Abstract
Tumor-induced immune suppression involves the accumulation of immune-suppressive infiltrates in the microenvironment. This study demonstrates increased numbers of CD4+CD25+FoxP3+ regulatory T cells (Tregs) in the lungs of C57BL/6 mice bearing a metastatic Lewis lung carcinoma (LLC) variant. These Tregs suppressed the proliferation of endogenous CD4+CD25− cells and expressed higher levels of the chemokine receptor CCR4 than other types of T cells. LLC-bearing lungs secreted elevated levels of the CCR4-associated chemokine CCL22 compared with normal lungs. However, CCL22 was not secreted by LLC or normal epithelial controls, suggesting that CCL22 is secreted by a nonepithelial component of the microenvironment. Migration assays revealed that medium conditioned by LLC-bearing lungs selectively recruited Tregs at higher frequencies than did medium conditioned by normal lungs. Neutralization of CCL22 significantly reduced this selective recruitment toward both conditioned media. A series of immunomagnetic isolations, FACS, and flow cytometric analyses were used to isolate different cellular fractions from both normal and LLC-bearing lungs. When isolated, only the NK-containing fractions secreted CCL22, and the same fraction isolated from LLC-bearing lungs secreted higher levels. Depletion of NK cells from both normal and LLC-bearing lung tissue significantly reduced CCL22 secretion, suggesting that a large portion of secreted CCL22 is NK cell dependent. Flow cytometric analysis of the lung NK compartments revealed no significant increase in NK cell numbers across LLC-bearing lung tissue as a whole as compared with normal tissue. However, immunofluorescent staining revealed an increased frequency of NK cells at the tumor periphery that were closely associated with the elevated FoxP3+ infiltrate.
Tumor-induced immune suppression is vital to the survival and progression of cancer. It impedes both adaptive and innate immunity, which could otherwise mount a protective anti-umor response. Likewise, tumor-induced immune suppression could be responsible for the failure of many promising cell-based vaccine attempts (1, 2) and other immune-based therapies (for review, see Ref. 3) against cancer. Tumor-induced immune suppression is caused by numerous mechanisms, many of which involve the accumulation of immune-suppressive infiltrates in the tumor microenvironment. One of the most potent and wellstudied suppressive phenotypes found in the tumor microenvironment is the regulatory T cell (Treg).4 Elucidating the mechanisms by which these cells traffic and accumulate in the tumor microenvironment could provide attractive therapeutic targets with which to combat tumor-induced immune suppression.
Tregs can be classified as either naturally occurring Tregs or inducible Tregs. Naturally occurring Tregs are matured in the thymus and constitutively express the IL-2 receptor CD25 (4). These cells preexist in basal levels throughout the body to restrict autoimmunity. Without these cells, severe autoimmune disorders rapidly occur in both mice (5–9) and humans (10). Although naturally occurring Tregs produce type II-associated cytokines such as IL-10 (11), their suppressive capacities are largely mediated via contact-dependent mechanisms through CTLA-4 (12) and membrane-bound TGF-β (13). This cell contact-mediated suppression is what largely separates naturally occurring Tregs from inducible Tregs. Inducible Tregs are not generated in the thymus, but rather are induced in the periphery upon activation. These cells suppress effecter cell function through contact-independent cytokine release. Inducible Tregs consist of an ever-growing list of subtypes (for review of Treg subsets, see Ref. 14). The best indicator of suppressive Treg populations is the expression of the transcription factor FoxP3. All naturally occurring Tregs express FoxP3 as well as many inducible populations. As such, the expression of CD4, CD25, and FoxP3 has been the traditional hallmark of the Treg phenotype.
The trafficking of T cells is mediated in part by chemokines and the expression of their receptors. Higher expression of the chemokine receptor CCR4 was previously reported on Tregs as compared with other types of T cells (15). Likewise, the CCR4-associated chemokines, thymus and activation-regulated chemokine (CCL17), and macrophage-derived chemokine (CCL22), have been correlated with higher frequencies of Tregs in gastric cancer (16). In ovarian cancer, tumor-derived CCL22 was shown to recruit Tregs in vivo and predicts a negative prognosis (17). Similarly, CCR4 has been implemented in the recruitment of Treg toward cerebral spinal fluid of patients with lymphomatous and carcinomatous meningitis (18) and toward gliomas (19) and the lymph nodes of patients with Hodgkin’s lymphoma (20).
The present study examines Treg levels and changes in chemokine secretion in a murine model of Lewis lung carcinoma (LLC). This aggressive squamous cell carcinoma readily establishes tumors in the lungs of injected mice and shares many pathological characteristics common to most squamous cell carcinomas. Although these studies reiterate the involvement of CCR4-associated chemokines in Treg recruitment, these studies also identify NK cells as a major source of CCL22 in the LLC microenvironment, providing a completely novel link between infiltrating NK cells and Treg recruitment.
Materials and Methods
Cell culture
A metastatic cell line of LLC (LN7) was used in all experiments. LLC and normal mouse lung epithelial cells (MLE-12) were kept in culture medium (RPMI 1640 medium (Invitrogen) supplemented with 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, 0.02 M HEPES buffer, and 5 × 10−5 M 2-ME). Cells were passed as needed just before confluence. MLE-12 cultures were detached before passage by brief trypsin treatment. LLC-LN7 cells are nonadherent in culture.
Generation of LLC-bearing lung tissue
Single-cell suspensions of 1.0 × 107 cells/ml washed in HBSS (Invitrogen) were prepared from LLC cell cultures that were in log phase. Female C57BL/6 mice were injected i.v. in the tail vein with 0.2 ml of PBS or LLC cell suspension, resulting in the delivery of 2.0 × 106 LLC cells/animal. Mice were euthanized ~16–18 days later when signs of labored breathing first appeared in the LLC-injected animals. Lung tissue was harvested and placed in HBSS before subsequent assays or was frozen into blocks in OCT (Sakura) compound using liquid N2.
Generation of conditioned medium
For the generation of conditioned medium from cell cultures, the cells were washed with HBSS three times before reconstitution in serum-free culture medium. Cultures were left at 37°C overnight (16 h). The following morning, the medium was collected and filtered. For the generation of conditioned medium from intact tissue, whole intact lungs were rinsed in PBS and placed overnight (16 h) at 37°C in 12 ml of serum-free culture medium. The following morning, the medium was collected and filtered.
ELISA
CCL22, CCL17, and CXCL12 were measured in various conditioned media using respective Quantikine Immunoassay kits (R&D Systems) according to the manufacturer’s instructions. IFN-γ levels were measured using an IFN-γ BD Biosciences OptEIA assay kit according to the manufacturer’s instructions.
Dissociation of solid lung tissues
Following harvest, entire sets of both normal and LLC-bearing lungs were placed in an enzyme digest (1 µg/ml collagenase IV, 0.6 µg/ml hyaluronidase, 0.1 µg/ml DNase; Sigma-Aldrich) dissolved in HBSS for 90 min at room temperature. Each specimen was then homogenized in a Stomacher 80 homogenizer (Seward) set on medium for 120 s. The resulting homogenate was then rinsed with HBSS and filtered to remove debris, resulting in single-cell suspensions.
Immunomagnetic isolation
CD11b and CD11c fractions were isolated from lung tissue homogenates using isolation kits (Miltenyi Biotec) according to the manufacturer’s instructions. CD3 and CD31 fractions were isolated from lung tissue homogenates by incubating with biotin-conjugated primary Abs for 15 min on ice (biotin-CD3, biotin-CD31; eBioscience), followed by a secondary incubation with streptavidin microbeads (Miltenyi Biotec) for 15 min on ice. The labeled cells were then positively collected using LS magnetic columns (Miltenyi Biotec). NK and T cell populations were negatively isolated from normal splenocytes using NK and PanT isolation kits, respectively (Miltenyi Biotec), according to the manufacturer’s instructions. Biotinylated NK1.1 was used to deplete ex vivo dissociates of NK cells using streptavidin microbeads and LS magnetic columns (Milenyi Biotec). For the immune suppression assays, Tregs were isolated from dissociated single-cell suspensions using Treg isolation kits (Miltenyi Biotec) according to the manufacturer’s instructions.
Transwell migration assay
Twelve-well Transwell plates with 6.5-mm insert filters (8–µm pore size; Costar) were used to measure migration. Seventy-five microliters of growth factor-reduced Matrigel (BD Biosciences) was coated on each insert and allowed to set for 2 h before being washed with serum-free culture medium. T cells isolated from normal splenocytes were reconstituted in culture medium with 1% FBS and seeded in the upper chamber (inserts) of the Transwell. Media placed in the bottom chambers contained 50% conditioned medium (serum free) and 50% culture medium with 2% FBS. In some cases, various amounts of neutralizing anti-CCL22 (R&D Systems) were added to the conditioned medium before the assay. The T cells were left to migrate for 18–24 h at 37°C. Both the migratory and nonmigratory cell fractions were collected and analyzed using flow cytometry after immunofluorescent staining with CD4, CD8, CD25, and FoxP3.
Flow cytometry
Nonspecific staining was blocked with FBS and 1 µl per 1.0 × 106 cells of CD16/32 (BD Pharmingen) before staining with 5 µl of the following Abs: PE-NK1.1, PerCP-CD4, PE-Cy7-CD8, PE-CD3 (BD Pharmingen); PE-CD19, FITC-CD27, PE-CD122, PE-CD3, PE-CD25, FITC-FoxP3, FITC-F4/80, PE-Ly-6G/Gr-1, FITC-CD31, allophycocyanin-CD11b, PECD11c, FITC-CD49a (DX5), and FITC-NK1.1 (eBioscience). Intracellular staining for FoxP3 required overnight fixation with fixation/permeabilization solution (eBioscience). CCR4 staining was achieved using a goat polyclonal primary Ab (Abcam), followed by a secondary incubation with PEdonkey F(ab′)2 anti-goat IgG (Jackson ImmunoResearch Laboratories). The extent and frequency of labeled cells was visualized using flow cytometry (FACSCanto; BD Biosciences).
Isolation using FACS
CD11b+ subpopulations were first immunofluorescently labeled as described above for flow cytometry and then washed with PBS containing 1 µg/ml propidium iodide (Molecular Probes). Sorting was performed using a MoFlo High-Speed Cell Sorter (Dako).
