Abstract
Backround
Reactive oxygen species (ROS) mediate the effects of anesthetic precondition to protect against ischemia and reperfusion injury, but the mechanisms of ROS generation remain unclear. In this study, we investigated if mitochondria-targeted antioxidant (mitotempol) abolishes the cardioprotective effects of anesthetic preconditioning. Further, we investigated the mechanism by which isoflurane alters ROS generation in isolated mitochondria and submitochondrial particles.
Methods
Rats were pretreated with 0.9% saline, 3.0 mg/kg mitotempol in the absence or presence of 30 min exposure to isoflurane. Myocardial infarction was induced by left anterior descending artery occlusion for 30 min followed by reperfusion for 2h and infarct size measurements. Mitochondrial ROS production was determined spectrofluorometrically. The effect of isoflurane on enzymatic activity of mitochondrial respiratory complexes was also determined.
Results
Isoflurane reduced myocardial infarct size (40±9 % = mean±SD) compared to control experiments (60±4 %). Mitotempol abolished the cardioprotective effects of anesthetic preconditioning (60±9%). Isoflurane enhanced ROS generation in submitochondrial particles with NADH, but not with succinate, as substrate. In intact mitochondria, isoflurane enhanced ROS production in the presence of rotenone, antimycin A, or ubiquinone when pyruvate and malate were substrates, but isoflurane attenuated ROS production when succinate was substrate. Mitochondrial respiratory experiments and electron transport chain complex assays revealed that isoflurane inhibited only complex I activity.
Conclusions
The results demonstrated that isoflurane produces ROS at complex I and III of the respiratory chain via the attenuation of complex I activity. The action on complex I decreases unfavorable reverse electron flow and ROS release in myocardium during reperfusion.
Introduction
Volatile anesthetics such as isoflurane have cardioprotective effects when administered before a period of myocardial ischemia and reperfusion and this phenomenon is referred to as anesthetic preconditioning (APC).1,2 It is accepted that mitochondria play a crucial role in the mechanism of APC.3-5 Volatile anesthetics increase signaling quantities of reactive oxygen species (ROS) production that serve as a mandatory step during triggering of APC, and scavengers of ROS block APC-induced cardioprotection.6-8 Mitochondrial K+ influx through mitochondrial ATP-sensitive K+ channels9 and/or Ca2+-activated K+ channels,10 uncoupling,3,5 mild depolarization11 and inhibition of mitochondrial respiration12,13 have been suggested as possible mechanisms responsible for mitochondrial ROS generation during APC. On the other hand, a burst of ROS generation during reperfusion exacerbates ischemic injury, and this may be ameliorated by APC or by application of anesthetic at the onset of reperfusion (anesthetic postconditioning).7,14 Thus, volatile anesthetics have apparent directionally opposite effect on mitochondrial ROS production when applied prior to ischemia or after reperfusion. The major sites responsible for mitochondrial ROS generation are complex I and complex III of the electron transport chain (ETC).15 Our previous study has demonstrated that ROS could be generated at complex III during APC16 while direct inhibitory effect of volatile anesthetics on complex I has also been elucidated.12,13 Thus, the detailed molecular mechanism and sites of ROS generation during APC are controversial.
In the present study, we investigated if mitotempol, a mitochondria-targeted superoxide dismutase mimetic, abolished APC. We hypothesized that inhibition of ETC complex I by isoflurane accounts for an increase of signaling ROS both, at complex I and at complex III, and for a decrease of detrimental ROS at the time of reperfusion through inhibition of reverse electron flow.
Methods and Materials
All experimental procedures and protocols used in the present study were reviewed and approved by the Animal Care and Use Committee of the Medical College of Wisconsin, and all were conformed to the Guiding Principles in the Care and Use of Animals of the American Physiologic Society and were in accordance with the Guide for the Care and Use of Laboratory Animals.
Surgical procedure and Experimental protocol
Male Wistar rats (270-320g) were randomly assigned to pretreatment with 0.9% saline (control) or super oxide dismuatase mimetics, tempol (30 mg/kg) or mitotempol (3.0 mg/kg). Mitotempol was synthesized by Dr. Joy Joseph (Ph.D., Associate Professor of Biophysics, Medical College of Wisconsin).
