Summary
Orthogonal high-resolution separations are critical for attaining improved analytical dynamic range and protein coverage in proteomic measurements. High pH reversed-phase liquid chromatography (RPLC) followed by fraction concatenation affords better peptide analysis than conventional strong-cation exchange (SCX) chromatography applied for the two-dimensional proteomic analysis. For example, concatenated high pH reversed-phase liquid chromatography increased identification for peptides (1.8-fold) and proteins (1.6-fold) in shotgun proteomics analyses of a digested human protein sample. Additional advantages of high pH RPLC with fraction concatenation include improved protein sequence coverage, simplified sample processing, and reduced sample losses, making this an attractive alternative to SCX chromatography in conjunction with the second dimension low pH RPLC for two-dimensional proteomics analyses.
Keywords: Two dimensional chromatographic separation, shotgun proteomics analysis, SCX, Fraction concatenation, High pH RP
Expert Commentary
1) The need for highly efficient 2D LC in proteomics analysis
Proteomics studies benefit from analytical capabilities that provide high proteome and protein sequence coverage. However, even with state of the art, high duty cycle instruments, the number of proteins identified with a conventional single liquid chromatography-tandem MS (LC-MS/MS) analysis is typically limited and fractionating protein samples prior to LC-MS/MS analysis is crucial for increasing both the analytical dynamic range and proteome coverage [1–4]. Two-dimensional gel electrophoresis (2D-PAGE) and multidimensional chromatographic separation etc. are often used to reduce sample complexity.[5–8] While 2D-PAGE has limitations in identification of low-abundance proteins, membrane proteins, as well as proteins with extreme physicochemical properties (i.e. molecular weight or isoelectric point),[3,6] first stage LC separation offers better compatibility with subsequent RPLC-MS analysis, the integration with high throughput methodologies, and ease of automation.[9–13] Towards this end, a high efficiency two-dimensional liquid chromatography (2D LC) separation is most often used, with low pH reversed phase (RP) LC as the second dimension, prior to tandem mass spectrometric analysis [1,3,4,14]. The effectiveness of a 2D LC separation depends on the compatibility of the two separations, the separation efficiency in both dimensions, as well as on the separation orthogonality [15]. In the past, achieving good orthogonality to the RP separation has governed the choice of a first dimension separation. Because it employs a different peptide separation mechanism than RP, strong cation exchange (SCX) liquid chromatography has been the most widely adopted first dimension for 2D LC separations in proteomics [6,16–18]. However, SCX has its limitations including the capability for resolving peptides, reduced sample recovery, and sample losses due to the required sample desalting prior to and/or after fractionation. As a result, methods that provide more effective separation than SCX, generate cleaner fractions, and that reduce sample processing steps and loss are desired.
2) Why use a concatenated high pH RPLC fractionation approach?
Typically, RPLC resolves peptides better and achieves higher peak capacities than SCX, primarily due to the faster chromatographic partitioning [19]. Another advantage of RPLC is that the use of low salt or salt-free buffers generates cleaner samples for downstream LC-MS(/MS) analyses, whereas often required sample desalting for SCX can have a negative impact on analytical sensitivity. When operated at widely different pH values (e.g., 10 and 3), 2D RPLC-RPLC provides separation orthogonality comparable to that of SCX-RPLC [15], [10,20,21]. The difference of separation selectivity between the low and high pH RPLC comes from the changes in the charge distribution within peptide chains upon altering pH of the eluent [22]. The 2D RPLC strategy has been employed in various proteomics applications to separate stable isotopic labeled [23–26] or non-labeled peptides [27,28]. The orthogonality of RPLC-RPLC can be further improved [5,11] by using different reversed phase packing materials, such as polyRP and octadecylsilanized particles that lead to different LC retention behaviors of peptides.
