Abstract
Clinical debate has arisen over the consequences of antioxidant supplementation during cancer chemotherapy. While antioxidants may impede the efficacy of chemotherapy by scavenging reactive oxygen species and free radicals, it is also possible that antioxidants alleviate unwanted chemotherapy-induced toxicity, thus allowing for increased chemotherapy doses. These contradictory assertions suggest that antioxidant supplementation during chemotherapy treatment can have varied outcomes depending on the cellular context. To gain a more robust understanding of the role that antioxidants play in chemotherapy, we investigated the dose-dependent effects of the antioxidant, N-acetylcysteine (NAC), on the redox-mediated regulation of intracellular signaling. In this study, we systematically evaluated the effect of Dox-induced ROS on the NF-κB pathway in a pediatric acute lymphoblastic leukemia (ALL) cell line by measuring the thiol-based oxidative modifications of redox-sensitive proteins within the pathway. We report a functional consequence of NAC supplementation during doxorubicin (Dox) chemotherapy administration via the NF-kappa B (NF-κB) signal transduction pathway. The ability of NAC to alter Dox-induced NF-κB activity is contingent on the ROS-mediated S-glutathionylation of IKK-β. Moreover, the NAC-dependent alteration of intracellular glutathione redox balance, through pro-oxidant and antioxidant mechanisms, can be exploited to either promote or inhibit Dox-induced NF-κB activity in an NAC-concentration-dependent manner. We developed an electron-transfer-based computational model that predicts the effect of NAC pretreatment on Dox-induced NF-κB signaling for a range of NAC and Dox treatment combinations.
Introduction
It is estimated that anywhere from 13% to 87% of cancer patients are prescribed antioxidant supplements in conjunction with standard chemotherapy.1,2 This wide range of percentages is most likely due to the fact that a consensus on the clinical effect of antioxidant supplementation during chemotherapy treatment has yet to be reached. While some published studies support the idea that antioxidants hinder the efficacy of chemotherapy treatment by interfering with oxidative mechanisms of action,3 other published studies advise that antioxidant supplementation can actually potentiate chemotherapy-mediated cancer cell death by promoting non-oxidative mechanisms of chemotherapy-induced apoptosis.4–6
The contradictory nature of these findings motivates a more targeted investigation of the mechanisms of action of antioxidants and how these mechanisms can vary for different chemotherapy doses. Because antioxidants comprise a wide variety of chemical compounds ranging from the endogenous antioxidants (such as the SH-compounds, NAC, glutathione, and antioxidant enzymes) to the dietary antioxidants (such as vitamins A, C, and E), the functional effects of combining antioxidants with chemotherapy cannot be assumed to be conserved across a variety of antioxidant and chemotherapy combinations.
When intracellular levels of reactive oxygen species (ROS) are high, ROS can inflict irreparable damage to cellular biomolecules and cause subsequent cell death.7 Because antioxidants, both endogenous and dietary, can react with and eliminate intracellular free radicals and reactive oxygen species (ROS), the presence of antioxidants in this high ROS environment would most likely be beneficial for cell survival. However, it has also been shown extensively in the literature that when ROS levels are low, ROS are more likely to alter cellular signal transduction through the reversible modification of intracellular redox-sensitive proteins.8–13 The cellular protein modifications caused by low levels of ROS do not necessarily result in cell death, and may even promote cell viability in some instances.14 Thus, the presence of antioxidants in this low ROS environment could either promote or inhibit cell survival depending on the intracellular signaling pathways that are affected.
If antioxidants and certain chemotherapeutic agents can eliminate and generate ROS, respectively, and if ROS can modulate intracellular signal transduction pathways, then antioxidant supplementation during chemotherapy likely affects signal transduction pathways regulating cancer cells. In this study, we have focused on the functional effects of a specific antioxidant, NAC, when used in combination with a widely-prescribed chemotherapeutic agent, doxorubicin (Dox). We systematically analyzed the biochemical mechanisms by which NAC, in the presence of low levels of ROS induced by the redox recycling of Dox, alters the redox-sensitive NF-κB signal transduction pathway. The NF-κB signal transduction pathway was chosen for analysis because it is extremely well-characterized, multiple points of redox susceptibility have been previously reported to exist within the pathway,14 and Dox treatment has been shown to modify the pathway in a redox-dependent manner.15 Moreover, the NF-κB signal transduction pathway is an oncogenic pathway believed to be involved in cellular growth, proliferation, and drug resistance development.16,17
While the ability of antioxidants to modulate NF-κB transcriptional activation has been reported previously in the literature,18 the mechanistic details behind the regulation of NF-κB by a combination of antioxidants and chemotherapy are incompletely defined.19 To our knowledge, this is the first attempt to use the systematic application of NAC in combination with Dox to monitor the effect of antioxidant supplementation during chemotherapy on a particular redox sensitive signal transduction network. The results of this study reveal that (a) protein thiols in the NF-κB activation pathway are differentially sensitive to ROS induced by clinically relevant concentrations of Dox, (b) NAC, in a concentration-dependent manner, can selectively alter the redox sensitivity of certain protein cysteines identified in (a), and (c) semi-quantitative descriptions based on basic electron-transfer reactions can be used to predict Dox-induced NF-κB activity as a function of varying extracellular NAC concentrations. The protocols and methodologies presented in this body of work offer an archetype for how antioxidants in combination with chemotherapeutic drugs can be investigated, with a targeted focus on a particular and relevant signal transduction pathway.
Experimental
Materials, cell culture and treatment conditions
All reagents were from Sigma-Aldrich unless otherwise specified. A B-cell precursor ALL cell line derived from a pediatric patient (EU1) has been previously characterized.20,21 EU1 cells were cultured in RPM1-1640 medium supplemented with 10% FBS and 100 U/ml of penicillin/streptomycin. For all experiments, unless otherwise stated, EU1 cells were resuspended in fresh media (1 × 106 cells ml−1) and treated with various concentrations of Dox (Enzo Life Sciences), the IKK-β inhibitor, SC-514 (Enzo Life Sciences), or NAC (Alfa Aesar, MA, USA), protected from light and incubated at 37 °C in a humidified atmosphere of 5% CO2. Growth media was made by supplementing phenol-red-free RPMI-1640 medium with 10% FBS and 100 U/ml of penicillin/streptomycin. Antibodies were used to quantify intracellular proteins before and after various Dox treatment regimens: mouse anti-IκB-α primary antibody (Genetex, CA, USA), rabbit anti-IKK-γ primary antibody (Cell Signaling, MA, USA), rabbit anti-IKK-β primary antibody (Santa Cruz Biotechnology, CA, USA), rabbit anti-p65 primary antibody (Abcam) and rabbit anti-actin primary antibody (Sigma-Aldrich, MO, USA).
Luciferase transduction
EU1 cells were transduced with Cignal™ lentiviral particles expressing Renilla (control) and Luciferase (NF-κB reporter) binding elements according to manufacturer’s protocol (SA Biosciences, MD, USA). EU1 cells were seeded, in triplicate, in a 96-well plate format at a starting concentration of 1 × 105 cells ml−1 in 100 μl of complete RPMI growth media. Cells were allowed to incubate for 24 h. After 24 h, growth media was removed from the wells and Renilla and Luciferase lentiviral particles, diluted in a mixture of SureEntry Trans-duction Reagent and growth media without antibiotics, was added to the cells at a multiplicity of infection (MOI) of 25. Cells were incubated for 18 h, then the lentiviral-containing growth media was removed and 100 μl of fresh growth media was added to the cells. On day 4 of the transduction protocol, the growth media was removed and replaced with 100 μl of fresh growth media containing 2 μg ml−1 of puromycin antibiotic. On day 7 of the transduction protocol, live cells were harvested and assayed for expression of the NF-κB reporter gene. Cells were maintained in culture using growth media supplemented with 2 μg ml−1 of puromycin antibiotic.
NF-κB activation (luciferase reporter assay)
Luciferase reporter activity was quantified using the Dual-Luciferase Reporter Assay System (Promega, WI, USA) according to manufacturer’s protocol. Transduced EU1 cells, suspended in 100 μl of growth media without puromycin antibiotic, were plated in 96-well plate format (1 × 106 cells ml−1). Cells were exposed to various treatment conditions (see below) for indicated times. After treatment, cells were lysed and luciferase protein expression was quantified using a Synergy 4 hybrid microplate reader (Biotek).
Intracellular ROS determination
EU1 cells were incubated in phenol red-free growth media with (Dox/NAC samples) or without (Dox samples) 1 mM NAC. After pretreatment, Dox, at a final concentration of 5 μM, was added to the cells and allowed to incubate for the indicated times. After Dox treatment, cells were spun down and the growth media removed. For determination of Dox- induced ROS formation, H2DCFDA resuspended in DMSO was added to the cells after Dox treatment to a final concentration of 5 μM and cells were incubated for 15 min. After incubation, the cells were pelleted and resuspended in fresh PBS at roo m temperature and the plate was assayed for DCF fluorescence signal using the microplate reader (Ex = 500 nm, Em = 535 nm). Unstimulated cells, pre-incubated with and without H2DCFDA dye, and phenol red-free media, pre-incubated with and without H2DCFDA dye and Dox, respectively, were used as controls.
