Abstract
Mucosal transmission of HIV predominately occurs during sexual intercourse or breast-feeding and generally results in a successful infection from just one or few founder virions. Here we assessed the impact of viral inoculum size on both viral and immune events within two groups of Rhesus macaques that were non-traumatically, orally inoculated with either multiple low (1000 to 4000 TCID50) or high (100,000 TCID50) doses of SIV. In agreement with previous studies, more diverse SIV variants were observed in macaques following infection with high dose oral SIV compared to a low dose challenge. In peripheral blood cells, the immune gene transcript levels of CXCL9, IFNγ, TNFα and IL10 remained similar to uninfected macaques. In contrast, OAS and CXCL10 were upregulated following SIV infection in both the high and low dosed macaques, with a more rapid kinetics (detectable by 7 days) following the high SIV dose challenge. In peripheral lymph nodes, an increase in CXCL10 was observed irrespective of viral dose while CXCL9 and OAS were differentially regulated in the two SIV dosed groups. Magnetic bead sorting of CD3+, CD14+ and CD3−/CD14− cells from peripheral blood identified the increase in OAS expression primarily within CD14+ monocytes, whereas the CXCL10 expression was primarily in CD3+ T cells. These findings provide insights into the impact of SIV challenge dose on viral and innate immune factors, which has the potential to inform future SIV/HIV vaccine efficacy trials in which vaccinated hosts have the potential to be infected with a range of viral challenge doses.
Keywords: AIDS, HIV, Innate immune responses, Low Dose, Oral transmission, Rhesus macaques, SIV
BACKGROUND
Results from the RV144 HIV vaccine trial, utilizing ALVAC-AIDSVAX [1–3], suggest that HIV vaccine success will depend on its ability to prevent acquisition of HIV during mucosal transmission of the virus [1]. One factor inhibiting vaccine development is that relatively little is known about the earliest viral and immune events during and immediately following HIV transmission. Current data indicates that successful transmission of HIV via a mucosal route is dependent on multiple factors, including the viral dose present in the inoculating fluid (semen, vaginal fluid, breast milk), the integrity of the mucosa, and the number of susceptible target cells at the mucosal site [4–19]. Due to the difficulties inherent in assessing these earliest events in humans, the SIV-macaque model has been successfully utilized by many investigators to elucidate the early events following vaginal, rectal and oral transmission of SIV [6, 13, 20–29].
Oral transmission is an important route of HIV transmission for infants who can become infected while breast feeding due to virus in breast milk [30–33], as well as adults who can be infected via genital-oral sex due to virus in semen [34–38]. Oral transmission studies using Rhesus macaques have been utilized for pathogenesis as well as for vaccine studies [6, 26–29, 38–44]. Initial studies assessing oral transmission in the macaque model utilized a high dose challenge, while more recent studies have utilized low dose challenge to better mimic a natural HIV exposure. The viral dose used for a mucosal challenge is an important consideration in SIV/HIV vaccine study design, as a higher number of virions contacting mucosal tissue may be more difficult for vaccine-mediated immune responses to control. Studies to date have determined that in sexual transmission of HIV, the majority of infections are initiated by a small number of transmitted viral variants [45–51]. Therefore, a vaccine would theoretically need to block only these few transmitted virions before they can disseminate and establish systemic infection. However, individuals that participate in high-risk behavior with HIV-infected individuals have an increased per-exposure transmission risk and higher viral dose per exposure [52, 53], resulting in a greater number of founder virus variants following HIV acquisition [54, 55]. We have developed and utilized a model of nontraumatic oral SIV transmission in Rhesus macaques to assess the effect of repeated low and high dose SIV administration on viral diversity and immune responses to SIV. As expected, we generally observed higher diversity of SIV variants in high dose oral SIV challenge macaques compared to low dose challenge. Innate immune responses were assessed by quantifying mRNA transcripts of six to eight immune modulators in both peripheral blood and lymph node cells. In peripheral blood cells the immune gene transcript levels were generally similar for the high and low dose infected macaques, although the kinetics of the CXCL10 and OAS increase was faster (by seven days postinfection) in the high dose macaques following successful SIV infection. In peripheral lymph nodes an increase in CXCL10 was observed irrespective of viral dose while CXCL9 and OAS were differentially regulated in the two SIV dosed groups. Magnetic cell sorting and analysis of peripheral blood cells identified the cellular sources of OAS as primarily CD14+ monocytes while CXCL10 was primarily produced in CD3+ T cells. These findings regarding viral and immune factors emphasize the importance of the challenge dose and its potential impact for SIV vaccines studies. These studies also shed light on how HIV vaccine efficacy might be impacted by the infecting HIV dose and how the immune response would vary when different dosing levels are encountered.
MATERIALS AND METHODS
Animal subjects and viral administrations
The macaques used in these studies were colony-bred Rhesus macaques (Macaca mulatta) housed at the California (CNPRC), Southwest (SNPRC), or Yerkes (YNPRC) National Primate Research Centers. Oral administration of the virus in both the high and low dose groups was undertaken by dripping the inoculum onto the gingival mucosa and tooth interface. The following macaques received high-dose SIV inoculations and were housed at CNPRC: RM11 (33291), RM12 (32167), RM13 (32174), RM14 (32296), and RM15 (33353). These macaques were non-traumatically, orally inoculated with two 100,000 50% tissue culture infective doses (TCID50) of SIVmac251 [56, 57]. Viral and immunological changes in these high-dosed macaques were described previously [28] and some data are re-analyzed here for comparison. The slow-progressing macaque RM16 was omitted from this study to focus on viral and immunological changes in macaques progressing to AIDS. In addition, Rhesus macaques housed at SNPRC were non-traumatically, orally infected three times with low-dose SIVmac251 that ranged between 1000 and 4000 TCID50. Macaques 17742 and 18984 received 4000 TCID50 while macaques 18412, 18414 and 19147 received 2000 TCID50 and macaque 18981 received 1000 TCID50. One low dose of SIV was administered to each macaque at days 0, 2 and 4 and these macaques were followed up for 6 months. Rhesus macaques infected intravenously at the YNPRC were also assessed. These macaques, RCe8, RCo8, RDo8, REi9, RHk8, RIf8, RJj8, RJl9, RKb9, RNr8, ROu8, RUn8, RWi8, RWu8 and RZz8, were intravenously inoculated with 10,000 TCID50 of SIVmac239 [58], and peripheral blood cells obtained at day 7 post-infection were analyzed in this study. All animals were cared for in accordance with National Institutes of Health guidelines and local Animal Care and Use Committees.
Tissue collection and processing
Peripheral blood mononuclear cells (PBMCs) were obtained from high and low-dose challenged, SIV-infected macaques at time points that reflect the acute phase (days 7 to 15 post-inoculation (pi)), acute to chronic transition (days 21 and 35 pi), early chronic phase (days 45 to 56 pi), and chronic phase (days 70 to 112 pi). The time points obtained varied slightly between macaques and can be summarized as follows for macaques inoculated with low dose inoculum (low dose macaques): 17742 - days 7, 14, 35 and 85; 18984 - days 7, 14, 35, and 85; 18412 - days 7, 14, 28 and 77; 18414 - days 7, 14, 28 and 77; 18981 -days 8, 15, 35, 70 and 112. For the macaques inoculated with high dose inoculum (high dose macaques), plasma was obtained as depicted in Fig. (1), and PBMCs were assessed from selected time points summarized as follows: RM11, RM14 and RM15- days 7, 21, 45 and 84; RM12 and RM13 - days 7, 28, 56 and 85 to represent acute and chronic stage of infection. PBMCs were extracted through density centrifugation with Ficoll-Paque Plus (GE Healthcare, Uppsala, Sweden).
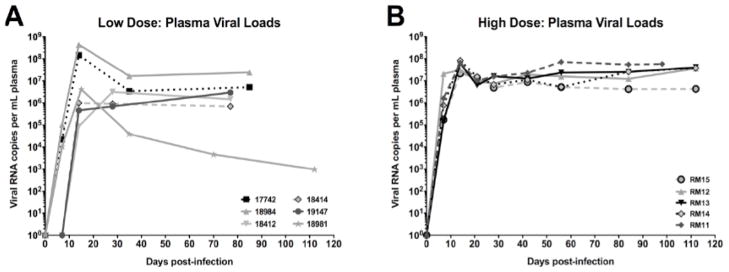
Fig. 1.