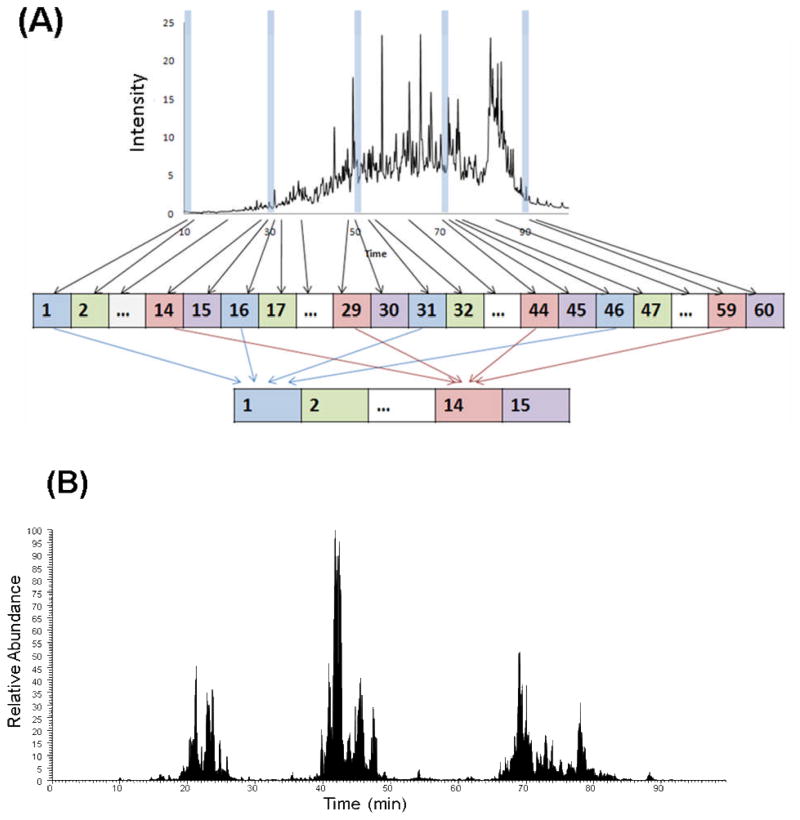
A concatenation approach offers further benefits in terms of proteome coverage [29]. First introduced to reduce the time required for 2D HPLC [22], concatenation involves combining multiple early, middle, and late RPLC fractions eluted over equal time intervals and with little overlap in the first dimension into a single concatenated fraction (Figure 1A). In addition to reducing the number of peptide fractions in the first dimension, concatenation effectively compensates for the imperfect orthogonality of the two separation dimensions, and makes more efficient use of a wider elution window in the second dimension separation compared to that of an individual fraction.
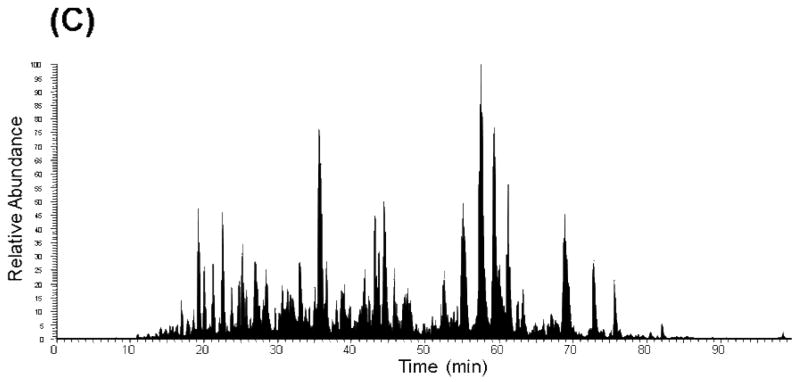
Figure 1.
A) Schematic depicting the concatenation strategy applied to a first dimensional low pH RP and high pH RP fractionation. Example RPLC-MS base peak chromatograms of fraction 10 following concatenation: B) low pH RPLC as a first dimension separation and C) high pH RPLC as a first dimension separation. Reprinted with permission from Proteomics 11:2019–2016 (2011). Copyright 2011 WILEY-VCH Verlag GmbH & Co.
