Abstract
Rationale
Atherosclerosis is a disease of large and medium sized arteries that is characterized by chronic vascular inflammation. While the role of Th1, Th2 and T-regulatory subsets in atherogenesis is established, the involvement of IL-17A-producing cells remains unclear.
Objective
To investigate the role of the IL-17A/IL-17RA axis in atherosclerosis.
Methods and Results
We bred Apolipoprotein-E-deficient (Apoe−/−) mice with IL-17A-deficient and IL-17 receptor A-deficient mice to generate Il17a−/−Apoe−/− and Il17ra−/−Apoe−/− mice. Western diet fed Il17a−/−Apoe−/− and Il17ra−/−Apoe−/− mice had smaller atherosclerotic plaques in the aortic arch and aortic roots, but showed little difference in plaque burden in the thoracoabdominal aorta compared with Apoe−/− controls. Flow cytometric analysis of Il17a−/−Apoe−/− and Il17ra−/−Apoe−/− aortas revealed that deficiency of IL-17A/IL-17RA preferentially reduced aortic arch, but not thoracoabdominal aortic T cell, neutrophil and macrophage content in comparison to Apoe−/− aortic segments. In contrast to ubiquitous IL-17RA expression throughout the aorta, IL-17A was preferentially expressed within the aortic arch of WD fed Apoe−/− mice. Deficiency of IL-17A or IL-17RA reduced aortic arch, but not thoracoabdominal aortic TNFα and CXCL2 expression. Aortic vascular IL-17RA supports monocyte adherence to explanted aortas in ex vivo adhesion assays. Short–term homing experiments revealed that the recruitment of adoptively transferred monocytes and neutrophils to the aortas of Il17ra−/−Apoe−/− mice is impaired compared with Apoe−/− recipients.
Conclusions
The IL-17A/IL-17RA axis increases aortic arch inflammation during atherogenesis through the induction of aortic chemokines, and the acceleration of neutrophil and monocyte recruitment to this site.
Keywords: Atherosclerosis, Inflammation, Leukocytes, Chemokines, Migration
Introduction
Atherosclerosis, a chronic inflammatory disease of large and medium sized arteries, continues to be the leading cause of cardiovascular disease and one of the most common causes of mortality worldwide. As a part of the adaptive immune response, T cells actively participate in regulating local and systemic inflammation during atherogenesis. While the respective roles of T helper 1 (Th1), T helper 2 (Th2), and T-regulatory (Treg) cells in atherosclerosis are well established,1,2 the role of T helper 17 (Th17) cells has only recently come under consideration.3,4
Th17 cells represent a novel lineage of T cells that are characterized by IL-17A, IL-17F, IL-21, IL-22, and RORγt expression.5,6 Other cells including γδ+T cells, lymphoid tissue inducer (LTi) cells, NK and NKT cells also produce IL-17A.5 Under non-inflamed conditions the expression of IL-17A is low; however, IL-17A is quickly induced following bacterial and fungal infections and promotes leukocyte recruitment to the site of inflammation through the production of chemokines and cytokines.7,8 In many inflammatory conditions Th17 cells release not only IL-17A, but also IL-17F.9,10 These cytokines can form IL-17A and IL-17F homodimers, as well as IL-17A/IL-17F heterodimers.9,10 The IL-17RA/IL-17RC complex serves as a receptor for IL-17A and IL-17F.8 IL-17A-producing cells are major contributors to the immune response against several pathogens, as well as, participants in autoimmune diseases such as multiple sclerosis, inflammatory bowel disease and arthritis.6,7
In line with the involvement of IL-17-producing cells in the regulation of the immune response during acute and chronic inflammation, Th17 cells11–14 and IL-17+γδ+T cells13 have recently been detected within human and mouse atherosclerotic vessels. Elevated plasma IL-17A was found in patients with coronary artery disease (CAD) in comparison with healthy controls,11 and in the carotid plaques of symptomatic patients undergoing endarterectomy.15 Together, these results suggest an association between atherosclerosis and increased production of IL-17A in mice and humans.
Several studies have recently investigated the role of IL-17A in atherogenesis, but have yielded inconsistent results. Administration of rat IL-17A neutralizing antibodies resulted in the reduction of Mac-2+ MΦ, and CD3+ T cells and the attenuation of aortic root lesions of Apoe−/− mice.12,16 Similarly, the blockade of IL-17A via an adenoviral soluble IL-17RA-construct led to decreased aortic and aortic root lesions,13 suggesting a pro-atherogenic role for IL-17A. In contrast, in vivo administration of IL-17A reduced plaque burden within the aortic roots of Ldlr−/− mice.14 Interestingly, the administration of rat anti-IL-17A Abs, but not mouse anti-IL-17 Abs reduced aortic root plaque development.17 Recently, an intriguing phenotype was observed in IL-17A-deficient Apoe−/− (Il17a−/−Apoe−/−) mice that demonstrated no difference in plaque burden within the descending aorta or aortic roots of Il17a−/−Apoe−/− mice, but a decrease in aortic MΦ, CD11b+CD11c+ cell, and T cell cellularity.18 Thus the role of IL-17A in atherosclerosis is currently not well understood. The possibilities of site-specific effects of the IL-17A/IL-17RA axis on atherogenesis, on the regulation of the aortic immune content and the immune response within the aorta remain to be determined.
To investigate the involvement of IL-17A and IL-17RA in atherogenesis, we bred IL-17A-deficient and IL-17RA-deficient mice with Apoe−/− mice. Here we report that IL-17A and IL-17RA deficiency attenuates atherosclerosis by reducing the overall cellularity of aortas through decreased aortic chemokine-dependent monocyte and neutrophil homing to aortas. Importantly, we demonstrate that, at the time point studied, deficiency of the IL-17A/IL-17RA axis preferentially affects atherosclerosis and leukocyte cellularity within the aortic arch, but not the thoracoabdominal aorta.
Materials and Methods
Animals
Il17ra−/− mice (a kind gift from Amgen, Inc) and Il17a−/− mice (kindly provided by Dr. Y.Iwakura, University of Tokyo, Tokyo, Japan) on the C57/BL6 background were crossed with Apoe−/− mice to generate Il17ra−/−Apoe−/− and Il17a−/−Apoe−/− mice. Six week-old female and male Apoe−/−, Il17ra−/ Apoe−/−, and Il17a−/−Apoe−/− mice were fed a chow diet for 20 weeks or Western diet (21% fat and 0.15% cholesterol, Harlan Taklad, Harlan Laboratories) for 12 or 15 weeks and used at 18 or 21 weeks of age unless otherwise noted. All animals were kept in specific-pathogen-free conditions, and animal experiments were approved by the Eastern Virginia Medical School Animal Care and Use Committee.
An expanded Methods section is available in the Online Data Supplement at http://circres.ahajournals.org.
Results
Deficiency of IL-17A and IL-17RA reduces atherosclerosis in aortas of Apoe−/− mice
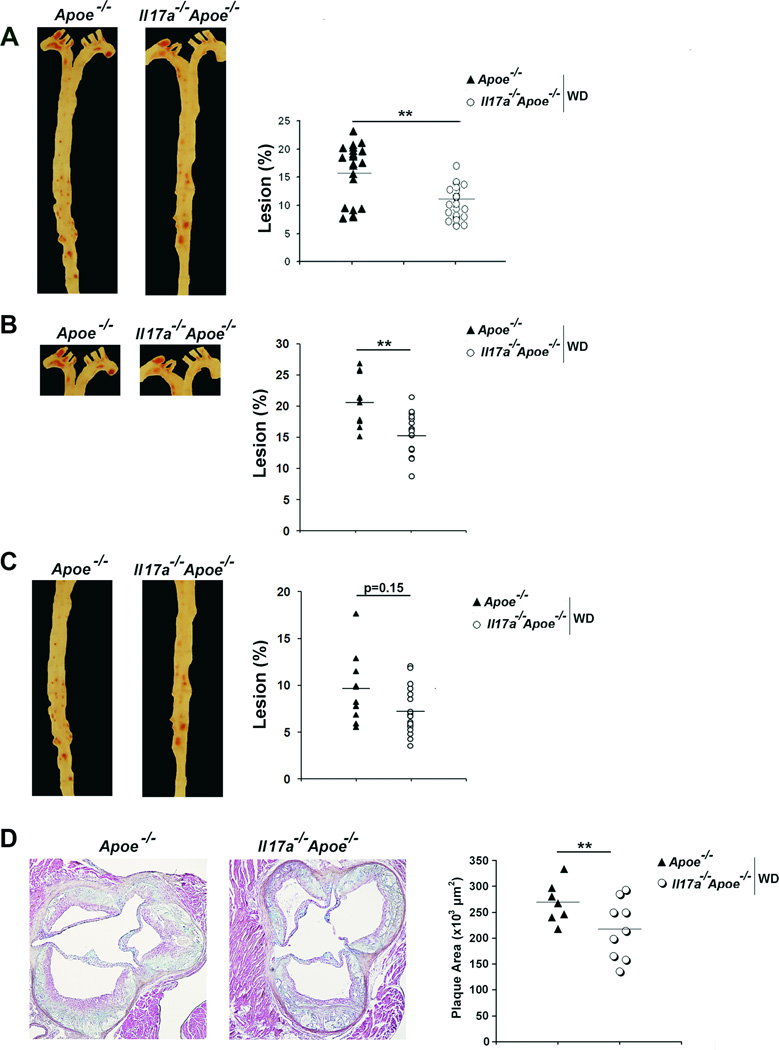
To directly assess the role of the IL-17A/IL-17RA axis in atherosclerosis, we generated Il17a−/−Apoe−/− and Il17ra−/−Apoe−/− mice. Il17a−/−Apoe−/−, Il17ra−/−Apoe−/−, and age and diet-matched Apoe−/− mice showed no difference in body weight, total cholesterol, and triglyceride levels (data not shown). The aortas of Il17a−/−Apoe−/− mice fed a Western diet (WD) for 15 weeks developed 35% smaller aortic lesions in comparison with Apoe−/− mice (Figure 1A). We also examined the aortic arches (Figure 1B) and thoracoabdominal aortas (Figure 1C) of Il17a−/−Apoe−/− and Apoe−/− mice separately. Plaque development was diminished within the aortic arch (Figure 1B), but not in the thoracoabdominal aortas (Figure 1C) of Il17a−/−Apoe−/− mice. We also detected a 19% reduction in aortic root plaque burden within Il17a−/−Apoe−/− mice in comparison with Apoe−/− mice (Figure 1D).