Plasma viral loads. Viral RNA copies per ml of plasma are shown for macaques inoculated orally with low doses (A) or high doses (B) of SIV.
Inguinal or axillary lymph node (LN) biopsies were obtained to provide representative sampling of peripheral lymph nodes. LN biopsies were at three time points in the post-acute phase for both groups of macaques; days 28 or 35, days 70 or 85 and day 112 for low dose macaques and days 21 or 28, days 45 or 56 and day 85 dpi for high dose macaques. All LN biopsies were immediately placed in RNAlater (Ambion, TX) and stored at −80 °C prior to mRNA extraction and cDNA synthesis.
Magnetic cell sorting
CD14+ monocytes and CD3+ T cells were sorted from PBMCs by MACS magnetic cell separation, using positive selection, according to the manufacturer’s protocol (Miltenyi Biotec, Auburn, CA). Briefly, CD14+ cells were first isolated by positive selection, and the CD14− subsets were then subjected to CD3+ isolation. In addition, the remaining flow-through from CD3 selection was collected as CD14−CD3− subsets. The sorted, CD14+ and CD3+ cell populations were found to be >90% pure via flow cytometric analysis. Magnetically purified CD14+, CD3+ and CD14−CD3− cells were lysed with RLT buffer from the RNeasy Mini Kit (Qiagen, Valencia, CA) and total RNA was extracted.
RNA extraction and cDNA synthesis
Total RNA from lymph node mononuclear cells (LNMC) and PBMCs were extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA) per manufacturer’s instructions. SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad CA) was used according to manufacturer’s protocol to synthesize cDNA.
Quantification of plasma viral RNA
Viral RNA was extracted from plasma using QIAamp Viral RNA Mini kit (Qiagen, Valencia, CA). Plasma viral RNA was quantified and is reported as copies of viral RNA per milliliter of plasma. For the SIV high dosed macaques, the Chiron Corporation branch DNA (bDNA) signal amplification assay, version 4.0, specific for SIV [59, 60], with a limit of detection of 125 copies/mL of plasma was utilized. For the SIV low dosed macaques the RNA Ultrasense One-Step Quantitative RT-PCR System (Invitrogen, Carlsbad, CA) with a limit of detection of approximately 500 copies/mL of plasma was utilized. The ability to quantify SIV RNA loads via these two methods has been demonstrated to be comparable and reproducible [61].
Quantitative real-time PCR analysis of immune effector genes
Real-time PCR utilizing gene-specific primers/probes were performed on an ABI 7700 or ABI 7300 (Applied Biosystems, Foster City CA) sequencer, as described previously [62, 63]. Changes in expression of eight immune modulators (IFN-α, IFN-γ, IL10, IL12, CXCL9, CXCL10, tumor necrosis factor alpha [TNF-α], and OAS) and glyceraldehyde-3- phosphate dehydrogenase (GAPDH) housekeeping gene were calculated by the delta delta Ct (ΔΔCT) method [27, 28, 63].
Cloning and Sequencing
SIVenv (V1-V2 region) was amplified via nested PCR [64] from genomic DNA from PBMC samples taken at the earliest SIV-positive time point (7 or 14 dpi). Fresh PCR product was cloned using the TOPO TA cloning kit for subcloning (Invitrogen, Carlsbad, CA). Plasmid DNA containing SIVenv clones were extracted using the QIAprep Spin Miniprep Kit (Qiagen, Valencia, CA), according to the manufacturer’s protocol, and sequenced. Sequences were proof read and double-checked, using the programs 4peak (mekentosj.com, Amsterdam, Netherlands), and Bioedit (Ibis Therapeutics, Carlsbad, CA). Verified sequences were analyzed, using neighbor-joining phylogenetic analysis with kimura-2 parameter model and MEGA version 4.1 (www.megasoftware.net, Center for Evolutionary Functional Genomics, Tempe, AZ).
Heteroduplex mobility assay
Heteroduplex mobility assay (HMA) was used to determine the genetic variability of transmitted viral variants in the orally inoculated Rhesus macaques as previously described [63, 64].
TRIM5 genotyping
TRIM5 genotypes of Rhesus macaques were determined by isolating genomic DNA from PBMCs and directly sequencing the PCR fragments from the C-terminal domain of TRIM5 as previously described [65]. TRIM5 gene polymorphisms were grouped into TRIM5CypA, TRIM5TFP, and TRIM5Q, representing genotypes as three different classes and six possible genotypes. Macaques 17742, 18412, 18414 and 18984 were identified as genotype TRIM5Q/Q, macaques 18981, 19147 and RM15 as TRIM5TFP/Q, RM 12,13, 14 as TRIM5TFP/TFP and RM11 as TRPM5CypA/CypA.
RESULTS
Plasma Viral Load in Orally Inoculated Rhesus Macaques
We performed a comparative study in eleven Rhesus macaques that became infected following a non-traumatic, oral administration of SIVmac251; Six of these animals were inoculated 3 times with a low dose challenge with two days between each dose (low dosed macaques): 17742 and 18984 (4000 TCID50); 18412, 18414 and 19147 (2000 TCID50); 18981 (1000 TCID50); and five (RM11, RM12, RM13, RM14, RM15) were administered two high doses (100,000 TCID50) one hour apart. All five high dosed macaques became infected and developed detectable viremia by 7 days post-infection (Fig. 1B). For macaques receiving low doses, oral SIV administrations occurred on day 0 (Monday), day 2 (Wednesday) and day 4 (Friday) of a chosen week and the first blood sample was obtained on day 7. Therefore, detection of SIV at day 7 would be expected if the infection occurred during the day 0 or 2 oral SIV administration (depending on the level of viral replication), and unlikely if the SIV infection occurred during the day 4 SIV administration (as would result in only a 3 day period for the virus to replicate to high enough levels to be detectable in the plasma at day 7 [6, 13, 20–29]). SIV was detected at day 7 in the two macaques that received 4000 TCID50, as well as one macaque that received 1000 TCID50 (Fig. 1A). In contrast, none of the macaques that received the 2000 TCID50 dosage had detectable SIV at 7 dpi (Fig. 1A). While the peak viremia during acute SIV infection was generally lower in the low dosed macaques, this measure was not useful in comparing the two groups as this difference could be due to inability to capture the true peak of viremia with our sampling interval. However, no significant difference in viral set point (viremia at days 70 to 85 post-infection) was observed between the two groups of macaques (student t test, p = 0.121). Some differences could be observed in viral setpoints within the low as well as high dosed groups, and TRIM5 genotyping was utilized to identify any association between genotype and viral levels [65]. Interestingly, the low dosed macaque with the lowest viral set point (<104 copies per ml of plasma), 18981, shared a TRIM5 genotype (TRIMTFP/Q) with the high dosed macaque with the lowest viral set point in that group, RM15. However, this genotype did not always result in a low set-point, as low dosed macaque 19147 also shared the TRIMTFP/Q genotype but had a viral load set point comparable to that of the other low (as well as high) dosed macaques indicating that this TRIM5 genotype does not have dramatic effect on SIVmac251 replication in vivo.
