels within the HNSCC tissue. The present study showed that a 3-week course of treatment with 1,25(OH)2D3 increased levels of CD4+ T cells and, to a somewhat more significant extent, increased intratumoral levels of CD8+ cells. Furthermore 1,25(OH)2D3 treatment increased levels of CD69+ cells, which represent mainly T cells and monocytes expressing this early activation marker.
Increased levels of vitamin D analogues have been shown in multiple studies to be associated with reduced cancer development [32,33]. Whether this is a direct effect on the developing malignant cells or occurs via immune modulatory effects of vitamin D analogs is not known. Although vitamin D analogs have been shown to stimulate immune reactivity in cancer patients, other studies have shown immune inhibitory effects of vitamin D analogs [34,35]. The immune dampening effects of vitamin D analogs has typically been tested either in models in which there is no immune dysfunction or in instances of chronic immune activation, such as in inflammatory bowel disease [36]. Whether the increase in immune cells infiltrating into HNSCC after treatment with 1,25(OH)2D3 reflected immune activation within the HNSCC tissue, and whether it was a consequence of our prior observation of diminished levels of immune inhibitory CD34+ cells and increased levels of mature dendritic cells, cannot be determined through the present studies, as they were immunohistochemical analyses of HNSCC tissues and were not designed to study function or causality. Of interest would be whether the observed increase in the intratumoral T-cell infiltration after 1,25(OH)2D3 treatment was a consequence of increased infiltration or increased intratumoral proliferation. Although it is not feasible to determine this in a clinical trial, it would be expected that the increased T-cell content could be due to enhanced infiltration, as vitamin D has previously been shown to modulate levels of inflammatory chemokines [37].
As a part of the analyses in this pilot study, the clinical effectiveness of 1,25(OH)2D3 treatment for newly diagnosed HNSCC patients was assessed by measuring the time to cancer recurrence after surgical treatment. In addition to the demonstration of an increase in infiltration by immune cells showing markers of activation within the HNSCC tumor of 1,25(OH)2D3-treated patients, results also suggested a postsurgical increase in the time to recurrence. Patients receiving 1,25(OH)2D3 treatment showed a lengthier time to recurrence, with a median time that was almost 3.5-fold that for the control patients who were untreated before surgery. Although this lengthier time to recurrence in 1,25(OH)2D3-treated HNSCC patients coincided with its immune enhancing effect, it cannot be definitively determined that these effects are causally related. It is important to note that these results suggesting a clinical response are from a small study that needs to be expanded to a larger cohort of patients before making definitive conclusions about clinical effectiveness. A larger study would also allow analysis of the effect of presurgical treatment with 1,25(OH)2D3 on correlations between the extent of the increase in immune infiltration and time to cancer recurrence or to patient responsiveness to salvage treatments following recurrence.
HNSCC patients are known for the multitude of their immune inhibitory cell populations that are induced by the HNSCC, with CD34+ cells being among these inhibitory cells. Thus it was surprising that 1,25(OH)2D3 treatment aiming to diminish levels of these CD34+ suppressor cells to stimulate immune infiltration within the HNSCC was sufficient to suggest a clinical response. Not known is the impact of the extent of vitamin D deficiency on responsiveness to the 1,25(OH)2D3 treatment. Future studies with a larger patient cohort could assess whether patients who are more profoundly vitamin D deficient have a greater increase in immune infiltration and time to cancer recurrence than patients who are less deficient in vitamin D. Whether the 1,25(OH)2D3 treatment also had an impact on other tumor-induced immune inhibitory populations, such as regulatory T cells (Treg) or tumor-associated macrophages, needs to be determined in subsequent studies. Also of interest for future studies to assess the immune enhancing effectiveness of 1,25(OH)2D3 would be an analysis of the functional activity of the intratumoral immune infiltrate. This would include assessment of whether there is not just an increase in the number of tumor-infiltrating T cells but whether there is stimulation of tumor-specific reactivity by these T cells or by the T cells of the regional lymph nodes. Such analyses of the effect of 1,25(OH)2D3 treatment on immune inhibitory cell populations other than CD34+ cells and on functional activity of intratumoral T cells are ongoing in a new trial that we have recently initiated with HNSCC patients. Finally, future studies might consider a combination immunotherapeutic approach with 1,25(OH)2D3 to stimulate intratumoral immune infiltration, plus a second form of immunotherapy involving a tumor antigen-containing vaccine for stimulating dendritic cells to trigger tumor-specific immune reactivity against any residual HNSCC cells.