Fig.1. Deficiency of IL-17A attenuates atherosclerosis in Apoe−/− mice.
(A–C) Il17a−/−Apoe−/− and Apoe−/− mice were fed a WD for 15 weeks and assessed for atherosclerotic plaques (n=18 mice/group). (A) Representative en face Oil Red O staining and lesion sizes (% of whole aorta) from Il17a−/−Apoe−/− and Apoe−/− mice. (B) Representative en face staining and lesion sizes (% of whole aorta) of the aortic arch and (C) thoracoabdominal aortic segments of Il17a−/−Apoe−/− and Apoe−/− mice. (D) Representative Movat staining of Il17a−/−Apoe−/− and Apoe−/− aortic root sections (n=8–12). The data depicts the mean±SEM. Each symbol represents 1 animal; horizontal bars represent means. ***-P<0.001, **-P<0.01, *-P<0.05.
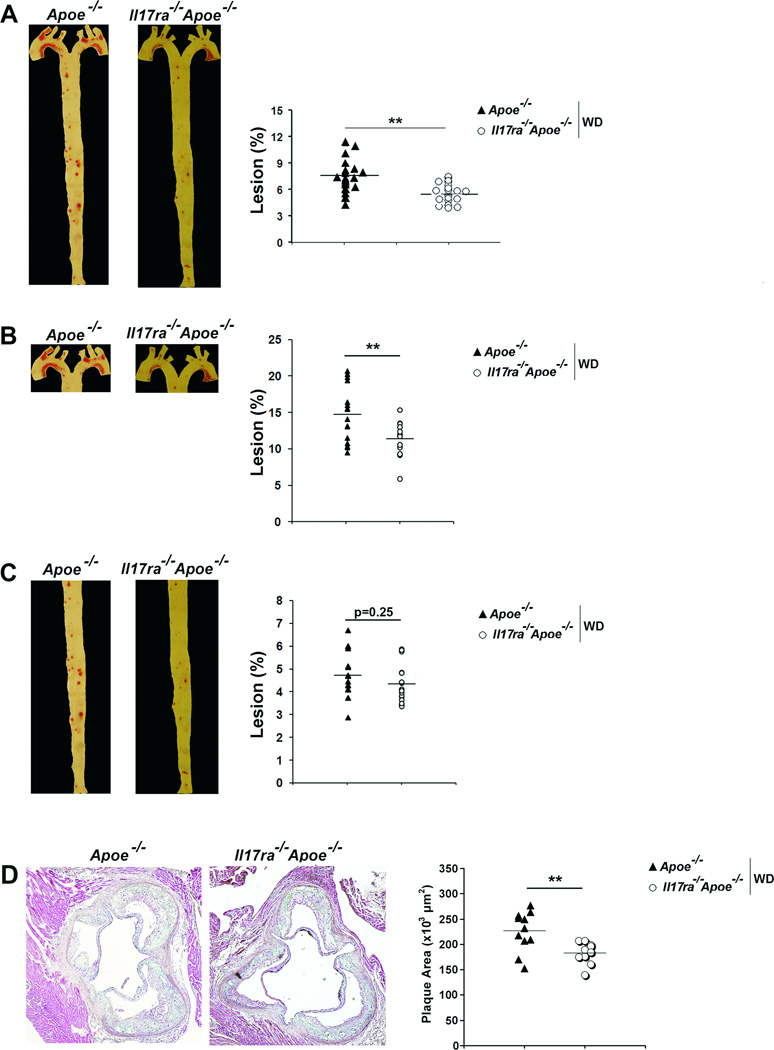
To further delineate the role of the IL-17A/IL-17RA axis in atherosclerosis, we examined atherogenesis in IL-17RA-deficient Apoe−/− mice. Il17ra−/−Apoe−/− mice developed 25% smaller lesions within whole aortas (Figure 2A) and aortic roots (Figure 2D) compared with Apoe−/− mice. IL-17RA deficiency resulted in reduced lesions within the aortic arch (Figure 2B), but not in the thoracoabdominal aortas (Figure 2C) of Il17ra−/−Apoe−/− mice. We also assessed the cross-sectional area of Il17ra−/−Apoe−/− and Apoe−/− aortic arch and thoracoabdominal aortic plaques. In agreement with the en face data, deficiency of IL-17RA yielded a 41% reduction in aortic arch lesions and had no effect on thoracoabdominal lesions (Figure 3A). Interestingly, aortic plaque burden throughout the aorta and within the aortic roots of Il17a−/−Apoe−/−, Il17ra−/−Apoe−/−, and Apoe−/− mice fed a 20 week chow diet were not significantly altered (4.3%±0.2, 4.2%±0.2, and 4.7%±0.5, n=12–14 mice/per group, p=0.3).
Fig.2. Deficiency of IL-17RA attenuates atherosclerosis in Apoe−/− mice.
(A–C) Il17ra−/−Apoe−/− and Apoe−/− mice (n=16 mice) were fed a WD for 12 weeks. (A) Representative Oil Red O staining and lesion sizes (% of whole aortas) of Il17ra−/−Apoe−/− and Apoe−/− aortas. (B) Representative aortic arch and (C) thoracoabdominal aortic segements from Il17ra−/−Apoe−/− and Apoe−/− mice. (D) Representative Movat staining of Il17a−/−Apoe−/− and Apoe−/− aortic root sections. The data depicts the mean±SEM. Each symbol represents 1 animal; horizontal bars represent means. ***-P<0.001, **-P<0.01, *-P<0.05.
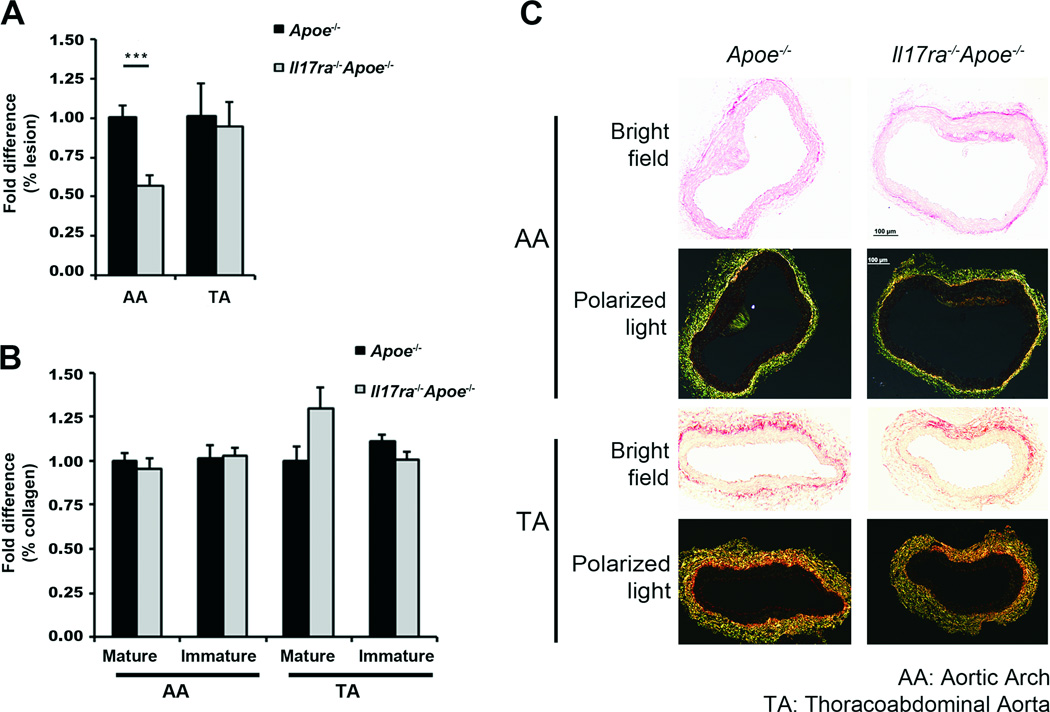
Fig.3. IL-17RA promotes lesion development in the aortic arch without affecting thoracoabdominal aortic lesions or collagen content.