Assessment of SIV Genetic Diversity at Early Time Points Following Low or High Dose SIV Oral Administration
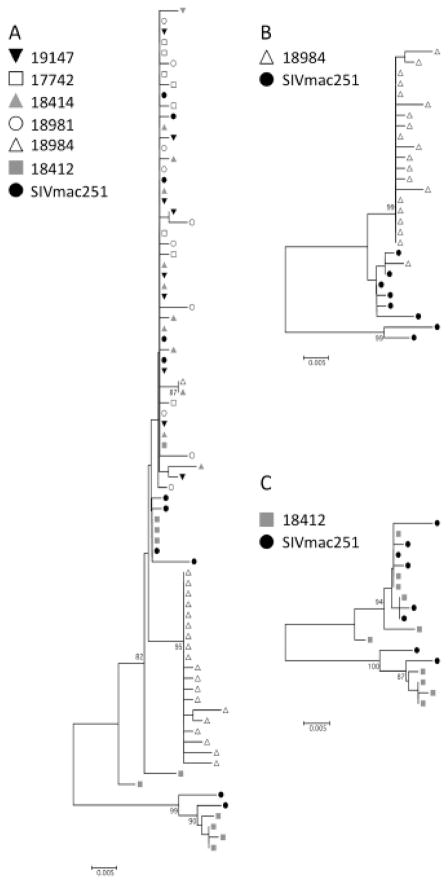
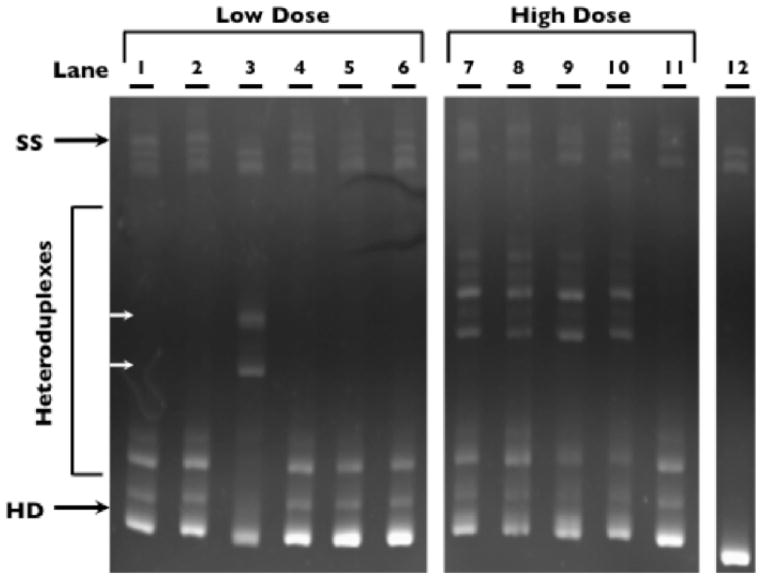
The V1-V2 region of the SIV env gene exhibits a high level of sequence heterogeneity in this region that provides an estimate for the viral diversity at early times following the oral SIV administration [49, 51, 66–68]. Studies from Keele et al., and others have determined that the number of infecting variants can be inferred through the HIV or SIV diversity at early timepoints by an assessment of phylogenetic branching patterns [45, 48, 51]. To determine the impact of viral dose on the founding viral population in low dosed orally infected macaques, we sequenced the SIV V1-V2 region of the env gene following PCR amplification of the viral DNA isolated from peripheral blood mononuclear cells (PBMC). Sequencing of SIVmac251 inoculum SIV RNA (21 cDNA clones sequenced) identified diverse quasispecies in the SIVmac251 viral inoculum. Eleven unique isolates, as determined by a neighbor-joining phylogenetic tree, were chosen to represent the diversity present (represented as closed circles in the phylogenetic tree, Fig. 2). Eight to eleven env V1-V2 clones were obtained for each low dose macaque at the earliest env PCRpositive time-point (days 7–14 post-infection) and assessed phylogenetically in comparison to the eleven sequences identified in the SIVmac251 inoculum used at Southwest primate center. Sequence analyses from all low dosed macaques along with the eleven representative SIVmac251 viral variants determined that sequences from four of the six low dosed macaques clustered into one branch of the phylogenetic tree, indicating that likely one viral variant initiated SIV infection in these macaques (Fig. 2A). Two of the low dose macaques had more diverse viral repertoires and had V1-V2 sequences appearing on two or more branches of the phylogenetic tree, likely indicating that multiple variants initiated the infection. For example, phylogenetic assessment indicates that macaque 18984 (infected with 4000 TCID50) was infected with a major SIV variant (where the majority of env sequences grouped together) and a minor variant (one env sequence on separate branch) (Fig. 2B). Sequences from macaque 18412 (infected with 2000 TCID50 of SIVmac251) exhibited high viral diversity with evidence for approximately 4 different viral variants initiating infection in this macaque (Fig. 2C). In addition to clonal sequencing analysis, heteroduplex mobility assay (HMA) was utilized to assess the diversity of the SIV env V1-V2 region in DNA isolated from PBMC at 7 or 14 days to obtain a general representation of the overall quasispecies diversity within both the low as well as the high dosed macaques. HMA assessment of SIVmac239 env V1- V2 region amplified from the plasmid clone produced a single homoduplex band (Fig. 3, lane 12). By HMA (lanes 1 to 6), only one macaque from the low-dose group, 18412 (inoculated with 2000 TCID50), displayed slowly migrating heteroduplexes (white arrows, lane 3), suggesting that multiple SIV variants initiated infection in this macaque, which is consistent with the results of sequence analysis (Fig. 2C). The absence of slowly migrating heteroduplex bands in the five other low-dosed macaques suggests that few (or homogenous) SIV species were transmitted in these five macaques, in agreement with the phylogentic analysis. In contrast, four of five macaques receiving the high dose inoculum exhibited slowly migrating heteroduplexes, indicating that multiple SIV variants initiated the infection in these four macaques (Fig. 3, lanes 7 to 11). The fifth macaque, RM15, displayed a pattern that was similar to the pattern observed in the majority of low dosed macaques, indicating relatively few virions initiated the infection. Interestingly, RM15 was the macaque with the lowest viral setpoint in the high dosed group although it is unlikely those two observations are directly related based on published studies [69].
Fig. 2.
Phylogenetic tree. Neighbor-joining phylogenetic trees were constructed from the sequences of clones obtained after PCR amplification of the SIV env V1-V2 region from day 7 or 14 PBMCs of low dose inoculated macaques (earliest SIV-positive sample). Bootstrap values from 500 replicates are indicated on each node if greater than 75%. Dots of the same shape and shade represent clones from the same animal. SIV Env V1-V2 sequences are depicted from all six low dose macaques as well as the SIV inoculum (A). Sequences from macaques 18984 and 18412 with the SIV inoculum are shown separately to demonstrate the higher diversity of transmitted virions in these two macaques (B and C respectively).
Fig. 3.
Heteroduplex Mobility Assay (HMA). Viral diversity in macaques administered low or high doses of SIV. Genomic DNA was obtained from PBMCs from the first SIV-positive sample of all orally inoculated macaques (day 14 for low dose macaques, and day 7 or 14 for high dose macaques) and the V1-V2 region of SIVenv was PCR amplified. Lanes 1 to 6 show the six macaques that were inoculated with low doses of SIVmac251 (lane 1: 17742, 2: 18984, 3: 18412, 4: 18414, 5: 18981, 6: 19147). Lanes 7 to 11 show the five macaques inoculated with high doses of SIVmac251 (lane 7: RM11, 8: RM12, 9: RM13, 10: RM14, 11: RM15). Lane 12 shows HMA for V1-V2 region of a SIVmac239 plasmid, to indicate the pattern when only one clonal sequence is present. HD indicates the homoduplex bands which is the brightest and fastest migrating band on the gel.
Assessment of mRNA Expression of Immune Modulators in PBMCs and Peripheral Lymph Nodes Following Low or High Dose Oral SIV Administration
The level of mRNA expression of immune modulators (cytokines, chemokines, and interferon response genes) was assessed in peripheral lymph nodes (LN) and blood following low or high dose oral SIV transmission. Quantitative real-time PCR analysis was utilized to assess gene expression levels of eight immune modulators in early events following SIV infection: IFN-α, OAS, IL-12, TNF-α, IFN-γ, CXCL9, CXCL10 and IL-10 [27, 28]. mRNA expression levels in PBMCs and lymph node cells was determined by fold changes in the mRNA levels of SIVinfected macaques comparison to uninfected macaques. The uninfected macaque samples were obtained from both primate centers at which these studies were undertaken (Southwest and California) to establish expression level range in uninfected macaques and to identify the two standard deviation confidence intervals depicted (preinfection timepoints from the same macaques enrolled in these studies were utilized to represent the uninfected samples when available). Both acute (2 to 28 dpi) and chronic (45 to 112 dpi) time points were assessed, and data is depicted as fold changes of all samples for each gene (Fig. 4). Symbols within boxes represent gene expression that is more than two standard deviations higher than the average expression of the same gene in uninfected macaques.