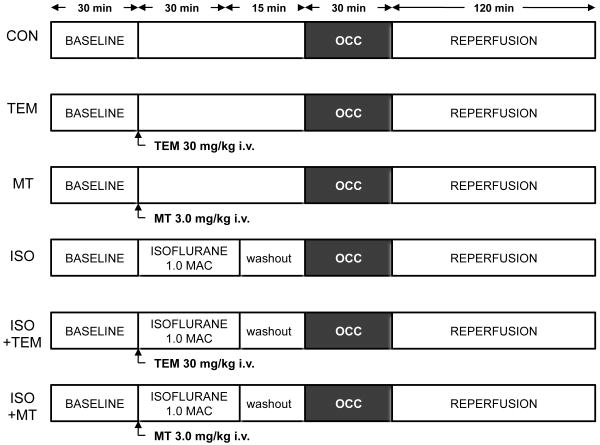
The surgical procedure was performed as previously described. 17 Briefly, after rats were anesthetized, the trachea was intubated for artificial ventilation. A thoracotomy was performed and a suture was placed around the left anterior descending artery for coronary artery occlusion and reperfusion. Systemic hemodynamics were recorded continuously on a polygraph and digitized using a computer connected to an analog-to-digital converter. The experimental design for in vivo experiments is shown in Figure 1. Baseline hemodynamics and arterial blood gas tensions were recorded for 30 min. All rats underwent 30 min of left anterior descending artery occlusion followed by 2 h of reperfusion, in the absence or presence of 30 min exposure to 1 minimum alveolar concentration of isoflurane in six separate experimental groups (n=8 per group). After reperfusion, myocardial infarct size was measured as previously described.18 Infarct size was expressed as percentage of the left ventricular area at risk.
Figure 1.
Experimental protocols used for the production of coronary artery occlusion (OCC) and reperfusion in rats treated with saline vehicle (CON), tempol (TEM), mitotempol (MT), and with and without 1.0 minimum alveolar concentration (MAC) isoflurane (ISO). n=8/group.
Isolation of rat heart mitochondria
Mitochondria were isolated from rat heart by differential centrifugation as previously described.3 Hearts were excised and washed in isolation buffer [200 mM mannitol, 50 mM sucrose, 5 mM KH2PO4, 5 mM 3-(N-morpholino) propanesulfonic acid, 1 mM EGTA, 0.1% bovine serum albumin (BSA), pH 7.3 at 25°C adjusted with 5 M KOH] and minced into small pieces. The suspension was homogenized in the presence of 5 U/mL of protease (from Bacillus licheniformis) by using a T 25 dispenser (IKA-Werke, Staufen, Germany). The mitochondrial pellets were resuspended in isolation buffer at a concentration of 10-20 mg/mL, stored on ice and used for experiments within 4 h. Protein concentration was determined using a modified Lowry assay kit (Bio-Rad, Hercules, CA).
The ROS scavenging effects of mitotempol and tempol
In order to assess the effectiveness of mitotempol and tempol to scavenge ROS, superoxide anion generation was measured using the chemiluminescent probe 2-methyl-6-(p-methoxyphenyl)-3,7-dihydroimidazo[1,2-α]pyrazin-3-one (MCLA) in the presence of mitotempol or tempol. 0.5mg mitochondria were added to 1 ml respiration buffer containing 5 μM 2-methyl-6-(p-methoxyphenyl)-3,7-dihydroimidazo[1,2-α]pyrazin-3-one (MCLA) and 5 mM pyruvate and malate were added to initiate superoxide generation. Antimycin A (10 μM) was used to maximize ROS production rate. Chemiluminescence was measured in a Modulus luminometer (Turner Biosystems, Sunnyvale, CA) at room temperature.
Measurement of mitochondrial ROS production
Mitochondrial ROS production was measured spectrofluorometrically using the fluorescent probe amplex red (12.5 μM) in the presence of 0.1 U/ml horseradish peroxidase at 30°C. Amplex red reacts with H2O2 in the presence of peroxidase and produces the red-fluorescent product resorufin. Resorufin fluorescence was measured using a spectrofluorometer (Photon Technology International, Birmingham, NJ) with excitation and emission wavelengths set at 530 and 583 nm, respectively. Measurements were performed in respiration buffer (130 mM KCl, 5 mM K2HPO4, 20 mM 3-(N-morpholino) propanesulfonic acid, 2.5 mM EGTA, 1 μM Na4P2O7, and 0.1% BSA, pH 7.4) containing mitochondria at a final concentration of 0.5 mg protein/ml. ROS generation was stimulated with complex I substrates pyruvate (5 mM) and malate (5 mM) or complex II substrate succinate (5 mM) in the presence and absence of isoflurane (0.5 mM). Rotenone (1 μM) and antimycin A (1 μM) were used to inhibit the activities of complex I and complex III, respectively. Decylubiquinone (50 μM) was used to estimate whether an increase of the ubiquinone/ubiquinol ratio affects ROS production.19
Measurement of ROS production in submitochondrial particles (SMPs)
Submitochondrial particles (SMPs) were prepared by sonicating mitochondria on ice (10 bursts of 10 s at 20 watt). The sonicated suspension was centrifuged at 10,000 g for 10 min (4 °C) and the supernatant was then centrifuged for 1 h at 100,000 g (4°C). The pellet was resuspended in mitochondrial isolation buffer, and protein content determined. Measurements were performed in respiration buffer containing SMPs at a final concentration of 0.5 mg protein/ml. ROS generation was stimulated with complex I substrate NADH (0.5 mM) or complex II substrate succinate (5 mM) in the presence and absence of isoflurane. Super oxide dismutase (100 units/ml) treatment was required when NADH was used as substrate to reduce the substrate-dependent background rate of the assay.20
Measurement of mitochondrial oxygen consumption
Mitochondrial oxygen consumption was measured in the presence or absence of isoflurane (0.5 mM) by using an oxygen electrode (Hansatech Instruments, Norfolk, United Kingdom) maintained at 30°C and experiments were conducted in respiration buffer containing 0.5 mg/ml of mitochondrial protein. State 2 respiration was initiated with complex I substrates pyruvate (5 mM) and malate (5 mM) or complex II substrate succinate (5 mM) in the presence and absence of complex I inhibitor rotenone (1 μM). State 3 respiration was measured in the presence of 250 μM ADP, and state 4 was monitored after complete ADP consumption.