(A) Average fold difference in aortic arch (AA) and thoracoabdominal aortic (TA) lesion area of 12 week WD Il17ra−/−Apoe−/− and Apoe−/− mice (% of aorta, n=6–8 mice/genotype). (B) Average fold difference of collagen maturation of Il17ra−/−Apoe−/− and Apoe−/− mice (% of aorta section, n=6–9). (C) Representative picrosirious red staining from the AA and TA segments of Il17ra−/−Apoe−/− and Apoe−/− mice (mature: red/orange, immature: yellow/green). The data depicts the mean±SEM. ***-P<0.001.
To further characterize the impact of the IL-17A/IL-17RA axis on lesion composition, we examined the collagen fiber content of the aortic arch and thoracoabdominal aortas of Il17ra−/−Apoe−/− and Apoe−/− mice using picrosirius red staining. The immature, mature, and total collagen fiber content was similar between Il17ra−/−Apoe−/− and Apoe−/− mice (Figure 3B and 3C). These results suggest that the IL-17A/IL-17RA pathway does not affect the maturation of aortic collagen after a 15 week WD regimen. To test the impact of the IL-17A/IL-17RA axis on aortic smooth muscle cell (SMC) content, we performed immunostaining for α-smooth muscle actin. The aortic arch and thoracoabdominal aortic SMC content of Il17ra−/− Apoe−/− and Apoe−/− mice were similar (aortic arch: 1.02±0.02 fold Il17ra−/−Apoe−/− vs Apoe−/−, 1.00±0.03 fold Apoe−/−vs Apoe−/−, p=0.80, thoracoabdominal aortas: 0.99±0.04 fold Il17ra−/−Apoe−/−vs Apoe−/−, 1.00±0.02 fold Apoe−/− vs Apoe−/−, p=0.89), suggesting that IL-17A does not significantly affect total aortic SMC content.
Disruption of the IL-17A/IL-17RA axis decreases the cellularity of atherosclerotic aortas
The development of atherosclerosis is accompanied by marked recruitment of leukocytes into the aortic wall.19 As IL-17A supports the induction of epithelial6,20 and vascular11–13,21 chemokines, we assessed the effects of IL-17A/IL-17RA on the cellularity and immune cell composition of the aorta during atherogenesis. We detected a 50% reduction in CD45+ leukocytes and CD3+ T cells within the whole aortas of 15 week WD-fed Il17a−/−Apoe−/− and Il17ra−/−Apoe−/− mice compared with Apoe−/− mice (Figure 4A). Next, to determine if disruption of the IL-17A/IL-17RA axis alters the aortic T cell response, we examined Th1 and Th17 cells within the aortas of Il17a−/−Apoe−/−, Il17ra−/−Apoe−/−, and Apoe−/− mice. While the relative percentage of IFNγ+ cells was unaltered (Figure 4C), the overall number of Th1 cells were significantly diminished in Il17ra−/−Apoe−/− and Il17a−/−Apoe−/− aortas (Figure 4D). In agreement with these data, we detected a significant reduction in aortic Ifng, Il6, and Tnfα mRNA expression (Figure 4B). Interestingly, deficiency of IL-17RA in Il17ra−/−Apoe−/− mice had no effect on the percentage (Figure 4C), total number of aortic IL-17A+ T cells (Figure 4D), or the percentage of IL-17A+ T cells within the spleen, peripheral lymph nodes and blood (data not shown) compared to Apoe−/− mice.
Fig.4. Disruption of the IL-17A/IL-17RA axis decreases the overall T cell content of the aortic arch, but not the thoracoabdominal aorta.
(A) Aortic cell suspensions from 15 week WD fed Il17a−/−Apoe−/−, Il17ra−/−Apoe−/−, and Apoe−/− mice were stained and analyzed by flow cytometry (n=7 mice/genotype, five independent experiments; white bars-Il17a−/−Apoe−/−, gray bars-Il17ra−/−Apoe−/−, and black bars-Apoe−/− mice). (B) mRNA expression of Il6, Ifnγ, and Tnf from the aortas Il17a−/−Apoe−/−, Il17ra−/−Apoe−/−, and Apoe−/− mice (n=12 aortas/genotype, three independent experiments). (C) Representative flow cytometry plots of IFNγ+CD3+ and IL-17A+CD3+ cells in the aortas of 15 week WD fed Il17a−/−Apoe−/−, Il17ra−/−Apoe−/−, and Apoe−/− mice. Plots are gated on CD45+CD3+ T cells (n=5 mice/genotype, four experiments). (D) Total number of IFNγ+CD3+ and IL-17A+CD3+ cells in the aortas of Il17a−/−Apoe−/−, Il17ra−/−Apoe−/−, and Apoe−/− mice. (E–F) Aortic arch and thoracoabdominal aortic cell suspensions from Apoe−/−, Il17a−/−Apoe−/−, and Il17ra−/−Apoe−/− mice were stained with anti-CD45 and TCRαβ Abs (n=9 aortas/genotype, four independent experiments). (E) Representative flow cytometry plots from Apoe−/−, Il17a−/−Apoe−/−, and Il17ra−/−Apoe−/− aortic arch and thoracoabdominal aortas. (F) Total number of aortic arch and thoracoabdominal aortic TCRαβ T cells from Apoe−/−, Il17a−/−Apoe−/−, and Il17ra−/−Apoe−/− mice. The data depicts means±SEM. ***-P<0.001, **-P<0.01, *-P<0.05.
To determine if the reduction of aortic leukocytes, like the reduction of plaques, occurred specifically in the aortic arch, the total CD45+ leukocyte cellularity of Il17a−/−Apoe−/− and Il17ra−/−Apoe−/− aortic segments was determined. The number of CD45+ leukocytes was significantly reduced in Il17a−/−Apoe−/−, Il17ra−/−Apoe−/− aortic arches in comparison to Apoe−/− segments (1.5±0.1×105, 1.2±0.1×105, 2.4±0.2×105 cells/aortic arch, respectively, p<0.01). Deficiency of IL-17A or IL-17RA significantly reduced the T cell content of Il17a−/−Apoe−/− and Il17ra−/−Apoe−/− aortic arches (Figure 4E and 4F). In contrast, we found no difference in the numbers of T cells isolated from the thoracoabdominal aortas of Il17a−/−Apoe−/− and Il17ra−/−Apoe−/− mice compared to Apoe−/− controls (1.5±0.2×105, 1.4±0.2×105, 1.3±0.1×105 cells/thoracoabdominal aorta, respectively, p=0.7, Figure 4E and 4F).