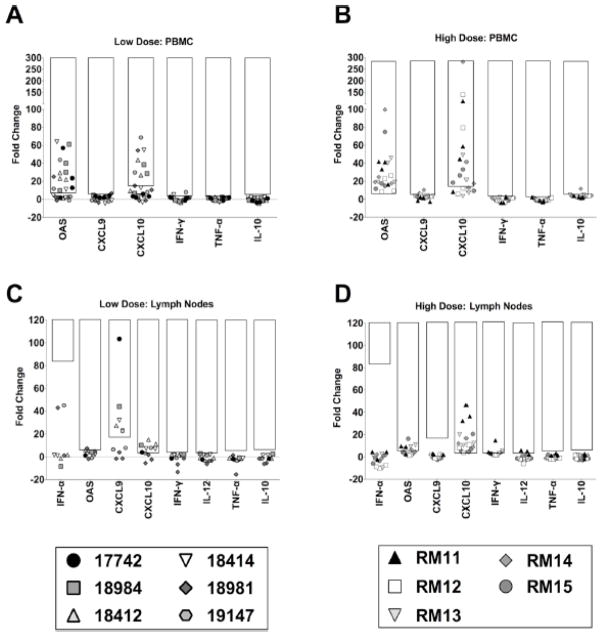
Fig. 4.
Fold changes in mRNA expression of immune modulating genes in LN and PBMCs. Fold changes are shown for mRNA expression of six immune response genes (from left: OAS, CXCL9, CXCL10, IFNγ, TNFα, IL-10) in PBMCs (A, B) as well as eight immune response genes (from left: IFN-α, OAS, CXCL9, CXCL10, IFN-γ, IL-12, TNF-α, IL-10) in LN (C, D) of macaques infected orally with low doses (A, C) or high doses (B, D) of SIV. Symbols of the same shape represent the same macaque and each symbol represents a different time point of sampling. Symbols within the black boxes represent mRNA expression levels that are increased more than two standard deviations away from the baseline gene expression. For PBMC, gene expression in uninfected macaques was determined by average gene expression levels of preinfection timepoint from both high and low dose macaques. For lymph nodes, the average of gene expression levels of preinfection time point from low dose macaques and 4 uninfected macaques from California primate center was used to determine baseline gene expression level at peripheral lymph nodes. For low dosed macaques, PBMCs were obtained at four time points between days 7 and 14, 28 and 35, 70 and 85, and at day 112; LN biopsies were obtained at three time points for the low dose macaques between days 28 and 35, day 70 and 85 and also at day 112. Immune gene expression in LN biopsies and PBMCs shown here for high dose macaques include PBMCs obtained at days 7 or 15, 21 or 28, 45 or 56 as well as 85 dpi and lymph node biopsies taken at three time points on days 21 or 28, 45 or 56 as well as 85 dpi as previous described [28].
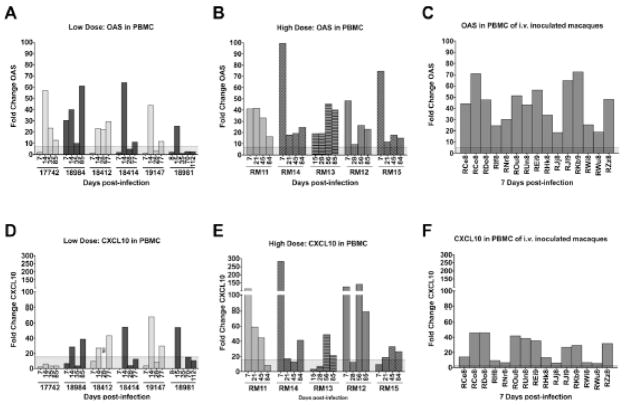
Assessment of PBMCs from the low and high dosed macaques determined that transcript levels of CXCL9, IFN-γ, TNF-α and IL-10 did not differ from uninfected macaques (Fig. 4A, B). IFN-α and IL-12 could not be reliably detected in the uninfected control PBMC samples and, therefore, fold changes could not be determined. OAS and CXCL10 gene expression were upregulated in both the high dosed and low dosed macaques to comparable levels, indicating that the viral challenge dose did not impact the overall response of these immune modulators in the peripheral blood (Figs. 4, 5). Furthermore, all 15 macaques infected intravenously with high doses of SIVmac239 had increased levels of OAS (Fig. 5C) and CXCL10 (Fig. 5F) on day 7 post-infection, indicating that OAS and CXCL10 up-regulation in PBMCs from SIV-infected animals is not restricted to the oral route of infection. However it is interesting to note that a distinction could be observed in the kinetics of the response through an assessment of the day 7 timepoints that were available for five of the low dosed and four of the high dosed macaques (Fig. 5A, B). The low dosed macaques had only one macaque (18984) with increased OAS expression on day 7 (Fig. 5A), and no macaques with increased CXCL10 on day 7 (Fig. 5D), while 4 out of 4 of the high dosed macaques had increased OAS expression (Fig. 5B) and 3 out of 4 exhibited increased CXCL10 expression at this early timepoint (Fig. 5E). The difference in dosing strategy in the low and high dosed animals may have played a role in the delayed kinetics, however this delay was also observed in low dosed macaques 17742 and 18981 which had detectable plasma viral load by the day 7 timepoint.
Fig. 5.
OAS and CXCL10 mRNA expression changes in peripheral blood. OAS (A, B, C) and CXCL10 (D, E, F) expression from acute to chronic SIV infection time points are shown for PBMCs of Rhesus macaques orally inoculated with low (A, D) or high doses (B, E) of SIVmac251. Also, OAS (C) and CXCL10 (F) expression in PBMCs are shown for macaques 7 days after i.v. infection with high doses of SIVmac239. Each bar represents the fold change of expression at one time point compared to uninfected macaques. The shaded area across the x-axis represents two standard deviations of the average expression of the corresponding gene in uninfected macaques. ND – not determined.
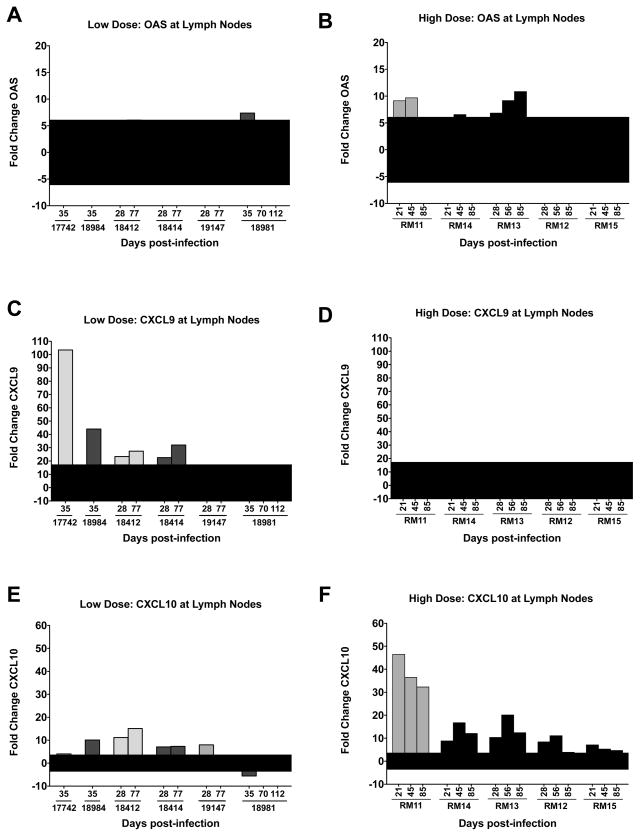
In peripheral lymph nodes (axillary and inguinal obtained from 21 to 112 days post-infection), gene expression of four immune modulators IFN-γ, IL-12, TNF-α and IL-10 remained stable (comparable to uninfected macaques), following successful low or high dose oral infection (Fig. 4C, D). CXCL10 expression was increased following SIV infection of both the low and high dosed groups. In PBMCs, OAS expression was also upregulated, however, in lymph nodes OAS was only upregulated in 3 of the 5 high dosed macaques and none of the low dosed macaques (student t test, p = 0.013) (Fig. 4C, D). In contrast, CXCL9 was only upregulated in the lymph nodes of the low dosed macaques (4 of the 6 macaques) with no upregulation in the high dosed macaques (student t test, p=0.024) (Fig. 4C, D). Assessment of the lymph node OAS, CXCL9 and CXCL10 transcripts is also presented such that each time point can be assessed in the high and low dosed oral SIV infected macaques (Fig. 6). OAS and CXCL10 upregulation in the high dosed macaques as well as CXCL9 and CXL10 upregulation in low-dosed macaques appears to be macaque specific and is equally prevalent at acute, chronic (21 to 35 days post-infection) or late (45 to 112 days post-infection) time points (Fig. 6). The differential gene expression pattern between the two oral SIV dosing groups suggests that SIV infection dosage can have long-term effects on immune responses that can be observed in peripheral lymph nodes up to 112 days postinfection.