Treg suppression assay
CD4+CD25+ cells were immunomagnetically isolated from normal and LLC-bearing lung tissue and were used to measure their immune inhibitory activity toward CD4+CD25− cells. The CD4+CD25− cells were isolated from dissociated tissue and labeled with CFSE (Molecular Probes). Ninety-six-well plates were coated for 90 min with 10 µg/ml anti-CD3 Ab in Ca2+/Mg2+-free PBS (Life Technologies) and then washed with PBS. T cells were plated at 2.0 × 105 cells/well in culture medium with 10% FBS at CD4+CD25+ cell to CD4+CD25+ cell ratios of 1:1, 1:10, 1:100, and 1:1000. Control wells contained CD4+CD25− cells without CD4+CD25+ cells. After 3 days, proliferation of the CD4+CD25− cells was measured using flow cytometry and reported as a percentage of cells that have undergone at least one division as defined by CFSE fluorescent intensity.
Immunofluorescent staining of frozen tissue sections
Lung tissue was frozen in OCT and cryosectioned into 10-µm sections and placed onto glass slides. Tissue sections were fixed in ice-cold methanol for 10 min, and nonspecific staining was then blocked using 2% donkey serum in PBS for 1 h at room temperature. After being rinsed in PBS, 1/100 dilutions in PBS of primary Abs (biotin-NK1.1, FoxP3; eBioscience) were added to the slides and allowed to incubate overnight at room temperature in a moist environment. The slides were then washed in PBS and donkey F(ab′)2 anti-rat FITC (The Jackson Laboratory) and streptavidin-Texas Red (Vector Laboratories) were added at a dilution of 1/100 each in PBS and allowed to incubate for 45 min at room temperature in the dark. For the immunofluorescent detection of Tregs, each primary Ab was raised in the same animal (rat) and thus could not be incubated simultaneously. Instead, the slides were incubated with anti-mouse FoxP3 (eBioscience) overnight in a warm moist environment. The slides were then washed in PBS and donkey Fab anti-rat FITC was added at a dilution of 1/100 and allowed to incubate for 45 min in the dark. Then, the slides were washed in PBS and the second primary Ab (anti-mouse CD4; eBioscience) was added at a dilution of 1/100 and allowed to incubate for 1 h in the dark. The slides were then washed in PBS and donkey F(ab′)2 anti-rat rhodamine was added at a dilution of 1/100 and allowed to incubate for 45 min in the dark. Following secondary incubations, slides were then rinsed in PBS, counterstained with Hoechst dye (Sigma-Aldrich), and then washed with distilled H2O and mounted using Vectashield fluorescent mount medium (Vector Laboratories). Fluorescent staining was visualized microscopically.
Generation of activated NK cells
NK cells were isolated from normal splenocytes via negative immunomagnetic separation (Miltenyi Biotec). The NK cells were then subjected to a range of IL-2 doses for 48 h in RPMI 1640 culture medium. IFN-γ ELISA was used to confirm NK cell activation.
Statistics
Data are reported using the mean as a measure of central tendency ± SEM. To compare one variable condition between groups, the two-tailed Student’s t test was used. Significance was reported in the 95% confidence interval.
Results
Immunosuppressive Treg levels are increased in LLC-bearing lung tissue
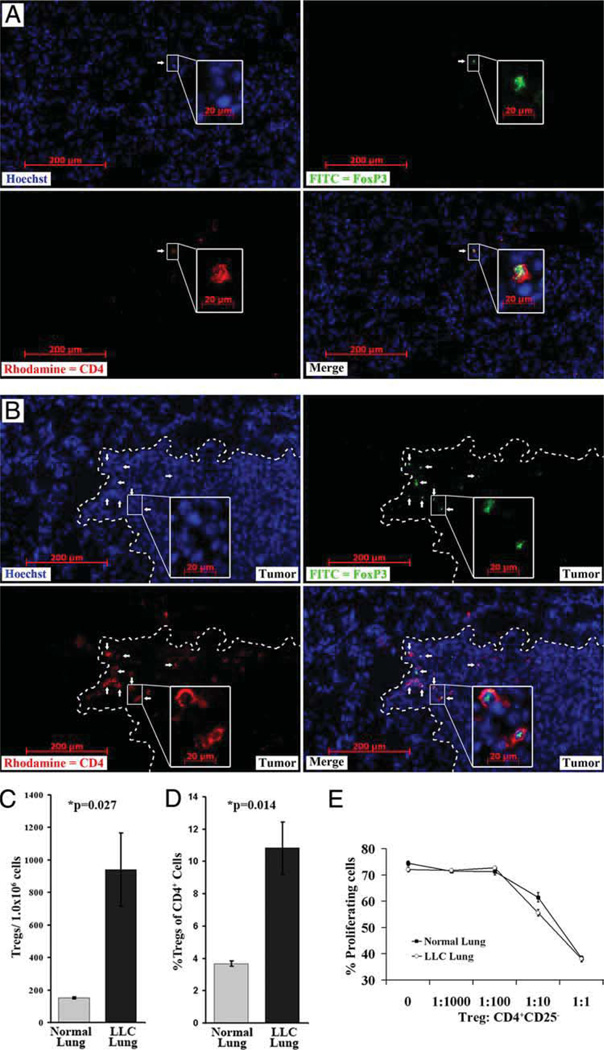
A large percentage of immune suppressive mechanisms used by solid tumors involve the accumulation of immune-suppressive infiltrates such as Tregs. Excised normal and LLC-bearing lungs were cryosectioned and stained with Hoechst dye (blue), anti-CD4 (PE, red), and anti-FoxP3 (FITC, green). Tregs are evidenced by FoxP3+ nuclei and CD4 cell surface staining (Fig. 1A and B). The distribution of CD4+Foxp3+ cells appeared fairly homogenous in normal lung tissue. However, in LLC-bearing lung tissue, an increased frequency of CD4+Foxp3+ cells was more evident at the tumor periphery, just inside the tumor mass (Fig. 1, **white line marks tumor periphery, white arrows mark examples of Tregs). Flow cytometric analysis of dissociated normal and LLC-bearing lungs showed significantly higher levels of CD4+CD25+FoxP3+ cells (Fig. 1C and D) inside the lungs bearing LLC as compared with normal controls. The frequency of these cells was low in normal lungs (151 ± 5 Tregs/1.0 × 106 lung cells) but was higher in LLC-bearing lungs (940 ± 225 Tregs/1.0 × 106 lung cells; p = 0.027). These CD4+CD25+FoxP3+ cells also comprised a greater percentage of the endogenous CD4+ cells (3.7% ± 0.17 in normal tissue vs 10.8% ± 1.6 in LLC-bearing tissue; p = 0.013). Although Foxp3 expression is a good indicator of the Treg phenotype, some studies have found rare populations of T cells that express FoxP3, but are not immune suppressive (21). To address this, we confirmed the suppressive capacity of these cells by immunomagnetically isolating CD4+CD25+ cells from both normal and LLC-bearing lungs and coculturing in various ratios with CFSE-labeled CD4+CD25− cells isolated from the same lung tissue samples. Proliferation was then stimulated using anti-CD3 and IL-2. Although Treg levels were increased in LLC-bearing lungs, CD4+CD25+ cells from both normal and LLC-bearing lungs suppressed the proliferation of endogenous CD4+CD25− cells in a dose-dependent manner (Fig. 1E). This validated their identity as Tregs.
FIGURE 1.
Treg levels are increased in LLC-bearing lung tissue. Immunofluorescent staining was performed on frozen sections from normal lung tissue (A) and LLC-bearing lung tissue (B) for nuclei (Hoechst, blue), CD4 (rhodamine, red) and FoxP3 (FITC, green). Tregs (white arrows) are considered CD4+ cells that also contain FoxP3 staining in the nucleus (merge). Tumor periphery is marked with a dashed white line. Insets are of a higher magnification of the indicated field. Immunofluorescent staining of CD4, CD25, and FoxP3 was also preformed on normal and LLC-bearing lung tissue dissociates. Flow cytometric analysis shows an increase in total Treg numbers (C) and an increase in the Treg percentage of CD4 infiltrate (D) in LLC-bearing lung tissue. Tregs that were immunomagnetically isolated from both normal and LLC-bearing lung tissue displayed the ability to suppress the proliferation of endogenous CD4+CD25− cells isolated from the respective tissue types (E).
Tregs express more CCR4 than other types of T cells
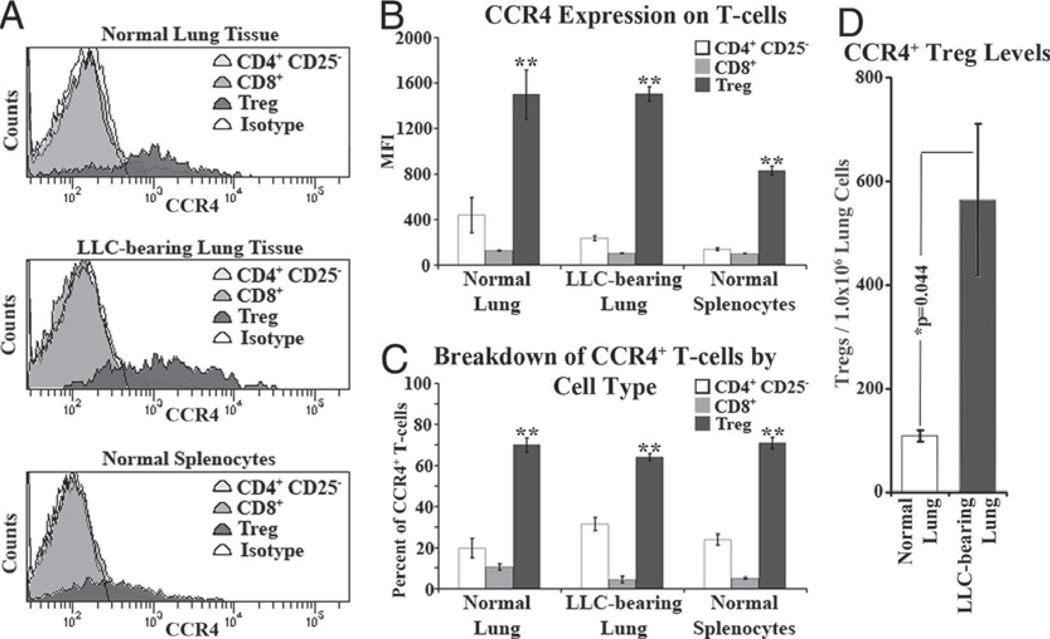
Because previous studies had shown that chemokines that bind to CCR4 are able to chemoattract Tregs, cells from dissociated normal and LLC-bearing lungs, along with normal splenocytes, were immunofluorescently stained for CD4, CD8, CD25, and CCR4 in an effort to profile T cell subpopulations in terms of their CCR4 expression. The mean fluorescent intensity (MFI) of CCR4 staining was significantly higher on Tregs compared with their CD8+ and CD4+CD25− counterparts in both normal and LLC-bearing lung tissue and in normal splenocytes (Fig. 2A and B). Although this increased CCR4 expression on Tregs was not as pronounced in normal splenocytes as it was in normal and LLC-bearing lungs, splenic Treg CCR4 MFI was still between 7- and 8-fold higher than for CD8+ and CD4+CD25− cells. Regardless of CCR4 staining intensity, Tregs comprised the majority of CCR4+ T cells in both normal and LLC-bearing lungs and in normal splenocytes (Fig. 2C). Taken together, these data suggest that Tregs inherently express higher levels of CCR4 than do other T cells and that this CCR4 expression is unaffected by LLC tumor presence. However, the number of CCR4+ Tregs in LLC-bearing lungs (564 ± 146 Tregs/1.0 × 106 lung cells) was greater than in normal lungs (109 ± 11 Tregs/1.0 × 106 lung cells; Fig. 2D).