Cell fractionation
Cytoplasmic fractions were obtained by cell lysing in non-reducing 2% Triton X buffer containing 50 mM β-glycero-phosphate, 10 mM NaPP, 30 mM NaF, 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 nM benzamidine, 20 mM N-ethylmaleimide, and 2 nM EGTA. Cells were lysed on ice for 1 h, followed by centrifugation for 10 min at 300×g.
Immunoprecipitation (IP)
Cytosolic lysates (150 μl) were incubated with 50 μl of magnetic protein A beads (Millipore, MA, USA) and placed on a rotating plate for 3 h at 4 °C to minimize non-specific binding. After incubation, beads were removed and the supernatant transferred to a new tube. To the supernatant, 1 μl of anti-GSH antibody was added (Virogen, MA, USA) and the mixture was allowed to incubate overnight at 4 °C. After overnight incubation, 50 μl of magnetic protein A beads were added to the sample mixture and allowed to incubate overnight at 4 °C. After the secondary overnight incubation, samples were pelleted and the supernatant was discarded. The pellet was washed 3 times with RIPA buffer. After the final wash step, the pellet was resuspended in 25 μl of resuspension buffer (a mixture of RIPA buffer and lysis buffer used for cell fractionation) then boiled for 10 min at 100 °C.
Western blotting
To analyze the redox state of IKK-γ, also known as NEMO, and to quantify S-glutathionylation of IKK-β and IκB-α, cytosolic and IP fractions were separated by native polyacrylamide gel electrophoresis and transferred to PVDF membranes. Membranes were incubated overnight at 4 °C in 10 ml of primary antibody solution (in blocking buffer) at a dilution of 1 : 1000. Following overnight incubation, membranes were incubated at room temperature with secondary antibodies at a dilution of 1 : 10 000 in 15 ml of blocking buffer for 1 h. After secondary antibody incubation, membranes were washed three times in TBS-T. Imaging and image analysis were conducted using the Li-Cor Odyssey Infrared Imaging System with the Odyssey 2.1 software. Where appropriate, β-actin was used as a loading control for normalization. Disulfide-bonded NEMO (IKK-γ), or NEMO dimer, was distinguishable from NEMO monomer based on the fact that NEMO dimers have a higher molecular weight than NEMO monomers. The IP protocol described in the prior section enriched for GSH-associated proteins. The IP fractions were probed by western blotting by antibodies specific for the proteins of interest (p65, IKK-β or IκB-α) to detect the S-glutathionylated form of the protein.
Intracellular GSH and GSSG quantification
Glutathione/glutathione disulfide assay (Oxford Biomedical, MI, USA) was used to measure GSH and GSSG levels in EU1 cytoplasmic lysate samples obtained after various Dox treatment conditions. GSH/GSSG quantification was achieved by the enzymatic recycling assay according to manufacturer’s protocol.
Statistical analysis
All values reported are the average of three or more independent biological replicates × standard error. Statistical significance is based upon the criteria of p < 0.05 for a Student’s t-test (two-tailed, equal variance).
A computational model of IKK-β S-glutathionylation
To better understand the effect of the NAC-altered glutathione redox potential on chemotherapy-induced IKK-β S-glutathionylation, a biochemical model of pertinent reactions was constructed. Previous models of ROS-induced protein thiol oxidation have characterized the rate constants for the oxidation reactions that result in general protein thiol modification.22–26 The identification of oxidant targets based on the relative chemical reactivity of potential substrates12 provided the quantitative basis by which a protein-specific S-glutathionylation model could be developed.
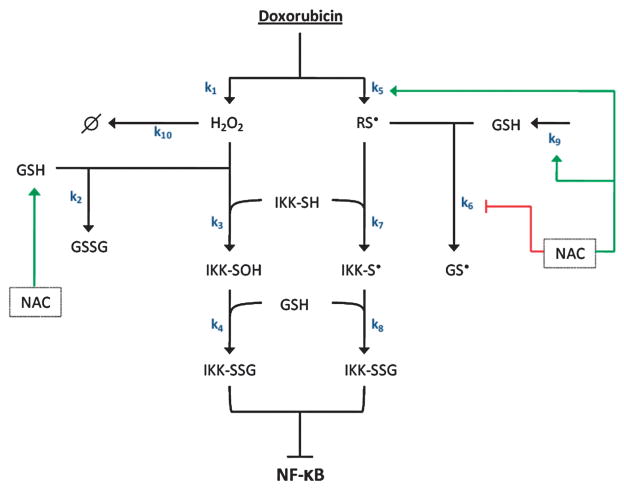
A schematic diagram of the model is shown in Fig. 1. The model describes the relevant literature-reported processes that control oxidant-induced IKK-β S-glutathionylation. An in-depth discussion on the choice of reactions and components to include in this model can be found in Supplementary Information. The IKK-β S-glutathionylation model consists of a series of ordinary differential equations27 that describe the rate of change of each of the species that comprise the network (Table 1). The initial conditions of the 11 compounds and the parameter values of the 10 kinetic parameters utilized in the model are shown in Tables 2 and 3, respectively. The IKK-β S-glutathionylation model was designed and numerically integrated using the ode15s solver within MATLAB R2009b (Mathworks Inc., Natick, MA, USA).
Fig. 1. Mechanistic model of glutathione-dependent IKK-β S-glutathionyl-ation.
Schematic representation of the proposed reactions involved in glutathione-dependent IKK-β S-glutathionylation. Doxorubicin treatment promotes the formation of hydrogen peroxide (H2O2) and protein thiyl radicals (RS•). Once formed, H2O2 mediates the S-glutathionylation of reduced IKK-β via the peroxide-dependent oxidation of IKK-β. Concurrently, doxorubicin-induced RS• formation induces IKK-β S-gluta-thionylation via the radical-dependent oxidation of IKK-β. However, increased thiyl radical levels simultaneously promote the radical oxidation of GSH into the glutathione thiyl radical (GS•) which serves to effectively diminish reduced GSH levels from the intracellular environment. NAC, the GSH precursor, regulates the peroxide-dependent and the radical-dependent mechanisms of IKK-β S-glutathionylation via its ability to promote glutathione synthesis as well as its ability to contribute to free-radical formation in the presence of H2O2.
Table 1.
Ordinary differential equation expressions utilized in the mechanistic model of IKK-β S-glutathionylation
| d[Doxe−]/dt = 3.8 × 10−7(t) + 1.5 × 10−5 |
| d[H2O2]/dt = k1([Doxe−]) − k2([GSH])([H2O2]) − k3([IKK-SH])([H2O2]) − k10([H2O2]) |
| d[RS•]/dt = k5([Dox e−]) k6([GSH])([RS•]) − k7 ([IKK-SH])([RS•]) |
| d[GSH]/dt = −k2([GSH])([H2O2]) − k6([GSH])([RS•]) − k4([GSH])([IKK-SOH]) − k8([GSH])([IKK-S•]) + k9 |
| d[GSSG]/dt = k2([GSH])([H2O2]) |
| d[GS•]/dt = k6([GSH])([RS•]) |
| d[IKK-SH]/dt = −k3([IKK-SH])([H2O2]) − k7([IKK-SH])([RS•]) |
| d[IKK-SOH]/dt = k3([IKK-SH])([H2O2]) − k4([GSH])([IKK-SOH]) |
| d[IKK-S•]/dt = k7([IKK-SH])([RS•]) − k8([GSH])([IKK-S•]) |
| d[IKK-SSG]/dt = k4([GSH])([IKK-SOH]) + k8([GSH])([IKK-S•]) |
Table 2.
Initial concentrations of the components that comprise the mechanistic model of IKK-β S-glutathionylation
Table 3.