Figure 6. OAS, CXCL9, CXCL10 mRNA expression changes in peripheral lymph node.
OAS (A, B), CXCL9 (C, D) and CXCL10 (E, F) expression during chronic SIV infection at lymph node are shown from Rhesus macaques orally inoculated with low (A, C, E) or high doses (B, D, F) of SIVmac251. Each bar represents the fold change of gene expression at one time point compared to uninfected macaques. The shaded area across the x-axis represents two standard deviations of the average expression of the corresponding gene in uninfected macaques.
OAS and CXCL10 Expression in Purified T Cells and Monocytes from PBMCs
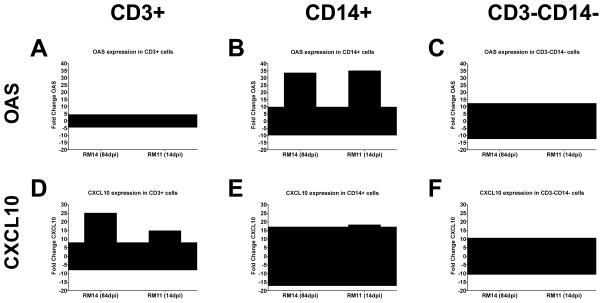
To determine which cell types in the PBMCs express OAS and CXCL10 mRNA, magnetic bead separation of PBMCs was used to isolate three cell populations from SIVuninfected and SIV-infected Rhesus macaques: CD3+ T cells, CD14+ monocytes (macrophage and dendritic cell precursors), and the remaining CD3−CD14− cell population, representing B cells, and NK cells. The sorting of these three cell subsets yielded greater than 90% pure cell populations. Fold changes in OAS and CXCL10 gene expression in SIVinfected macaques was calculated by comparing them to the average expression in the same cell subsets from six uninfected macaques. The assessment of OAS mRNA in two SIV-infected macaques indicated that OAS expression was increased 35-fold in CD14+ monocytes from both infected macaques (Fig. 7B), but was comparable to uninfected macaques in CD3+ T cells (Fig. 7A) and in CD3−CD14− cells subsets (Fig. 7C). Expression of CXCL10 in the two infected macaques was increased 25-and 15-fold, respectively, in CD3+ T cells (Fig. 7D), while expression of this gene in CD14+ monocytes from one macaque (Fig. 7E) and CD3−CD14− cells from both macaques (Fig. 7F) was within the range of uninfected macaques. These results suggest that the up-regulation of OAS in PBMCs occurs primarily in monocytes while up-regulation of CXCL10 can be attributed to CD3+ lymphocytes.
Figure 7. Expression of OAS and CXCL10 in PBMC cell subsets.
Fold changes of expression of OAS (A, B, C) and CXCL10 (D, E, F) are shown in isolated CD3+ T cells (A, D), CD14+ monocytes (B, E) and cells that do not have either of these markers (CD3−CD14−) (C, F). PBMCs were taken from macaque RM14 (84 dpi, left bar) and RM11 (14 dpi, right bar). Expression of OAS or CXCL10 in each cell subset is compared to gene expression in the same cell subset of uninfected Rhesus macaques. The fold change is considered increased or decreased in SIV infected compared to uninfected macaques when it is greater than two standard deviations (shaded area) away from the average expression in uninfected animals.
DISCUSSION
While high dose mucosal challenge of SIV provides greater assurance that the SIV administration will result in successful infection, these doses exceed naturally occurring viral titers in body fluids [14–19]. Due to the large number of virions in high dose inoculums, host defenses at mucosal sites may be overwhelmed, reducing our ability to detect the beneficial effects of vaccines or microbicides. Low dose viral administration more closely mimics naturally occurring viral inocula and, therefore, would be predicted to be more appropriate for vaccine, microbicide or transmission studies, although these experiments present additional issues both in designing and comparing studies (including inoculation timing, viral dose and optimal samples collection time points). In this study, the SIV-macaque model was utilized to evaluate viral and immune changes following either high or low dose SIV transmission via the oral route. Virologic analyses revealed that plasma viral set-points during chronic phases were comparable between the low and high dosed macaques indicating that irrespective of the oral challenge dose the resultant viral replication is comparable during the chronic phase of the infection.
In general, the transcription changes of key immune modulators such as IFN-γ, TNF-α, IL-12 and IL-10 in PBMC following SIV infection in the low and high dosed orally SIVinfected macaques are in agreement with published studies [29, 70, 71]. For example, the lack of an upregulation of the cytokines TNF-α and IL-10 during the chronic phase of the SIV infection in IV inoculated macaques has also been reported, although a transient upregulation of these cytokines can be observed in some macaques [70, 72, 73]. Also, IFN-γ gene expression level in PBMCs is not upregulated following oral SIV-infected infant macaques [29], in agreement with our findings. In lymph nodes, other studies have described an increase in IFN-γ, TNF-α, IL-12 and IL-10 gene expression within the first week post-SIV infection in orally or vaginally exposed macaques [29, 71]. However, the most dramatic upregulation was observed in lymph nodes near the sites of virus administration whereas those distal lymphoid tissues (similar to the ones assessed here) were less affected or unchanged [29, 71].
Assessment of immune modulators in PBMCs of the low and high dosed macaques identified two genes, OAS and CXCL10, that were upregulated. Overall, the mRNA levels of OAS and CXCL10 were similar in low and high dosed macaque PBMCs, although we did observe a more rapid increase in the high dosed (and intravenously inoculated) macaques in comparison to the low dosed macaques. These delayed immune responses occurred in low dosed macaques that had detectable plasma viral loads at the day 7 time point (Fig. 1 compared to Fig. 4), and, therefore, can not be attributed solely to a delay in SIV replication. Indeed, a recent study by Liu et al. assessed the impact of SIV dosage on viral and immunologic factors following rectal SIV infection of macaques [74] and, although CXCL10 and OAS were not evaluated in that study, this group also found that low SIV dosages results in a delayed, as well as a reduced expression of a number of plasma cytokines and chemoki nes (IFN-α, IFN-γ, IL-1R α, IL-15, IL-18 and MCP-1) [74]. Together, these data suggest a model in which low doses of SIV delivered mucosally result in a delayed innate immunologic response in peripheral blood when compared to macaques administered a high mucosal dose of SIV.
Assessment of peripheral lymph nodes following SIV infection in the low and high dosed macaques identified three immune modulators that were upregulated (OAS, CXCL9 and CXCL10). The immunologic differences between the differentially dosed macaques was most obvious within the lymph nodes in which high dosed macaques had increased OAS and CXCL10 expression while low dosed macaques upregulated CXCL9 and CXCL10 (Figs. 4, 6). Interestingly, these two immune modulators are differentially regulated; OAS is produced in response to IFN-α stimulation [75] and CXCL9 principally induced by IFN-γ [76]. Interestingly, CXCL10 can be induced by both IFN-α and IFN-γ [76, 77]. Therefore, the differential immune modulator transcript expression may be driven by a strong IFN-α mediated response in high dosed macaques and a strong IFN-γ mediated response in low dosed macaques. Since OAS is an antiviral protein [75] and the CXCL9 and CXCL10 are chemokines that bind the receptor CXCR3 [78, 79] expressed on Th1 (CD4+ and CD8+ T cells) as well as NK cells [80, 81], this differential expression has the potential to elicit different immune responses both within the peripheral lymph node as well as other lymphatic tissues. Our previous studies demonstrated that increased mRNA expression of OAS and CXCL10 at mucosal sites was associated with slower disease progression, whereas increased mRNA expression of these same immune factors in peripheral blood cells and lymph nodes was associated with more rapid disease progression [27, 28]. Therefore, the location of these immune modulators likely plays an important in determining early events following infection which has the potential to impact disease outcome.
