Abstract
OBJECTIVES
Prior studies showed that immune inhibitory CD34+ progenitor cells, whose numbers are increased in head and neck squamous cell carcinoma (HNSCC) patients, can be differentiated into immune stimulatory dendritic cells by culture with 1α,25-dihydroxyvitamin D3 (1,25[OH]2D3). This was extended to a pilot study to diminish intratumoral levels of CD34+ progenitor cells by inducing their maturation into dendritic cells with 1,25(OH)2D3.
STUDY DESIGN
Newly diagnosed HNSCC patients were untreated for 3 weeks or received 3 weeks of 1,25(OH)2D3 treatment befoer surgical treatment.
SUBJECTS AND METHODS
HNSCC tissue was collected by biopsy from six patients who had no prior 1,25(OH)2D3 treatment and at the time of surgical treatment from six untreated patients and 11 patients who completed 1,25(OH)2D3 treatment. Tissues were analyzed by immunohistochemistry for levels of CD34+ cells and dendritic cells.
RESULTS
After 1,25(OH)2D3 treatment, intratumoral levels of CD34+ cells and levels of immature dendritic cells declined. However, levels of intratumoral mature dendritic cells increased. Clinical effects of 1,25(OH)2D3 treatment are premature to analyze.
CONCLUSIONS
Treatment of HNSCC patients with 1,25(OH)2D3 reduced levels of immune inhibitory CD34+ cells while increasing maturation of dendritic cells. This supports added studies to determine the effect of 1,25(OH)2D3 on intratumoral immune competence.
Head and neck squamous cell carcinoma (HNSCC) is an aggressive disease with a 5-year mortality rate of approximately 50 percent. Patients with HNSCC have profound immune defects that are associated with increased recurrence.1 Previous work investigated the contribution of CD34+ progenitor cells, whose levels are increased in HNSCC patients, to this immune dysfunction.2 CD34+ cells are hematopoietic progenitor cells that intensely express the CD34 marker as opposed to the dimmer level of expression by endothelial cells. Their numbers are elevated in patients with HNSCC, and they exhibit nonspecific suppression of T-cell function. In healthy individuals, CD34+ cell levels are less than 1 percent of the peripheral blood mononuclear leukocyte population in contrast to patients with HNSCC in whom they compose approximately 5 percent of the peripheral blood mononuclear leukocyte population.3 HNSCC-derived granulocyte-macrophage colony stimulating factor (GM-CSF) stimulates the expansion and mobilization of CD34+ cells into the peripheral blood and tumor tissue from bone marrow. CD34+ cells mediate suppression of T-cell functions by secreting transforming growth factor-beta (TGF-β).4
The CD34+ cells from HNSCC patients are progenitor cells because they can grow in soft agar containing colonystimulation factors into colonies containing monocytes and neutrophils.2 Further studies examined the functional and clinical significance of GM-CSF and CD34+ cells in patients with HNSCC. The depletion of CD34+ cells from the peripheral blood of HNSCC patients increased the proliferative capacity of intratumoral T cells.2 Patients with high levels of GM-CSF, the CD34+-mobilizing cytokine, had a corresponding increase in intratumoral CD34+ cells. Examination of 27 patients with HNSCC showed 19 of them to have readily detectable CD34+ cell levels in the peripheral blood, whereas seven control patients did not have detected CD 34+ cells.3 When GM-CSF and CD34+ levels were analyzed in relation to patients’ T stage and nodal status, GM-CSF and CD34+ levels were increased as T stage increased.5 These levels increased more prominently at all T stages with cervical nodal involvement. Twenty HNSCC patients with high levels of GM-CSF and CD34+ cells and 17 HNSCC patients with low levels of GM-CSF and CD34+ cells were evaluated for whether GM-CSF production and the presence of CD34+ cell within primary HNSCC tissue translated into increased recurrence or metastasis during the 2 years after surgical excision.6 This study found a four-fold increase in the level of GM-CSF and a two-and-a-half-fold increase in CD34+ cell levels in the patients who subsequently developed recurrences or metastatic disease. This suggests that increased levels of immune inhibitory CD34+ cells could be decreasing the intratumoral T-cell defenses, leading to decreased disease-free survival.