Acknowledgments
The authors thank Bridgette Ransom, Kiwana Gibbs, and Kim Sutton for their technical contributions to the described analyses. These studies were supported by the Clinical Science and Biomedical Laboratory Research and Development Services of the Department of Veterans Affairs and by grants R01CA85266 and R01CA128837 from the National Institutes of Health to MRIY.
References
- 1.Heimdal JH, Aarstad HJ, Klementsen B, Olofsson J. Peripheral blood mononuclear cell (PBMC) responsiveness in patients with head and neck cancer in relation to tumour stage and prognosis. Acta Otolaryngol. 1999;119:281–4. doi: 10.1080/00016489950181828. [DOI] [PubMed] [Google Scholar]
- 2.Cho Y, Miyamoto M, Kato K, Fukunaga A, Shichinohe T, Kawarada Y, et al. CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res. 2003;63:1555–9. [PubMed] [Google Scholar]
- 3.De Stefani A, Valente G, Forni G, Lerda W, Ragona R, Cortesina G. Treatment of oral cavity and oropharynx squamous cell carcinoma with perilymphatic interleukin-2: Clinical and pathologic correlations. J Immunother Emphasis Tumor Immunol. 1996;19:125–33. doi: 10.1097/00002371-199603000-00005. [PubMed] [DOI] [PubMed] [Google Scholar]
- 4.van Herpen CM, Looman M, Zonneveld M, Scharenborg N, de Wilde PC, van de LL, et al. Intratumoral administration of recombinant human interleukin 12 in head and neck squamous cell carcinoma patients elicits a T-helper 1 profile in the locoregional lymph nodes. Clin Cancer Res. 2004;10:2626–35. doi: 10.1158/1078-0432.ccr-03-0304. [DOI] [PubMed] [Google Scholar]
- 5.Karcher J, Dyckhoff G, Beckhove P, Reisser C, Brysch M, Ziouta Y, et al. Antitumor vaccination in patients with head and neck squamous cell carcinomas with autologous virus-modified tumor cells. Cancer Res. 2004;64:8057–61. doi: 10.1158/0008-5472.CAN-04-1545. [DOI] [PubMed] [Google Scholar]
- 6.To WC, Wood BG, Krauss JC, Strome M, Esclamado RM, Lavertu P, et al. Systemic adoptive T-cell immunotherapy in recurrent and metastatic carcinoma of the head and neck: A phase 1 study. Arch Otolaryngol Head Neck Surg. 2000;126:1225–31. doi: 10.1001/archotol.126.10.1225. [DOI] [PubMed] [Google Scholar]
- 7.Hadden J, Verastegui E, Barrera JL, Kurman M, Meneses A, Zinser JW, et al. A trial of IRX-2 in patients with squamous cell carcinomas of the head and neck. Int Immunopharmacol. 2003;3:1073–81. doi: 10.1016/S1567-5769(03)00029-8. [DOI] [PubMed] [Google Scholar]
- 8.Wanebo HJ, Riley T, Karz D, Pace RC, Johns ME, Cantrell RW. Indomethacin sensitive suppressor-cell activity in head and neck cancer patients. The role of the adherent mononuclear cell. Cancer. 1988;61:462–74. doi: 10.1002/1097-0142(19880201)61:3<462::aid-cncr2820610310>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 9.Lathers DMR, Achille NJ, Young MRI. Incomplete Th2 skewing of cytokines in plasma of patients with squamous cell carcinoma of the head and neck. Hum Immunol. 2003;64:1160–6. doi: 10.1016/j.humimm.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 10.Strauss L, Volland D, Kunkel M, Reichert TE. Dual role of VEGF family members in the pathogenesis of head and neck cancer (HNSCC): Possible link between angiogenesis and immune tolerance. Med Sci Monit. 2005;11:BR280–92. [PubMed] [Google Scholar]
- 11.Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, et al. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–66. [PubMed] [Google Scholar]