Analysis of purified populations of CD3+ T cells and CD14+ monocytes determined that in PBMCs CD3+ T cells were the major producer of CXCL10 whereas OAS was produced mainly by CD14+ monocytes. Because CD4+ T cells can potentially be infected with SIV, it is possible that the increase in CXCL10 expression is due to direct viral infection, although indirect cytokine effects cannot be ruled out. In contrast, monocytes are rarely found to be infected with HIV. Accordingly, as monocytes produce OAS at higher levels than lymphocytes in response to interferon [82], this suggests that interferon may be responsible for the monocyte-specific upregulation of OAS. These immunemodulators might be induced to defend against SIV infection, yet they could also potentially increase the activation state of CD4+ target cells, which would be predicted to facilitate HIV spread and increase the rate of disease progression.
In summary, the studies presented here are the first to provide a detailed assessment of immune and viral changes in Rhesus macaques inoculated orally with low and high doses of SIV. These studies provide evidence that infection after low dose SIV administration has more in common with natural HIV infection than does high dose SIV administration, particularly with regard to the limited number of virions establishing infection [45, 46, 83, 84]. Overall, the similarities in viral and immune parameters between the two SIV dosing strategies were more evident than the differences. The potential for an extended eclipse phase (delay in viral and innate immune responses) likely provides an additional opportunity for a vaccine to be protective when oral low dose SIV inoculations are administered. We also found differential patterns of gene expression observed in the lymph nodes of the high (upregulation of OAS and CXCL10) and low dosed (upregulation of CXCL9 and CXCL10) orally SIV inoculated macaques. These data would indicate that SIV challenge doses influence the innate immune response at least through the establishment of the chronic phase of the infection. Overall, these data support the use of low dose challenges in the SIV-macaque model as they establish virologic outcomes that are more comparable to what has been observed during mucosal HIV infections [45, 46, 49–51, 84, 85] and therefore represents a better model for evaluating efficacy of vaccines and microbicides. Finally, these studies have the potential to inform future SIV/HIV vaccine efficacy trials in which vaccinated hosts have the potential to be infected with a range of viral challenge doses. The authors do not have any commercial or other considerations that might be interpreted as a conflict of interest with regard to the data presented herein.
Supplementary Material
Acknowledgments
FUNDING
We acknowledge the excellent animal care and veterinary staff at the Southwest National Primate Research Center (SNPRC), the California National Primate Research Center (CNPRC) and the Yerkes National Primate Research Center (YNPRC), where the monkey experiments were performed. We also thank Kiran Mir for critical reading of the manuscript. Work at the SNPRC was supported by Public Health Service grants P51 RR013986 from the National Center for Research Resources, and was conducted in a facility constructed with support from Research Facilities Improvement Program Grant number C06 RR12087 from National Center for Research Resources, National Institutes of Health. Work was also supported by Public Health Service Grants P51 RR000169 to the CNPRC, P51 RR013986 to the SNPRC and P51 RR000165 to the YNPRC. These studies were supported by the Viral Pathogenesis Training grant awarded to MAG, an F32 fellowship (AI084556) awarded to VS, as well as the NIH NIDCR (R01 DE017541) and the Pendleton Trust grant awarded to DLS.
References
- 1.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 2.Vaccari M, Poonam P, Franchini G. Phase III HIV vaccine trial in Thailand: a step toward a protective vaccine for HIV. Expert Rev Vaccines. 2010;9:997–1005. doi: 10.1586/erv.10.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benmira S, Bhattacharya V, Schmid ML. An Effective HIV Vaccine: A Combination of Humoral and Cellular Immunity? Curr HIV Res. 2010;8:441–9. doi: 10.2174/157016210793499286. [DOI] [PubMed] [Google Scholar]
- 4.Jotwani R, Cutler CW. Multiple dendritic cell (DC) subpopulations in human gingiva and association of mature DCs with CD4+ Tcells in situ. J Dent Res. 2003;82:736–41. doi: 10.1177/154405910308200915. [DOI] [PubMed] [Google Scholar]
- 5.Chou LL, Epstein J, Cassol SA, West DM, He W, Firth JD. Oral mucosal Langerhans’ cells as target, effector and vector in HIV infection. J Oral Pathol Med. 2000;29:394–402. doi: 10.1034/j.1600-0714.2000.290805.x. [DOI] [PubMed] [Google Scholar]
- 6.Milush JM, Kosub D, Marthas M, Schmidt K, Scott F, Wozniakowski A, et al. Rapid dissemination of SIV following oral inoculation. AIDS. 2004;18:2371–80. [PubMed] [Google Scholar]
- 7.Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, Reimann KA, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–7. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 8.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis. 2008;198:687–93. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- 9.Kaul R, Pettengell C, Sheth PM, Sunderji S, Biringer A, MacDonald K, et al. The genital tract immune milieu: an important determinant of HIV susceptibility and secondary transmission. J Reprod Immunol. 2008;77:32–40. doi: 10.1016/j.jri.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Chakraborty H, Sen PK, Helms RW, Vernazza PL, Fiscus SA, Eron JJ, et al. Viral burden in genital secretions determines maleto- female sexual transmission of HIV-1: a probabilistic empiric model. AIDS. 2001;15:621–7. doi: 10.1097/00002030-200103300-00012. [DOI] [PubMed] [Google Scholar]
- 11.Hirbod T, Broliden K. Mucosal immune responses in the genital tract of HIV-1-exposed uninfected women. J Intern Med. 2007;262:44–58. doi: 10.1111/j.1365-2796.2007.01822.x. [DOI] [PubMed] [Google Scholar]
- 12.Greenhead P, Hayes P, Watts PS, Laing KG, Griffin GE, Shattock RJ. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J Virol. 2000;74:5577–86. doi: 10.1128/jvi.74.12.5577-5586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chenine AL, Siddappa NB, Kramer VG, Sciaranghella G, Rasmussen RA, Lee SJ, et al. Relative transmissibility of an R5 clade C simian-human immunodeficiency virus across different mucosae in macaques parallels the relative risks of sexual HIV-1 transmission in humans via different routes. J Infect Dis. 2010;201:1155–63. doi: 10.1086/651274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willumsen JF, Newell ML, Filteau SM, Coutsoudis A, Dwarika S, York D, et al. Variation in breastmilk HIV-1 viral load in left and right breasts during the first 3 months of lactation. AIDS. 2001;15:1896–8. doi: 10.1097/00002030-200109280-00026. [DOI] [PubMed] [Google Scholar]
- 15.Shepard RN, Schock J, Robertson K, Shugars DC, Dyer J, Vernazza P, et al. Quantitation of human immunodeficiency virus type 1 RNA in different biological compartments. J Clin Microbiol. 2000;38:1414–8. doi: 10.1128/jcm.38.4.1414-1418.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vernazza PL, Gilliam BL, Dyer J, Fiscus SA, Eron JJ, Frank AC, et al. Quantification of HIV in semen: correlation with antiviral treatment and immune status. AIDS. 1997;11:987–93. [PubMed] [Google Scholar]
- 17.Rousseau CM, Nduati RW, Richardson BA, Steele MS, John- Stewart GC, Mbori-Ngacha DA, et al. Longitudinal analysis of human immunodeficiency virus type 1 RNA in breast milk and of its relationship to infant infection and maternal disease. J Infect Dis. 2003;187:741–7. doi: 10.1086/374273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Semba RD, Kumwenda N, Hoover DR, Taha TE, Quinn TC, Mtimavalye L, et al. Human immunodeficiency virus load in breast milk, mastitis, and mother-to-child transmission of human immunodeficiency virus type 1. J Infect Dis. 1999;180:93–8. doi: 10.1086/314854. [DOI] [PubMed] [Google Scholar]