We and others have shown that concatenation (pooling equal interval RPLC fractions) improves orthogonality and proteome coverage in 2D RPLC-RPLC shotgun analysis compared to pooling adjacent RPLC fractions that is routinely applied [29,30]. In an earlier study, concatenated pH 7.5 RP (pooling two equal interval fractions) approach yielded a >30% increase in the number of phosphopeptide identifications compared to the adjacent fraction pooling method from a digested mouse liver sample [30]. We recently showed that low pH RPLC-low pH RPLC with concatenation provides a ~2-fold increase in both peptide and protein identifications from a trypsin-digested human protein sample compared to those identified using the same 2D LC scheme without concatenation [29]. With concatenation, the second dimensional separation is better utilized as evidenced by the peptide clusters spread across a wide LC elution time window (Figure 1B). However, the gaps between the peptide clusters indicate that the separation space is still underutilized. The use of a concatenated high pH RP-low pH RPLC separation scheme affords better utilization of the separation window, as evidenced by the broad coverage of chromatographic space in Figure 1C.
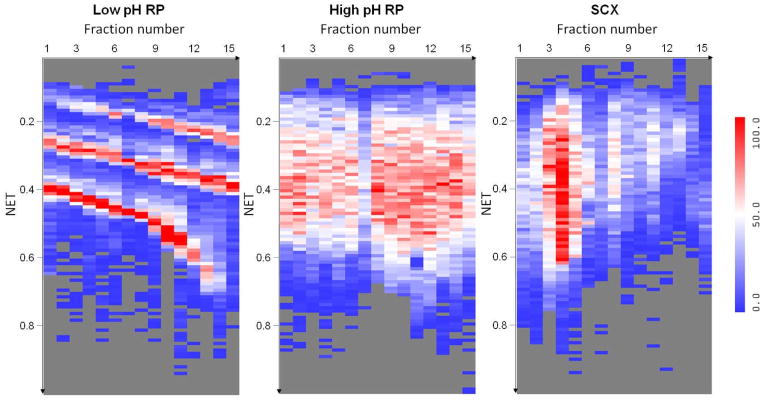
Figure 2 illustrates the separation orthogonality provided by concatenated RPLC-RPLC when compared to SCX-RPLC for the 2D LC-MS/MS of trypsin-digested human proteins. The distribution of peptides across the concatenated fractions (first dimension) and the low pH RPLC elution time (second dimension) illustrate the orthogonality of the first dimension separation (i.e., concatenated low pH RPLC, concatenated high pH RPLC, or SCX) with the second dimension (low pH) RPLC separation. As expected, concatenated low pH RPLC has the smallest orthogonality to the second dimensional low pH RPLC. In principle, SCX provides good orthogonality to RPLC; however, most tryptic peptides carry 2+, 3+ and 4+ charges and tend to group during SCX fractionation, which leads to a non-uniform use of the 2D space and a reduction in overall separation efficiency. In contrast, concatenated high pH RPLC-low pH RPLC much more uniformly covers the 2D space. The concatenated high pH RPLC-low pH RPLC [29] has resulted in 37633 unique peptides and 4363 unique proteins (with ≥ 2 unique peptides) from trypsin-digested human MCF10A cell sample, ~80% more peptides and ~60% more protein identifications than obtained using traditional SCX-RPLC.
Figure 2.
Heat maps illustrating the distinct number of unique peptides identified in each LC normalized elution time (NET) bin for each fraction of three different approaches. The x-axis represents the fraction number in the first dimension separation (concatenated low pH RPLC, concatenated high pH RPLC, and SCX) and the y-axis is the normalized elution time (NET) of the second dimension low pH RPLC. The number of peptides (low to high) is indicated by the blue-white-red color scale.