FIGURE 2.
CCR4 expression on Tregs is unique among T cells. Homogenates from both normal and LLC-bearing lung tissue along with normal splenocytes were immunofluorescently stained for CD8, CD4, CD25, FoxP3, and CCR4. A, Representative histograms of CCR4 expression among T cell types. B, Across all types of homogenate, Tregs had significantly higher MFI of CCR4 staining compared with both CD8+ and CD4+CD25− cells (**, p < 0.05 vs CD8+ and CD4+CD25−). Similarly, of all CCR4+ T cells in the homogenates, Tregs comprised a greater percentage as compared with the CD8+ and CD4+CD25− populations (C). In addition, LLC-bearing tissue contains a significantly increased number of CCR4+ Tregs (D).
LLC-bearing lung tissue secretes elevated levels of CCL22
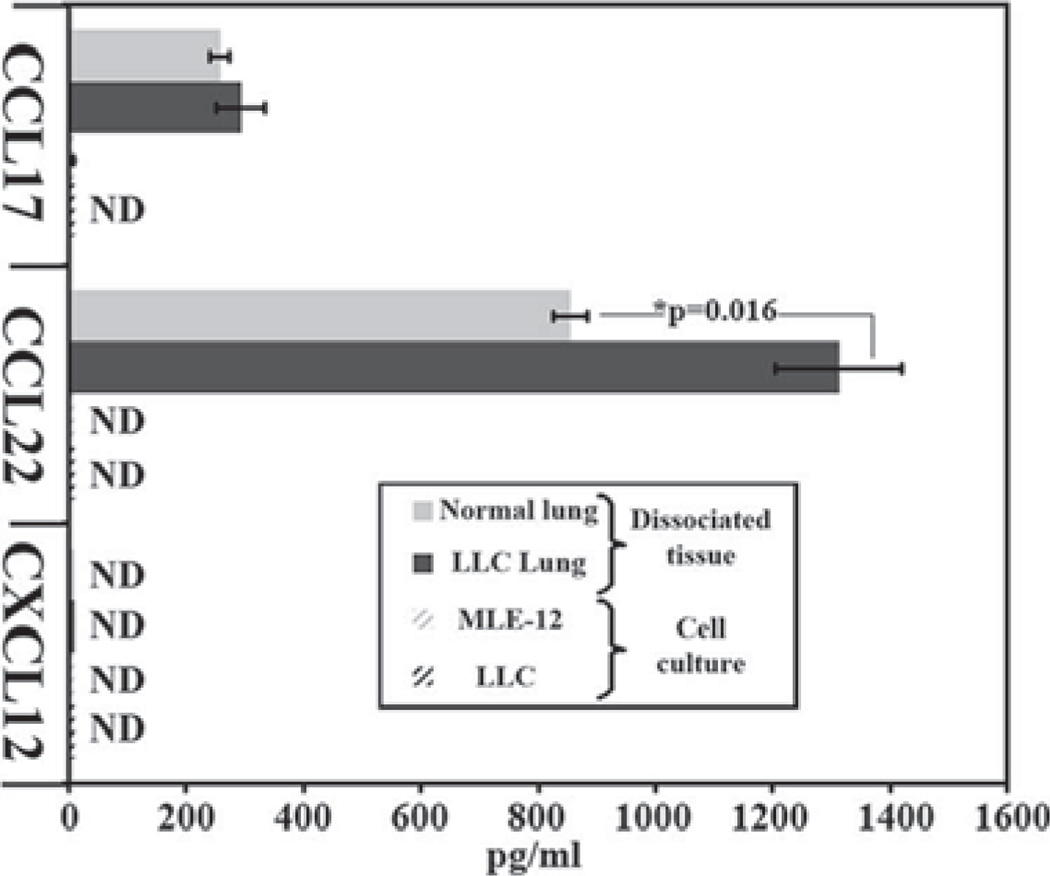
Having shown that Tregs express more CCR4 than other T cell populations, ELISA was used to measure the levels of the CCR4-associated chemokines CCL22 and CCL17 in medium conditioned by both LLC and normal mouse lung epithelial (MLE-12) cultures in addition to medium conditioned by intact normal and LLC-bearing lung tissue. No detectable levels of either CCL22 or CCL17 were secreted by MLE-12 or LLC cell cultures (Fig. 3). However, the conditioned medium taken off of normal lung tissue secreted both CCL17 (257 ± 17 pg/ml) and CCL22 (855 ± 29 pg/ml). LLC-bearing lung tissue secreted similar levels of CCL17 (292 ± 4 1pg/ml) compared with normal tissue, but secreted significantly higher amounts of CCL22 (1313 ± 108 pg/ml; p = 0.016). Another chemokine, CXCL12, has been implemented in the chemoattraction of Tregs toward bone marrow (22). However, no CXCL12 was detected in any of the conditioned medium.
FIGURE 3.
LLC-bearing lung tissue secretes higher levels of CCL22. Medium conditioned on normal mouse epithelial cell cultures (MLE-12), LLC cell cultures, normal lung tissue, or LLC-bearing lung tissue was tested for CCL17, CCL22, and CXCL12 using ELISA.
CCL22 secreted from LLC-bearing lung tissue selectively attracts more Tregs than normal lung tissue
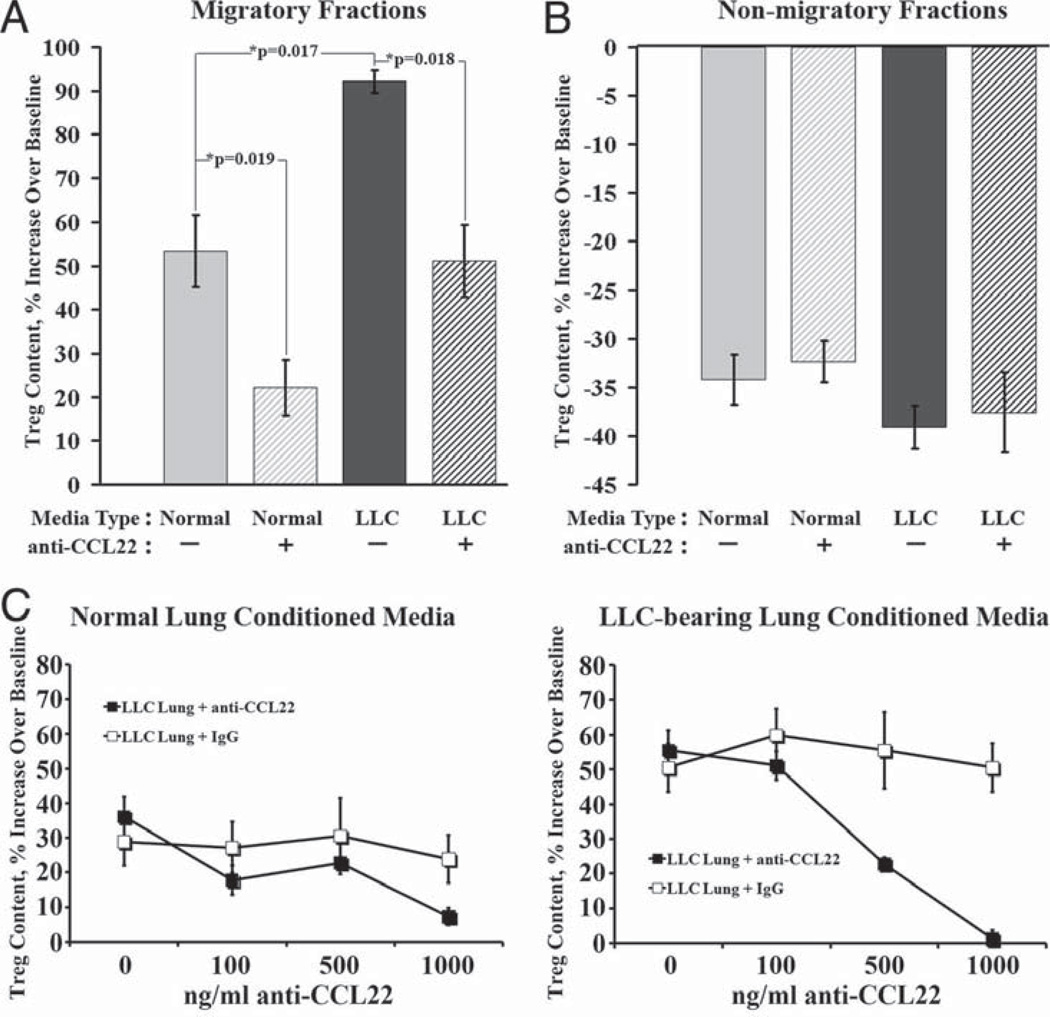
After identifying CCL22 as a candidate factor for selective Treg recruitment, migration assays determined whether the elevated level of CCL22 secreted from LLC-bearing lungs selectively stimulates the migration of Tregs. Transwell migration assays were used with splenic T cells seeded in the upper chamber and medium conditioned by normal or LLC-bearing lungs placed in the lower. Following the overnight migration, flow cytometry was used to analyze the Treg content in the cell fractions that migrated into the lower chambers. The baseline Treg percentage of the normal CD4+ splenocytes used in the assay was 6.7 ± 0.2%. Therefore, migratory cell fractions whose Treg percentage of CD4+ cells was higher than this baseline would indicate selective Treg recruitment toward those conditioned media. The fraction of cells that migrated toward normal lung conditioned medium contained a 53.4 ± 8.1% increase in Treg content over the assay’s baseline (Fig. 4A). The fraction of cells that migrated toward LLC-bearing lung conditioned medium contained a significantly higher increase in Treg content over baseline at 92.1 ± 2.6% (p = 0.018). Addition of a neutralizing Ab against CCL22 into the conditioned medium reduced the selective Treg recruitment toward normal lung-conditioned medium to only 22.2 ± 6.4% (p = 0.019) and reduced the selective Treg recruitment toward LLC-bearing lung-conditioned medium to only 51.1 ± 8.2% (p = 0.018). The ratio of CD4+ cells to CD8+ cells remained unchanged in all fractions (data not shown). The nonmigratory cell fractions left in the top of the Transwell assay were also analyzed for Treg content. The change from baseline in Treg percentage of CD4+ cells in the nonmigratory fractions were all between −30 and −40%. In all cases, the Treg content in these fractions was less than the assay’s baseline level, suggesting that Tregs moved away from these fractions and toward the conditioned medium (Fig. 4B).