Rate expressions and rate constants for the ODEs that comprise the mechanistic IKK-β S-glutathionylation modela
| Rxn | Expression | Parameter value at various [NAC](mM)
|
|||
|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 5 | ||
| R1 | k1 ([Dox sq]) → H2O2 | k1 = 6.0 × 10−3 | 6.0 × 10−3 | 6.0 × 10−3 | 6.0 × 10−3 |
| R2 | k2 ([GSH]) ([H2O2]) → GSSG | k2 = 2.0 × 10−3 | 2.0 × 10−3 | 2.0 × 10−3 | 2.0 × 10−3 |
| R3 | k3 ([IKK-SH]) ([H2O2]) → IKK-SOH | k3 = 5.0 × 10−5 | 5.0 × 10−5 | 5.0 × 10−5 | 5.0 × 10−5 |
| R4 | k4 ([IKK-SOH]) ([GSH]) → IKK-SSG | k4 = 6.0 × 10−3 | 6.0 × 10−3 | 6.0 × 10−3 | 6.0 × 10−3 |
| R5 | k5 ([Dox sq]) → RS•]) | k5 = 1.2 × 10−3 | 3.7 × 10−3 | 3.7 × 10−3 | 3.7 × 10−3 |
| R6 | k6 ([GSH]) ([RS •]) → GS• | k6 = 5.0 × 10−3 | 5.0 × 10−3 | 5.0 × 10−3 | 2.5 × 10−3 |
| R7 | k7 ([IKK-SH]) ([RS •]) → IKK-S• | k7 = 5.0 × 10−4 | 5.0 × 10−4 | 5.0 × 10−4 | 5.0 × 10−4 |
| R8 | k8 ([GSH]) ([IKK-S•]) → IKK-SSG | k8 = 5.0 × 10−3 | 5. ×10−3 | 5.0 × 10−3 | 5.0 × 10−3 |
| R9 | Null → [GSH] | k9 = 6.0 × 10−2 | 1.8 × 10−1 | 3.7 × 10−1 | 6.0 × 10−1 |
Reaction rate constants highlighted in bold represent the rate constants that were fitted to experimentally-determined IKK-β-SSG behavior for the various NAC pretreatment conditions investigated. The rate constants are given in units of inverse a.u. × inverse time (a.u.−1 s−1).
The IKK-β S-glutathionylation process is promoted by Dox administration. Upon Dox administration, intracellular enzymes convert the anthracycline drug into a semiquinone free radical.28–30 The differential equation describing the rate of change of intracellular semiquinone Dox as a function of time is modeled by the following equation:
| (1) |
where t represents time in seconds. This equation was fit to the semiquinone Dox profile for a 500 nM Dox treatment condition, as predicted by a previous model of Dox bioactivation in EU1 cells.21 When Dox is activated to its semiquinone form, it can react with molecular oxygen to produce superoxide, which is converted to hydrogen peroxide (H2O2) by superoxide dismutase.31–33 Semiquinone Dox can also react with intra-cellular protein thiols leading to the production of protein thiyl radicals (RS•).31–33 The rate constants that describe the formation of H2O2 and RS• from intracellular semiquinone Dox were estimated from the literature-reported reaction rates of molecular oxygen and protein thiols, respectively, with semiquinone Dox.12,34 Once formed, H2O2 and RS• can subsequently react with reduced IKK-β (IKK-SH) leading to the formation of IKK-SSG (Fig. 1).
The rate constants for the reactions used in the mathematical model are shown in Table 3. Although these rates have units of inverse arbitrary unit × inverse time (a.u.−1 s−1), the literature-reported proportionalities between the rate constants for the different reactions are conserved.12,22,35,36 Correspondingly, the initial concentrations of the species utilized in the model (Table 2) also mirror the literature-reported proportionalities between each of the components accounted for in the model.22,37,38 In constructing the IKK-β S-glutathionylation model it was assumed that Dox degradation and efflux was negligible for the timescales being simulated39 and that all oxidation reactions occurred in the absence of target recycling. The reactions used in the mathematical model are not inherently irreversible; however, they require the actions of intracellular antioxidants for their reversibility. The explicit incorporation of these additional intracellular antioxidants was precluded by a lack of quantitative experimental data pertaining to their kinetic rates of reactions. As such, the oxidative effects predicted by this simplified preliminary model should be taken as approximate representations of what would typically occur in vivo.
Modeling the effect of NAC administration on IKK-β S-glutathionylation
Once a mathematical network model of IKK-β S-glutathionyl-ation had been constructed, we opted to simulate the effects of NAC administration within the network as follows. Previous studies highlight three processes that are both relevant to IKK-β S-glutathionylation and susceptible to NAC-dependent modulation: glutathione synthesis (k9), protein thiyl radical formation (k5), and glutathione thiyl radical formation (k6).40–43 Since concentration-specific quantitative details on the effect of NAC pre-treatment on GSH synthesis were available in the literature,40 these quantitative effects were directly implemented for the various NAC pretreatment conditions that were simulated.
Although concentration-specific quantitative information on the effect of NAC treatment on the formation of protein thiyl and glutathione thiyl radicals was unavailable in the literature, we were able to obtain generalized quantitative details on the directional effects of NAC treatment on these processes. The Fenton reaction of transition metals (Fe2+) with H2O2 to produce Fe3+ and OH• radical and the Haber-Weiss reaction of low molecular weight thiols (such as NAC) with Fe3+ to produce protein thiyl radicals illustrate the processes by which increased NAC availability will promote the formation of protein thiyl radicals:41
| (2) |
| (3) |
NAC administration increases thiyl radical formation and thiyl-based oxidation by approximately 3-fold;40–43 this process is highly dependent on the concentration of oxidants within the environment and can therefore vary from one condition to the next.43 Moreover, a sigmoidal relationship exists between the extracellular NAC pretreatment concentration applied and the extracellular NAC concentration at which this 3-fold-change increase in thiyl radical formation is observed (Fig. S1).43 From these literature-reported findings, it was concluded that the effect of NAC pretreatment could be modeled as a 3-fold increase in the rate of RS• formation (k5) for a given NAC pretreatment condition; once this 3-fold increase in protein thiyl radical formation rate is fit to a particular NAC pretreatment condition, all subsequent NAC pretreatment conditions would maintain the same effect.
The effect of NAC pretreatment on glutathione thiyl formation was deduced from the following radical-related reactions:42–44
| (4) |
| (5) |
Eqn (4) and (5) represent the potential fates of a protein thiyl radical upon reaction with NAC or GSH thiols. In the presence of NAC, the rate of formation of GS• is decreased because NAC and GSH effectively compete over the limited pool of available intracellular radicals.12
The rate at which NAC reacts with radicals is comparable to the rate at which GSH reacts with radicals.11,12 If it is assumed that the intracellular concentration of NAC at which NAC begins to compete with GSH is equivalent to the intracellular concentration of GSH, then NAC pretreatment will decrease GSH thiyl radical formation by a factor of 2. Intracellular NAC is efficiently converted to GSH and cysteine (Cys),40 and the levels of these species are regulated by transport mechanisms37 that ensure the maximum level of free intra-cellular NAC will not exceed the intracellular levels of GSH within the cell.45 Assuming that NAC pretreatment will only affect GS• formation when the intracellular NAC concentration is at or above a certain threshold imposes a sigmoidal relationship between NAC pretreatment and NAC-induced effects on GS• formation rates. This type of sigmoidal behavior, as a function of NAC-pretreatment, is not unprecedented.43
Model simulations were carried out for various combinations of the potentially-relevant NAC-dependent effects described above (Fig. S2). The combination that best fit the experimental data for Dox-induced IKK-β S-glutathionylation, for each NAC pretreatment condition, was chosen as the proposed mechanistic description of glutathione-dependent IKK-β S-glutathionylation. The rate constants predicted from this model-fitting procedure are highlighted in bold and shown in Table 2.
Results and discussion
Due to a lack of consensus on the functional effects of anti-oxidant supplementation during chemotherapy, there is a great need for the accurate and systematic characterization of the role that specific antioxidants play during specific chemotherapy treatment regimens. To begin addressing this issue, we applied varying doses of doxorubicin chemotherapy in combination with varying doses of the antioxidant NAC. We then monitored the effects of these dose combinations on several of the redox-sensitive cytosolic protein components that lie upstream in the NF-κB transcriptional network.
This present work focuses on the critical redox-sensitive proteins that lie upstream of NF-κB because previous studies have already elucidated the effect that antioxidant administration has on NF-κB and other potential sites of redox regulation within the NF-κB activation pathway. Redox regulation of NF-κB activation occurs via doxorubicin-mediated inhibition of proteasome activity46 for example, and NAC supplementation during hypoxia, a condition that is analogous to Dox treatment, affects the S-glutathionylation status of the p65 subunit of the NF-κB molecule itself.47 These studies provided significant insight into the many diverse ways in which the NF-κB activation pathway can be sensitive to redox-regulation as mediated by Dox administration. We measured NF-κB thiol modification under a combination of Dox and NAC concentrations, but did not observe significant changes in S-glutathionylation of the p65 subunit (Fig S3). We note that while the p65 S-glutathionylation detected may indicate artefacts of oxidation during the lysing procedure, relative to control conditions, the presence of NAC and/or Dox had no effect on the protein. Therefore, to effectively understand the effect of antioxidant supplementation during chemotherapy on the entire NF-κB activation pathway, analysis of the oxidative modifications of proteins that lie upstream in the NF-κB activation network is necessary. We focused specifically on the three protein components that are directly upstream of the NF-κB dimer: IKK-γ (NEMO), IKK-β, and IκB-α.