Bone marrow–derived CD34+ progenitor cells have the capacity to differentiate into immune stimulatory dendritic cells.7 Dendritic cells are professional antigen-presenting cells, and they can activate naive T cells. They present antigens in the context of class II MHC and secrete interleukin (IL)-12, a T-cell activating cytokine. Unfortunately, dendritic cell maturation is defective in cancer patients.8 Although immature dendritic cells can induce T-cell tolerance, mature dendritic cells are potent stimulators of the primary immune system and can stimulate antitumor immunity.9
Because of the potent immune stimulatory effects of mature dendritic cells, studies determined if the immune inhibitory CD34+ cells that are mobilized in HNSCC patients could be differentiated into immune stimulatory dendritic cells. CD34+ cells from the peripheral blood of HNSCC patients that were grown in a media containing GM-CSF, stem cell factor, and tumor necrosis factor differentiated into cells that were phenotypically homologous to dendritic cells.10 The efficiency of the differentiation was approximately 25 percent. The functional competence of these cells was shown by their ability to present the tetanus toxoid antigen to autologous T cells and stimulate their intracellular expression of interferon-gamma (IFN-γ).5 Additional studies showed that the supplementation of the CD34+ cell cultures with 1α,25-dihydroxyvitamin D3 (1,25[OH]2D3) further doubled the number of cells that differentiated into dendritic cells.3 In mice bearing tumors, vitamin D3 reduced the levels of CD34+ cells and stimulated antitumor immunity.10 In a phase 1B clinical trial with patients having advanced HNSCC, 25-hydroxyvitamin D3 was shown to reduce the number of peripheral blood CD34+ cells, increase HLA-DR expression (marker for antigen presenting cells), increase plasma IL-12 (a marker of active antigen-presenting cells), increase plasma IFN-γ, and improve activation of patients’ peripheral blood T cells.11 However, this clinical trial was not designed to analyze the effect of vitamin D3 analogs on the composition of CD34+ cells or antigen-presenting cells within the tumor tissue. The present study extended these prior studies by analyzing how 1,25(OH)2D3 affects intratumoral levels of CD34+ cells and dendritic cells. This study found that 1,25(OH)2D3 significantly dropped intratumoral levels of CD34+ cells and levels of immature dendritic cells. In contrast, levels of mature dendritic cells within the HNSCC tissue significantly increased.
MATERIALS AND METHODS
Patients with HNSCC
Recruitment of patients into this study was approved by the Institutional Review Board of record. Patients who were newly diagnosed with stage II-IV HNSCC who were being scheduled for surgery were eligible for enrollment into this randomized trial with preoperative treatment of 1,25(OH)2D3 (calcitriol). Control patients with HNSCC did not receive 1,25(OH)2D3 treatment. Patients were excluded if they had received prior immunotherapy or radiation treatment in the previous 3 weeks or had concurrent malignancies. Patients’ clinical pathologic data were reviewed in regards to cancer stage, presence of perineural invasion, lymphovascular invasion, and cervical nodal disease. This trial is currently ongoing and, thus, it is too early to allow determination of whether 1,25(OH)2D3 treatment impacts on HNSCC recurrence or metastasis, either at the 1-year or 2-year follow-up time points.
Sample-size calculations were estimated from preliminary studies in animal models. Because these preliminary studies led to the anticipation of large effect sizes, a sample of 10 subjects in each group was estimated to provide 90 percent power to detect a difference of 1 standard deviation.
1,25(OH)2D3 Treatment and Collection of Specimens
Patients were treated with 4 μg of enteric 1,25(OH)2D3 for each of 3 sequential days followed by 4 days of no treatment. This treatment schedule has previously been shown to have minimal toxicity.12 Nevertheless, serum calcium and parathyroid hormone levels were measured weekly to monitor toxicity. At the conclusion of 3 cycles of this treatment, patients underwent resective surgery. Surgically excised HNSCC tissue was collected from 11 patients who received 1,25(OH)2D3 treatment and six untreated patients. In addition, HNSCC tissue biopsies were obtained with 4-mm cupped forceps from six of these patients at the time of HNSCC diagnosis before randomization into the untreated or 1,25(OH)2D3-treated arms and were used as tissues from untreated patients. Thus, the results of immunohistochemical analyses were compared among tissues from 11 1,25(OH)2D3-treated patients and 12 untreated patients.