Reprinted with permission from Proteomics 11:2019–2016 (2011). Copyright 2011 WILEY-VCH Verlag GmbH & Co.
An additional advantage of high pH RPLC consist of higher tolerance towards the samples in which salts or other reagents have not been removed. Our results from high pH RPLC-LC-MS/MS analysis of peptide samples containing 1M urea and SPE-cleaned samples indicate that desalting is not necessary prior to high pH RPLC fractionation[29]. The combined desalting-fractionation operation can reduce sample preparation time and decrease sample losses, which is critical for small-sized biological/clinical samples.
3) Practical considerations when employing concatenation
When employing a concatenation strategy, one should determine the number of fractions to be concatenated. The strategy employed depends on the RPLC gradient time in the first dimension separation and the number of desirable LC-MS/MS analyses. Concatenation should be performed for fractions that have minimal overlap and also spread across the whole range of the elution profile in the first dimensional separation (species in each concatenated fraction should elute at least minutes apart to minimize their overlap). Fundamentally, the more fractions that are concatenated from the first dimension separation, the wider range of elution time the concatenated fraction can cover in the second dimensional separation. Another factor to be considered is the overlap between the neighboring fractions in the first dimensional separation, which contributes to the overlap of the neighboring concatenated fractions in the second dimensional separation. To combine fractions into the same number of post-concatenation fractions, a longer gradient that can more effectively resolve peptides in the neighboring fractions reduces the overlap between post-concatenation fractions. Under the experimental condition we have investigated [29] (~ 60 min effective gradient from which 60 fractions were collected and concatenated into 15 post-concatenation fractions), only minor overlap between neighboring post-concatenation fractions was observed (the average standard deviation of the overlap is 0.14 ± 0.01 for concatenated high pH RPLC).
Five-year view
High pH RPLC followed by fraction concatenation provides an efficient separation for improving proteome coverage and an attractive alternative to conventional SCX for 2D LC shotgun proteomics. In particular, use of this methodology eliminates the need to desalt samples prior to and/or following the first dimension separation, which reduces both sample losses (that are often around 50% or more in our routine proteomics sample preparation) and sample processing times. Reduced sample loss is especially important in studies where samples are valuable and their size limited, e.g. clinical biopsies. For smaller samples (<10 μg), on-line micro-SCX-RPLC [31] can provide greater sensitivity, while with larger samples RPLC-RPLC with concatenation can be automated in an off-line format, to provide improve proteome coverage and overall high sensitivity. For comprehensive post-translational modification analyses, reduced sample losses translate to a reduction in starting materials that are typically on the order of 8–10 mg [32,33] to allow for both fractionation and subsequent enrichment of low level modified peptides. Therefore, we expect that the simple experimental procedure and excellent chromatographic performance of the concatenated high-pH reversed phase LC separation methodology will drive it adoption for broad, sensitive, and reproducible shotgun proteomics applications over the next five years.
Key issues.
A high efficiency two-dimensional liquid chromatography separation, with low pH reversed phase liquid chromatography most often used as the second dimension, can enhance the ability to resolve peptides from a complex mixture in a shotgun proteomics analyses.
The effectiveness of the two-dimensional liquid chromatography separations depends on the chromatographic resolving power of each separation stage, as well as on the orthogonality of the two separations.
Strong-cation exchange chromatography, which is the current popular choice for the first dimension separation approach in shotgun proteomics is orthogonal to a reversed phase separation, but has limited resolving power.
High pH reversed phase chromatography has high resolving power, but is semi-orthogonal to the second dimensional reversed phase separation.
Concatenating multiple fractions that have minimal overlap and cover a wide separation window, improves the effective orthogonality of a high pH reversed phase first dimension separation to the second dimensional reversed phase separation.
High pH reversed phase liquid chromatography with fraction concatenation is a better alternative to strong cation change chromatography in two dimensional separations, in that it results in greatly improved peptide and protein coverage, as well as analytical sensitivity.