Dox treatment induces ROS in EU1 cells that can be attenuated by NAC
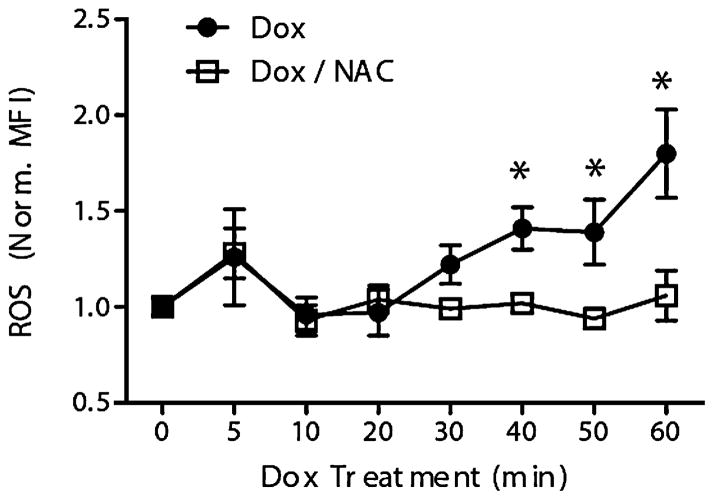
To characterize the capacity for Dox treatment to induce ROS generation in leukemic cells, the EU1 cell line was treated with Dox (5 μM) for various time points, after which Dox-induced ROS generation was quantified by plate reader measurement of DCF fluorescence (Fig. 2). A significant increase in ROS production was seen at 40 min of Dox treatment. After 40 min of Dox treatment, ROS levels had increased by 50% above baseline and the Dox-induced ROS levels continued to rise for the duration of the Dox treatment regimen (Fig. 2).
Fig. 2. Doxorubicin treatment induces ROS in EU1 cells that can be attenuated by N-acetylcysteine (NAC) pre-treatment.
Time-dependent doxorubicin-induced H2O2 in EU1 cells, with and without NAC pre-treatment, quantified by plate reader measurement of DCF fluores-cence (MFI = mean fluorescent intensity). ([NAC] = 1 mM, 30 min pre-treatment; [Dox] = 5 μM, 1 h treatment; *p o 0.05)
Next, the ability of NAC to prevent Dox-induced ROS generation was assessed. EU1 cells were pretreated with NAC (1 mM) for 30 min followed by Dox administration (5 μM) for various time points up to 1 h (Fig. 2). Results show that NAC pretreatment was able to inhibit Dox-induced ROS in the EU1 cells. The deviation between the ROS profiles for EU1 cells with and without NAC pretreatment was evident by 30 min of Dox treatment and became more pronounced for the remainder of the treatment duration (Fig. 2). While the EU1 cells without NAC pretreatment experienced increased Dox-induced ROS levels up to 2x their baseline values, the NAC pretreated EU1 cells were able to maintain their baseline ROS levels for the majority of the treatment.
Differential sensitivities of NF-κB-related proteins result in selective ROS-induced IKK-β S-glutathionylation
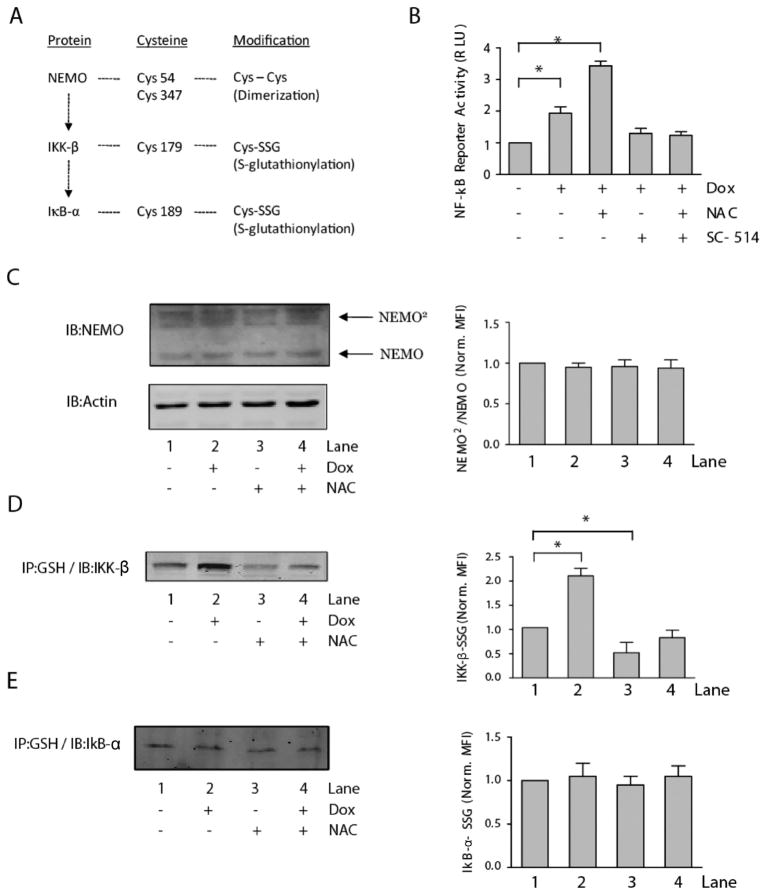
The role that ROS play in regulating NF-κB activation has been addressed previously in the literature and multiple points of redox regulation have been reported, such as the disulfide-based dimerization of NEMO, the S-glutathionylation of IKK-β, and the S-glutathionylation of IκB-α14 (Fig. 3A). However, if a greater understanding of the mechanism by which Dox alters this pathway is to be achieved, it is necessary to determine the specific components in the pathway that are susceptible to Dox-induced ROS. Luciferase-transduced EU1 cells were treated for 1 h with various combinations of NAC, Dox, and the IKK-β inhibitor, SC-514; the effects of these varied treatment conditions on NF-κB activation were quantified by plate reader measurement of luciferase luminescence (Fig. 3B). Dox treatment of luciferase-transduced EU1 cells led to a significant increase in NF-κB reporter activity. NAC pretreatment prior to Dox administration resulted in an even greater response in NF-κB reporter activity; luciferase luminescence values were almost 2x that of the Dox-only treatment group. This is in direct contrast to the effects of NAC on the NF-κB pathway due to other stimulatory conditions, such as TNF-α and phorbol 12-myristate 13-acetate.48 However, when luciferase-transduced cells were pretreated with SC-514 (100 μM) prior to Dox administration, the Dox treatment regimen was no longer able to induce NF-κB reporter activity (Fig. 3B), even after pretreatment with NAC. These results support previous data showing that H2O2 activation of NF-κB is IKK-β-dependent and controlled by redox-mediated mechanisms.9,14
Fig. 3. Differential sensitivities of NF-κB-related proteins result in selective ROS-induced IKK-β S-glutathionylation.
(A) Schematic of a subset of the proteins in the NF-κB activation pathway, their redox sensitive cysteine residues, and the oxidative modifications of the cysteine residues that have been reported to alter NF-κB activity. (B) NF-κB activity, quantified by relative luciferase induction (RLU = relative light units), in untreated and doxorubicin-treated EU1 cells with and without pre-treatment with NAC or IKK-β inhibitor, SC-514. Representative immunoblot analysis, with accompanying densitometry quantification normalized to lane 1, of (C) doxorubicin-induced NEMO dimerization, (D) doxorubicin-induced IKK-β S-glutathionylation, and (E) doxorubicin-induced IκB-α S-glutathionylation in EU1 cells, with and without NAC pretreatment. ([NAC] = 1 mM, 30 min pre-treatment; [SC-514] = 100 μM, 1 h pre-treatment; [Dox] = 5 μM, 4 h treatment; *p < 0.05).
NEMO has been shown to undergo disulfide bond formation during periods of oxidative stress and this process is thought to positively contribute to NF-κB activation;49 therefore the effect of Dox treatment and NAC pretreatment on NEMO dimerization was assessed. Dox treatment did not result in any significant changes to the levels of disulfide bonded NEMO protein in EU1 cells; the same results were seen in the NAC-pretreated cells (Fig. 3C). The addition of a glutathione moiety to an active cysteine residue of a protein can result in structural changes to the protein or blocked access to critical amino acid residues, two processes that can potentially inhibit DNA binding, enzyme attachment, or protein phosphorylation.36 Cys 179 of IKK-β9,14,50 and Cys 189 of IκB-α51 have been reported as potential targets of protein S-glutathionylation with functional consequences on catalytic activity and protein–protein interactions. To assess the effect of Dox treatment on the S-glutathionylation of IKK-β and IκB-α, EU1 cells, with or without NAC pretreatment, were exposed to Dox for 1 h and then lysed. Protein-glutathione mixed disulfides were immuno-precipitated from cytoplasmic lysates with an anti-GSH antibody then run on a western blot to quantify the relative amounts of IKK-β and IκB-α (Fig. 3D and E). Doxorubicin treatment induced increased S-glutathionylation of IKK-β but had insignificant effects on the S-glutathionylation of IκB-α. NAC pretreatment was able to rescue the EU1 cells from Dox-induced IKK-β S-glutathionylation; however, NAC pretreatment had no effect on the low levels of Dox-induced IκB-α S-glutathionylation that were quantified (Fig. 3D and E). Interestingly, NAC pre-treatment was able to decrease the level of S-glutathionylated IKK-β independent of Dox administration.