- 19.O’Byrne P, MacPherson PA. Understanding HIV viral load: implications for counselling. Can J Public Health. 2008;99:189–91. doi: 10.1007/BF03405471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Q, Duan L, Estes J, Ma ZM, Rourke T, Wang Y, et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–52. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 21.Letvin NL, King NW. Immunologic and pathologic manifestations of the infection of rhesus monkeys with simian immunodeficiency virus of macaques. J Acquir Immune Defic Syndr. 1990;3:1023–40. [PubMed] [Google Scholar]
- 22.Hirsch VM, Lifson JD. Simian immunodeficiency virus infection of monkeys as a model system for the study of AIDS pathogenesis, treatment, and prevention. Adv Pharmacol. 2000;49:437–77. doi: 10.1016/s1054-3589(00)49034-4. [DOI] [PubMed] [Google Scholar]
- 23.Desrosiers RC. The simian immunodeficiency viruses. Annu Rev Immunol. 1990;8:557–78. doi: 10.1146/annurev.iy.08.040190.003013. [DOI] [PubMed] [Google Scholar]
- 24.Hirsch VM, Johnson PR. Pathogenic diversity of simian immunodeficiency viruses. Virus Res. 1994;32:183–203. doi: 10.1016/0168-1702(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 25.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–7. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 26.Kosub DA, Durudas A, Lehrman G, Milush JM, Cano CA, Jain MK, et al. Gamma/Delta T cell mRNA levels decrease at mucosal sites and increase at lymphoid sites following an oral SIV infection of macaques. Curr HIV Res. 2008;6:520–30. doi: 10.2174/157016208786501490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durudas A, Milush JM, Chen H-L, Engram JC, Silvestri G, Sodora DL. Elevated levels of innate immune modulators in lymph nodes and blood are associated with more-rapid disease progression in simian immunodeficiency virus-infected monkeys. J Virol. 2009;83:12229–40. doi: 10.1128/JVI.01311-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milush JM, Stefano-Cole K, Schmidt K, Durudas A, Pandrea I, Sodora DL. Mucosal innate immune response associated with a timely humoral immune response and slower disease progression after oral transmission of simian immunodeficiency virus to rhesus macaques. J Virol. 2007;81:6175–86. doi: 10.1128/JVI.00042-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abel K, Pahar B, Van Rompay KK, et al. Rapid virus dissemination in infant macaques after oral simian immunodeficiency virus exposure in the presence of local innate immune responses. J Virol. 2006;80:6357–67. doi: 10.1128/JVI.02240-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scarlatti G. Mother-to-child transmission of HIV-1: advances and controversies of the twentieth centuries. AIDS Rev. 2004;6:67–78. [PubMed] [Google Scholar]
- 31.Mofenson LM. Interaction between timing of perinatal human immunodeficiency virus infection and the design of preventive and therapeutic interventions. Acta Paediatr Suppl. 1997;421:1–9. doi: 10.1111/j.1651-2227.1997.tb18311.x. [DOI] [PubMed] [Google Scholar]
- 32.Nduati R, John G, Mbori-Ngacha D, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA. 2000;283:1167–74. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 33.Fawzi W, Msamanga G, Spiegelman D, et al. Transmission of HIV-1 through breastfeeding among women in Dar es Salaam, Tanzania. J Acquir Immune Defic Syndr. 2002;31:331–8. doi: 10.1097/00126334-200211010-00010. [DOI] [PubMed] [Google Scholar]
- 34.Bratt GA, Berglund T, Glantzberg BL, Albert J, Sandström E. Two cases of oral-to-genital HIV-1 transmission. Int J STD AIDS. 1997;8:522–5. doi: 10.1258/0956462971920695. [DOI] [PubMed] [Google Scholar]
- 35.Rothenberg RB, Scarlett M, del Rio C, Reznik D, O’Daniels C. Oral transmission of HIV. AIDS. 1998;12:2095–105. doi: 10.1097/00002030-199816000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257–64. doi: 10.7326/0003-4819-125-4-199608150-00001. [DOI] [PubMed] [Google Scholar]
- 37.Vittinghoff E, Douglas J, Judson F, McKirnan D, MacQueen K, Buchbinder SP. Per-contact risk of human immunodeficiency virus transmission between male sexual partners. Am J Epidemiol. 1999;150:306–11. doi: 10.1093/oxfordjournals.aje.a010003. [DOI] [PubMed] [Google Scholar]
- 38.Ruprecht RM, Baba TW, Liska V, et al. Oral SIV, SHIV, and HIV type 1 infection. AIDS Res Hum Retroviruses. 1998;14 (Suppl 1):S97–103. [PubMed] [Google Scholar]
- 39.Baba TW, Koch J, Mittler ES, et al. Mucosal infection of neonatal rhesus monkeys with cell-free SIV. AIDS Res Hum Retroviruses. 1994;10:351–7. doi: 10.1089/aid.1994.10.351. [DOI] [PubMed] [Google Scholar]
- 40.Baba TW, Trichel AM, An L, et al. Infection and AIDS in adult macaques after nontraumatic oral exposure to cell-free SIV. Science. 1996;272:1486–9. doi: 10.1126/science.272.5267.1486. [DOI] [PubMed] [Google Scholar]
- 41.Ferrantelli F, Rasmussen RA, Buckley KA, et al. Complete protection of neonatal rhesus macaques against oral exposure to pathogenic simian-human immunodeficiency virus by human anti- HIV monoclonal antibodies. J Infect Dis. 2004;189:2167–73. doi: 10.1086/420833. [DOI] [PubMed] [Google Scholar]
- 42.Van Rompay KK, Greenier JL, Cole KS, et al. Immunization of newborn rhesus macaques with simian immunodeficiency virus (SIV) vaccines prolongs survival after oral challenge with virulent SIVmac251. Journal of Virol. 2003;77:179–90. doi: 10.1128/JVI.77.1.179-190.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Rompay KK, Abel K, Lawson JR, Singh RP, Schmidt KA, Evans T, et al. Attenuated poxvirus-based simian immunodeficiency virus (SIV) vaccines given in infancy partially protect infant and juvenile macaques against repeated oral challenge with virulent SIV. J Acquir Immune Defic Syndr. 2005;38:124–34. doi: 10.1097/00126334-200502010-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hansen SG, Vieville C, Whizin N, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15:293–9. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keele BF, Giorgi EE, Salazar-Gonzalez JF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci USA. 2008;105:7552–7. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salazar-Gonzalez JF, Bailes E, Pham KT, et al. Deciphering human immunodeficiency virus type 1 transmission and early envelope diversification by single-genome amplification and sequencing. J Virol. 2008;82:3952–70. doi: 10.1128/JVI.02660-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goonetilleke N, Liu MK, Salazar-Gonzalez JF, et al. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J Exp Med. 2009;206:1253–72. doi: 10.1084/jem.20090365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stone M, Keele BF, Ma ZM, et al. A limited number of simian immunodeficiency virus (SIV) env variants are transmitted to rhesus macaques vaginally inoculated with SIVmac251. J Virol. 2010;84:7083–95. doi: 10.1128/JVI.00481-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller C, Li Q, Abel K, et al. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol. 2005;79:9217–27. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haaland R, Hawkins P, Salazar-Gonzalez J, et al. Inflammatory genital infections mitigate a severe genetic bottleneck in heterosexual transmission of subtype A and C HIV-1. PLoS Pathog. 2009;5:e1000274. doi: 10.1371/journal.ppat.1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keele BF, Li H, Learn GH, et al. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J Exp Med. 2009 doi: 10.1084/jem.20082831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boily MC, Baggaley RF, Wang L, et al. Heterosexual risk of HIV- 1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis. 2009;9:118–29. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Powers KA, Poole C, Pettifor AE, Cohen MS. Rethinking the heterosexual infectivity of HIV-1: a systematic review and metaanalysis. Lancet Infect Dis. 2008;8:553–63. doi: 10.1016/S1473-3099(08)70156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bar KJ, Li H, Chamberland A, Tremblay C, Routy JP, Grayson T, et al. Wide variation in the multiplicity of HIV-1 infection among injection drug users. J Virol. 2010;84:6241–7. doi: 10.1128/JVI.00077-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li H, Bar KJ, Wang S, et al. High Multiplicity Infection by HIV-1 in Men Who Have Sex with Men. PLoS Pathog. 2010;6:e1000890. doi: 10.1371/journal.ppat.1000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Greenier JL, Miller CJ, Lu D, et al. Route of simian immunodeficiency virus inoculation determines the complexity but not the identity of viral variant populations that infect rhesus macaques. J Virol. 2001;75:3753–65. doi: 10.1128/JVI.75.8.3753-3765.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marthas ML, Lu D, Penedo MC, Hendrickx AG, Miller CJ. Titration of an SIVmac251 stock by vaginal inoculation of Indian and Chinese origin rhesus macaques: transmission efficiency, viral loads, and antibody responses. AIDS Res Hum Retroviruses. 2001;17:1455–66. doi: 10.1089/088922201753197123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Engram J, Dunham R, Makedonas G, et al. Vaccine-induced, Simian Immunodeficiency Virus-specific CD8+ T cells reduce virus replication but do not protect from Simian Immunodeficiency Virus disease progression. J Immunol. 2009;183:706–17. doi: 10.4049/jimmunol.0803746. [DOI] [PubMed] [Google Scholar]