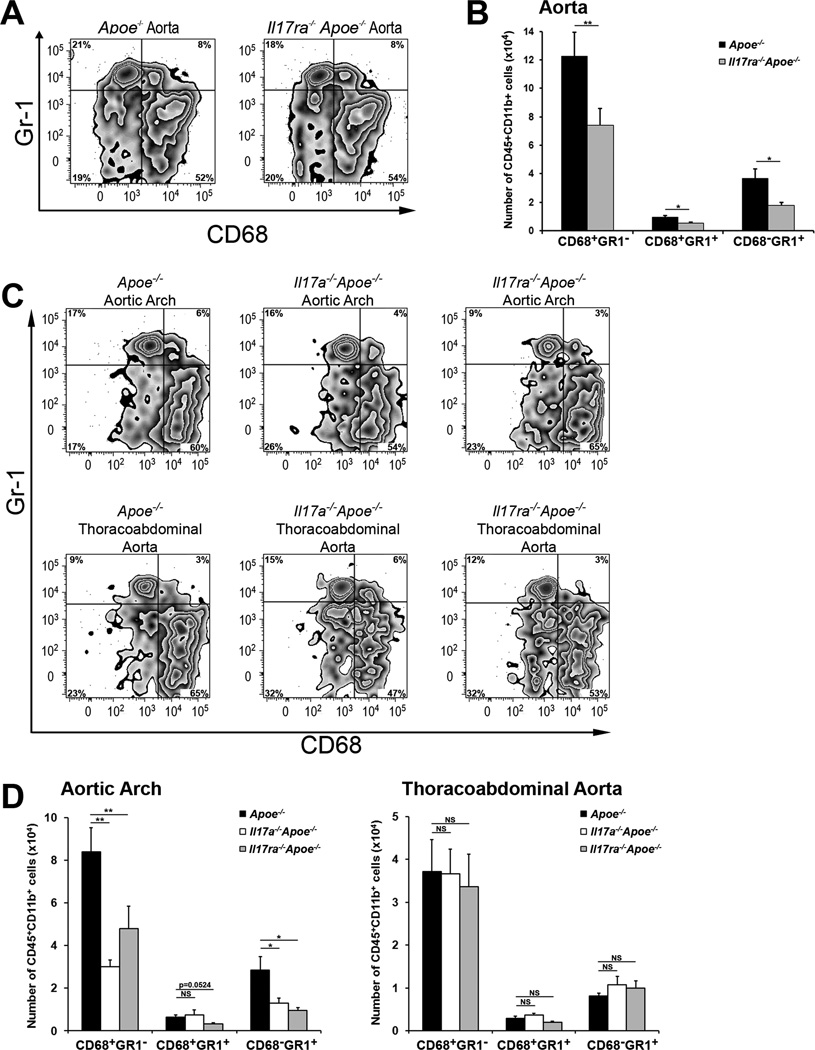
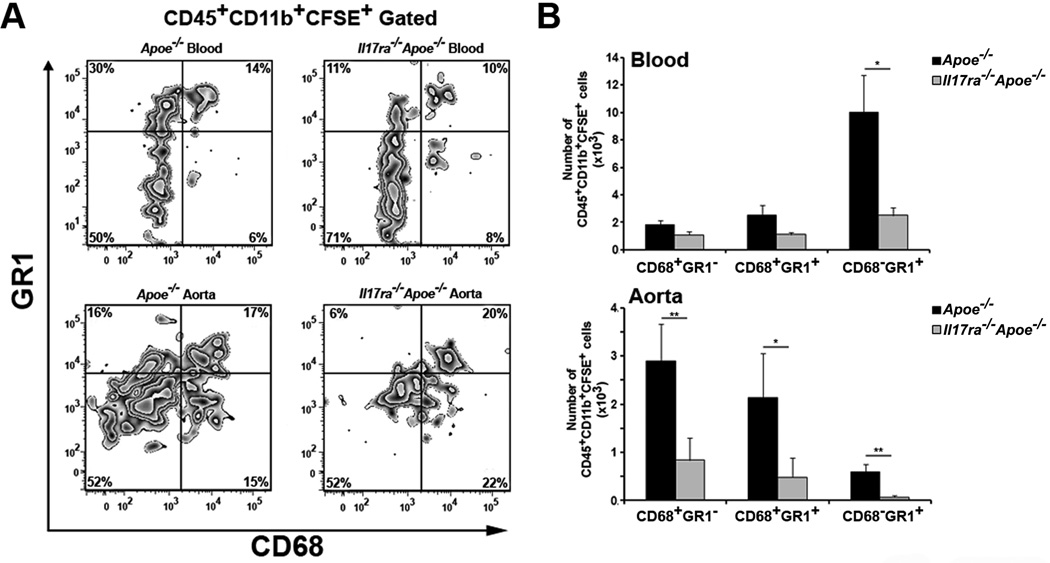
To further understand the role of IL-17A/IL-17RA in the regulation of aortic leukocyte numbers, we next examined the myeloid cell content of the aortas, PLNs, spleens and blood of WD fed Il17a−/−Apoe−/−, Il17ra−/−Apoe−/−, and Apoe−/− mice. CD45+CD11b+ cells were notably reduced in Il17a−/−Apoe−/− and Il17ra−/−Apoe−/− aortas compared to Apoe−/− aortas (2.6±0.4×105, 2.3±0.4×105, and 4.1±0.8×105 cells, respectively, p<0.05). To examine myeloid cell subsets, we performed staining for CD68 and the neutrophil/monocyte marker Gr-1, which recognizes the Ly6C and Ly6G sites. We found three major populations: CD68+Gr-1− MΦ, CD68−Gr-1+ neutrophils and a subset of CD68+Gr-1+ leukocytes within the aortas (Figure 5). To better characterize CD68+Gr-1+ cells, we analyzed the expression of CCR1, L-selectin, and Ly6C. CD68+Gr1+ cells were found to express CCR1, L-selectin and intermediate levels of LyC6 (Online Figure I). While the two major subsets of monocytes: pro-inflammatory (Ly-6Chi Gr1+CCR2+CX3CR1low) and patrolling (Ly-6Clo Gr1−CCR2−CX3CR1hi) monocytes, are well characterized, little is known about Ly6Cinterm cells. Our results suggest that Ly6Cinterm cells are CD68+Gr-1+ with some features (L-selectin+CCR2+) of pro-inflammatory monocytes (Online Figure I). Although the relative percentages of aortic MΦ and neutrophils were unaltered between Il17ra−/−Apoe−/− and Apoe−/− mice (Figure 5A), we found a significant decrease in the number of aortic CD68+Gr-1− MΦ, CD68+Gr-1+ leukocytes and CD68−Gr-1+ neutrophils in Il17ra−/−Apoe−/− aortas (Figure 5B). There was no difference in splenic or peripheral blood myeloid cell subsets in Il17ra−/−Apoe−/− and Apoe−/− mice (unpublished data).
Fig.5. Disruption of the IL-17A/IL-17RA axis decreases MΦ and neutrophil cellularity in the aortic arch, but not in the thoracoabdominal aorta.
(A–B) 15 week WD Il17a−/−Apoe−/−, Il17ra−/−Apoe−/−, and Apoe−/− aortic cell suspensions were stained and analyzed by flow cytometry (n=9 mice/genotype). (A) Representative flow cytometry plots are gated on CD45+CD11b+ cells (n=9 mice/genotype, seven independent experiments). (B) The total number of CD68+ MΦ, CD68+Gr-1high leukocytes and Gr-1+CD68− neutrophils were decreased in the aortas of WD fed Il17ra−/−Apoe−/− (grey bars) compared with Apoe−/− (black bars) mice. (C) Representative flow cytometry plots are gated on CD45+CD11b+ cells (n=9 mice/genotype, four independent experiments). (D) Total numbers of aortic arch (left) and thoracoabdominal aortic (right) CD68+Gr1− MΦ, CD68+Gr1+ leukocytes, and CD68−Gr1+ neutrophils (white bars-Il17a−/−Apoe−/−, gray bars-Il17ra−/−Apoe−/−, and black bars-Apoe−/− mice). The data depicts means±SEM. ***-P<0.001, **-P<0.01, *-P<0.05.
To determine if the IL-17A/IL-17RA dependent-reduction of MΦ and neutrophils occurred predominantly in the aortic arch, the aortic arches and thoracoabdominal aortas of Il17a−/−Apoe−/−, Il17ra−/−Apoe−/− and Apoe−/− mice were assessed by flow cytometry. We found distinct effects of IL-17A and IL-17RA deficiency on the myeloid cell content in the aortic arch, but not in the thoracoabdominal aortas of Il17a−/−Apoe−/− and Il17ra−/−Apoe−/− mice. While the relative percentage of MΦ, and neutrophils were unaltered (Figure 5C), the total number of aortic arch MΦ and neutrophils were reduced by ~44% in Il17ra−/−Apoe−/− and Il17a−/−Apoe−/− mice (Figure 5D). These findings further implicate the IL-17A/IL-17RA axis as a regulator of aortic myeloid cell content. Interestingly, despite the prevalent effects of IL-17RA deficiency within the aortic arch, the reduction of MΦs in the aortic arch (~44%, Figure 5D,) was smaller in comparison to MΦ reduction in the whole aorta (~63%, Figure 5B) of Il17ra−/−Apoe−/− mice suggesting that other pro-atherogenic factors, in addition to MΦ, are regulated by the IL-17A/ IL-17RA axis within the aortic arch.