Immunohistochemistry
Tissue blocks that were frozen in O.C.T. embedding medium (Miles, Elkhart, IN) were cryosectioned into 10-mm thickness slices and placed on slides. Approximately 50 slides were made per frozen block. Slides were stored at −80°C until use.
After tumor tissue had been cryosectioned, intratumoral CD34+ cells and dendritic cells were recognized by immunohistochemistry. The cryosections were fixed onto slides with 100 percent acetone for 10 minutes. Slides were allowed to dry, and tissue was outlined with a PAP pen (Invitrogen, Carlsbad, CA). Slides were then washed and incubated in phosphate-buffered saline for 10 minutes. Endogenous peroxidase was quenched by incubating tissue in a 0.3 percent H2O2/phosphate-buffered saline solution for 5 minutes. This step was repeated two additional times. Nonspecific antibody was added for 20 minutes so as to bind to Fc epitopes and reduce background staining. Next, the primary antibody and corresponding isotype control antibodies were added to slides and allowed to incubate for 1 hour. Primary antibodies that were used were against CD34 to detect the progenitor cells, DC-LAMP to detect mature dendritic cells, and DC-SIGN to detect immature dendritic cells. Slides were rewashed with buffer for 5 minutes, and then positive staining was visualized using Vecastain ABD immunoperoxidase (Vector Labs, Burlingame, CA) and counterstaining with hematoxylin.
Quantification of Data and Analysis
The number of positive staining cells in each microscopic field was quantitated by individual cell counts. Three random areas of slides were identified and graded by four independent graders. The same four graders were used for each of the primary antibodies. The graders were blinded as to the identity of the tissues. Data for the treated and untreated patients were statistically compared by using a two-tailed Student t test.
Role of the Funding Source
The funding sources, which were the Department of Veterans Affairs and the National Institutes of Health, had no involvement in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
RESULTS
Patient Population
Tissue specimens were collected for immunohistochemical analyses from 11 HNSCC patients who received 1,25(OH)2D3 treatment and six HNSCC patients who were untreated during the 3-week time interval (Table 1). In addition, biopsies were collected from 6 of these patients at the time of diagnosis before randomization into study arms. Patient ages ranged from 42 to 77, and the majority of the cancers originated from the oral cavity. Many of these patients had aggressive disease and a multitude of high risk pathologic risk factors. Eleven patients had stage IV disease.
Table 1.
Patient population
| Age | Sex | Primary | TMN | Stage | Perineural invasion |
ECS | Lymphovascular spread |
Positive margins |
|---|---|---|---|---|---|---|---|---|
| Untreated | ||||||||
| 68 | M | Oral cavity | T4N2b | 4 | No | No | No | Yes |
| 57 | M | Nasal cavity | T1Nx | 1 | Yes | Unknown | Yes | Yes |
| 55 | M | Oral cavity | T3N2b | 4 | No | No | No | Yes |
| 48 | M | Oral cavity | T4N2b | 4 | No | No | No | No |
| 54 | M | Oral cavity | T4N3 | 4 | No | Yes | No | No |
| 77 | F | Oral cavity | T4Nx | 4 | Yes | Unknown | No | No |
| 1,25(OH)2D3 treated | ||||||||
| 58 | M | Oral cavity | T3N0 | 3 | Yes | No | No | No |
| 61 | M | Oral cavity | T4N0 | 4 | Yes | No | Yes | Yes |
| 44 | F | Oral cavity | T4N0 | 4 | Yes | No | No | No |
| 44 | M | Oral cavity | T1N0 | 1 | No | No | No | No |
| 69 | M | Oral cavity | T4N1 | 4 | Yes | No | No | No |
| 75 | F | Oral cavity | T1N0 | 1 | Yes | No | No | No |
| 72 | M | Oral cavity | T3N2C | 4 | No | No | No | Yes |
| 42 | M | Oral cavity | T4N0 | 4 | Yes | No | Yes | No |
| 58 | M | Oral cavity | T2N0 | 2 | Yes | No | Yes | No |
| 74 | F | Oropharynx | T2N0 | 2 | Yes | No | No | Yes |
| 60 | F | Oropharynx | T2N2 | 4 | No | Yes | Yes | No |
M, male; F, female.