For very small samples (<10 μg), SCX-RPLC can presently provide greater sensitivity; however, given larger samples RPLC-RPLC with concatenation can be automated in an off-line format, and provide improved proteome coverage.
Acknowledgments
We thank NIH NCRR (Grant RR018522 to RDS) for support of this research. Work was performed in the NIH NCRR P41 Biomedical Technology Research Center for Proteomics located in the Environmental Molecular Sciences Laboratory (EMSL), a U.S. Department of Energy (DOE) Office of Biological and Environmental Science national scientific user facility at Pacific Northwest National Laboratory (PNNL) in Richland, Washington. PNNL is a multiprogram national laboratory operated by Battelle for the DOE under Contract DE-AC05-76RL01830.
Footnotes
Financial and competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
*=of interest
**=of considerable interest
- 1.Wang H, Chang-Wong T, Tang H-Y, Speicher DW. Comparison of Extensive Protein Fractionation and Repetitive LC-MS/MS Analyses on Depth of Analysis for Complex Proteomes. Journal of Proteome Research. 2009;9(2):1032–1040. doi: 10.1021/pr900927y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fang Y, Robinson DP, Foster LJ. Quantitative Analysis of Proteome Coverage and Recovery Rates for Upstream Fractionation Methods in Proteomics. Journal of Proteome Research. 2010;9(4):1902–1912. doi: 10.1021/pr901063t. [DOI] [PubMed] [Google Scholar]
- 3.Fournier ML, Gilmore JM, Martin-Brown SA, Washburn MP. Multidimensional Separations-Based Shotgun Proteomics. Chemical Reviews. 2007;107(8):3654–3686. doi: 10.1021/cr068279a. [DOI] [PubMed] [Google Scholar]
- 4.Washburn MP, Wolters D, Yates JR. Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotech. 2001;19(3):242–247. doi: 10.1038/85686. [DOI] [PubMed] [Google Scholar]
- 5.Lambert J-P, Ethier M, Smith JC, Figeys D. Proteomics: from Gel Based to Gel Free. Analytical Chemistry. 2005;77(12):3771–3788. doi: 10.1021/ac050586d. [DOI] [PubMed] [Google Scholar]
- 6.Slebos RJC, Brock JWC, Winters NF, et al. Evaluation of Strong Cation Exchange versus Isoelectric Focusing of Peptides for Multidimensional Liquid Chromatography-Tandem Mass Spectrometry. Journal of Proteome Research. 2008;7(12):5286–5294. doi: 10.1021/pr8004666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Balgley BM, DeVoe DL, Lee CS. Capillary Isoelectric Focusing-Based Multidimensional Concentration/Separation Platform forProteome Analysis. Analytical Chemistry. 2003;75(13):3145–3152. doi: 10.1021/ac034014+. [DOI] [PubMed] [Google Scholar]
- 8.Malmström J, Lee H, Nesvizhskii AI, et al. Optimized Peptide Separation and Identification for Mass Spectrometry Based Proteomics via Free-Flow Electrophoresis. Journal of Proteome Research. 2006;5(9):2241–2249. doi: 10.1021/pr0600632. [DOI] [PubMed] [Google Scholar]