- 12.Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 13.Garrity T, Pandit R, Wright MA, Benefield J, Young MRI. Increased presence of CD34+ cells in the peripheral blood of head and neck cancer patients and their differentiation into CD1a+ cells. Int J Cancer. 1997;73:663–9. doi: 10.1002/(sici)1097-0215(19971127)73:5<663::aid-ijc9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 14.Young MRI, Wright MA, Vellody K, Lathers DMR. Skewed differentiation of bone marrow CD34+ cells of tumor bearers from dendritic toward monocytic cells, and the induction of differentiation toward dendritic cells by 1a,25-dihydroxyvitamin D. 3. Int J Immunopharmacol. 1999;21:675–88. doi: 10.1016/s0192-0561(99)00044-2. [DOI] [PubMed] [Google Scholar]
- 15.Benvenuti F, Lagaudriere-Gesbert C, Grandjean I, Jancic C, Hivroz C, Trautmann A, et al. Dendritic cell maturation controls adhesion, synapse formation, and the duration of the interactions with naive T lymphocytes. J Immunol. 2004;172:292–301. doi: 10.4049/jimmunol.172.1.292. [DOI] [PubMed] [Google Scholar]
- 16.Asai T, Storkus WJ, Mueller-Berghaus J, Knapp W, DeLeo AB, Chikamatsu K, Whiteside TL. In vitro generated cytolytic T lymphocytes reactive against head and neck cancer recognize multiple epitopes presented by HLA-A2, including peptides derived from the p53 and MDM-2 proteins. Cancer Immun. 2002;2:3. [PubMed] [Google Scholar]
- 17.Young MR, Neville BW, Chi AC, Lathers DM, Boyd GM, Day TA. Oral premalignant lesions induce immune reactivity to both premalignant oral lesions and head and neck squamous cell carcinoma. Cancer Immunol Immunother. 2007;56:1077–86. doi: 10.1007/s00262-006-0242-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohm JE, Shurin MR, Esche C, Lotze MT, Carbone DP, Gabrilovich DI. Effect of vascular endothelial growth factor and FLT3 ligand on dendritic cell generation in vivo. J Immunol. 1999;163:3260–8. [PubMed] [Google Scholar]
- 19.Kulbersh JS, Day TA, Gillespie MB, Young MR. 1a,25-Dihydroxyvitamin D3 to skew intratumora levels of immune inhibitory CD34+ progenitor cells into dendritic cells. Otolaryngol Head Neck Surg. 2009;140:235–40. doi: 10.1016/j.otohns.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muindi JR, Peng Y, Potter DM, Hershberger PA, Tauch JS, Capozzoli MJ, et al. Pharmacokinetics of high-dose oral calcitriol: Results from a phase 1 trial of calcitriol and paclitaxel. Clin Pharmacol Ther. 2002;72:648–59. doi: 10.1067/mcp.2002.129305. [DOI] [PubMed] [Google Scholar]
- 21.Caras I, Grigorescu A, Stavaru C, Radu DL, Mogos I, Szegli G, Salageanu A. Evidence for immune defects in breast and lung cancer patients. Cancer Immunol Immunother. 2004;53:1146–52. doi: 10.1007/s00262-004-0556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuss I, Hathaway B, Ferris RL, Gooding W, Whiteside TL. Imbalance in absolute counts of T lymphocyte subsets in patients with head and neck cancer and its relation to disease. Adv Otorhinolaryngol. 2005;62:161–72. doi: 10.1159/000082506. [DOI] [PubMed] [Google Scholar]
- 23.Molling JW, Langius JA, Langendijk JA, Leemans CR, Bontkes HJ, van der Vliet HJ, et al. Low levels of circulating invariant natural killer T cells predict poor clinical outcome in patients with head and neck squamous cell carcinoma. J Clin Oncol. 2007;25:862–8. doi: 10.1200/JCO.2006.08.5787. [DOI] [PubMed] [Google Scholar]