FIGURE 4.
CCL22 mediates selective recruitment of Tregs toward conditioned medium. Whole T cells were isolated from normal splenocytes and used in Transwell migration assays to measure selective Treg recruitment toward normal (light bars) and LLC-bearing lung (dark bars)-conditioned medium with (striped bars) or without (solid bars) neutralizing Ab against CCL22. Following the assay, the Treg percentage of CD4+ cells was measured using flow cytometry in both the migratory fractions in the bottom chamber (A) and the nonmigratory fractions left in the top chambers (B). Inhibition of selective Treg recruitment using neutralizing Ab against CCL22 in a Transwell migration assay is dose dependent in both normal and LLC-bearing lung-conditioned medium and Ab specific (C).
To ensure that the impact of CCL22 neutralization on selective Treg recruitment is anti-CCL22 dose dependent, increasing concentrations of neutralizing anti-CCL22 Ab and isotype control were added to both normal and LLC-bearing lung-conditioned medium and used in Transwell migration assays as described above (Fig. 4C). Increasing concentrations of anti-CCL22 reduced selective Treg recruitment toward both normal and LLC-bearing lung-conditioned medium. Increasing concentrations of isotype control had no effect.
CD11b+ cells isolated from LLC-bearing lungs secrete more CCL22 than those isolated from normal lungs
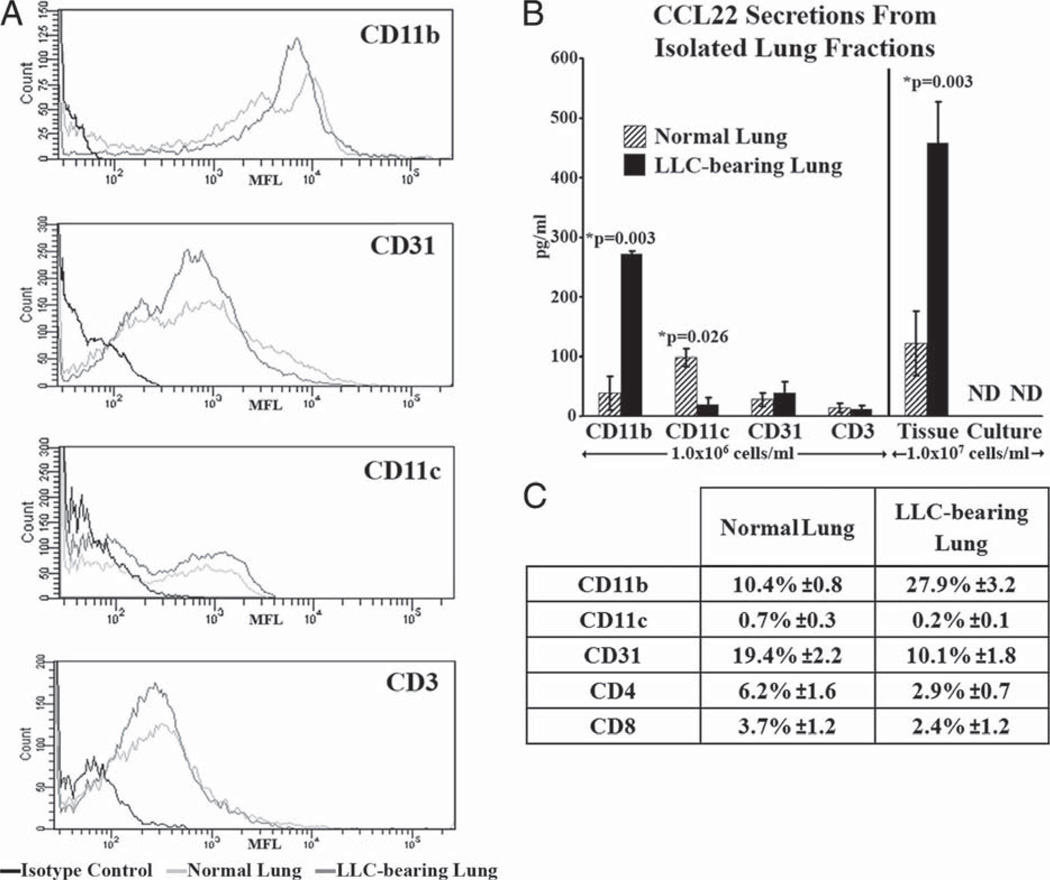
CCL22 is secreted from normal and, to a greater extent, from LLC-bearing lung tissue. However, it is not secreted by normal epithelial cells or by the primary LLC cells in culture, suggesting that some other component of the microenvironment is responsible for the secretion seen in the solid lung tissues. In an effort to identify the CCL22-secreting components of the microenvironment, both normal and LLC-bearing lungs underwent immunomagnetic fractionation based on several broad-spectrum cellular markers: CD11b+, CD11c+, CD31+, and CD3+. The resulting cellular fractions were enriched for each cellular marker (Fig. 5A) and were plated overnight. ELISA was then used to measure CCL22 secretion into the medium. The CD31+ and CD3+ fractions isolated from both normal and LLC-bearing lungs secreted low levels of CCL22 (Fig. 5B). Interestingly, the CD11c+ cells isolated from normal lungs secreted moderate amounts of CCL22 (98.31 ± 14.75 pg/ml), but this secretion was significantly reduced when compared with CD11c+ cells isolated from LLC-bearing lungs (19.42 ± 11.15 pg/ml; p = 0.026). In contrast, the CD11b+ cells isolated from normal lungs secreted low levels of CCL22 (38.43 ± 28.74 pg/ml), while CD11b+ cells isolated from LLC-bearing lungs secreted elevated levels of CCL22 (271.94 ± 5.25 pg/ml; p = 0.003).
FIGURE 5.
CD11b+ cells isolated from LLC-bearing lung tissue secrete increased levels of CCL22. A, Following immunomagnetic isolation, CD11b+, CD11c+, CD31+, and CD3+ fractions from both normal and LLC-bearing tissue each contained a population enriched for each cell type as determined by immunofluorescent staining. B, These populations were then incubated overnight at 1.0 × 106 cells/ml to create conditioned medium. ELISA was then used on the various conditioned media to measure the levels of secreted CCL22. C, Immunofluorescent staining and flow cytometric analysis was used to measure the endogenous levels of each cell type found in the lung homogenates before the isolations.
All of the fractionated cells were plated equally at 1.0 × 106 cells/ml, allowing for the direct comparison of CCL22 secretion between the cell types in this assay. However, CCL22 secretion in vivo may also be impacted by the relative numbers of each cell type found in both normal and LLC-bearing lungs. To address this, flow cytometry was used to analyze the relative frequency of each cellular fraction in both normal and LLC-bearing lungs (Fig. 5C). Although moderate amounts of CD31+, CD4+, and CD8+ cells were present in both tissue types, the most abundant cell type was CD11b+ cells. This fraction comprised 10.4 ± 0.8% of cells from dissociated normal lungs and 27.9 ± 3.2% of cells from dissociated LLC-bearing lungs. Taken together, not only are CD11b+ cells the most abundant cellular fraction, but they display CCL22 secretion abilities consistent with the observed increases in total CCL22 levels seen in LLC-bearing lungs. Hence, any increase in CCL22 secretion seen from LLC-associated CD11b+ cells would be magnified by their increase in cell number inside LLC-bearing lung tissue.
CD11b+ cell subpopulations in normal and LLC-bearing lung tissue
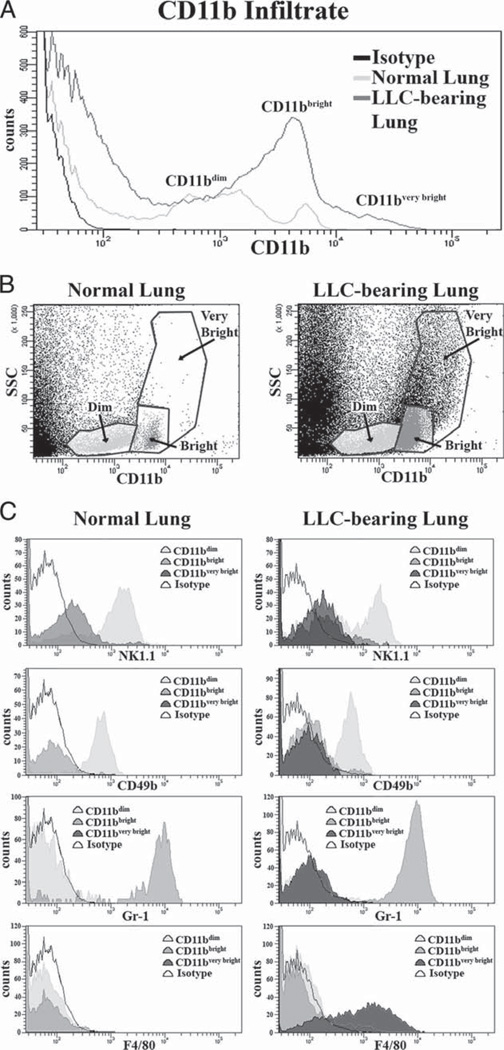
CD11b is expressed on many different cell types. After identifying CD11b+ cells as the primary cellular components responsible for the observed increase in CCL22 secretion from LLC-bearing lungs, studies were conducted to characterize the CD11b+ populations found in cell dissociates from both normal and LLC-bearing lungs. Immunofluorescent staining of CD11b alone shows two distinct populations of CD11b+ cells in normal lung tissue (CD11bdim, and CD11bbright). The number of CD11bbright cells was increased in LLC-bearing tissue, along with the appearance of another CD11b+ population (CD11bvery bright; Fig. 6A). Notably, this CD11bvery bright population somewhat overlaps the CD11bbright population in terms of CD11b staining intensity. However, the CD11bbright and CD11bvery bright populations are distinctly different from each other in terms of side scatter (Fig. 6B).