- 9.Motoyama A, Xu T, Ruse CI, Wohlschlegel JA, Yates JR. Anion and Cation Mixed-Bed Ion Exchange for Enhanced Multidimensional Separations of Peptides and Phosphopeptides. Analytical Chemistry. 2007;79(10):3623–3634. doi: 10.1021/ac062292d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delmotte N, Lasaosa M, Tholey A, Heinzle E, Huber CG. Two-Dimensional Reversed-Phase × Ion-Pair Reversed-Phase HPLC: An Alternative Approach to High -Resolution Peptide Separation for Shotgun Proteome Analysis. Journal of Proteome Research. 2007;6(11):4363–4373. doi: 10.1021/pr070424t. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura T, Kuromitsu J, Oda Y. Evaluation of Comprehensive Multidimensional Separations Using Reversed-Phase, Reversed-Phase Liquid Chromatography/Mass Spectrometry for Shotgun Proteomics. Journal of Proteome Research. 2008;7(3):1007–1011. doi: 10.1021/pr7005878. [DOI] [PubMed] [Google Scholar]
- 12.Marshall J, Jankowski A, Furesz S, et al. Human Serum Proteins Preseparated by Electrophoresis or Chromatography Followed by Tandem Mass Spectrometry. Journal of Proteome Research. 2004;3(3):364–382. doi: 10.1021/pr034039p. [DOI] [PubMed] [Google Scholar]
- 13.Motoyama A, Yates JR. Multidimensional LC Separations in Shotgun Proteomics. Analytical Chemistry. 2008;80(19):7187–7193. doi: 10.1021/ac8013669. [DOI] [PubMed] [Google Scholar]
- 14.Fang Y, Robinson DP, Foster LJ. Quantitative Analysis of Proteome Coverage and Recovery Rates for Upstream Fractionation Methods in Proteomics. Journal of Proteome Research. 2010 doi: 10.1021/pr901063t. [DOI] [PubMed] [Google Scholar]
- 15*.Gilar M, Olivova P, Daly AE, Gebler JC. Orthogonality of Separation in Two-Dimensional Liquid Chromatography. Analytical Chemistry. 2005;77(19):6426–6434. doi: 10.1021/ac050923i. Comparison of different orthogonal liquid chromatography approaches. [DOI] [PubMed] [Google Scholar]
- 16.Opiteck GJ, Lewis KC, Jorgenson JW, Anderegg RJ. Comprehensive On-Line LC/LC/MS of Proteins. Analytical Chemistry. 1997;69(8):1518–1524. doi: 10.1021/ac961155l. [DOI] [PubMed] [Google Scholar]
- 17.Peng J, Elias JE, Thoreen CC, Licklider LJ, Gygi SP. Evaluation of Multidimensional Chromatography Coupled with Tandem Mass Spectrometry (LC/LC MS/MS) for Large-Scale Protein Analysis: The Yeast Proteome. Journal of Proteome Research. 2002;2(1):43–50. doi: 10.1021/pr025556v. [DOI] [PubMed] [Google Scholar]
- 18.Ishihama Y, Rappsilber J, Mann M. Modular Stop and Go Extraction Tips with Stacked Disks for Parallel and Multidimensional Peptide Fractionation in Proteomics. Journal of Proteome Research. 2006;5(4):988–994. doi: 10.1021/pr050385q. [DOI] [PubMed] [Google Scholar]
- 19**.Gilar M, Olivova P, Daly AE, Gebler JC. Two-dimensional separation of peptides using RP-RP-HPLC system with different pH in first and second separation dimensions. Journal of Separation Science. 2005;28(14):1694–1703. doi: 10.1002/jssc.200500116. Comparing high pH reversed phase and strong cation exchange as first dimensional separation. [DOI] [PubMed] [Google Scholar]
- 20.Gilar M, Olivova P, Chakraborty AB, Jaworski A, Geromanos SJ, Gebler JC. Comparison of 1-D and 2-D LC MS/MS methods for proteomic analysis of human serum. ELECTROPHORESIS. 2009;30(7):1157–1167. doi: 10.1002/elps.200800630. [DOI] [PubMed] [Google Scholar]
- 21.Toll H, Oberacher H, Swart R, Huber CG. Separation, detection, and identification of peptides by ion-pair reversed-phase high-performance liquid chromatography-electrospray ionization mass spectrometry at high and low pH. Journal of Chromatography A. 2005;1079(1–2):274–286. doi: 10.1016/j.chroma.2005.03.121. [DOI] [PubMed] [Google Scholar]