IL-17A/IL-17RA supports TNFα and aortic chemokine production within the aortic arch of Apoe−/− mice
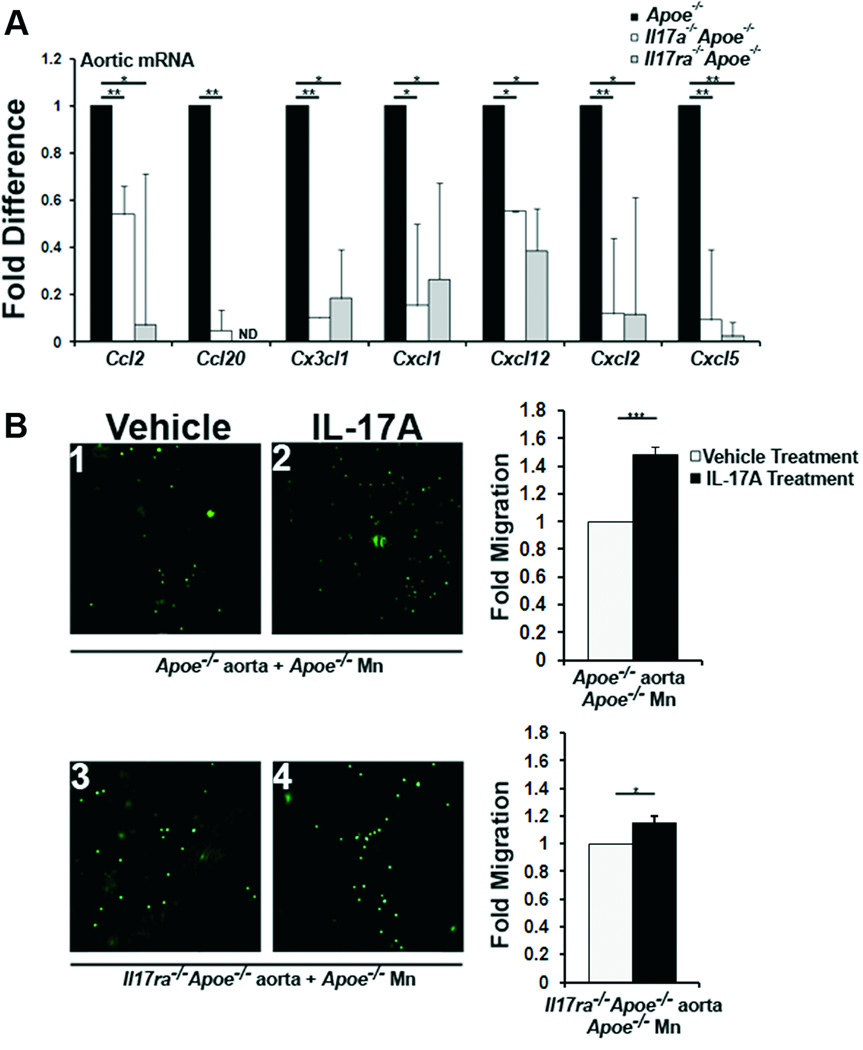
As IL-17A induces the production of reactive oxygen species (ROS),22 pro-inflammatory chemokines, and cytokines at sites of inflammation, including the vasculature11–13,21, we hypothesized that IL-17A may potentially regulate the homing of leukocytes to atherosclerotic aortas. In keeping with these findings, we evaluated aortic chemokine expression in Il17ra−/−Apoe−/−, Il17a−/−Apoe−/−, and Apoe−/− mice (Figure 6A). Ccl2, Ccl20, Cx3cl1, Cxcl1, Cxcl12, Cxcl2, and Cxcl5 mRNA expression was reduced in WD fed Il17a−/−Apoe−/− and Il17ra−/−Apoe−/− aortas compared with Apoe−/− aortas (Figure 6A), indicating that the IL-17A/IL-17RA axis regulates the expression of several chemokines within the aorta. As CCL2 and CX3CL1 support monocyte migration,23 and CXCL1, CXCL2 and CXCL5 participate in neutrophil recruitment into the aorta,24 these data implicate the IL-17A/IL-17RA axis as a strong potential regulator of monocyte and neutrophil migration via aortic chemokine induction.
Fig.6. Vascular IL-17RA supports monocyte recruitment in an IL-17A dependent manner.
(A) mRNA expression of chemokines in 15 week WD fed Il17a−/−Apoe−/−, Il17ra−/−Apoe−/−, and Apoe−/− aortas (n=12 aortas/genotype, three independent experiments). (B) Aortas from Apoe−/− and Il17ra−/−Apoe−/− mice were treated with 100 ng/ml IL-17A (black bars) or a vehicle (white bars). CFSE-labeled Apoe−/− monocytes (green) were added to aortas for an adhesion assay, and adherent monocytes were counted (n=5 experiments/condition).
Monocytes express IL-17RA, IL-17RC and chemotactically migrate towards IL-17A both in vitro and in vivo models of rheumatoid arthritis.25,26 We previously found that IL-17A supported monocyte adherence to explanted Apoe−/− aortas in an ex vivo adhesion assay.13 To further determine the role of vascular IL-17RA in monocyte adhesion, whole aortas from WD fed Il17ra−/−Apoe−/− mice were explanted and co-incubated with CFSE-labeled Apoe−/− monocytes in the presence or absence of IL-17A (Figure 6B) to assess the contribution of aortic IL-17RA to IL-17A/IL-17RA-dependent monocyte adherence. Consistent with our prior data,13 IL-17A increased the adhesion of Apoe−/− monocytes to Apoe−/− aortas by 48±4% (Figure 6B1 and 6B2). Co-cultures of Apoe−/− monocytes and Il17ra−/−Apoe−/− aortas resulted in a 13±5% (p<0.05) increase in adhesion in response to IL-17A (Figure 6B3 and 6B4). Thus as IL-17A-induced adhesion was significantly reduced when IL-17RA-deficient Apoe−/− aortas were used (Figure 6B), vascular IL-17RA likely supports monocyte adherence to the aortic lumen.
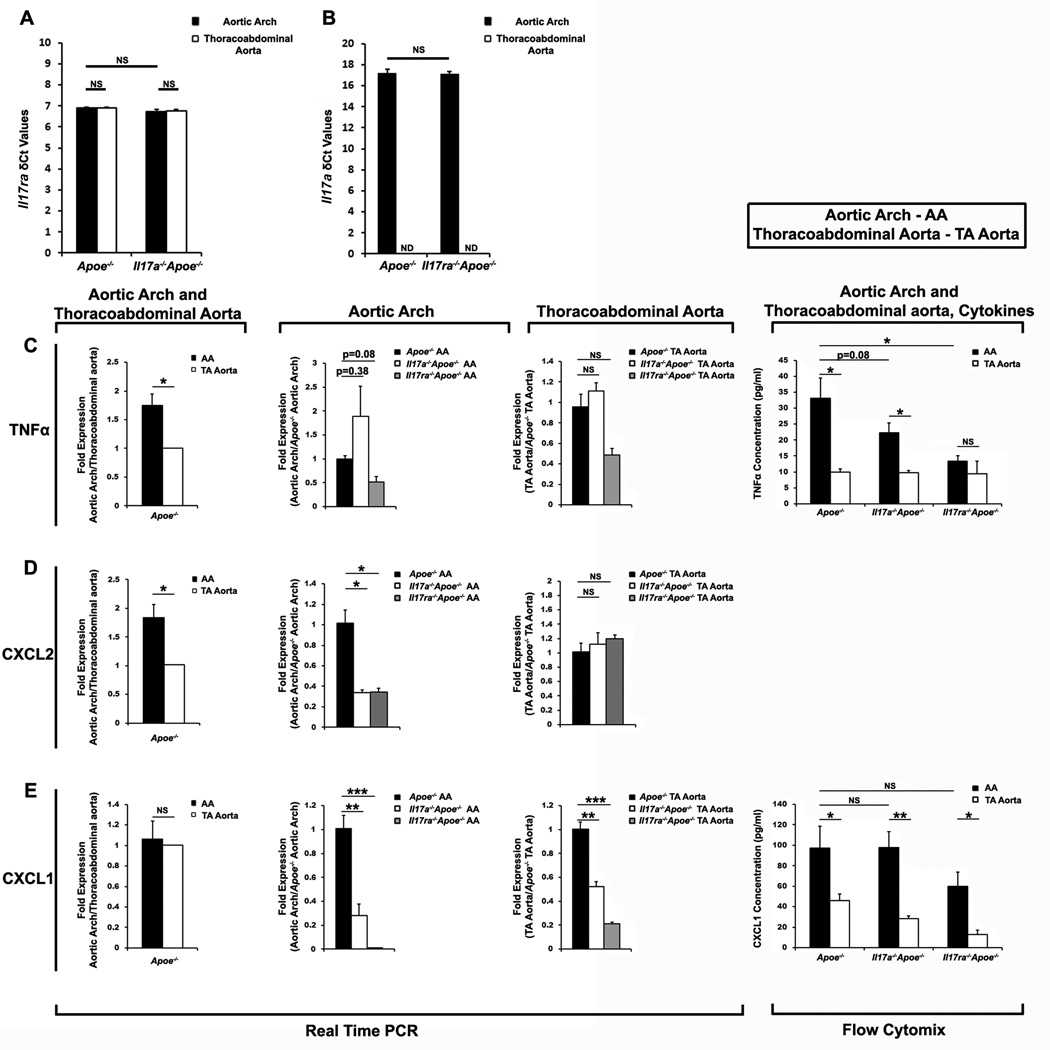
To further understand the potential effects of the IL-17A/IL-17RA axis in leukocyte recruitment into the aorta, we next examined the expression of IL-17A, IL-17RA and several chemokines within the aortic arch and thoracoabdominal aortas of Il17ra−/−Apoe−/− or Il17a−/−Apoe−/−, and Apoe−/− mice. While IL-17RA mRNA was expressed throughout the aorta with no significant differences between the aortic arch and thoracoabdominal aorta (Figure 7A), IL-17A was preferentially detected within the aortic arch of Apoe−/− mice (Figure 7B). These data highlight the distinct arch-specific expression of IL-17A and suggest a reason for the preferential effects of IL-17A/IL-17RA deficiency on the aortic arch at this time point. Additionally, we detected elevated levels of TNFα and CXCL2 in Apoe−/− aortic arches in comparison to Apoe−/− thoracoabdominal aortas (Figure 7C and D, respectively).
Figure 7. Aortic arch IL-17A affects the regional production of pro-inflammatory TNF-α, Cxcl1, and Cxcl2.
mRNA expression levels (δCt) of Il17ra (A) and Il17a (B) within the aortic arch (AA) and thoracoabdominal (TA) aortas of 12 week WD-fed Apoe−/− and Il17ra−/−Apoe−/− mice (A) or Apoe−/− and Il17ra−/−Apoe−/− mice (B), (n=9 mice/genotype, 3 independent experiments). (C–E) Combined aortic arch (AA) and thoracoabdominal (TA) aortic mRNA expression and protein levels of TNFα (C), CXCL2 (D), CXCL1 (E) within 12 week WD-fed Apoe−/−, Il17a−/−Apoe−/−, and Il17ra−/−Apoe−/− mice. RT-PCR: n=9 mice/genotype, 3 independent experiments; Flow cytomix: n=6 mice/genotype, two independent experiments.