Electron transport chain (ETC) assay
ETC enzyme activity was measured spectrophotometrically as specific donor-acceptor oxidoreductase activity.21,22 Isolated mitochondria were solubilized with 2% cholic acid and diluted to a final concentration of 100 μg/ml in the experimental buffer [220 mM D-mannitol, 70 mM sucrose, 5 mM 3-(N-morpholino) propanesulfonic acid, 2 mM EDTA, and 0.2% BSA (pH 7.4)]. In the isoflurane-treated group, isoflurane (0.5 mM) dissolved in DMSO was added just before the reaction.
Complex I (NADH-ubiquinone oxidoreductase) activity assay
Complex I activity was determined by the rotenone-sensitive reduction of NADH absorbance using decylubiquinone as acceptor. The assay mixture contained 20 μg/mL mitochondrial protein, 50 mM KH2PO4, 0.1 mM EDTA, 0.1% BSA, 0.15 mg/mL asolectin, 2 mM antimycin A, and 0.2 mM NADH in a spectrophotometer cuvette. The reaction was initiated by adding 75 mM decylubiquinone, and the change in absorbance of NADH was measured at 340 nm (ε = 6.22 mM-1cm-1).
Complex II (succinate-ubiquinone oxidoreductase) activity assay
The activity of complex II was determined by dichlorophenolindophenol reduction. The assay mixture contained 20 μg/mL mitochondrial protein, 50 mM KH2PO4, 0.1 mM EDTA, 0.1% BSA, 5 mM NaN3, 0.5 mM duroquinone, and 25 mM dichlorophenolindophenol. The reaction was initiated by adding 20 mM succinate, and the change in absorbance of dichlorophenolindophenol was measured at 600 nm (ε = 21 mM-1cm-1).
Complex III (ubiquinol-cytochrome c reductase) activity assay
The activity of complex III was determined by antimycin A sensitive reduction of cytochrome c in the presence of decylubiquinol. Decylubiquinol was synthesized according to the method described by Trumpower.23 The assay mixture contained 5 μg/mL of mitochondrial protein, 50 mM KH2PO4, 0.1 mM EDTA, 0.1% BSA, 3 mM NaN3, and 60 μM oxidized cytochrome c. The reaction was initiated by adding 100 μM decylubiquinol, and the change in absorbance of cytochrome c was measured at 550 nm (ε = 18.5 mM-1cm-1).
Complex IV (cytochrome c oxidase) activity assay
The activity of complex IV was assessed by the decrease in the rate of absorbance of reduced cytochrome c at 550 nm (ε = 18.5 mM-1cm-1). The assay mixture contained 50 mM KH2PO4, 0.15 mg/mL asolectin, and 40 μM reduced cytochrome c, and the reaction was initiated by adding 1 μg/mL of mitochondrial protein.
Administration of isoflurane in vitro experiments
The appropriate volume of isoflurane stock solution dissolved in DMSO was added to the experimental buffer to obtain the desired concentration (0.5 mM corresponding to approximately 1 minimum alveolar concentration for rats). In control experiments, we confirmed that our final DMSO concentration (below 0.2 % v/v) had no effect on mitochondrial ROS production, oxygen consumption and electron transport chain assays. At the end of each experiment, the isoflurane concentration was analyzed by gas chromatography. The concentration of isoflurane varied by ±10% from the reported value.
Data analysis
All data are presented as mean± SD. Statistical analysis was performed with Origin 7 (OriginLab, Northampton, MA) software. Comparisons were performed using two-tailed hypothesis testing. One-way analysis of variance was used for statistical analysis of differences between groups, with Bonferroni's post hoc test. Student't t-test was used to test for a difference between two groups. Heart rate, mean arterial pressure and rate-pressure product were analyzed by one-way repeated measures analysis of variance. A P-value less than 0.05 was considered statistically significant.