Our results suggest that in EU1 cells, NEMO dimerization and IκB-α S-glutathionylation are two processes which are insensitive to ROS induced by Dox (Fig. 3C and E). While it has been shown in the literature that NEMO dimerization does occur within mammalian cells,49 and is a necessary step for some methods of NF-κB activation,52 our results are consistent with reports that in some cell lines NEMO dimer formation remains unaltered upon stimulation.52 This insensitivity may be the result of the spatial nature of cellular ROS production by Dox, or of higher than normal basal levels of oxidants within the EU1 cells. Certain cancer cells are considered to be under oxidative stress conditions even in the absence of external stimuli.53,54 This basal oxidative condition could potentially induce substantial amounts of NEMO dimerization in the absence of external stimuli, which would preclude additional NEMO dimer formation during cell stimulation. This dependency of NEMO dimerization on the intracellular redox environment is further supported by the fact that cells of different lineages exhibit significantly different levels of NEMO dimerization under basal conditions.49 Alternatively, it may be possible that while IKK-β-SSG formation is dependent on disulfide covalent adduct formation, the S-glutathionylation of IκB-α may result from non-disulfide covalent addition of GSH via a lipid-peroxidation-based mechanism.55 Additional investigation will be necessary to determine if the anti-GSH antibody utilized in this study is able to recognize non-disulfide covalent adducts involving GSH.
The administration of Dox to the EU1 cells did not result in significant increases in the level of IκB-α-SSG, but did result in increases in the level of IKK-β-SSG (Fig. 3D and E). While both of these processes involve protein-S glutathionylation,51 it appears that the sensitivity of IKK-β to this modification is substantially greater than that of IκB-α. In fact, previous work by Shelton et al. highlights the formation of S-glutathionylated IKK-β under basal conditions,56 further supporting the idea that IKK-β is readily sensitive to oxidative modification. Differential sensitivity of cysteine residues is not an unprecedented idea; however, most of the studies that have provided insight into differential sensitivity have utilized the exogenous administration of oxidants in an in vitro system to accurately characterize the relative susceptibility of certain protein thiol species to oxidation.11,12 This study provides evidence that ROS induced endogenously by the metabolic bioactivation of Dox in a cellular system can also result in differential protein oxidation. While IκB-α S-glutathionylation at Cys 189 has been proposed as a potential mechanism by which NF-κB is regulated,51 the results of our study suggest that this is not a relevant mechanism by which NF-κB is regulated in EU1 cells under the treatment conditions applied.
NAC can modulate glutathione redox status and control Dox-induced IKK-β S-glutathionylation and NF-κB activity in a dose-dependent manner
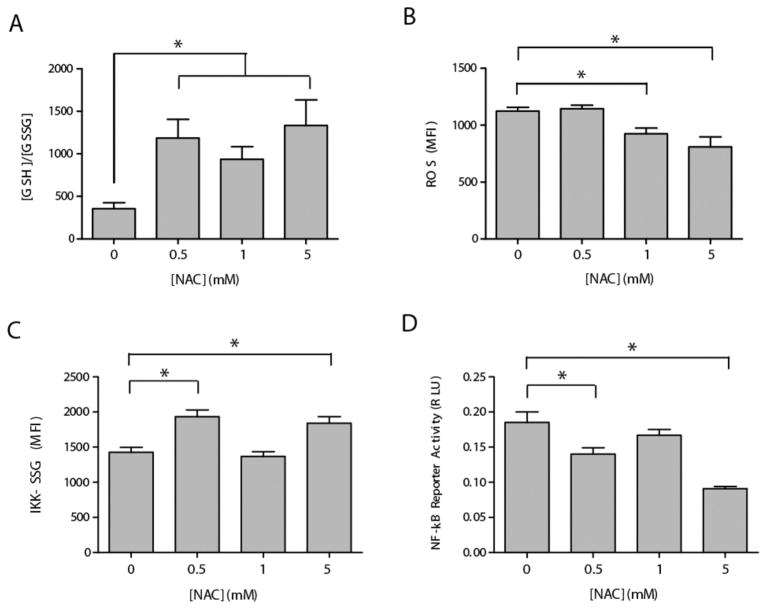
To examine the effect of NAC pretreatment on Dox-induced glutathione-based redox signaling, EU1 cells were pretreated with various concentrations of NAC (0.5–5 mM) to alter the intracellular GSH/GSSG redox state prior to Dox treatment. The EU1 cells were pretreated with designated concentrations of NAC for 30 min followed by Dox (500 nM) administration for 1 h. A Dox concentration of 500 nM was utilized because a previous model of Dox bioactivation21 suggested a more substantial role for Dox-mediated signaling at low Dox concentrations. After Dox treatment, the intracellular glutathione redox balance was quantified (Fig. 4A). NAC pretreatment prior to Dox administration promoted increased ratios of GSH/GSSG. The 0.5 and 5 mM NAC pretreatment conditions resulted in GSH/GSSG values that were approximately 3× higher than the GSH/GSSG values measured in the absence of NAC pretreatment. The 1 mM NAC pretreatment condition, though it too resulted in increased GSH/GSSG values compared to the Dox-only controls, showed a slightly decreased GSH/GSSG compared to the 0.5 mM and 5 mM treatment groups (Fig. 4A). Dox-induced ROS formation was also quantified as a function of NAC pretreatment condition (Fig. 4B). The 0.5 mM NAC pretreatment group showed no significant difference in Dox-induced ROS formation compared to the Dox-only controls. Significant decreases in Dox-induced ROS, compared to the Dox-only controls, were seen only in the 1 mM and 5 mM NAC pretreatment groups.
Fig. 4. NAC can modulate glutathione redox status and control Dox-induced IKK-β S-glutathionylation and NF-κB activity in a dose-dependent manner.
(A) and (B) EU1 cells were treated with various concentrations of NAC prior to doxorubicin administration. (A) the intracellular GSH/GSSG ratio as quantified by an enzymatic recycling assay, and (B) intracellular ROS measurements of DCF fluorescence. (C) and (D) Luciferase-transfected and non-transfected EU1 cells, respectively, were pretreated with various concentrations of NAC prior to doxorubicin administration and then lysed. (C) intracellular levels of S-glutathionylated IKK-β quantified by IP and western blot, and (D) NF-κB reporter activity as detected by luciferase luminescence.
To examine the effect of altered glutathione redox balance on Dox-induced NF-κB activity, non-transduced and luciferase-transduced EU1 cells were pretreated for 30 min with various concentrations of NAC (0.5–5 mM) prior to Dox (500 nM) administration for 1 h. After treatment, the intra-cellular concentration of S-glutathionylated IKK-β was quantified in the non-transduced EU1 cells, while Dox-induced NF-κB was quantified in the luciferase-transduced EU1 cells (Fig. 4C and D). Unexpectedly, none of the NAC pretreatment conditions were able to inhibit Dox-induced IKK-β S-glutathionylation at the 500 nM [Dox] condition (Fig. 4C), as was previously seen for the 5 μM [Dox] treatment condition (Fig. 3D). Both the 0.5 mM and the 5 mM NAC pretreatment groups led to an increased level of Dox-induced IKK-β S-glutathionylation. The 1 mM NAC pretreatment group exhibited the same amount of Dox-induced IKK-β S-glutathionylation as the Dox-only treatment group (Fig. 4C). Correspondingly, none of the NAC pretreatment groups were able to promote Dox-induced NF-κB activity to levels that were significantly higher than the Dox-only group (Fig. 4D), a phenomenon that was exhibited at the 5 μM [Dox] treatment condition (Fig. 3A). Both the 0.5 mM and the 5 mM NAC pretreatment groups experienced significantly less Dox-induced NF-κB activity compared to the Dox-only group. NAC pretreatment at the 1 mM level did not significantly affect Dox-induced NF-κB activity compared to the Dox-only controls (Fig. 4D).