Reduced Intratumoral CD34+ Cell Levels in HNSCC Patients Treated with 1,25(OH)2D3
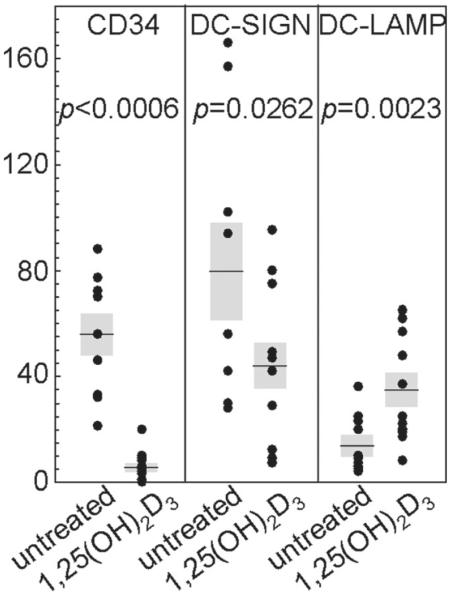
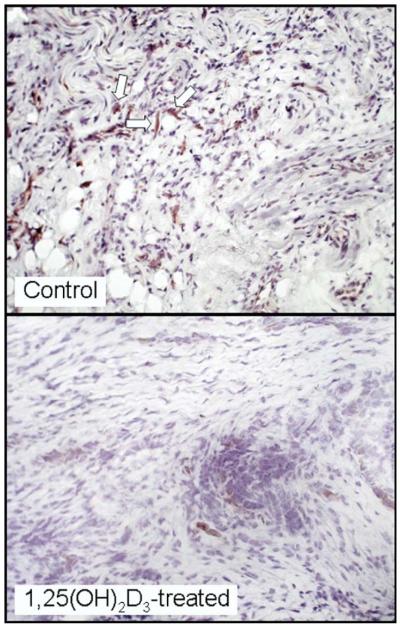
CD34+ progenitor cells have been shown to be potent mediators of immune suppression in patients with HNSCC.13,14 Previous in vitro studies showed that 1,25(OH)2D3 accentuates the differentiation of HNSCC-mobilized CD34+ cells into dendritic cells that can stimulate T-cell reactivity.3,5 Thus, the effect of 1,25(OH)2D3 treatment on intratumoral CD34+ cell levels was assessed. This was accomplished by immunohistochemical staining. The results show that levels of intratumoral CD34+ cells in patients receiving 1,25(OH)2D3 treatment were statistically significantly lower than within HNSCC tissue of untreated patients (P = .006, Fig 1). Figure 2 is a representative microscopic image of the intense staining of CD34+ progenitor cells in HNSCC tissue from an untreated patient and the limited number of CD34+ cells in a 1,25(OH)2D3-treated patient.
Figure 1.
Reduced intratumoral levels of CD34+ cells and immature DC-LAMP+ dendritic cells and increased levels of mature DC-SIGN+ dendritic cells in HNSCC tissue from patients treated with 1,25(OH)2D3. The numbers of immunostained cells per microscopic field were enumerated in HNSCC tissues from untreated and 1,25(OH)2D3-treated patients. Shown are the averages of the numbers of immunostained cells for each patient. The shaded areas represent the SEM.
Figure 2.
Representative microscopic images of reduced intratumoral levels of CD34+ cells in HNSCC tissues from a patient treated with 1,25(OH)2D3 as compared with levels in HNSCC tissue of an untreated patient. Arrows indicate examples of positive-stained cells.