Results
Effects of MT on infarct size in vivo
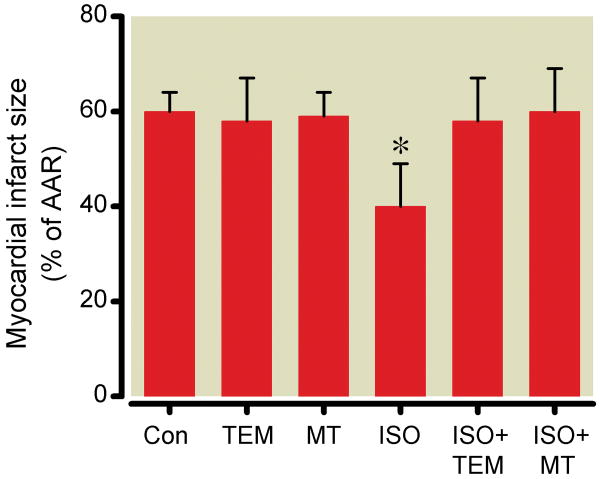
There were no differences in baseline heart rate, mean arterial pressure and rate pressure product between groups (Table 1). Mitotempol and tempol had no effect on hemodynamics. Due to its cardiodepressant effects, heart rate, mean arterial pressure and rate pressure product were briefly decreased by isoflurane, however, these values returned to baseline before coronary artery occlusion. Isoflurane (Fig.2) significantly decreased infarct size (40±9% of area at risk) compared to control experiments (60±4%). Mitotempol and tempol alone had no effect on infarct size (58±7% and 59±5%, respectively) but abolished the cardioprotection afforded by isoflurane (60±9% and 58±9%, respectively). The actions of mitotempol and tempol to scavenge mitochondrial ROS were confirmed in isolated mitochondria subjected to antimycin A. ROS production was significantly reduced in the presence of mitotempol (3.7±1.3 relative light units/mg protein/sec) and tempol (3.4±1.2 relative light units/mg protein/sec) compared to the absence (59.5±6.4 relative light units/mg protein/sec) of antioxidants.
Table 1. Systemic Hemodynamics.
| Reperfusion (h) | ||||||
|---|---|---|---|---|---|---|
| Baseline | ISO | Preocclusion | 30 min CAO | 1 | 2 | |
| HR (beats/min) | ||||||
| CON | 384±46 | --- | 351±23 | 383±43 | 362±39 | 346±36 |
| TEM | 374±40 | --- | 343±48 | 337±48 | 314±33 | 305±47 |
| MT | 401±40 | --- | 380±28 | 382±36 | 347±30 | 345±25 |
| ISO | 386±42 | 306±65* | 341±41 | 377±52 | 350±57 | 346±56 |
| TEM + ISO | 383±28 | 294±32* | 344±23 | 369±35 | 369±38 | 365±46 |
| MT + ISO | 368±19 | 296±17* | 361±26 | 373±32 | 362±31 | 342±33 |
| MAP (mmHg) | ||||||
| CON | 103±17 | --- | 108±18 | 107±18 | 80±14 | 76±15 |
| TEM | 114±16 | --- | 102±19 | 91±20 | 72±8 | 67±12 |
| MT | 126±10 | --- | 115±8 | 101±16 | 77±7 | 69±5 |
| ISO | 114±15 | 63±13* | 106±13 | 105±12 | 75±20 | 73±24 |
| TEM + ISO | 114±10 | 64±6* | 102±8 | 102±8 | 85±8 | 79±13 |
| MT + ISO | 119±15 | 69±8* | 117±15 | 117±16 | 98±21 | 86±20 |
| RPP (min/mmHg /10-3) | ||||||
| CON | 47.8±11.7 | --- | 45.2±8.8 | 46.7±8.4 | 36.3±5.4 | 33.2±5.8 |
| TEM | 51.3±10.6 | --- | 42.4±11.4 | 36.8±10.2 | 29.6±5.0 | 26.9±6.7 |
| MT | 60.1±7.4 | --- | 49.3±8.5 | 46.4±7.4 | 35.6±4.8 | 33.4±3.7 |
| ISO | 52.3±10.4 | 26.3±9.5* | 43.2±8.8 | 45.9±9.3 | 33.2±10.2 | 32.7±13.0 |
| TEM+ISO | 51.9±7.5 | 25.8±4.6* | 42.5±4.3 | 42.7±6.4 | 38.9±6.3 | 36.7±7.2 |
| MT+ISO | 54.4±9.0 | 28.7±3.0* | 52.2±6.9 | 50.9±8.6 | 43.5±8.4 | 37.4±8.9 |
Abbreviations: CON=control; TEM=tempol; MT=mitotempol; ISO=isoflurane; HR=heart rate; MAP=mean arterial pressure; RPP=rate-pressure product; CAO=coronary artery occlusion; h=hour. Data are presented as mean±standard deviation (SD). n=8/group.
P<0.05 versus baseline.
Figure 2.
Myocardial infarct size expressed as a percentage of the left ventricular area at risk (AAR) in rats pretreated with saline vehicle (Con), tempol (TEM) and mitotempol (MT), and in the absence or presence of isoflurane (ISO). Data are presented as mean±standard deviation . n=8/group. *P<0.05 versus Con.