FIGURE 6.
Endogenous CD11b+ cells are comprised of multiple populations in normal and LLC-bearing tissue. A, Normal and LLC-bearing tissue homogenates were immunofluorescently stained with CD11b and analyzed with flow cytometry. CD11bdim, CD11bbright, and CD11bvery bright populations were gated as depicted in B. C, Representative histograms of NK1.1, CD49a, Gr-1, and F4/80 expression across the gated CD11b populations.
All of the CD11bbright cells in normal and LLC-bearing lung tissue coexpress the myeloid/granulocyte marker Gr-1 (Fig. 6C). Although normal granulocytes may express both CD11b (23) and Gr-1 (24), these markers are also suggestive of myeloid-derived suppressor cells (25). Additionally, previous reports of increased myeloid-derived suppressor cells in the tumor microenvironment (26–31) are consistent with our observed increases in this population. The CD11bvery bright cells, found only in LLC-bearing lungs, coexpress the macrophage marker F4/80, suggesting that these cells are tumor-associated macrophages Finally, the CD11bdim cells express both CD49b and NK1.1, suggesting that they are NK cells. None of the CD11b+ cells stained positive for CD31+ (data not shown).
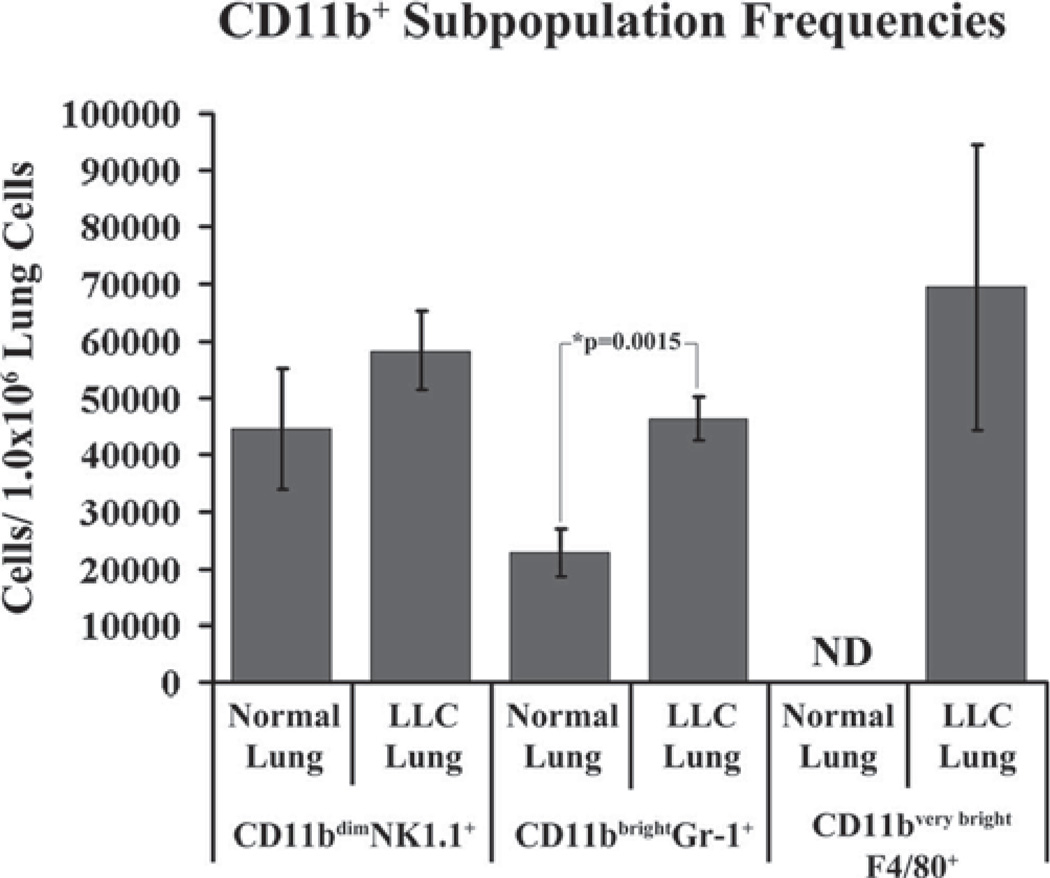
Finally, each CD11b subpopulation was quantified in both normal and LLC-bearing lung dissociates using flow cytometry (Fig. 7). There is a significant increase in the frequency of CD11bbrightGr-1+ cells in LLC-bearing lung dissociates as compared with normal lung dissociates (p = 0.0015). A large influx of CD11bvery brightF4/80+ cells is seen in LLC-bearing lung dissociates. No detectable levels of CD11bvery brightF4/80+ cells were found in normal lung dissociates. Although the average frequency of CD11bdimNK1.1+ cells was higher in LLC-bearing lung dissociates (58,272 cells/1.0 × 106 lung cells) as compared with normal lung dissociates (44,518 cells/1.0 × 106 lung cells), this difference was not statistically significant.
FIGURE 7.
Normal and LLC-bearing lung dissociates were immunofluorescently stained with CD11b, NK1.1, Gr-1, and F4/80 and the frequency of each population was determined using flow cytometry.
Isolation of CD11b+ populations using FACS
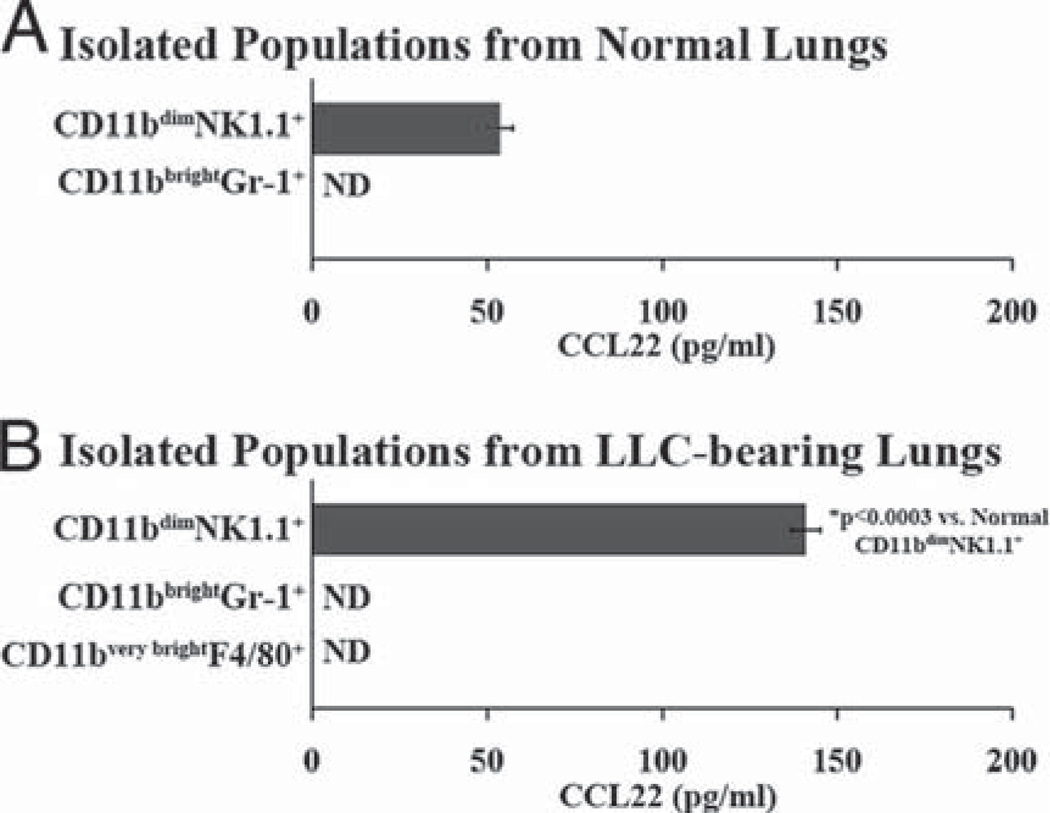
To identify which of the CD11b+ populations are able to secrete CCL22, FACS was used to isolate CD11bdimNK1.1+, CD11bbrightGr-1+, and CD11bvery brightF4/80+ populations from dissociated normal and LLC-bearing lungs. Following FACS isolation, each fraction was plated overnight and ELISA was used to measure secreted CCL22 levels in the medium. No CCL22 secretion was detected from the CD11bbrightGR-1+ cells isolated from either normal or LLC-bearing lungs. Likewise, no CCL22 was secreted from the isolated CD11bvery brightF4/80+ cells that appear in the LLC-bearing lung tissue (Fig. 8). Of all the CD11b+ cells found in both normal and LLC-bearing lungs, the only detectable amounts of CCL22 secretion came from the CD11bdimNK1.1+ populations. Furthermore, the CD11bdimNK1.1+ cells isolated from dissociated LLC-bearing lungs secreted significantly higher levels of CCL22 than did the CD11bdimNK1.1+ cells isolated from dissociated normal lungs. These data indicate that these CD11bdimNK1.1+ populations are capable of CCL22 secretion and may significantly contribute to the increased CCL22 secretion seen in LLC-bearing lungs.
FIGURE 8.
Immunofluorescently stained normal (A) and LLC-bearing (B) dissociates were first gated on CD11b+ cells, and FACS was preformed to isolate the CD11bdimNK1.1+, CD11b+Gr-1+, and CD11b+F4/80 populations. The isolated fractions were then plated overnight at 5.0 × 105 cells/ml and ELISA was used to measure secreted CCL22 in the medium.