Increased Numbers of Intratumoral Mature Dendritic Cells in HNSCC Tumor Tissues from 1,25(OH)2D3-Treated Patients
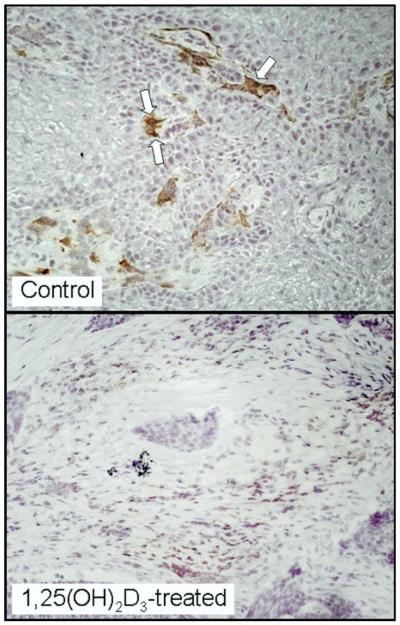
Dendritic cells originate in the bone marrow as progenitor cells and then migrate in the peripheral blood. Once in contact with a foreign antigen, such as neoplastic cells, their ability to express MHC-II antigens and secrete IL-12 makes them potent stimulators of the immune response and stimulators of antitumor immunity. Although immature dendritic cells can be immune inhibitory, mature dendritic cells, identified by their surface marker DC-LAMP, are particularly potent stimulators of T-cell reactivity and their presence has been associated with reduced tumor progression.8,15,16 Because 1,25(OH)2D3 has previously been shown to facilitate dendritic cell maturation,13,14 its effect on dendritic cell levels within HNSCC tissue was tested. Results of immunohistochemical analyses showed a significant presence of dendritic cells expressing the immature marker DC-SIGN in HNSCC tissue from untreated patients. Although these cells were detectable in HNSCC tissue from patients treated with 1,25(OH)2D3, their levels were reduced (P = .0262, Figs 1 and 3).
Figure 3.
Representative microscopic images of reduced intratumoral levels of DC-SIGN+ immature dendritic cells in HNSCC tissues from a patient treated with 1,25(OH)2D3 as compared with levels in HNSCC tissue of an untreated patient. Arrows indicate examples of positive-stained cells.
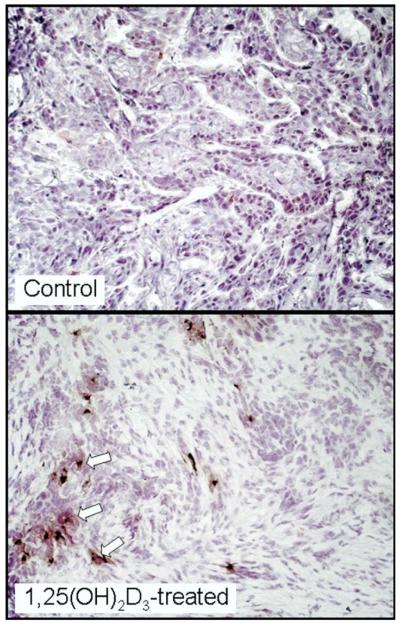
Evaluation of HNSCC tissues for the presence of mature dendritic cells expressing DC-LAMP showed only low levels of positive staining cells in tissues from untreated patients (Fig 4). There was a striking increase in intratumoral mature dendritic cells in HNSCC tissue of 1,25(OH)2D3-treated patients (P = .0023, Figs 1 and 4).
Figure 4.
Representative microscopic images of increased intratumoral levels of DC-LAMP+ mature dendritic cells in HNSCC tissues from a patient treated with 1,25(OH)2D3 as compared with levels in HNSCC tissue of an untreated patient. Arrows indicate examples of positive-stained cells.
DISCUSSION
Patients with HNSCC are particularly deficient in their immune competence.1 One mediator of this profound immune suppression is the CD34+ progenitor cell population.2 CD34+ cells’ immunosuppressive effects had been shown by enzymatically dissociating tumor tissue and immunomagnetically removing the CD34+ cells. This resulted in increased activity of intratumoral T cells, as seen by an increase in their production of IL-2.2 CD34+ cells are increased in the peripheral blood and tumor tissue of over two thirds of patients with HNSCC.13 These suppressor cells can differentiate into a variety of immune stimulatory cells, including dendritic cells.3 In patients with HNSCC, dendritic cells have been shown to have defects in their maturation.17 The lack of mature dendritic cells in the tumor environment and ineffective activation of antitumor responses by dendritic cells is a failure of their function rather than of their recruitment into the tumor.18 Additionally, these studies found that an elevated level of dendritic cells expressing the immature marker DC-SIGN was associated with a decrease in overall survival. Based on prior in vitro studies,3 the present study hypothesized that the treatment of HNSCC patients with 1,25(OH)2D3 would drive the differentiation of immune inhibitory CD34+ progenitor cells whose levels are increased in HNSCC patients into mature dendritic cells. If 1,25(OH)2D3 could drive the differentiation of an immune suppressive cell into mature immune stimulatory dendritic cells intratumorally, it could be an adjunctive therapy in the treatment of HNSCC.