ROS production in isolated mitochondria and submitochondrial particles
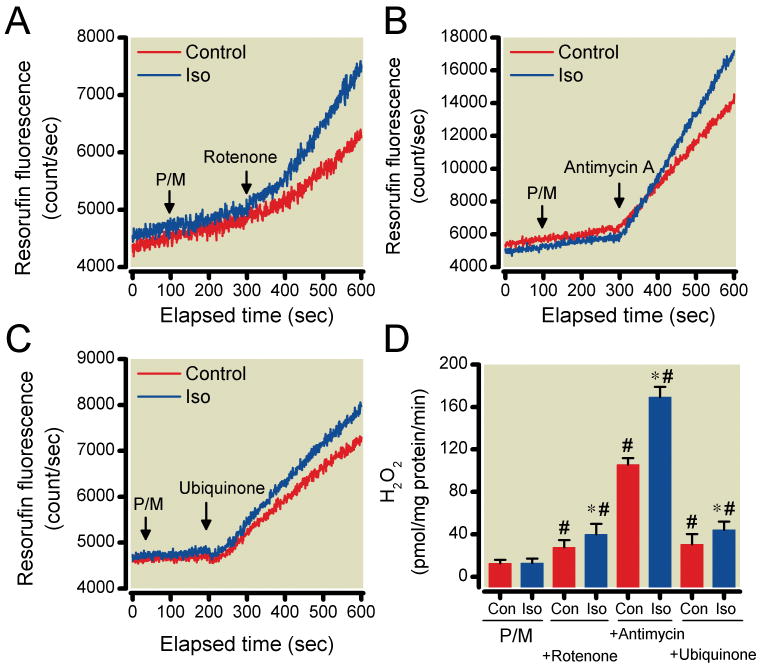
Isoflurane-dependent changes in resorufin fluorescence observed during state 2 respiration in isolated mitochondria initiated by complex I-linked substrates pyruvate and malate or complex II-linked substrate succinate are shown in Fig. 3A-D and 4A-C, respectively. Rotenone, antimycin A and ubiquinone alone increased ROS generation when complex I-linked substrates were used (Fig. 3A-C). While isoflurane alone did not alter ROS production rate with complex I-linked substrates, it enhanced ROS generation in the presence of rotenone (27.6±6.9 and 39.9±9.7 pmol H2O2/mg protein/min for the control and isoflurane-treated groups, respectively), antimycin A (105.7±6.0 and 169.3±9.8 pmol H2O2/mg protein/min) or ubiquinone (30.6±9.5 and 44.2±7.8 pmol H2O2/mg protein/min) (Fig. 3D). However, when electrons were delivered to complex II by succinate, isoflurane alone decreased ROS production rate (400.9±18.3 and 135±21.7 pmol H2O2/mg protein/min for the control and isoflurane-treated groups, respectively), whereas isoflurane had no effect on rotenone, antimycin A and ubiquinone-modulated ROS production (Fig. 4C).
Figure 3.
Reactive oxygen species (ROS) production in isolated mitochondria with pyruvate and malate (P/M) as substrates. Representative traces show that rotenone (A), antimycin-A (B), and ubiquinone (C) induced ROS production to a larger extent in the presence compared with the absence (Con) of isoflurane (Iso). (D) Summary of the recordings. Summary data are mean±SD. P/M alone; n=10/group. Rotenone, antimycin-A and ubiquinone; n=8/group. *P<0.05 versus corresponding Con, #P<0.05 versus respective Con with P/M alone.
Figure 4.
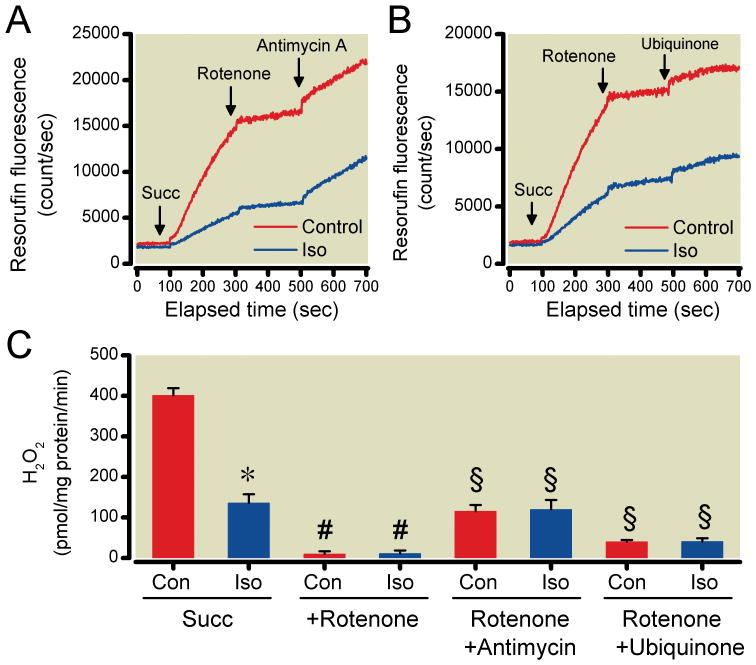
Reactive oxygen species (ROS) production in isolated mitochondria with succinate (Succ) as substrate. The effects of antimycin-A (A) and ubiquinone (B) on complex II-linked ROS production in the presence or absence (Con) of isoflurane (Iso) were analyzed after reverse electron flow induced-ROS was blocked by rotenone. Iso reduced reverse electron flow induced-ROS production in the absence of rotenone, however, Iso had no effect in the presence of rotenone, and antimycin A or ubiquinone. (C) Summary of recordings. Data are presented as mean±SD, n=8/group, *P<0.05 versus corresponding Con, #P<0.05 versus the respective Con with Succ alone, §P<0.05 versus respective Con with rotenone.