Next, we investigated the effects of IL-17A or IL-17RA deficiency on the expression of TNFα and several chemokines within the aortic arch and thoracoabdominal aorta. Deficiency of IL-17A or IL-17RA led to reduced TNFα protein expression in the aortic arch, without affecting the thoracoabdominal aorta, suggesting that the IL-17A/IL-17RA axis is a regulator of TNFα synthesis in atherosclerosis-prone sites of the aorta (Figure 7C). Deficiency of IL-17A or IL17RA also reduced Cxcl2 expression within the aortic arch compared with Apoe−/− mice (Figure 7D). In contrast, no difference in Cxcl2 expression was detected between Il17a−/−Apoe−/−, Il17ra−/−Apoe−/− and Apoe−/− thoracoabdominal aortas (Figure 7D). Together, these data highlight an important role of the IL-17A/IL-17RA axis in the upregulation of TNFα and several chemokines within the aortic arch during atherogenesis. Interestingly, we also found that not all chemokines were regulated by the IL17A/IL-17RA axis exclusively in the aortic arch. Cxcl1 mRNA expression, but not CXCL1 protein expression was attenuated in the aortic arches of Il17a−/−Apoe−/−, Il17ra−/−Apoe−/− compared with Apoe−/− mice (Figure 7E).
Recruitment of neutrophils and monocytes to atherosclerotic aortas is reduced in IL-17A- or IL-17RA- deficient Apoe−/− mice
To investigate the effects of the IL-17A/IL-17RA axis on in vivo monocyte and neutrophil homing into aortas, short-term adoptive transfer experiments were performed. CFSE-labeled peripheral blood leukocytes were adoptively transferred into Il17ra−/−Apoe−/− and Apoe−/− mice to directly investigate the migration of monocytes and neutrophils in the recipients. To identify subsets of CD45+CFSE+CD11b+ cells containing CD68+Gr-1− monocytes, Gr-1+CD68+ leukocytes and Gr-1+CD68− neutrophils, circulating blood was used to characterize myeloid cell populations and analyze the distribution of emigrating cells in the aortas (Figure 8A). Although the percentage of emigrated CFSE+CD11b+ monocytes and neutrophils was unaltered between Il17ra−/−Apoe−/− and Apoe−/− aortas, (Figure 8A) the absolute number of CD45+CD11b+CFSE+ CD68+Gr-1− MΦ, Gr-1+CD68− neutrophils, and Gr-1+CD68+ leukocytes was significantly lower in the aortas of Il17ra−/−Apoe−/− recipients (Figure 8B). Interestingly, while the absence of IL-17RA reduced monocyte and neutrophil homing into the aortas, it did not alter myeloid cell trafficking into the spleen and PLN (unpublished data). The number of circulating CFSE+ monocytes in the blood were unaltered between the recipients, however, the number of circulating CFSE+ Gr-1+CD68− neutrophils and Gr-1+CD68+ leukocytes were slightly decreased in Il17ra−/−Apoe−/− recipients (P<0.05, Figure 8B). To assess the role of IL-17RA in the migration of myeloid cells to the aorta, we performed competitive homing experiments to examine the migration of IL-17RA-deficient and IL-17RA-sufficient monocytes and neutrophils to Apoe−/− aortas. We found no difference between the migration of Il17ra−/−Apoe−/− and Apoe−/− monocytes and neutrophils to the aortas of Apoe−/− recipients (unpublished data). Overall these in vitro and in vivo results suggest that the IL-17A/IL-17RA axis plays a pro-inflammatory role during atherogenesis through the induction of aortic chemokines and the recruitment of neutrophils and monocytes to atherosclerotic plaque
Fig. 8. Reduced recruitment of neutrophils and monocytes to atherosclerotic plaques of Il17a−/−Apoe−/− and Il17ra−/−Apoe−/− mice.
(A–B) Apoe−/− peripheral blood leukocytes were adoptively transferred to 15 week WD-fed recipient Apoe−/− and Il17ra−/−Apoe−/− mice. After 12 hours, recipient aortas, blood and spleens and PLNs (not shown) were stained for CD45, CD11b, CD68, Gr-1 and evaluated by flow cytometry. (A) Representative flow cytometry plots of emigrated myeloid cells: MΦ/monocytes (CD68+Gr-1−), CD68+Gr-1+ cells, and neutrophils (CD68−Gr-1+). Numbers in quadrants are a percentage of positive cells. All plots are gated on CD45+CD11b+CFSE+ cells. (B) Homing of Apoe−/− leukocytes (as numbers of CD45+CD11b+CFSE+ cells) in blood and the aortas of Il17ra−/−Apoe−/− (gray bars, n=11) and Apoe−/− (back bars, n=11) recipients from 5 independent experiments. The data depicts means±SEM. ***-P<0.001, **-P<0.01, *-P<0.05.
Discussion
The development and persistence of atherosclerosis depends on chronic inflammation mediated by both the innate and adaptive immune responses. Several recent publications have convincingly shown elevated levels of circulating and arterial Th17 and IL-17A+ T cells in atherosclerosis-prone Apoe−/− and Ldlr−/− mice and CAD patients.3 Despite a clear correlation between elevated levels of IL-17A and atherosclerosis, to date, the functions of IL-17A-producing cells and IL-17A in atherosclerosis remain poorly defined.
In this study, we sought to delineate the role of IL-17A+ cells and the IL-17A/IL-17RA pathway in atherosclerosis through the use of newly generated Il17a−/−Apoe−/− and Il17ra−/−Apoe−/− mice. We previously reported a reduction in atherosclerotic plaques in soluble IL-17RA blockaded Apoe−/− mice.13 In the present study, we expanded our focus and investigated the impact of IL-17A or IL-17RA deficiency on atherogenesis. We found a significant decrease in the aortic lesions of WD, but not chow diet fed Il17a−/−Apoe−/− and Il17ra−/−Apoe−/− mice compared with Apoe−/− mice. To further dissect the roles of the IL-17A/IL-17RA axis, several potential sites of atherogenesis including the aortic arch, thoracoabdominal aorta, and aortic roots of mice fed WD were examined. IL-17A/IL-17RA deficiency had striking inhibiting effects on atherosclerotic plaque burden in the aortic arch, but only minor effects within the thoracoabdominal compartment. The observation that IL-17A exerts limited effects on the descending aorta is in line with the findings of Madhur et.al. that IL-17A-deficient Apoe−/− mice display slight non-significant reductions in thoracoabdominal aortic plaques in comparison to Apoe−/− controls.18 There are several examples of the site-specific development and progression of atherosclerosis.28 Deficiency of IL-4 within the bone marrow resulted in no change in aortic-root lesion area, despite reduced en face lesion areas.29 The deficiency of p47 NADPH oxidase subunit in Apoe−/− mice reduced aortic atherosclerosis to a greater extent than the aortic root.30 In the present study, we characterized the aortic arch-specific influence of the IL-17A/IL-17RA axis.
Histological examination of the aortic arch and thoracoabdominal aortic collagen content surprisingly revealed no differences between Il17ra−/−Apoe−/− and Apoe−/− mice at this time point. Interestingly, we also found no difference in SMC content between Il17ra−/−Apoe−/− and Apoe−/− mice, indicating that the IL-17A/IL-17RA axis does not significantly influence SMC proliferation and migration within the atherosclerotic aorta. While several recent studies have suggested that IL-17A may support SMC proliferation in vitro,11,31 Danzaki, et. al. demonstrated elevated aortic root SMC content in 8 week WD-fed Il17a−/−Apoe−/− mice.32 Further examination of the effects of the IL-17A/IL-17RA axis on aortic SMC and endothelial cell functions are ultimately necessary.
One of the striking observations of this study was the differences in atherosclerotic lesions between the aortic arch and thoracoabdominal aorta of 15 week WD-fed mice. To assess the potential mechanisms for this phenomenon, we analyzed the expression of IL-17A and IL-17RA at these different anatomical sites of the aorta. IL-17RA was expressed ubiquitously in the aorta, whereas IL-17A expression was preferentially found within the aortic arch. Interestingly, we detected no difference in the expression of IL-17A in the TA and AA in 5 week-old Apoe−/− mice, indicating that the specific up-regulation of IL-17A does not occur in relatively healthy aortas. Altogether, our findings suggest that the aortic expression of IL-17A may depend on the stage of lesion development, which varies at different anatomical locations. The stage of lesion development also depends on the local inflammation that accompanies atherogenesis. Indeed, elevated expression of the pro-inflammatory cytokine TNFα and several chemokines that are involved in aortic leukocyte migration were preferentially detected in the aortic arches of 15 week WD-fed Apoe−/− mice. Further temporal studies will be required to determine if IL-17A-producing cells may be similarly recruited into advanced thoracoabdominal aortas.