- 22*.Dwivedi RC, Spicer V, Harder M, et al. Practical Implementation of 2D HPLC Scheme with Accurate Peptide Retention Prediction in Both Dimensions for High-Throughput Bottom-Up Proteomics. AnalyticalChemistry. 2008;80(18):7036–7042. doi: 10.1021/ac800984n. Showing the concatenation of high pH reversed phase fractions can reduce analysis time. [DOI] [PubMed] [Google Scholar]
- 23.Dwivedi RC, Krokhin OV, Cortens JP, Wilkins JA. Assessment of the reproducibility of random hexapeptide peptide library-basedprotein normalization. J Proteome Res. 2010;9(2):1144–1149. doi: 10.1021/pr900608z. [DOI] [PubMed] [Google Scholar]
- 24.Dwivedi RC, Dhindsa N, Krokhin OV, Cortens J, Wilkins JA, El-Gabalawy HS. The effects of infliximab therapy on the serum proteome of rheumatoid arthritis patients. Arthritis Res Ther. 2009;11(2):R32. doi: 10.1186/ar2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coombs KM, Berard A, Xu W, et al. Quantitative proteomic analyses of influenza virus-infected cultured human lung cells. J Virol. 2010;84(20):10888–10906. doi: 10.1128/JVI.00431-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lau E, Lam MP, Siu SO, et al. Combinatorial use of offline SCX and online RP-RP liquid chromatography for iTRAQ-based quantitative proteomics applications. Mol Biosyst. 2011;7(5):1399–1408. doi: 10.1039/c1mb05010a. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh D, Lippert D, Krokhin O, Cortens JP, Wilkins JA. Defining the membrane proteome of NK cells. J Mass Spectrom. 2010;45(1):1–25. doi: 10.1002/jms.1696. [DOI] [PubMed] [Google Scholar]
- 28.Francois I, Cabooter D, Sandra K, Lynen F, Desmet G, Sandra P. Tryptic digest analysis by comprehensive reversed phasextwo reversed phase liquid chromatography (RP-LCx2RP-LC) at different pH’s. J Sep Sci. 2009;32(8):1137–1144. doi: 10.1002/jssc.200800578. [DOI] [PubMed] [Google Scholar]
- 29**.Wang Y, Yang F, Gritsenko MA, et al. Reversed-phase chromatography with multiple fraction concatenation strategy for proteome profiling of human MCF10A cells. Proteomics. 2011;11:2019–2016. doi: 10.1002/pmic.201000722. Comparing low and high pH reversed phase with fraction concatenation and strongcation exchange as first dimensional separation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song C, Ye M, Han G, et al. Reversed-Phase-Reversed-Phase Liquid Chromatography Approach with High Orthogonality for Multidimensional Separation of Phosphopeptides. Analytical Chemistry. 2009;82(1):53–56. doi: 10.1021/ac9023044. [DOI] [PubMed] [Google Scholar]
- 31.Gao M, Deng C, Yu W, Zhang Y, Yang P, Zhang X. Large scale depletion of the high-abundance proteins and analysis of middle-and low-abundance proteins in human liver proteome by multidimensional liquid chromatography. Proteomics. 2008;8(5):939–947. doi: 10.1002/pmic.200600099. [DOI] [PubMed] [Google Scholar]
- 32.Villen J, Beausoleil SA, Gerber SA, Gygi SP. Large-scale phosphorylation analysis of mouse liver. Proc Natl Acad Sci U S A. 2007;104(5):1488–1493. doi: 10.1073/pnas.0609836104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swaney DL, Wenger CD, Thomson JA, Coon JJ. Human embryonic stem cell phosphoproteome revealedby electron transfer dissociation tandem mass spectrometry. Proc Natl Acad Sci U S A. 2009;106(4):995–1000. doi: 10.1073/pnas.0811964106. [DOI] [PMC free article] [PubMed] [Google Scholar]