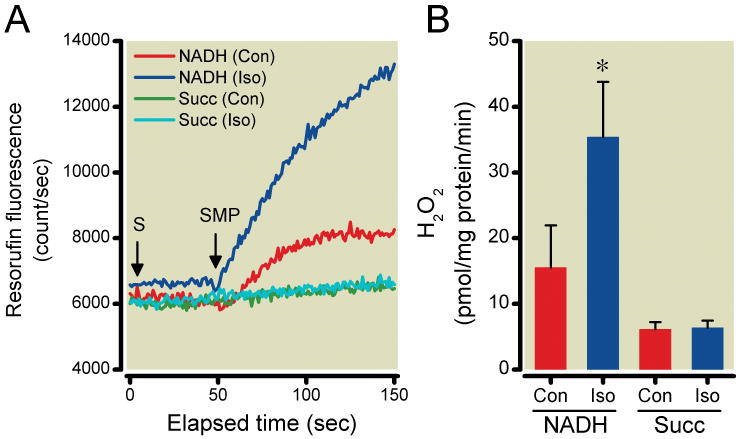
When using SMP instead of intact mitochondria, ROS production rate significantly increased in the presence of isoflurane alone with NADH as substrate (15.5±6.4 and 35.4±8.4 pmol H2O2/mg protein/min for the control and isoflurane-treated groups, respectively), but not with succinate (6.1±1.1 and 6.4±1.0 pmol H2O2/mg protein/min) (Fig. 5A, B).
Figure 5.
Reactive oxygen species (ROS) production in submitochondrial particles (SMP) with complex I (NADH) and complex II (succinate) substrates in the presence or absence (Con) of isoflurane (Iso). Representative traces (A) show the effects of Iso on ROS production using the different substrates (S). Iso induced ROS generation only when NADH was used as substrate, but had no effect when succinate (Succ) was used. (B) Summary data are mean±SD, n=8/group, *P<0.05 versus Con.
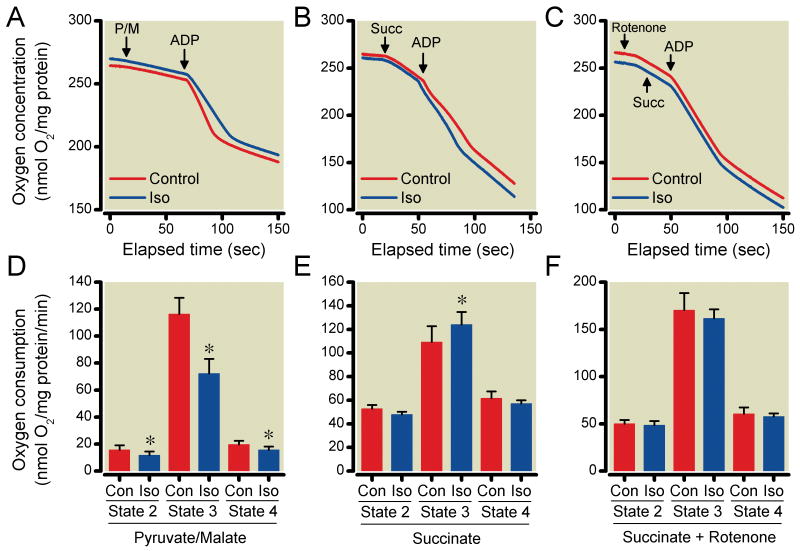
The effects of isoflurane on mitochondrial respiration
The effects of isoflurane on mitochondrial respiration initiated by pyruvate and malate or succinate in the absence and presence of rotenone are shown in Fig. 6A-C. Isoflurane significantly decreased state 3 respiration (72±11 nmol O2/min/mg protein) compared to the control group (116±12 nmol O2/mg protein/min) when pyruvate and malate were used (Fig. 6D). When succinate was used in the absence of rotenone, isoflurane significantly increased state 3 respiration (124±10 nmol O2/mg protein/min) compared to the control group (109±13 nmol O2/mg protein/min) (Fig. 6E). On the other hand, isoflurane had no significant effect on mitochondrial respiration when succinate was used in the presence of rotenone. (Fig. 6F)
Figure 6.
Effect of isoflurane (Iso) on mitochondrial respiration using substrates pyruvate/malate (P/M), succinate (Succ), and adenosine diphosphate (ADP) in the absence or presence of Iso. Representative traces demonstrate the effect of Iso on oxygen consumption using P/M (A), Succ (B) and Succ+rotenone (C). Iso decreased mitochondrial respiration compared to the control group (Con) when P/M were used (D). When Succ was used in the absence of rotenone, Iso significantly increased mitochondrial respiration (E). Iso had no significant effect on mitochondrial respiration when Succ was used in the presence of rotenone (F). Summary data are mean±SD. P/M; n=10/group. Succinate and Succinate+Rotenone; n=8/group. *P<0.05 versus Con.