Analysis of NK1.1+ cells in normal and LLC-bearing lung tissue
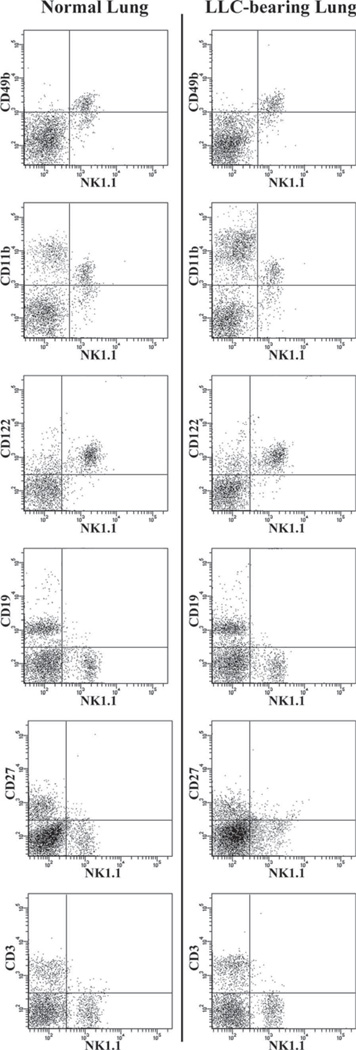
Although NK1.1 is one of the most specific NK markers available for murine NK cells, it can also be expressed on NKT cells, which themselves have reported immune-suppressive capabilities. Therefore, to confirm that the NK1.1+ cells isolated in these studies are true NK cells, we conducted a thorough analysis of the NK compartment in the lungs of both normal and LLC-bearing mice (Fig. 9). In both normal and LLC-bearing lung dissociates, NK1.1+ cells were also CD11bdim, CD49b+, CD122+, CD27+, CD19+, and CD3−, indicating that they are true NK cells. Additionally, this analysis demonstrates that no detectable levels of NKT cells reside in either normal or LLC-bearing lung tissue.
FIGURE 9.
Analysis of the NK compartments in normal and LLC-bearing lung tissue. Dissociates from both normal and LLC-bearing lung tissue were immunofluorescently stained for NK1.1 along with CD49b (DX5), CD11b, CD122, CD19,CD27, and CD3 and analyzed using flow cytometry.
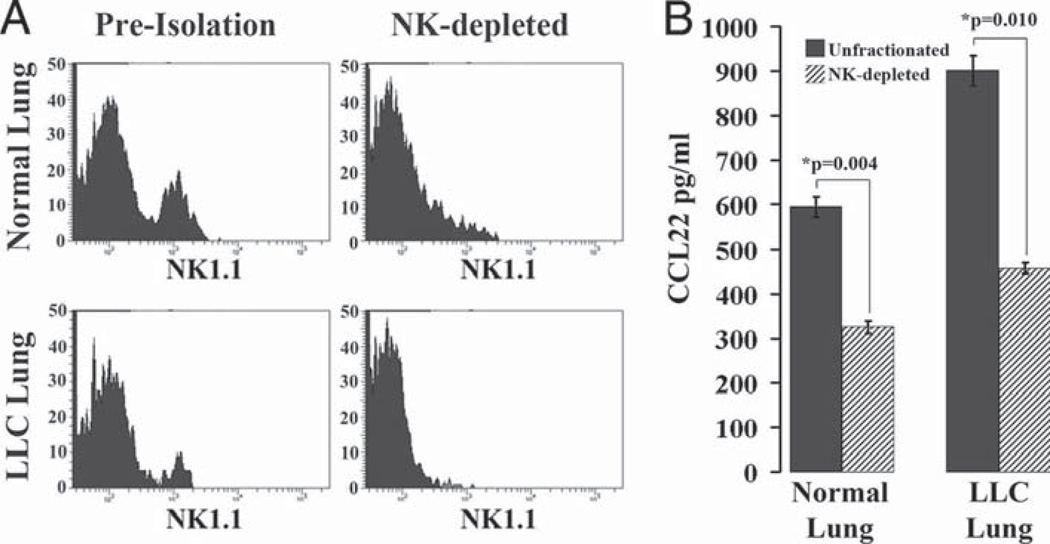
Depletion of NK1.1+ cells results in decreased CCL22 secretion from both normal and LLC-bearing lung dissociates
The fact that the isolated NK1.1+ cells from both normal and LLC-bearing lung dissociates were the only CD11b+ subpopulations to secrete CCL22 suggests that NK cells may be the major CCL22-secreting components of both normal and LLC-bearing lung tissues. To test this hypothesis, we immunomagnetically depleted NK1.1+ cells from both normal and LLC-bearing lung dissociates and incubated the resulting fractions alongside unfractionated dissociates overnight before analyzing the resulting medium for CCL22 secretion using ELISA (Fig. 10). Immunomagnetic depletion of NK1.1+ cells resulted in reduced numbers of NK cells in both normal and LLC-bearing lung dissociates (Fig. 10A). Unfractionated LLC-bearing lung dissociates secreted more CCL22 than did normal lung dissociates (902 ± 34 pg/ml vs 595 ± 22 pg/ml, respectively; Fig. 10B). Depletion of NK cells resulted in a significant reduction of CCL22 secretion for both normal and LLC-bearing lung dissociates (325 ± 14 pg/ml and 457 ± 12 pg/ml, respectively), indicating that a large portion of secreted CCL22 from both normal and LLC-bearing lungs is dependent on the presence of NK cells.
FIGURE 10.
NK-depleted lung dissociates secrete reduced levels of CCL22. A, NK1.1+ cells were immunomagnetically isolated from normal and LLC-bearing lung dissociates, and the isolated fractions each contained enriched populations. B, The unfractionated and depleted populations were incubated overnight to create conditioned medium and ELISA was then used to measure CCL22 levels.
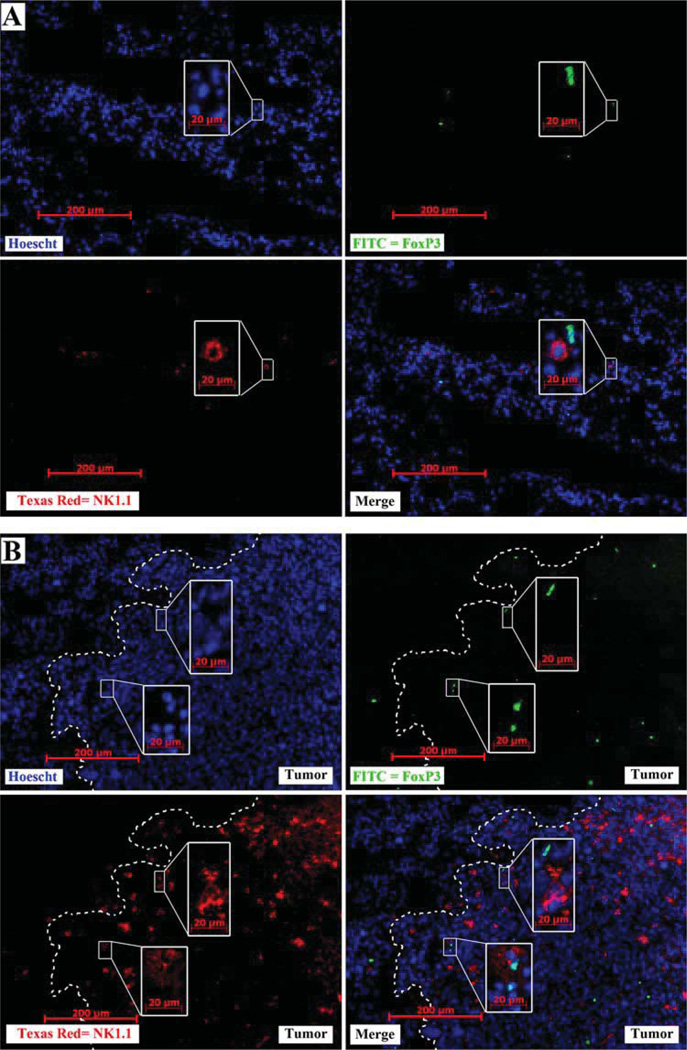
FoxP3+ cells colocalize with large numbers of NK cells at the tumor periphery
If NK cells play an active role in the selective recruitment of Tregs toward the tumor microenvironment, then it is reasonable to predict that NK cells and Tregs colocalize in the tumor microenvironment. Excised normal and LLC-bearing lungs were cryosectioned and stained with Hoechst dye (blue), anti-NK1.1 (PE, red), and anti-FoxP3 (FITC, green) (Fig. 11). Although both NK1.1+ and FoxP3+ cells can be found associated with each other in normal lung tissue (Fig. 11A), each cell type is distributed homogenously across the tissue section with modest frequency. In contrast, increased numbers of both FoxP3+ cells and NK1.1+ cells appear just inside the tumor periphery (white line, Fig. 11B). Furthermore, nearly all visible FoxP3+ cells were located next to NK1.1+ cell(s).
FIGURE 11.
Increased numbers of FoxP3+ cells associate with increased NK cell numbers at the tumor periphery. Immunofluorescent staining was performed on frozen sections from normal lung tissue (A) and LLC-bearing lung tissue (B) for nuclei (Hoechst Blue), NK1.1 (Texas Red, red), and FoxP3 (FITC, green). Most FoxP3+ cells are located adjacent to NK cells (merge). Tumor periphery is marked with a dashed white line. Insets are of a higher magnification of the indicated field.