The present study is the first to identify the effects of 1,25(OH)2D3 on the intratumoral levels of immune inhibitory CD34+ cells and mature dendritic cells in patients with HNSCC. This study showed that 1,25(OH)2D3 treatment decreased the levels of intratumoral CD34+ cells and decreased the levels of immature dendritic cells, which have previously been shown to be capable of stimulating T-cell tolerance.8 More prominent was the pronounced increase in intratumoral levels of mature dendritic cells in patients who received 1,25(OH)2D3 treatment. Although it is not possible to determine if the decline in CD34+ progenitor cells or in immature dendritic cells and the increase in mature dendritic cells were causally related, these results are consistent with prior in vitro studies showing that 1,25(OH)2D3 stimulates the differentiation of HNSCC patient-derived CD34+ cells into mature antigen-presenting dendritic cells. It was curious that the decline in the levels of immature dendritic cells was not as prominent as the decline in D34+ progenitor cells or the increase in mature dendritic cells. It is possible that this lesser level of decline in immature dendritic cell levels is a reflection of the transition of CD34+ progenitor cells to a mature dendritic state.
The significance of the observed skewing from increased levels of immune inhibitory CD34+ cells to increased levels of mature dendritic cells is that this mature dendritic cell population would be potent in stimulating immune reactivity. Studies are currently ongoing to determine if the increased levels of mature dendritic cells translates into increased anti-HNSCC T-cell reactivity.
CONCLUSION
The present study has shown the ability of 1,25-dihydroxyvitamin D3 to reduce the levels of intratumoral immune suppressive CD 34+ cells and immature dendritic cells and to increase intratumoral levels of mature dendritic cells. The patient population in this study will be followed for an additional 2 years to analyze if modulating intratumoral levels of CD34+ cells or levels of dendritic cells has clinical effectiveness in regards to locoregional control, distant metastasis, and ultimately disease-free survival. If so, this study would provide the foundation for a larger investigation to determine if 1,25(OH)2D3 could be an adjunctive treatment in HNSCC by helping to overcome the profound immune suppression in HNSCC patients so as to increase immune reactivity against autologous HNSCC.
ACKNOWLEDGEMENTS
The authors would like to thank Bridgette Ransom, Kiwana Gibbs, Kim Sutton, and Anna-Maria Clark for their technical contributions to the analyses of immunostained cells.
FINANCIAL DISCLOSURES Supported by the Research Service of the Department of Veterans Affairs and by grants R01CA85266 and R01CA97813 from the National Institutes of Health to MRIY.
Footnotes
Sponsorships or competing interests that may be relevant to this article are disclosed at the end, preceding the references.
Accepted for an oral resident research presentation by Dr J.S. Kulbersh at the 2008 meeting of the American Academy of Otolaryngology–Head and Neck Surgery.
AUTHOR CONTRIBUTIONS Jonathan S. Kulbersh, author; Terry A. Day, collaborator; M. Boyd Gillespie, coinvestigator; M. Rita I. Young, author.