To date, the exact role of IL-17A+ cells in atherosclerosis is unclear due to conflicting results from neutralizing antibody and bone marrow transfer experiments.12–14,16–18,27 However, some of the discrepant results may be attributable to differences in the experimental design, diets, or other confounding factors. In addition, most of these studies have examined aortic root atherosclerosis, but only few studies directly examined whole aortic plaque burden by en face analysis. The results reported here clearly demonstrate the aortic arch-specific effects of IL-17A/IL-17RA, and at least partially shed light on the controversy of reported effects of IL-17A/IL-17R axis blockade during atherogenesis.
Th17 cells play an important role in the immune response, and are major contributors to autoimmune diseases such as multiple sclerosis, inflammatory bowel disease and arthritis.5 There are several pathways by which IL-17A-producing T cells might affect local inflammation. IL-17A supports the production of IL-6 and IL-8, and the chemokines CCL5, CCL2, CXCL1 and CXCL10 in several cell types, including endothelial and vascular smooth muscle cells (VSMCs),33,34 fibroblasts and epithelial cells.6,20 The results reported here clearly demonstrate that the IL-17A/IL-17RA axis affects the expression of multiple aortic chemokines, including Ccl2, Ccl20, Cx3cl1, Cxcl1, Cxcl12, Cxcl2, and Cxcl5, thus accelerating leukocyte recruitment to atherosclerotic vessels.
In support of this notion, we detected reduced numbers of T cells, CD68+Gr-1− MΦ, CD68+Gr-1+ myeloid cells, and CD68−Gr-1+ neutrophils in WD fed Il17ra−/−Apoe−/− and Il17a−/−Apoe−/− aortas at steady-state conditions. Interestingly, the phenotype of reduced aortic MΦ content was also reported when anti-IL-17A Abs were used to block IL-17A functions in vivo.12,16 Importantly, additional separate examination of the aortic arch and thoracoabdominal segments of Il17ra−/−Apoe−/−, Il17a−/−Apoe−/−, and Apoe−/− aortas by flow cytometry revealed diminished numbers of T cells, MΦ, and neutrophils specifically within the aortic arches of Il17ra−/−Apoe−/− and Il17a−/−Apoe−/− mice. Thus, these results clearly emphasize a distinct role for the IL-17A/IL-17RA axis in the regulation of the number of T and myeloid cells within the aortic arch, and to a lesser extent, the thoracoabdominal aorta. It is interesting to note that the reduction in leukocyte cellularity within the aortic arch was relatively smaller compared with the reduction in plaque burden, suggesting that other IL-17/IL-17RA-dependent factors in addition to the total cellularity are involved in atherogenesis.
Monocytes express IL-17RA, and recent data suggests that IL-17A can also directly affect monocyte chemotaxis in vivo and in vitro.25 Antibody blockade of IL-17A in the synovial fluid of rheumatoid arthritis patients inhibited in vitro monocyte chemotaxis, and MΦ accumulation in the bronchoalveolar lavage fluid during allergic airway inflammation.25 Lethally irradiated low density lipoprotein receptor-deficient (Ldlr−/−) mice reconstituted with IL-17RA-deficient bone marrow resulted in reduced aortic root lesions, neutrophil, and mast cell content in comparison to Il17ra+/+ recipients suggesting a potential role of IL-17RA on hematopoetic populations during atherogenesis.27 Evidence have also demonstrated that vascular ECs21 and SMCs11,12,22 express IL-17RA, and are able to respond to IL-17A. To address the extent to which IL-17RA expression by vascular cells impacts IL-17A-induced adhesion to atherosclerotic aortas, we performed ex vivo adhesion assays. Supplementation of explanted aortas with rIL-17A strongly supported monocyte adherence in a manner that depended on vascular IL-17RA.
The recruitment of leukocytes into the aortas during the initial and established stages of atherosclerosis is one of the key components of the progression of atherosclerosis. To further establish the role of IL-17A in the regulation of leukocyte content in the aortas, we determined whether the IL-17A/IL-17RA axis affects the migration of monocytes and neutrophils to the aortas in short-term adoptive transfer experiments. We detected significant reductions of CFSE+ emigrated Apoe−/− monocytes and neutrophils within the aortas of Il17ra−/−Apoe−/− recipients highlighting an essential role of vascular IL-17RA in supporting myeloid cell migration. Of note, we found no difference in the migration of Il17ra−/−Apoe−/− and Apoe−/− monocytes and neutrophils to Apoe−/− aortas in the short-term homing experiments. These findings suggest that the reduction of monocyte and neutrophil content of the aorta in steady-state conditions is due to impaired capacity of myeloid cells to migrate into atherosclerotic aortas in the absence of IL-17A or vascular IL-17RA. Interestingly, as studies involving chemokine or chemokine receptor-deficient mice display distinct anatomical alterations in atherosclerotic plaque formation, the overt phenotype of Il17a−/−Apoe−/− and Il17ra−/−Apoe−/− mice can be partially attributed to the regulation of multiple aortic chemokines by IL-17A.
While several studies have suggested a pro-atherogenic role of IL-17A based on neutralizing strategies,12,13,16 and bone marrow transfers,27 Taleb, et al.4 and Danzaki, et al.32 recently proposed an atheroprotective role for IL-17A+ T cells through cross-regulation of Th1 cells and IFNγ. Danzaki, et. al. demonstrated elevated Th1 cell content and IFNγ production in 8 week WD-fed Il17a−/−Apoe−/− splenocytes.32 Although initial in vitro differentiation assays have implicated cross-regulation of Th17 cell differentiation by Th1 cells,5 recent data have shown that Th17 cells are induced in parallel to Th1 cells in some pathological conditions.3 Indeed, while Th1 cells represent the major T helper subset in atherosclerosis, both Th17, Th1, and Th1/Th17 cells are present in both murine and human atherosclerotic arteries.3,11–13,27 Therefore, we tested if the deficiency of IL-17A or IL-17RA would alter the Th1 response during atherogenesis. While the percentage and number of splenic and peripheral blood Th1 cells were unaltered, the numbers of aortic Th1 cells were diminished within Il17a−/−Apoe−/− and Il17ra−/−Apoe−/− mice. These data indicate that the deficiency of IL-17A or IL-17RA has no effect on the generation and maintenance of Th1 cells in atherosclerosis. Altogether, our data suggest that while the IL-17A/IL-17RA axis does not affect the percentage of Th1 cells, it does influence the number of aortic T cells and therefore, the total levels of T cell-derived IFNγ.
In summary, using IL-17A and IL-17RA deficient Apoe−/− mice, we demonstrate that the IL-17A/IL-17RA pathway plays a pro-inflammatory role during atherogenesis preferentially within the aortic arch. Upon atherogenesis, Th17 and other IL-17A-producing cells accumulate within the aortas and release IL-17A, which in turn, induces the production of TNFα and various chemokines - resulting in accelerated monocyte and neutrophil homing and further development of atherosclerosis preferentially within the aortic arch of the aorta.
Novelty and Significance.
What is known?
Elevated levels of Interleukin-17A (IL-17A), a hallmark cytokine of T helper 17 and IL-17A+ γδ T cells, are detectable within murine and human atherosclerotic plaques as well as the plasma of patients with coronary artery disease and artery disease (post-endarterectomy).
Attempts to neutralize IL-17A via neutralizing antibodies, decoy receptors, and bone marrow transfer have yielded inconsistent results, suggesting both pro- and anti-atherogenic roles for IL-17A.
Recent studies with Il17a−/−Apoe−/− mice revealed slight changes in aortic root and thoracoabdominal aortic lesions, despite a favorable trend towards decreased aortic leukocyte infiltration.
What new information does this article contribute?
We found that in mice placed on Western diet for 15 weeks, IL-17A plays a pro-inflammatory role by affecting aortic arch lesions, pro-inflammatory cytokines, and chemokine production.
IL-17A supports aortic inflammation by promoting vascular-IL-17RA dependent chemokine expression thereby stimulating monocyte and neutrophil recruitment to atherosclerotic plaques.
Deficiency of IL-17A reduces the number, but not the percentage, of aortic IFNγ+ T cells.
The role of IL-17A in atherosclerosis is currently unclear due to conflicting data obtained from IL-17A neutralization studies and differences in measurements of atherosclerosis. In other pathological states, IL-17A promotes leukocyte recruitment to sites of inflammation by supporting the production of stromal chemokines. In the present study, we sought to clarify the role of IL-17A in atherosclerosis using both IL-17A- and IL-17RA- deficient atherosclerosis-prone Apoe−/− mice. We demonstrate that IL-17A and IL-17RA deficient mice display reductions in aortic arch but not in extent of thoraco-abdominal aortic plaques, numbers of leukocytes, nor levels of chemokines. Additionally, examination of aortic arch and thoraco-abdominal aortic expression of Il17ra and Il17a within WD-fed mice revealed the exclusive expression of IL-17A within the aortic arch, which corresponded with elevated levels of pro-inflammatory TNFα, CXCL2, and CXCL1. Adoptive transfer experiments demonstrate reduced recruitment of monocytes and neutrophils to the aortas of IL-17RA-deficient Apoe−/− mice. These findings suggest that IL-17A plays a site-specific pro-inflammatory role and that it specifically stimulates chemokine and cytokine production, as well as monocyte and neutrophil recruitment in the aortic arch.