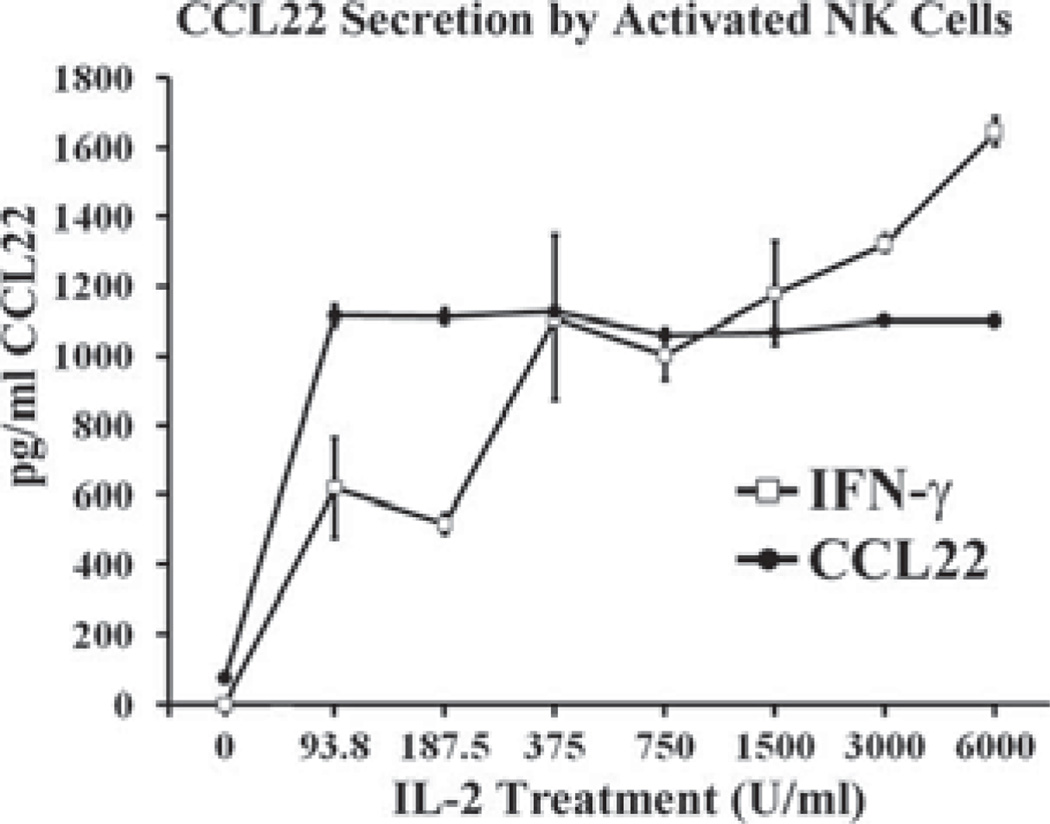
IL-2 induces CCL22 secretion from normal NK cells
Despite the apparent increase in NK cell numbers at the tumor periphery (Fig. 11B), large increases in NK cell numbers in LLC-bearing lung dissociates as a whole were not seen (Fig. 7). This fact suggests that an influx of NK cell numbers alone may not be responsible for increased NK-derived CCL22. Another explanation could be an increase in NK cell activation. As such, we treated normal splenic NK cells with increasing doses of IL-2 and measured CCL22 secretion responses to ascertain the effect of NK activation on CCL22 secretion. Without IL-2 treatment, NK cells did not secrete detectable levels of CCL22. Low-dose IL-2 induced appreciable amounts of CCL22 secretion, as did higher doses (Fig. 12). Interestingly, CCL22 secretion was the same in response to both low and high doses. This demonstrates that although CCL22 secretion is inducible by IL-2, it is not dose dependent. In addition to CCL22, IFN-γ secretion was monitored as a measure of NK activation.
FIGURE 12.
CCL22 secretion is induced by IL-2. Splenic NK cells were treated with increasing doses of IL-2. After 48 h, CCL22 and IFN-γ levels were measured using ELISA.
Discussion
Tumor-induced immune suppression continues to impede the effectiveness of immune-based therapies against cancer. Solid tumors utilize numerous mechanisms designed to evade host antitumor immune responses. Many of these mechanisms involve the accumulation of immune-suppressive infiltrates. Elucidation of the mechanisms by which these immune-suppressive phenotypes accumulate in the tumor microenvironment could produce attractive therapeutic targets with which to combat tumor-induced immune suppression. One of the most potent and well-studied immunesuppressive cell types is the Treg. The studies presented here demonstrate that elevated secretion of the chemokine CCL22 can elicit the selective attraction of Tregs toward LLC-bearing lung tissue. However, these studies also indicate that CCL22 is not secreted by either the LLC cells themselves or normal mouse lung epithelial controls. Instead, these studies show that NK cells not only have the ability to secrete CCL22, but display secretion patterns consistent with the overall increases in CCL22 levels seen in tumor-bearing lungs. Together with the fact that a large portion of the total CCL22 secretion from both normal and tumor-bearing lungs is NK dependent, these studies collectively suggest that NK cells may represent a major contributing source toward both basal CCL22 levels in normal lung tissue and toward the increased secretion levels of CCL22 seen in tumor-bearing lungs. This demonstration is a novel link between Treg recruitment and infiltrating NK cells.
The migration studies presented here demonstrate that selective Treg recruitment is higher toward medium conditioned by LLC-bearing lungs and that CCL22 contributes significantly to this recruitment. These studies were performed using unfractionated T cells isolated from normal splenocytes. It is possible that Tregs located in lung tissue may represent a slightly different Treg population, although both splenic and lung-associated Tregs have elevated levels of CCR4 expression. Although this elevated expression level is not as high as that of lung-associated Tregs, the fact that their CCR4 expression is significantly higher compared with other types of splenic T cells suggests that splenic Tregs are a conservative model for selective migration studies along the CCL22/CCR4 axis. It is noteworthy that even in excess concentrations of neutralizing anti-CCL22 Ab, selective Treg recruitment toward the conditioned medium was not entirely reduced to baseline levels. This suggests that other factors in the conditioned medium may also contribute to Treg recruitment. Recently, the chemokine MCP-1(CCL2) has been shown to have biologically significant signaling activity through CCR4 (19) and that this activity can attract Tregs in vitro (32). CCL2 has traditionally been associated with CCR2 and CCR5 due to its high affinity for these receptors. However, chemokines have long been described as “promiscuous” due to their tendency to have basal affinity with a wide range of receptors. Although these studies focused on the traditional CCR4 chemokines, it is possible that the selective recruitment of Tregs unaccounted by CCL22 is due to alternative CCR4 ligands such as CCL2.
CCL22 has previously been been shown to induce the chemotaxis of Tregs in vitro (15), as well as the selective recruitment of Tregs toward ovarian carcinoma (17). However, unlike ovarian carcinoma, the data presented here show that neither LLC cells nor normal epithelial lung cells secrete CCL22. CCL22 secretion from a solid tumor in which the tumor cells do not secrete CCL22 has not been described, raising the possibility that this scenario may be somewhat unique to LLC. However, NK infiltration is known to be common in most solid cancers (for review, see Ref. 33), meaning that NK cells may contribute toward CCL22 secretion in many cancer types. Although these studies suggest that a significant amount of CCL22 secretion is NK dependent in LLC, it is not known whether NK cells represent a significant source of CCL22 in cancers that contain CCL22-producing tumor cells.
The initial observation that LLC-bearing lungs secreted elevated levels of CCL22 was made using ELISA analysis of conditioned medium. Therefore, the focus of these studies was on CCL22 secretion. An alternative approach to measure the contribution of NK cells toward CCL22 levels would be to immunofluorescently stain for intracellular CCL22 production. However, it is important to distinguish between CCL22 production and CCL22 secretion. It has been previously observed that cell lines that stain positively for intracellular CCL22 did not secrete CCL22 (19). Hence, although immunofluorescent detection of CCL22 would provide information about CCL22 production, it may be misleading in terms of CCL22 secretion.
The aim of the present studies was to introduce NK cells as a significant source of elevated CCL22 levels in the lungs of LLCbearing mice and to demonstrate that increased CCL22 secretion leads to selective Treg recruitment. Future studies will focus on the direct impact that NK cells have on CCL22-mediated Treg recruitment in vivo through the use of depleting Abs against NK cells. Such studies will confirm the novel link between NK-secreted chemokines and Treg recruitment toward LLC introduced here. However, in vivo NK depletion has been shown to adversely affect other immune cell populations aside from NK cells, including T cells (34) and dendritic cells (35). This is likely due to the central role that NK cells have in establishing baseline levels and phenotypes of other immune cell populations (36). Similar disparities in infiltrate populations are seen in NK knockout mice (37–39). Therefore, extensive studies analyzing the impact of NK depletion on major groups of immune infiltrate in the lungs of both normal and LLC-bearing mice are also necessary to draw any conclusions gained from NK-depletion studies.
The idea that NK cells may play a role in the recruitment of Tregs to the tumor microenvironment is a novel yet troubling concept. NK cells are able to elicit rapid cytotoxic activity against certain tumors without prior exposure to any specific stimulus (40), and their activation is elicited by the lack of MHC class I on target cells (41). Along with the fact that the hallmark of many cancer types is the lack of MHC expression (42– 48), NK cells have great potential for use in immune-based therapies against cancer and indeed have well-documented antitumor activity (49, 50). However, NK cells are vulnerable to tumor-induced immune suppression, particularly from Tregs (51–54). The relationship between NK cells and Tregs is further complicated by the recent observation that NK cells are able to kill Tregs (55). The ultimate outcome of NK cell and Treg interaction in the tumor microenvironment is largely speculative at this point, but it is clear that a dynamic relationship exists with both parties able to adversely affect the other. If NK cells are able to up-regulate CCL22 secretion in the tumor microenvironment, then NK cells may have the unexpected side effect of indirectly contributing to tumor-induced immune suppression by recruiting Tregs.
Acknowledgments
We thank Dr. Amanda LaRue and HaiQun Zeng for technical expertise and assistance with cell sorting. We are also grateful to Jennifer Konopa-Mulligan and Jarrett Walsh for support and assistance.
Footnotes
This work was supported by the Medical Research Service of the Veteran’s Affairs and by National Institute of Health Grants MRIY CA97813 and CA8566.