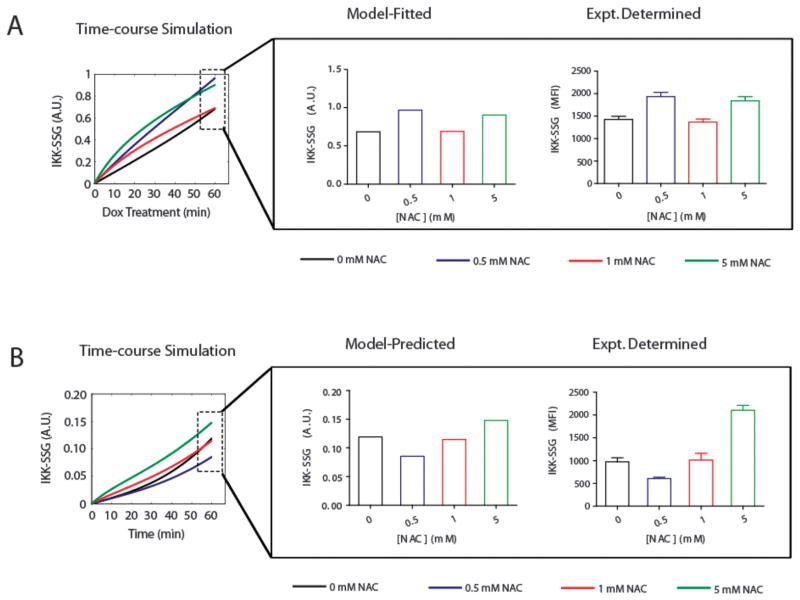
An electron-transfer-based mathematical model elucidates the pro-oxidant and antioxidant mechanisms by which NAC modulates Dox-induced IKK-β S-glutathionylation
The biphasic trends we observed with changing NAC dosage required the development of a mathematical model to help elucidate the pro-oxidant and antioxidant mechanisms by which NAC modulates Dox-induced IKK-β S-glutathionylation. Using the mathematical model, literature-reported pro-oxidant and antioxidant effects of NAC treatment were systematically simulated and tested for their ability to reproduce the complex IKK-β S-glutathionylation behavior under the various NAC pretreatment conditions that were applied.
NAC treatment is known to induce GSH synthesis, thus with increasing levels of NAC the rate constant for GSH synthesis was proportionally increased according to published data on the effect of NAC treatment on the rate of GSH synthesis in the erythrocyte cell.40 The rates of GSH synthesis for the four NAC treatment conditions are shown in Table 3. Literature-reported qualitative details describing NAC-dependent protein thiyl41 and GSH thiyl radical formation rates42–44 were translated into semi-quantitative rules that were used to determine the extracellular NAC pretreatment concentrations at which a 3-fold increase in the protein thiyl radical formation rate and a 2-fold decrease in the GSH thiyl radical formation rate would initially occur (Fig. S2). The 3-fold and 2-fold effects of NAC pretreatment on the RS• and GS• formation rates were deduced from literature-published studies and the related redox-reactions derived from those studies.12,40–44 An illustration of the systematic process by which the model-fitting was carried out is shown in Supplemental Fig. S2.
The results of the model-fitting simulations predict that the two processes susceptible to NAC modulation (apart from the well characterized GSH synthesis reactions (R9)) exhibit their NAC-susceptibility at different extracellular NAC concentrations (Table 3). The model confirms literature reports that show increasing concentrations of NAC can result in a threshold-dependent increase in the rate of protein thiyl radical formation (R5)43 and the model predicts that increasing concentrations of NAC can result in a threshold-dependent decrease in the rate of GSH thiyl formation (R6). Moreover, the model-predicted minimum [NAC] that produces the threshold-dependent decrease in the rate of GSH thiyl radical formation is higher than the model-predicted minimum [NAC] that produces the threshold-dependent increase in the rate of protein thiyl radical formation (Table 3). The dynamic mechanistic model behavior was obtained by fitting to the experimental dataset of Dox-induced IKK-β S-glutathionylation (Fig. 5A). To test the universality of the model-predicted mechanism of IKK-β S-glutathionylation, the model-predicted step-like responses of (R5) and (R6) and the graded response of (R9) to increasing NAC concentrations were tested in their ability to predict IKK-β S-glutathionylation as a result of a new experimental condition, NAC treatment only, independent of Dox. The mechanistic model was able to accurately predict NAC-dependent IKK-β S-glutathionylation in the absence of Dox treatment for a variety of NAC pretreatment conditions (Fig. 5B) without any additional model fitting.
Fig. 5. An electron-transfer-based computational model predicts pro-oxidant and anti-oxidant mechanisms by which NAC modulates Dox-induced IKK-β S-glutathionylation.<.
br>(A) Model-fitted and experimentally-derived values for doxorubicin-induced IKK-β S-glutathionylation after various NAC pretreatment regimens. (B) Model-predicted and experimentally-derived values for IKK-β S-glutathionylation after various NAC pretreatment regimens without doxorubicin treatment.
The RS• and GS• radical formation rates elicited different threshold responses to increasing concentrations of extra-cellular NAC (Table 3). The model predicts that the NAC-dependent 3-fold increase in the rate of RS• formation occurs at relatively low NAC pretreatment concentrations (0.5 mM), whereas, the NAC-dependent 2-fold decrease in the rate of GS• formation occurs at relatively high NAC pretreatment concentrations (5 mM). The predicted behavior of the RS• formation rate as a result of NAC pretreatment suggests that the level of H2O2 induced by a 500 nM Dox treatment regimen is high enough to promote the Fenton and Haber-Weiss reactions, which lead to increased protein thiyl radical formation in the presence of NAC.41 The predicted behavior of the GS• formation rate as a result of NAC pretreatment suggests that a relatively high extracellular concentration of NAC is needed to effectively compete with GSH for the quenching of intracellular thiyl radicals.
The varied effects of NAC on the behavior of the system may reflect differences in substrate abundance, or specificity, of the substrates involved in the NAC-dependent redox reactions (Haber-Weiss reaction and the reaction of protein thiyl radicals with GSH thiols). If a low concentration of NAC can promote the formation of thiyl radicals, it can be assumed that within the intracellular milieu oxidized transition metals are in excess compared to their respective protein substrates. On the other hand, if a relatively high concentration of NAC is needed to decrease the rate of formation of GS thiyl radicals, then potentially GSH substrates are in excess of the thiyl radicals with which GSH can react. The consequence of such a system would be the maintenance of equilibrium concentrations of oxidized transition metals that are higher than the equilibrium concentrations of protein thiyl radicals. The literature supports this hypothesis in that greater than 99% of pro-oxidant reactions within the cell are mediated by non-radical oxidants such as metal ions, whereas only 1% of the pro-oxidant reactions are mediated by free radical intermediates such as protein thiyl radicals.37
The effect of NAC supplementation on NF-κB signaling during Dox administration is complex and adaptable
The dynamic IKK-β S-glutathionylation responses observed for varying treatment combinations of Dox and NAC suggest that the pro-oxidant and antioxidant effects of NAC administration are dependent on the level of Dox to which the cells are exposed. We observed that for the 500 nM Dox treatment condition, none of the NAC pretreatment groups were able to promote Dox-induced NF-κB activity to levels that were significantly higher than the Dox-only group (Fig. 4D); this phenomenon was only observed at the 5 μM [Dox] treatment condition (Fig. 3B). This behavior suggests that at a high [Dox] condition, NAC’s antioxidant function is more dominant than its pro-oxidant function, whereas, at the low [Dox] condition, the opposite occurs. One way in which this concentration-dependent behavior could be realized is if the intracellular metabolism of Dox itself is concentration-dependent. Previous work conducted in our laboratory support the idea that Dox-metabolism in EU1 cells is a concentration-dependent process.21 The ability of NAC to promote or hinder S-glutathionylation of IKK-β may be dependent on intracellular ROS levels, a parameter that can be regulated by intracellular Dox concentration.21 This discrepancy suggests that the intracellular metabolism of Dox, a process that generates ROS, or perhaps even intracellular transport of doxorubicin, may dictate the ability of NAC to alter Dox-induced NF-κB activation in leukemia cells. If this is the case, then one would expect to see varied degrees of NAC-dependent protein oxidation as a function of varying Dox concentrations.