Supplementary Material
Acknowledgments
We thank Dr. Iwakura for providing breeding pairs of Il17a−/− mice and Amgen Inc. for providing breeding pairs of Il17ra−/− mice.
Sources of Funding This work was supported by the NHLBI HL107522 (E.G.) and AHA Pre-doctoral Fellowship grant 11PRE7520041 (M.B.).
Non-standard Abbreviations and Acronyms
- Apoe
Apolipoprotein E
- IL-17A
Interleukin-17A
- IL-17RA
Interleukin-17 Receptor A
- Ldlr
Low-density lipoprotein receptor
- WD
Western Diet
- MΦ
Macrophages
- PLN
Peripheral Lymph Node
- RORγt
Retinoic acid receptor-related orphan receptor-γt
- TA
Thoracoabdominal aorta
- AA
Aortic arch
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures. None
Reference List
- 1.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*) Annu Rev Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 3.Butcher M, Galkina E. Current views on the functions of interleukin-17A-producing cells in atherosclerosis. Thromb Haemost. 2011;106:787–795. doi: 10.1160/TH11-05-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taleb S, Tedgui A, Mallat Z. Interleukin-17: friend or foe in atherosclerosis? Curr Opin Lipidol. 2010;21:404–408. doi: 10.1097/MOL.0b013e32833dc7f9. [DOI] [PubMed] [Google Scholar]
- 5.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds JM, Angkasekwinai P, Dong C. IL-17 family member cytokines: regulation and function in innate immunity. Cytokine Growth Factor Rev. 2010;21:413–423. doi: 10.1016/j.cytogfr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang SH, Dong C. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res. 2007;17:435–440. doi: 10.1038/cr.2007.35. [DOI] [PubMed] [Google Scholar]
- 10.Wright JF, Guo Y, Quazi A, Luxenberg DP, Bennett F, Ross JF, Qiu Y, Whitters MJ, Tomkinson KN, Dunussi-Joannopoulos K, Carreno BM, Collins M, Wolfman NM. Identification of an interleukin 17F/17A heterodimer in activated human CD4+ T cells. J Biol Chem. 2007;282:13447–13455. doi: 10.1074/jbc.M700499200. [DOI] [PubMed] [Google Scholar]
- 11.Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, Sokol SI, Pfau S, Pober JS, Tellides G. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119:1424–1432. doi: 10.1161/CIRCULATIONAHA.108.827618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erbel C, Chen L, Bea F, Wangler S, Celik S, Lasitschka F, Wang Y, Böckler D, Katus HA, Dengler TJ. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J Immunol. 2009;183:8167–8175. doi: 10.4049/jimmunol.0901126. [DOI] [PubMed] [Google Scholar]
- 13.Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K, Galkina E. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2010;121:1746–1755. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taleb S, Romain M, Ramkhelawon B, Uyttenhove C, Pasterkamp G, Herbin O, Esposito B, Perez N, Yasukawa H, Van Snick J, Yoshimura A, Tedgui A, Mallat Z. loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J Exp Med. 2009;206:2067–2077. doi: 10.1084/jem.20090545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erbel C, Dengler TJ, Wangler S, Lasitschka F, Bea F, Wambsganss N, Hakimi M, Böckler D, Katus HA, Gleissner CA. Expression of IL-17A in human atherosclerotic lesions is associated with increased inflammation and plaque vulnerability. Basic Res Cardiol. 2011;106:125–134. doi: 10.1007/s00395-010-0135-y. [DOI] [PubMed] [Google Scholar]
- 16.Gao Q, Jiang Y, Ma T, Zhu F, Gao F, Zhang P, Guo C, Wang Q, Wang X, Ma C, Zhang Y, Chen W, Zhang L. A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J Immunol. 2010;185:5820–5827. doi: 10.4049/jimmunol.1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng X, Taleb S, Wang J, Tang TT, Chen J, Gao XL, Yao R, Xie JJ, Yu X, Xia N, Yan XX, Nie SF, Liao MY, Cheng Y, Mallat Z, Liao YH. Inhibition of IL-17A in atherosclerosis. Atherosclerosis. 2011;215:471–474. doi: 10.1016/j.atherosclerosis.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 18.Madhur MS, Funt SA, Li L, Vinh A, Chen W, Lob HE, Iwakura Y, Blinder Y, Rahman A, Quyyumi AA, Harrison DG. Role of Interleukin 17 in Inflammation, Atherosclerosis, and Vascular Function in Apolipoprotein E-Deficient Mice. Arterioscler Thromb Vasc Biol. 2011;31:1565–1572. doi: 10.1161/ATVBAHA.111.227629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galkina E, Ley K. Leukocyte influx in atherosclerosis. Curr Drug Targets. 2007;8:1239–1248. doi: 10.2174/138945007783220650. [DOI] [PubMed] [Google Scholar]
- 20.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen S, Shimada K, Zhang W, Huang G, Crother TR, Arditi M. IL-17A is proatherogenic in high-fat diet-induced and Chlamydia pneumoniae infection-accelerated atherosclerosis in mice. J Immunol. 2010;185:5619–5627. doi: 10.4049/jimmunol.1001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pietrowski E, Bender B, Huppert J, White R, Luhmann HJ, Kuhlmann CR. Pro-inflammatory effects of interleukin-17A on vascular smooth muscle cells involve NAD(P)H- oxidase derived reactive oxygen species. J Vasc Res. 2011;48:52–58. doi: 10.1159/000317400. [DOI] [PubMed] [Google Scholar]
- 23.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drechsler M, Megens RT, van Zandvoort M, Weber C, Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010;122:1837–1845. doi: 10.1161/CIRCULATIONAHA.110.961714. [DOI] [PubMed] [Google Scholar]
- 25.Shahrara S, Pickens SR, Dorfleutner A, Pope RM. IL-17 induces monocyte migration in rheumatoid arthritis. J Immunol. 2009;182:3884–3891. doi: 10.4049/jimmunol.0802246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Vietinghoff S, Koltsova EK, Mestas J, Diehl CJ, Witztum JL, Ley K. Mycophenolate mofetil decreases atherosclerotic lesion size by depression of aortic T-lymphocyte and interleukin-17-mediated macrophage accumulation. J Am Coll Cardiol. 2011;57:2194–2204. doi: 10.1016/j.jacc.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Es T, van Puijvelde GH, Ramos OH, Segers FM, Joosten LA, van den Berg WB, Michon IM, de Vos P, van Berkel TJ, Kuiper J. Attenuated atherosclerosis upon IL-17R signaling disruption in LDLr deficient mice. Biochem Biophys Res Commun. 2009;388:261–265. doi: 10.1016/j.bbrc.2009.07.152. [DOI] [PubMed] [Google Scholar]
- 28.VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol. 2004;24:12–22. doi: 10.1161/01.ATV.0000105054.43931.f0. [DOI] [PubMed] [Google Scholar]
- 29.King VL, Szilvassy SJ, Daugherty A. Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor−/− mice. Arterioscler Thromb Vasc Biol. 2002;22:456–461. doi: 10.1161/hq0302.104905. [DOI] [PubMed] [Google Scholar]
- 30.Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland S, Yeh E, Runge MS. p47phox is required for atherosclerotic lesion progression in ApoE(−/−) mice. J Clin Invest. 2001;108:1513–1522. doi: 10.1172/JCI11927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng G, Wei L, Xiurong W, Xiangzhen L, Shiguang Z, Songbin F. IL-17 stimulates migration of carotid artery vascular smooth muscle cells in an MMP-9 dependent manner via p38 MAPK and ERK1/2-dependent NF-kappaB and AP-1 activation. Cell Mol Neurobiol. 2009;29:1161–1168. doi: 10.1007/s10571-009-9409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Danzaki K, Matsui Y, Ikesue M, Ohta D, Ito K, Kanayama M, Kurotaki D, Morimoto J, Iwakura Y, Yagita H, Tsutsui H, Uede T. Interleukin-17A Deficiency Accelerates Unstable Atherosclerotic Plaque Formation in Apolipoprotein E-Deficient Mice. Arterioscler Thromb Vasc Biol. 2011 doi: 10.1161/ATVBAHA.111.229997. [DOI] [PubMed] [Google Scholar]
- 33.Rao DA, Eid RE, Rao DA, Eid RE, Qin L, Yi T, Kirkiles-Smith NC, Tellides G, Pober JS. Interleukin (IL)-1 promotes allogeneic T cell intimal infiltration and IL-17 production in a model of human artery rejection. J Exp Med. 2008;205:3145–3158. doi: 10.1084/jem.20081661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weaver CT, Hatton RD. Interplay between the TH17 and TReg cell lineages: a (co-)evolutionary perspective. Nat Rev Immunol. 2009;9:883–889. doi: 10.1038/nri2660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.