- 24.Aue G, Njuguna N, Tian X, Soto S, Hughes T, Vire B, et al. Lenalidomide-induced upregulation of CD80 on tumor cells correlates with T-cell activation, the rapid onset of a cytokine release syndrome and leukemic cell clearance in chronic lymphocytic leukemia. Haematologica. 2009;94:1266–73. doi: 10.3324/haematol.2009.005835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freeman CM, Martinez FJ, Han MK, Ames TM, Chensue SW, Todt JC, et al. Lung dendritic cell expression of maturation molecules increases with worsening COPD. Am J Respir Crit Care Med. 2009;180:1179–88. doi: 10.1164/rccm.200904-0552OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez-Albaitero A, Lee SC, Morgan S, Grandis JR, Gooding WE, Ferrone S, Ferris RL. Role of polymorphic Fc gamma receptor IIIa and EGFR expression level in cetuximab mediated, NK cell dependent in vitro cytotoxicity of head and neck squamous cell carcinoma cells. Cancer Immunol Immunother. 2009;58:1855–64. doi: 10.1007/s00262-009-0697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergmann C, Strauss L, Zeidler R, Lang S, Whiteside TL. Expansion of human T regulatory type 1 cells in the microenvironment of cyclooxygenase 2 overexpressing head and neck squamous cell carcinoma. Cancer Res. 2007;67:8865–73. doi: 10.1158/0008-5472.CAN-07-0767. [DOI] [PubMed] [Google Scholar]
- 28.Kacani L, Wurm M, Schennach H, Braun I, Andrle J, Sprinzl GM. Immunosuppressive effects of soluble factors secreted by head and neck squamous cell carcinoma on dendritic cells and T lymphocytes. Oral Oncol. 2003;39:672–9. doi: 10.1016/s1368-8375(03)00076-9. [DOI] [PubMed] [Google Scholar]
- 29.Pandit R, Lathers DM, Beal NM, Garrity T, Young MRI. CD34+ immune suppressive cells in the peripheral blood of patients with head and neck cancer. Ann Otol Rhinol Laryngol. 2000;109:749–54. doi: 10.1177/000348940010900809. [DOI] [PubMed] [Google Scholar]
- 30.Young MR, Lathers DM. Myeloid progenitor cells mediate immune suppression in patients with head and neck cancers. Int J Immunopharmacol. 1999;21:241–52. doi: 10.1016/s0192-0561(99)00008-9. [DOI] [PubMed] [Google Scholar]
- 31.Young MRI, Wright MA, Lozano Y, Matthews JP, Benefield J, Prechel MM. Mechanisms of immune suppression in patients with head and neck cancer: Influence on the immune infiltrate of the cancer. Int J Cancer. 1996;67:333–8. doi: 10.1002/(SICI)1097-0215(19960729)67:3<333::AID-IJC5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 32.Rejnmark L, Tietze A, Vestergaard P, Buhl L, Lehbrink M, Heickendorff L, Mosekilde L. Reduced prediagnostic 25-hydroxyvitamin D levels in women with breast cancer: A nested case-control study. Cancer Epidemiol Biomarkers Prev. 2009;18:2655–60. doi: 10.1158/1055-9965.EPI-09-0531. [DOI] [PubMed] [Google Scholar]
- 33.Goldstein BD, Kurt RA. Dietary vitamin D3 restriction influences tumor growth, but not the ability to generate an antigen-specific immune response in OTII transgenic mice. Immunol Invest. 2009;38:365–82. doi: 10.1080/08820130902861968. [DOI] [PubMed] [Google Scholar]
- 34.Lathers DM, Clark JI, Achille NJ, Young MR. Phase 1B study to improve immune responses in head and neck cancer patients using escalating doses of 25-hydroxyvitamin D3. Cancer Immunol Immunother. 2004;53:422–30. doi: 10.1007/s00262-003-0459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Enioutina EY, Bareyan D, Daynes RA. Vitamin D3-mediated alterations to myeloid dendritic cell trafficking in vivo expand the scope of their antigen presenting properties. Vaccine. 2007;25:1236–49. doi: 10.1016/j.vaccine.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol. 2003;17:2386–92. doi: 10.1210/me.2003-0281. [DOI] [PubMed] [Google Scholar]
- 37.Vulcano M, Struyf S, Scapini P, Cassatella M, Bernasconi S, Bonecchi R, et al. Unique regulation of CCL18 production by maturing dendritic cells. J Immunol. 2003;170:3843–9. doi: 10.4049/jimmunol.170.7.3843. [DOI] [PubMed] [Google Scholar]