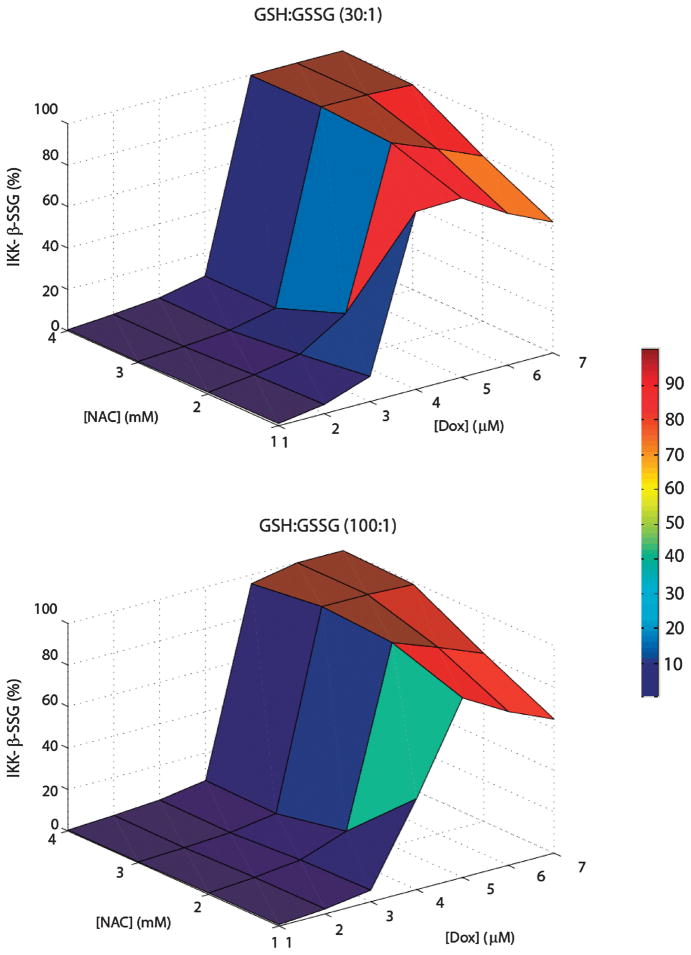
Using the fitted mathematical model of IKK-β S-glutathionylation, we tested if varying dose combinations of NAC/Dox would produce complex IKK-β S-glutathionylation profiles. in silico, we exposed EU1 cells to a range of Dox/NAC doses and predicted the percentage of IKK-β proteins that would undergo protein S-glutathionylation (Fig. 6). This mechanistic model implementation provides a qualitative description of the variability in Dox-induced IKK-β S-glutathionylation that can be realized in cancer cells that have been exposed to different doses of NAC prior to Dox administration (Fig. 6). If ROS is a critical factor determining the net effect of NAC supplementation during Dox administration, then variability in glutathione redox balance, a measure of a cell’s ROS buffering capacity, would likely alter the level of IKK-β S-glutathionylation that is experienced within a given cell. We tested this hypothesis by simulating and comparing the model-predicted IKK-β S-glutathionylation surfaces for EU1 cells with a GSH : GSSG redox balance of 30 : 1 and EU1 cells with a GSH : GSSG balance of 100 : 1, corresponding to a physiological range reported for mammalian cells.57 The model predicts different surface contours for these two GSH : GSSG redox potentials, suggesting that the effect of NAC supplementation during Dox treatment is not only complex, but also adaptable, based on the glutathione redox balance exhibited by the cell (Fig. 6).
Fig. 6. The combinatorial effect of NAC and Dox on NF-κB signaling is complex and adaptable.
In silico predictions of the degree of IKK-β S-glutathionylation (% of total) that would occur after 1 h treatments with various dose combinations of Dox and NAC under two distinctly different GSH : GSSG redox balances (30 : 1 and 100 : 1). Color scale represents the percentage of IKK-β-SSG.
This analysis illustrates that the effect of antioxidant supplementation during chemotherapy is highly dependent on the antioxidant and chemotherapy dosage that is applied. These in silico predictions on the effect of NAC supplementation for a range of initial Dox dosages offer some insight into the ability of NAC to affect multiple electron-transfer reactions in dynamic and uniquely independent ways. However, these results are only approximations of what may occur in vivo. The model contains only a few potential reactions, the concentrations of the species are not directly known, and the reactions are carried out in the absence of substrate recycling. Nonetheless, the concept that varied dose combinations of antioxidants and chemotherapy can produce variability in chemotherapy-induced intracellular signaling is wholly supported by this study.
The current model represents a proof of principle and as such provides a preliminary view of the consequences that certain pro- and antioxidant pathways can have on an upstream redox-sensitive protein within the NF-κB signaling pathway, namely IKK-β. It should be noted, however, that the model and the results generated from the model, are incomplete in their scope. Some of the explanations given for the reactions contained in the model are speculative in nature and based on limited availability of experimental data on reaction rates and protein interactions that occur in vivo. Future experimental perturbations of the system in question and additional quantification of pertinent reaction parameters will allow for further model refinement and more accurate descriptions of the pro- and antioxidant effects of NAC on the upstream protein components involved in NF-κB signaling.
Conclusions
Because the efficacy of standard cancer chemotherapy has reached a plateau,58 additional approaches must be developed to improve treatment. To this end, antioxidant supplementation during chemotherapy has emerged as a possible complementary strategy. The goal of this study was to begin addressing the functional consequences of antioxidant supplementation during chemotherapy administration by focusing on the role that NAC, an antioxidant, plays in modulating the chemotherapy-induced activity of a relevant signal transduction pathway known to regulate cancer cell growth and proliferation.
The results of this study provide a mechanistic rationale for the way in which NAC supplementation can regulate Dox-induced NF-κB signal transduction in ALL cells by coupling a redox-sensitive protein to an antioxidant/free radical network. If NF-κB activation is a mechanism by which cancer cells elude chemotherapy induced toxicity, then the systematic modulation of the glutathione redox balance through the controlled administration of NAC or other antioxidants, could potentially serve as a simple therapeutic measure to combat chemotherapy-induced drug resistance development. As NAC continues to be assessed for its cytoprotective capabilities in non-cancerous cell types during chemotherapy administration59 and its role in mitigating cancer cell proliferation and signaling,60 it is worth considering that the pro- and antioxidant effects of this molecule, induced by the competing reactions shown in this study, could prove to be deleterious in some cell types and beneficial or innocuous in others. The ability of NAC to modify NF-κB signal transduction during chemotherapy administration is dependent on the intracellular redox environment characterized by GSH/GSSG balance and this parameter varies from one cancer cell type to the next.
Although this study does not answer all questions related to antioxidant supplementation during chemotherapy, it does provide meaningful direction regarding the way in which researchers should approach the issue. The results of this study suggest that previous contradictory findings regarding antioxidant supplementation during chemotherapy were most likely due to the extrapolation of results from one experimental condition to the next, with little to no distinction between antioxidant type, antioxidant dose, or even chemotherapy treatment duration. The redox-based framework presented in this paper utilized logical analysis, based on simple redox-reactions, to determine the possible effects of antioxidant supplementation on a potentially important signaling pathway. In the future, the redox-based framework presented in this study could be modified to examine the role that clinically used antioxidants will have in combination with other frequently used chemotherapeutic agents.
Supplementary Material
Acknowledgments
The authors would like to express their thanks to Debika Mitra for her extensive help with the IP protocol utilized in this study.