Abbreviations used in this paper: Treg, regulatory T cell; LLC, Lewis lung carcinoma; MFI, mean fluorescence intensity; ND, not detected.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Peterson AC, Harlin H, Gajewski TF. Immunization with Melan-A peptide-pulsed peripheral blood mononuclear cells plus recombinant human interleukin-12 induces clinical activity and T-cell responses in advanced melanoma. J. Clin. Oncol. 2003;21:2342–2348. doi: 10.1200/JCO.2003.12.144. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Sherry RM, Morton KE, Scharfman WJ, Yang JC, Topalian SL, Royal RE, Kammula U, Restifo NP, Hughes MS, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J. Immunol. 2005;175:6169–6176. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- 3.Giarelli E. Cancer vaccines: a new frontier in prevention and treatment. Oncology. 2007;21:11–17. discussion 18. [PubMed] [Google Scholar]
- 4.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25): breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 5.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 6.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 7.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 8.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 9.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rβ-deficient mice: implications for the nonredundant function of IL-2. Immunity. 2002;17:167–178. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 10.Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr. Opin. Rheumatol. 2003;15:430–435. doi: 10.1097/00002281-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Strauss L, Bergmann C, Szczepanski M, Gooding W, Johnson JT, Whiteside TL. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-β1 mediates suppression in the tumor microenvironment. Clin. Cancer Res. 2007;13:4345–4354. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- 12.Paust S, Lu L, McCarty N, Cantor H. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc. Natl. Acad. Sci. USA. 2004;101:10398–10403. doi: 10.1073/pnas.0403342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface-bound transforming growth factor β. J. Exp. Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horwitz DA, Zheng SG, Gray JD. The role of the combination of IL-2 and TGF-β or IL-10 in the generation and function of CD4+CD25+ and CD8+ regulatory T cell subsets. J. Leukocyte Biol. 2003;74:471–478. doi: 10.1189/jlb.0503228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, D’Ambrosio D. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4+CD25+ regulatory T cells. J. Exp. Med. 2001;194:847–853. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, Fujii H. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int. J. Cancer. 2008;122:2286–2293. doi: 10.1002/ijc.23392. [DOI] [PubMed] [Google Scholar]
- 17.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 18.Haas J, Schopp L, Storch-Hagenlocher B, Fritzsching B, Jacobi C, Milkova L, Fritz B, Schwarz A, Suri-Payer E, Hensel M, Wildemann B. Specific recruitment of regulatory T cells into the CSF in lymphomatous and carcinomatous meningitis. Blood. 2008;111:761–766. doi: 10.1182/blood-2007-08-104877. [DOI] [PubMed] [Google Scholar]
- 19.Jordan JT, Sun W, Hussain SF, DeAngulo G, Prabhu SS, Heimberger AB. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol. Immunother. 2008;57:123–131. doi: 10.1007/s00262-007-0336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishida T, Ishii T, Inagaki A, Yano H, Komatsu H, Iida S, Inagaki H, Ueda R. Specific recruitment of CC chemokine receptor 4-positive regulatory T cells in Hodgkin lymphoma fosters immune privilege. Cancer Res. 2006;66:5716–5722. doi: 10.1158/0008-5472.CAN-06-0261. [DOI] [PubMed] [Google Scholar]
- 21.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-β dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou L, Barnett B, Safah H, Larussa VF, Evdemon-Hogan M, Mottram P, Wei S, David O, Curiel TJ, Zou W. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. 2004;64:8451–8455. doi: 10.1158/0008-5472.CAN-04-1987. [DOI] [PubMed] [Google Scholar]
- 23.Arnaout MA, Wang EA, Clark SC, Sieff CA. Human recombinant granulocyte-macrophage colony-stimulating factor increases cell-to-cell adhesion and surface expression of adhesion-promoting surface glycoproteins on mature granulocytes. J. Clin. Invest. 1986;78:597–601. doi: 10.1172/JCI112615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleming TJ, Fleming ML, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow: RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J. Immunol. 1993;151:2399–2408. [PubMed] [Google Scholar]
- 25.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, Bicciato S, Bronte V. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J. Clin. Invest. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bronte V, Serafini P, Apolloni E, Zanovello P. Tumor-induced immune dysfunctions caused by myeloid suppressor cells. J. Immunother. 2001;24:431–446. doi: 10.1097/00002371-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Bronte V, Wang M, Overwijk WW, Surman DR, Pericle F, Rosenberg SA, Restifo NP. Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells. J. Immunol. 1998;161:5313–5320. [PMC free article] [PubMed] [Google Scholar]
- 28.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat. Rev. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 29.Kusmartsev S, Gabrilovich DI. Inhibition of myeloid cell differentiation in cancer: the role of reactive oxygen species. J. Leukocyte Biol. 2003;74:186–196. doi: 10.1189/jlb.0103010. [DOI] [PubMed] [Google Scholar]
- 30.Kusmartsev SA, Li Y, Chen SH. Gr-1+ myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation. J. Immunol. 2000;165:779–785. doi: 10.4049/jimmunol.165.2.779. [DOI] [PubMed] [Google Scholar]
- 31.Melani C, Chiodoni C, Forni G, Colombo MP. Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice suppresses immune reactivity. Blood. 2003;102:2138–2145. doi: 10.1182/blood-2003-01-0190. [DOI] [PubMed] [Google Scholar]
- 32.Power CA, Meyer A, Nemeth K, Bacon KB, Hoogewerf AJ, Proudfoot AE, Wells TN. Molecular cloning and functional expression of a novel CC chemokine receptor cDNA from a human basophilic cell line. J. Biol. Chem. 1995;270:19495–19500. doi: 10.1074/jbc.270.33.19495. [DOI] [PubMed] [Google Scholar]
- 33.Yang Q, Goding SR, Hokland ME, Basse PH. Antitumor activity of NK cells. Immunol. Res. 2006;36:13–25. doi: 10.1385/IR:36:1:13. [DOI] [PubMed] [Google Scholar]
- 34.Byrne P, McGuirk P, Todryk S, Mills KH. Depletion of NK cells results in disseminating lethal infection with Bordetella pertussis associated with a reduction of antigen-specific Th1 and enhancement of Th2, but not Tr1 cells. Eur. J. Immunol. 2004;34:2579–2588. doi: 10.1002/eji.200425092. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida O, Akbar F, Miyake T, Abe M, Matsuura B, Hiasa Y, Onji M. Impaired dendritic cell functions because of depletion of natural killer cells disrupt antigen-specific immune responses in mice: restoration of adaptive immunity in natural killer-depleted mice by antigen-pulsed dendritic cell. Clin. Exp. Immunol. 2008;152:174–181. doi: 10.1111/j.1365-2249.2008.03601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shingu K, Helfritz A, Kuhlmann S, Zielinska-Skowronek M, Jacobs R, Schmidt RE, Pabst R, von Horsten S. Kinetics of the early recruitment of leukocyte subsets at the sites of tumor cells in the lungs: natural killer (NK) cells rapidly attract monocytes but not lymphocytes in the surveillance of micrometastasis. Int. J. Cancer. 2002;99:74–81. doi: 10.1002/ijc.10279. [DOI] [PubMed] [Google Scholar]
- 37.Baca ME, Mowat AM, Parrott DM. Immunological studies of NK cell-deficient beige mice: II. Analysis of T-lymphocyte functions in beige mice. Immunology. 1989;66:131–137. [PMC free article] [PubMed] [Google Scholar]
- 38.Baca ME, Mowat AM. Immunological studies of NK cell-deficient beige mice: I. Defective ability of beige lymphocytes to mediate local and systemic graft-versus-host reactions. Immunology. 1989;66:125–130. [PMC free article] [PubMed] [Google Scholar]
- 39.Saxena RK, Saxena QB, Adler WH. Defective T-cell response in beige mutant mice. Nature. 1982;295:240–241. doi: 10.1038/295240a0. [DOI] [PubMed] [Google Scholar]
- 40.Riccardi C, Santoni A, Barlozzari T, Puccetti P, Herberman RB. In vivo natural reactivity of mice against tumor cells. Int. J. Cancer. 1980;25:475–486. doi: 10.1002/ijc.2910250409. [DOI] [PubMed] [Google Scholar]
- 41.Ljunggren HG, Karre K. Host resistance directed selectively against H-2-deficient lymphoma variants: analysis of the mechanism. J. Exp. Med. 1985;162:1745–1759. doi: 10.1084/jem.162.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marincola FM, Shamamian P, Alexander RB, Gnarra JR, Turetskaya RL, Nedospasov SA, Simonis TB, Taubenberger JK, Yannelli J, Mixon A, et al. Loss of HLA haplotype and B locus down-regulation in melanoma cell lines. J. Immunol. 1994;153:1225–1237. [PubMed] [Google Scholar]
- 43.Miyazawa M, Nishio J, Wehrly K, David CS, Chesebro B. Spontaneous recovery from Friend retrovirus-induced leukemia: mapping of the Rfv-2 gene in the Q/TL region of mouse MHC. J. Immunol. 1992;148:1964–1967. [PubMed] [Google Scholar]
- 44.Polsky D, Lilly F. Suppression of H-2b-associated resistance to Friend erythroleukemia virus by a class I gene from the H-2d major histocompatibility complex haplotype. Proc. Natl. Acad. Sci. USA. 1991;88:9243–9247. doi: 10.1073/pnas.88.20.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Algarra I, Garcia-Lora A, Cabrera T, Ruiz-Cabello F, Garrido F. The selection of tumor variants with altered expression of classical and nonclassical MHC class I molecules: implications for tumor immune escape. Cancer Immunol. Immunother. 2004;53:904–910. doi: 10.1007/s00262-004-0517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bubenik J. Tumour MHC class I downregulation and immunotherapy (Review) Oncol. Rep. 2003;10:2005–2008. [PubMed] [Google Scholar]
- 47.Khong HT, Wang QJ, Rosenberg SA. Identification of multiple antigens recognized by tumor-infiltrating lymphocytes from a single patient: tumor escape by antigen loss and loss of MHC expression. J. Immunother. 2004;27:184–190. doi: 10.1097/00002371-200405000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rees RC, Mian S. Selective MHC expression in tumours modulates adaptive and innate antitumour responses. Cancer Immunol. Immunother. 1999;48:374–381. doi: 10.1007/s002620050589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ortaldo JR, Herberman RB. Heterogeneity of natural killer cells. Annu. Rev. Immunol. 1984;2:359–394. doi: 10.1146/annurev.iy.02.040184.002043. [DOI] [PubMed] [Google Scholar]
- 50.Trinchieri G. Biology of natural killer cells. Adv. Immuno. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barao I, Hanash AM, Hallett W, Welniak LA, Sun K, Redelman D, Blazar BR, Levy RB, Murphy WJ. Suppression of natural killer cell-mediated bone marrow cell rejection by CD4+CD25+ regulatory T cells. Proc. Natl. Acad. Sci. USA. 2006;103:5460–5465. doi: 10.1073/pnas.0509249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE, Novault S, Escudier B, Vivier E, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-β-dependent manner. J. Exp. Med. 2005;202:1075–1085. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smyth MJ, Teng MW, Swann J, Kyparissoudis K, Godfrey DI, Hayakawa Y. CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J. Immunol. 2006;176:1582–1587. doi: 10.4049/jimmunol.176.3.1582. [DOI] [PubMed] [Google Scholar]
- 54.Trzonkowski P, Szmit E, Mysliwska J, Dobyszuk A, Mysliwski A. CD4+CD25+ T regulatory cells inhibit cytotoxic activity of T CD8+ and NK lymphocytes in the direct cell-to-cell interaction. Clin. Immunol. 2004;112:258–267. doi: 10.1016/j.clim.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 55.Roy S, Barnes PF, Garg A, Wu S, Cosman D, Vankayalapati R. NK cells lyse T regulatory cells that expand in response to an intracellular pathogen. J. Immunol. 2008;180:1729–1736. doi: 10.4049/jimmunol.180.3.1729. [DOI] [PubMed] [Google Scholar]