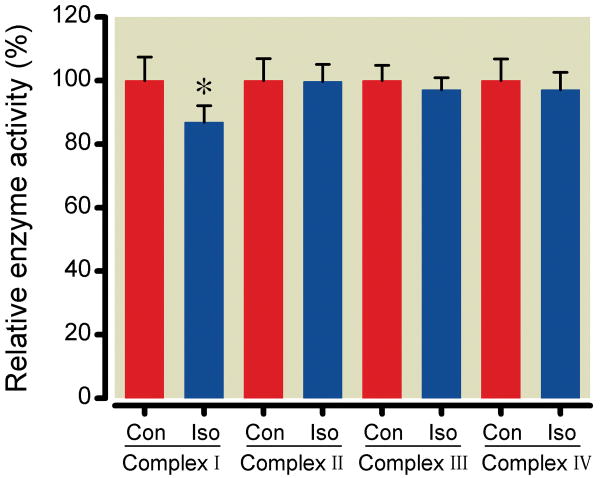
ETC complex activity assay in solubilized mitochondria
In order to further pinpoint the complexes targeted by isoflurane, the relative activities of the ETC complexes were quantified in cholic acid-solubilized mitochondria (Fig. 7). Isoflurane significantly reduced only complex I activity to 86±5% (356±18 nmol NADH/mg protein/min) of control (410±30 nmol NADH/mg protein/min); no changes in the activities of other complexes were observed (99±5, 97±3 and 97±5 % of control for Complex II, Complex III and Complex IV activities, respectively).
Figure 7.
The effects of (Iso) on the activity of the electron transport chain complexes. Enzymatic activity for each complex during Iso is expressed relative to the absence (Con) of Iso. Data are mean±SD, n=8/group, *P<0.05 versus (Con).
Discussion
The present study showed that (1) mitochondrial-targeted antioxidant abolished the cardioprotection of APC; (2) isoflurane enhanced ROS production in SMPs when complex I-linked substrates were used, however, this occurred in intact mitochondria only in the presence of rotenone, antimycin A or ubiquinone; (3) with a complex II-linked substrate, isoflurane did not alter ROS production in SMPs, but decreased ROS production in intact mitochondria; and (4) isoflurane directly attenuated complex I activity.
Low concentrations of ROS24,25 have been demonstrated to activate preconditioning pathways. Volatile anesthetics such as sevoflurane26 and isoflurane6,16 have also been shown to activate cardioprotective signaling through ROS-sensitive pathways. ROS produced by volatile anesthetics activate a variety of proteins such as protein kinase C, Akt, and glycogen synthase kinase-β, which ultimately causes a delay of permeability transition pore opening, thereby protecting the myocardium against ischemia and reperfusion injury.27 Previous studies demonstrated that ROS scavengers abolished volatile anesthetic-induced reduction in myocardial infarct size.6-8 Although mitochondria have been suggested as the source of protective signaling ROS in APC,27 direct evidence for this was lacking. Piperidine nitroxides such as tempol are well established antioxidants in vitro and in vivo.28 The antioxidant effect is based on their ability to catalyze the dismutation of superoxide and to detoxify redox-reactive forms of transition metal ions. Mitotempol, accumulated in mitochondria due to its cationic triphenylphosphonium moiety, is an effective antioxidant due to its proximity to ETC-generated ROS.29 The current results demonstrate that scavenging of ROS with tempol and, more importantly, mitotempol abolishes isoflurane-induced cardioprotection. While mitotempol is specific in mostly scavenging mitochondria-originating ROS, tempol indifferently scavenges all sources of cellular ROS. This directly implicates mitochondria as the source ROS responsible for protective signaling.