REFERENCES
- 1.Heimdal JH, Aarstad HJ, Klementsen B, et al. Peripheral blood mononuclear cell (PBMC) responsiveness in patients with head and neck cancer in relation to tumour stage and prognosis. Acta Otolaryngol. 1999;119:281–4. doi: 10.1080/00016489950181828. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt-Pak A, Wright MA, Matthews JP, et al. Mechanisms of immune suppression in patients with head and neck cancer: presence of CD34+ cells which suppress immune functions within cancers that secrete granulocyte-macrophage colony-stimulating factor. Clin Cancer Res. 1995;1:95–103. [PubMed] [Google Scholar]
- 3.Garrity T, Pandit R, Wright MA, et al. Increased presence of CD34+ cells in the peripheral blood of head and neck cancer patients and their differentiation into CD1a+ cells. Int J Cancer. 1997;73:663–9. doi: 10.1002/(sici)1097-0215(19971127)73:5<663::aid-ijc9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 4.Young MRI, Wright MA, Matthews JP, et al. Suppression of T cell proliferation by tumor-induced granulocyte-macrophage progenitor cells producing transforming growth factor-b and nitric oxide. J Immunol. 1996;156:1916–22. [PubMed] [Google Scholar]
- 5.Lathers DMR, Achille N, Kolesiak K, et al. Increased levels of immune inhibitory CD34+ progenitor cells in the peripheral blood of patients with node positive head and neck cancer and the ability of the CD34+ cells to differentiate into antigen presenting dendritic cells. Clin Cancer Res. 2001;125:205–12. doi: 10.1067/mhn.2001.117871. [DOI] [PubMed] [Google Scholar]
- 6.Young MRI, Wright MA, Lozano Y, et al. Increased recurrence and metastasis in patients whose primary head and neck squamous cell carcinomas secreted granulocyte-macrophage colony-stimulating factor and contained CD34+ natural suppressor cells. Int J Cancer. 1997;74:69–74. doi: 10.1002/(sici)1097-0215(19970220)74:1<69::aid-ijc12>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 7.Rossi M, Young JW. Human dendritic cells: potent antigen presenting cells at the crossroads of innate and adaptive immunity. J Immunol. 2005;175:1373–81. doi: 10.4049/jimmunol.175.3.1373. [DOI] [PubMed] [Google Scholar]
- 8.Almand B, Resser JR, Lindman B, et al. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–66. [PubMed] [Google Scholar]
- 9.Whiteside TL, Stanson J, Shurin MR, et al. Antigen-processing machinery in human dendritic cells: up-regulation by maturation and down-regulation by tumor cells. J Immunol. 2004;173:1526–34. doi: 10.4049/jimmunol.173.3.1526. [DOI] [PubMed] [Google Scholar]
- 10.Wiers K, Wright MA, Vellody K, et al. Failure of tumor-reactive lymph node cells to kill tumor in the presence of immune suppressive CD34+ cells can be overcome with vitamin D3 treatment to diminish CD34+ cell levels. Clin Exp Met. 1998;16:275–82. doi: 10.1023/a:1006501110857. [DOI] [PubMed] [Google Scholar]
- 11.Lathers DM, Clark JI, Achille NJ, et al. Phase 1B study to improve immune responses in head and neck cancer patients using escalating doses of 25-hydroxyvitamin D3. Cancer Immunol Immunother. 2004;53:422–30. doi: 10.1007/s00262-003-0459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muindi JR, Peng Y, Potter DM, et al. Pharmacokinetics of high-dose oral calcitriol: results from a phase 1 trial of calcitriol and paclitaxel. Clin Pharmacol Ther. 2002;72:648–59. doi: 10.1067/mcp.2002.129305. [DOI] [PubMed] [Google Scholar]
- 13.Pandit R, Lathers DM, Beal NM, et al. CD34+ immune suppressive cells in the peripheral blood of patients with head and neck cancer. Ann Otol Rhinol Laryngol. 2000;109:749–54. doi: 10.1177/000348940010900809. [DOI] [PubMed] [Google Scholar]
- 14.Young MR, Lathers DM. Myeloid progenitor cells mediate immune suppression in patients with head and neck cancers. Int J Immunopharmacol. 1999;21:241–52. doi: 10.1016/s0192-0561(99)00008-9. [DOI] [PubMed] [Google Scholar]
- 15.Ladanyi A, Kiss J, Somlai B, et al. Density of DC-LAMP+ mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol Immunother. 2007;56:1459–69. doi: 10.1007/s00262-007-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elliott B, Scolyer RA, Suciu S, et al. Long-term protective effect of mature DC-LAMP+ dendritic cell accumulation in sentinel lymph nodes containing micrometastatic melanoma. Clin Cancer Res. 2007;13:3825–30. doi: 10.1158/1078-0432.CCR-07-0358. [DOI] [PubMed] [Google Scholar]
- 17.Almand B, Clark JI, Nikitina E, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–89. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 18.O’Donnell RK, Mick R, Feldman M, et al. Distribution of dendritic cell subtypes in primary oral squamous cell carcinoma is inconsistent with a functional response. Cancer Lett. 2007;255:145–52. doi: 10.1016/j.canlet.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]