- 59.Marx PA, Spira AI, Gettie A, et al. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med. 1996;2:1084–9. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 60.Pachl C, Todd JA, Kern DG, et al. Rapid and precise quantification of HIV-1 RNA in plasma using a branched DNA signal amplification assay. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:446–54. doi: 10.1097/00042560-199504120-00003. [DOI] [PubMed] [Google Scholar]
- 61.Leutenegger CM, Higgins J, Matthews TB, et al. Real-time TaqMan PCR as a specific and more sensitive alternative to the branched-chain DNA assay for quantitation of simian immunodeficiency virus RNA. AIDS Res Hum Retroviruses. 2001;17:243–51. doi: 10.1089/088922201750063160. [DOI] [PubMed] [Google Scholar]
- 62.Abel K, Alegria-Hartman MJ, Rothaeusler K, Marthas M, Miller CJ. The relationship between simian immunodeficiency virus RNA levels and the mRNA levels of alpha/beta interferons (IFNalpha/beta) and IFN-alpha/beta-inducible Mx in lymphoid tissues of rhesus macaques during acute and chronic infection. J Virol. 2002;76:8433–45. doi: 10.1128/JVI.76.16.8433-8445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abel K, Compton L, Rourke T, Montefiori D, Lu D, Rothaeusler K, et al. Simian-human immunodeficiency virus SHIV89. 6- induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and alpha interferon responses. J Virol. 2003;77:3099–118. doi: 10.1128/JVI.77.5.3099-3118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sodora DL, Lee F, Dailey PJ, Marx PA. A genetic and viral load analysis of the simian immunodeficiency virus during the acute phase in macaques inoculated by the vaginal route. AIDS Research and Human Retroviruses. 1998;14:171–81. doi: 10.1089/aid.1998.14.171. [DOI] [PubMed] [Google Scholar]
- 65.Kirmaier A, Wu F, Newman RM, et al. TRIM5 suppresses crossspecies transmission of a primate immunodeficiency virus and selects for emergence of resistant variants in the new species. PLoS Biol. 2010:8. doi: 10.1371/journal.pbio.1000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Almond N, Jenkins A, Heath AB, Kitchin P. Sequence variation in the env gene of simian immunodeficiency virus recovered from immunized macaques is predominantly in the V1 region. J Gen Virol. 1993;74 (Pt 5):865–71. doi: 10.1099/0022-1317-74-5-865. [DOI] [PubMed] [Google Scholar]
- 67.Overbaugh J, Rudensey LM, Papenhausen MD, Benveniste RE, Morton WR. Variation in simian immunodeficiency virus env is confined to V1 and V4 during progression to simian AIDS. J Virol. 1991;65:7025–31. doi: 10.1128/jvi.65.12.7025-7031.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tao B, Fultz PN. Molecular and biological analyses of quasispecies during evolution of a virulent simian immunodeficiency virus, SIVsmmPBj14. J Virol. 1995;69:2031–7. doi: 10.1128/jvi.69.4.2031-2037.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sagar M, Lavreys L, Baeten JM, et al. Infection with multiple human immunodeficiency virus type 1 variants is associated with faster disease progression. J Virol. 2003;77:12921–6. doi: 10.1128/JVI.77.23.12921-12926.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Benveniste O, Vaslin B, Le Grand R, et al. Comparative interleukin (IL-2)/interferon IFN-gamma and IL-4/IL-10 responses during acute infection of macaques inoculated with attenuated neftruncated or pathogenic SICmac251 virus. Proc Natl Acad Sci U S A. 1996;93:3658–63. doi: 10.1073/pnas.93.8.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abel K, Rocke DM, Chohan B, Fritts L, Miller CJ. Temporal and anatomic relationship between virus replication and cytokine gene expression after vaginal simian immunodeficiency virus infection. J Virol. 2005;79:12164–72. doi: 10.1128/JVI.79.19.12164-12172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Benveniste O, Vaslin B, Le Grand R, et al. Interleukin 1 beta, interleukin 6, tumor necrosis factor alpha, and interleukin 10 responses in peripheral blood mononuclear cells of cynomolgus macaques during acute infection with SIVmac251. AIDS Res Hum Retroviruses. 1996;12:241–50. doi: 10.1089/aid.1996.12.241. [DOI] [PubMed] [Google Scholar]
- 73.Cheret A, Le Grand R, Caufour P, et al. Cytokine mRNA expression in mononuclear cells from different tissues during acute SIVmac251 infection of macaques. AIDS Res Hum Retroviruses. 1996;12:1263–72. doi: 10.1089/aid.1996.12.1263. [DOI] [PubMed] [Google Scholar]
- 74.Liu J, Keele BF, Li H, Keating S, Norris PJ, Carville A, et al. Lowdose mucosal simian immunodeficiency virus infection restricts early replication kinetics and transmitted virus variants in rhesus monkeys. J Virol. 2010;84:10406–12. doi: 10.1128/JVI.01155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chakrabarti A, Jha BK, Silverman RH. New insights into the role of RNase L in innate immunity. J Interferon Cytokine Res. 2011;31:49–57. doi: 10.1089/jir.2010.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol. 2011;89:207–15. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Farber JM. Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol. 1997;61:246–57. [PubMed] [Google Scholar]
- 78.Loetscher M, Loetscher P, Brass N, Meese E, Moser B. Lymphocyte-specific chemokine receptor CXCR3: regulation, chemokine binding and gene localization. Eur J Immunol. 1998;28:3696–705. doi: 10.1002/(SICI)1521-4141(199811)28:11<3696::AID-IMMU3696>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 79.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark- Lewis I, et al. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–9. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim CH, Rott L, Kunkel EJ, Genovese MC, Andrew DP, Wu L, et al. Rules of chemokine receptor association with T cell polarization in vivo. J Clin Invest. 2001;108:1331–9. doi: 10.1172/JCI13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yamamoto J, Adachi Y, Onoue Y, et al. Differential expression of the chemokine receptors by the Th1- and Th2-type effector populations within circulating CD4+ T cells. J Leukoc Biol. 2000;68:568–74. [PubMed] [Google Scholar]
- 82.Witt PL, Spear GT, Helgeson DO, Lindstrom MJ, Smalley RV, Borden EC. Basal and interferon-induced 2′,5′-oligoadenylate synthetase in human monocytes, lymphocytes, and peritoneal macrophages. J Interferon Res. 1990;10:393–402. doi: 10.1089/jir.1990.10.393. [DOI] [PubMed] [Google Scholar]
- 83.Delwart EL, Sheppard HW, Walker BD, Goudsmit J, Mullins JI. Human immunodeficiency virus type 1 evolution in vivo tracked by DNA heteroduplex mobility assays. J Virol. 1994;68:6672–83. doi: 10.1128/jvi.68.10.6672-6683.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhu T, Mo H, Wang N, et al. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261:1179–81. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 85.Abrahams MR, Anderson JA, Giorgi EE, et al. Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype C reveals a non-poisson distribution of transmitted variants. J Virol. 2009;83:3556–67. doi: 10.1128/JVI.02132-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.