Footnotes
Electronic supplementary information61 available. See DOI: 10.1039/c1mb05315a
Notes and references
- 1.Gupta D, Lis CG, Birdsall TC, Grutsch JF. Supportive Care Cancer. 2005;13:912–919. doi: 10.1007/s00520-005-0820-9. [DOI] [PubMed] [Google Scholar]
- 2.Richardson MA, Sanders T, Palmer JL, Greisinger A, Singletary SE. J Clin Oncol. 2000;18:2505–2514. doi: 10.1200/JCO.2000.18.13.2505. [DOI] [PubMed] [Google Scholar]
- 3.Labriola D, Livingston R. Oncology (Williston Park) 1999;13:1003–1008. discussion 1008, 1011-1002. [PubMed] [Google Scholar]
- 4.Chinery R, Brockman JA, Peeler MO, Shyr Y, Beauchamp RD, Coffey RJ. Nat Med. 1997;3:1233–1241. doi: 10.1038/nm1197-1233. [DOI] [PubMed] [Google Scholar]
- 5.Mediavilla MD, Cos S, Sanchez-Barcelo EJ. Life Sci. 1999;65:415–420. doi: 10.1016/s0024-3205(99)00262-3. [DOI] [PubMed] [Google Scholar]
- 6.Schmitt CA, Lowe SW. J Pathol. 1999;187:127–137. doi: 10.1002/(SICI)1096-9896(199901)187:1<127::AID-PATH251>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 7.Nordberg J, Arnér ESJ. Free Radical Biol Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 8.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 9.de Oliveira-Marques V, Cyrne L, Marinho HS, Antunes F. J Immunol. 2007;178:3893–3902. doi: 10.4049/jimmunol.178.6.3893. [DOI] [PubMed] [Google Scholar]
- 10.Ghezzi P. Free Radical Res. 2005;39:573–580. doi: 10.1080/10715760500072172. [DOI] [PubMed] [Google Scholar]
- 11.Winterbourn CC, Metodiewa D. Free Radical Biol Med. 1999;27:322–328. doi: 10.1016/s0891-5849(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 12.Winterbourn CC, Hampton MB. Free Radical Biol Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Jones DP, Go YM, Anderson CL, Ziegler TR, Kinkade JM, Jr, Kirlin WG. FASEB J. 2004;18:1246–1248. doi: 10.1096/fj.03-0971fje. [DOI] [PubMed] [Google Scholar]
- 14.Oliveira-Marques V, Marinho HS, Cyrne L, Antunes F. Antioxid Redox Signaling. 2009;11:2223–2243. doi: 10.1089/ars.2009.2601. [DOI] [PubMed] [Google Scholar]
- 15.Wang S, Kotamraju S, Konorev E, Kalivendi S, Joseph J, Kalyanaraman B. Biochem J. 2002;367:729–740. doi: 10.1042/BJ20020752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dolcet X, Llobet D, Pallares J, Matias-Guiu X. Virchows Arch. 2005;446:475–482. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- 17.Zhou M, Gu L, Zhu N, Woods WG, Findley HW. Oncogene. 2003;22:8137–8144. doi: 10.1038/sj.onc.1206911. [DOI] [PubMed] [Google Scholar]
- 18.Lin X, Li Q, Wang YJ, Ju YW, Chi ZQ, Wang MW, Liu JG. Biochem J. 2007;406:215–221. doi: 10.1042/BJ20070186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bednarski BK, Baldwin AS, Jr, Kim HJ. PLoS One. 2009;4:e6992. doi: 10.1371/journal.pone.0006992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou M, Gu L, Findley HW, Jiang R, Woods WG. Cancer Res. 2003;63:6357–6362. [PubMed] [Google Scholar]
- 21.Finn NA, Findley HW, Kemp ML. PLoS Comput Biol. 2011;7:e1002151. doi: 10.1371/journal.pcbi.1002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adimora NJ, Jones DP, Kemp ML. Antioxid Redox Signaling. 2010;13:731–743. doi: 10.1089/ars.2009.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antunes F, Cadenas E. FEBS Lett. 2000;475:121–126. doi: 10.1016/s0014-5793(00)01638-0. [DOI] [PubMed] [Google Scholar]
- 24.Makino N, Sasaki K, Hashida K, Sakakura Y. Biochim Biophys Acta, Gen Subj. 2004;1673:149–159. doi: 10.1016/j.bbagen.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki K, Bannai S, Makino N. Biochim Biophys Acta, Gen Subj. 1998;1380:275–288. doi: 10.1016/s0304-4165(97)00152-9. [DOI] [PubMed] [Google Scholar]
- 26.Seaver LC, Imlay JA. J Bacteriol. 2001;183:7182–7189. doi: 10.1128/JB.183.24.7182-7189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Chem-Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Bachur NR, Gordon SL, Gee MV, Kon H. Proc Natl Acad Sci U S A. 1979;76:954–957. doi: 10.1073/pnas.76.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cummings J, Allan L, Willmott N, Riley R, Workman P, Smyth JF. Biochem Pharmacol. 1992;44:2175–2183. doi: 10.1016/0006-2952(92)90344-i. [DOI] [PubMed] [Google Scholar]
- 30.Sinha BK, Mimnaugh EG, Rajagopalan S, Myers CE. Cancer Res. 1989;49:3844–3848. [PubMed] [Google Scholar]
- 31.Kostrzewa-Nowak D, Paine MJI, Wolf CR, Tarasiuk Br J. J Cancer. 2005;93:89–97. doi: 10.1038/sj.bjc.6602639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menna P, Recalcati S, Cairo G, Minotti G. Cardiovasc Toxicol. 2007;7:80–85. doi: 10.1007/s12012-007-0011-7. [DOI] [PubMed] [Google Scholar]
- 33.Ravi D, Das KC. Cancer Chemother Pharmacol. 2004;54:449–458. doi: 10.1007/s00280-004-0833-y. [DOI] [PubMed] [Google Scholar]
- 34.Kalyanaraman B, Perez-Reyes E, Mason RP. Biochim Biophys Acta, Gen Subj. 1980;630:119–130. doi: 10.1016/0304-4165(80)90142-7. [DOI] [PubMed] [Google Scholar]
- 35.D’Autreaux B, Toledano MB. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 36.Dalle-Donne I, Rossi R, Colombo G, Giustarini D, Milzani A. Trends Biochem Sci. 2009;34:85–96. doi: 10.1016/j.tibs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Jones DP. Am J Physiol Cell Physiol. 2008 doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koshkin V, Lotan O, Pick E. Biochim Biophys Acta, Bioenerg. 1997;1319:139–146. doi: 10.1016/s0005-2728(96)00154-5. [DOI] [PubMed] [Google Scholar]
- 39.Ozols RF, Locker GY, Doroshow JH, Grotzinger KR, Myers CE, Young RC. Cancer Res. 1979;39:3209–3214. [PubMed] [Google Scholar]
- 40.Raftos JE, Whillier S, Kuchel PW. J Biol Chem. 2010;285:23557–23567. doi: 10.1074/jbc.M109.067017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nappi AJ, Vass E. Biochim Biophys Acta, Gen Subj. 1997;1336:295–302. doi: 10.1016/s0304-4165(97)00039-1. [DOI] [PubMed] [Google Scholar]
- 42.O’Brien PJ. Free Radical Biol Med. 1988;4:169–183. doi: 10.1016/0891-5849(88)90025-1. [DOI] [PubMed] [Google Scholar]
- 43.Sagrista ML, Garcia AE, Africa De Madariaga M, Mora M. Free Radical Res. 2002;36:329–340. doi: 10.1080/10715760290019354. [DOI] [PubMed] [Google Scholar]
- 44.Halliwell B. Am J Med. 1991;91:S14–S22. [Google Scholar]
- 45.Burgunder JM, Varriale A, Lauterburg BH. Eur J Clin Pharmacol. 1989;36:127–131. doi: 10.1007/BF00609183. [DOI] [PubMed] [Google Scholar]
- 46.Tergaonkar V, Bottero V, Ikawa M, Li Q, Verma IM. Mol Cell Biol. 2003;23:8070–8083. doi: 10.1128/MCB.23.22.8070-8083.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qanungo S, Starke DW, Pai HV, Mieyal JJ, Nieminen AL. J Biol Chem. 2007;282:18427–18436. doi: 10.1074/jbc.M610934200. [DOI] [PubMed] [Google Scholar]
- 48.M. Staal Fj Fau - Roederer, L. A. Roederer M Fau - Herzenberg, L. A. Herzenberg La Fau - Herzenberg, L. A. Herzenberg, G. R. Schafer Fq Fau - Buettner and G. R. Buettner.
- 49.Herscovitch M, Comb W, Ennis T, Coleman K, Yong S, Armstead B, Kalaitzidis D, Chandani S, Gilmore TD. Biochem Biophys Res Commun. 2008;367:103–108. doi: 10.1016/j.bbrc.2007.12.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reynaert NL, van der Vliet A, Guala AS, McGovern T, Hristova M, Pantano C, Heintz NH, Heim J, Ho YS, Matthews DE, Wouters EF, Janssen-Heininger YM. Proc Natl Acad Sci U S A. 2006;103:13086–13091. doi: 10.1073/pnas.0603290103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kil IS, Kim SY, Park JW. Biochem Biophys Res Commun. 2008;373:169–173. doi: 10.1016/j.bbrc.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 52.Marienfeld RB, Palkowitsch L, Ghosh S. Mol Cell Biol. 2006;26:9209–9219. doi: 10.1128/MCB.00478-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marnett LJ. Carcinogenesis. 2000;21:361–370. doi: 10.1093/carcin/21.3.361. [DOI] [PubMed] [Google Scholar]
- 54.Mates JM, Perez-Gomez C, Nunez de Castro I. Clin Biochem. 1999;32:595–603. doi: 10.1016/s0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 55.Zhu X, Gallogly MM, Mieyal JJ, Anderson VE, Sayre LM. Chem Res Toxicol. 2009;22:1050–1059. doi: 10.1021/tx9000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shelton MD, Distler AM, Kern TS, Mieyal JJ. J Biol Chem. 2009;284:4760–4766. doi: 10.1074/jbc.M805464200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schafer FQ, Buettner GR. Free Radical Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 58.Prasad KN. Expert Rev Anti-Infect Ther. 2003;3:903–915. [Google Scholar]
- 59.Shi R, Huang CC, Aronstam R, Ercal N, Martin A, Huang YW. BMC Pharmacol. 2009;9:7. doi: 10.1186/1471-2210-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perera RM, Bardeesy N. Nature. 2011;475:43–44. doi: 10.1038/475043a. [DOI] [PubMed] [Google Scholar]
- 61.Lupetti A, Paulusma-Annema A, Senesi S, Campa M, Van Dissel J, Nibbering P. Antimicrob Agents Chemother. 2002;46:1634–1639. doi: 10.1128/AAC.46.6.1634-1639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.