Complex I and Complex III are the major sites of ROS generation in mitochondria;15 however, the site responsible for volatile anesthetic-induced ROS generation remains undefined. The following evidence supports the contention that complex I is a source of isoflurane-induced ROS: Isoflurane increases ROS generation with complex I-, but not complex II-linked substrates in SMPs (Fig. 5); and isoflurane only alters activity of complex I, but not of the other complexes in solubilized mitochondria. However, isoflurane-induced ROS generation in isolated mitochondria was not detected in our experiments when complex I-linked substrates were used. This apparent discrepancy observed in isolated mitochondria as compared with submitochondrial particles may have occurred because ROS generated at complex I site are released into the mitochondrial matrix where they rapidly react with manganese superoxide dismutase before interact with detection reagent in the buffer.15 Interestingly, in the presence of rotenone, antimycin A or ubiquinone, isoflurane enhanced ROS production with complex I-linked substrates. In the case of complex I inhibitor rotenone, the inhibitory effect of isoflurane on complex I is enhanced and thereby allows isoflurane-induced H2O2 detection in the buffer. The contribution of antimycin A (inhibitor of complex III) and ubiquinone (donates electrons to complex III in its reduced form) to isoflurane-enhanced ROS generation suggests a possible involvement of complex III. It is generally assumed that ROS at complex III is generated by oxygen reduction via electron transfer from ubisemiquinone which is formed at the ubiquinol oxidation center. Inhibition of electron flow downstream of this center, e.g. by antimycin A, increases ROS generation.15 However, this mechanism is not able to explain isoflurane-enhanced ROS generation at complex III because isoflurane did not affect electron flow at complex III as demonstrated by the ETC complex assay. Recently, Dröse et al. have proposed an alternate mechanism for ROS generation at complex III: They observed that ROS generation depends on the redox state of the ubiquinone pool. An increased ubiquinone/ubiquinol ratio enhances ROS generation at complex III.19 According to this mechanism, an electron is transferred to oxygen from reduced downstream cytochrome bL to oxygen in a reverse reaction via oxidized ubiquinone. We confirmed that exogenous ubiquinone enhanced ROS generation with complex I substrates. Interestingly, isoflurane further enhanced ROS generation in the presence of ubiquinone. Inhibition of complex I by isoflurane may further increase the ubiquinone/ubiquinol ratio by decreasing the electron flow complex I to form reduced ubiquinol, thereby enhancing ROS generation at the complex III site. Similarly, diazoxide, independent of its putative effect on mitochondrial ATP-sensitive K+ channels, attenuates ubiquinol level via inhibition of complex II, causing an increase in ROS at the ubiquinol oxidation.30 Thus, inhibition of electron flow at upstream complexes could contribute to ROS generation at complex III through changes in the ubiquinone/ubiquinol ratio. We suggest a mechanism whereby isoflurane interacts with complex I and causes an increase in ROS generation at the complex III site.
Ischemic or pharmacologic preconditioning-induced mitochondrial K+ influx through mitochondrial ATP-sensitive K+ channels and/or Ca2+-activated K+ channels and subsequent matrix alkalinization was previously suggested as underlying mechanism of increased ROS production.31,32 For example, diazoxide, a putative mitochondrial ATP-sensitive K+ channel agonist, produced pharmacological preconditioning by generating small quantities of ROS.33 However, several studies have demonstrated K+-independent action of diazoxide, specifically interaction with the ETC that might be also related to ROS production as mentioned before.30,34,35 APC has been associated with mild mitochondrial depolarization, possibly through mitochondrial K+ channel opening10,36 and mild mitochondrial uncoupling has been shown to be cardioprotective.3,37,38 While a parallel increase in ROS production and depolarization during APC seems counterintuitive in view of the general assumption that mitochondrial depolarization decreases ROS production,11 it may be the result of multiple actions of isoflurane on mitochondrial bioenergetics. Future studies will elucidate the interactive mechanism between volatile anesthetic-induced ROS generation via ETC interaction and K+ flux- or otherwise induced mitochondrial depolarization.
In agreement with our results from complex activity measurements, isoflurane inhibited oxygen consumption in the presence of complex I-linked substrates, but not when succinate, as complex-II linked substrate, was used in the presence of rotenone. When succinate was used in the absence of rotenone, isoflurane in fact increased the oxygen consumption. This again points to complex I as the target of isoflurane. Succinate is converted to fumarate and malate and then to oxaloacetate in the Krebs cycle without rotenone.39 Oxaloacetate participates in a direct feedback inhibition of complex II that results in decreased oxidation of succinate. When complex I activity is inhibited, NADH oxidation and, thereby, the levels of NAD+ are decreased, which in turn, impairs the oxidation of malate to oxaloacetate.40 Therefore, inhibitory effects of isoflurane on complex I could increase complex II-linked respiration via attenuation of oxaloacetate generation.
While a limited increase in ROS generation is critical for initiating prosurvival signaling pathways during APC, oxidative stress contributes to mitochondrial and cellular injury during reperfusion. With succinate as substrate, electrons entering the ETC at complex II can generate ROS by reverse electron flow to complex I. Rotenone that blocks complex I largely attenuates reverse flow-induced ROS generation.41 Similarly, isoflurane significantly reduces ROS generation induced by reverse electron flow when a complex II substrate is used. This action may be particularly relevant during myocardial ischemia, since succinate concentration significantly increases during hypoxia.42,43 Isoflurane applied at the onset of reperfusion (anesthetic postconditioning) may attenuate reverse electron flow-induced ROS generation, thereby delaying the opening of mitochondrial permeability transition pore and increasing cell survival.14 Thus, the source and mechanism of ROS generation observed during reperfusion is likely to be fundamentally different from that of “ triggering” ROS elicited during APC.
The current results should be interpreted within the constraints of several limitations. Experiments were conducted in an established model of myocardial infarction in rats; thus, these results may not be directly comparable to those obtained in humans. We were unable to measure the effect of tempol and mitotempol on the small amount of ROS generated by isoflurane directly in vitro experiments. Tempol and mitotempol convert superoxide anion to hydrogen peroxide similarly to superoxide dismutase. Therefore, the scavenging properties of those superoxide dismutase mimetics cannot be detected with the very sensitive amplex red-based assay that detects hydrogen peroxide. On the other hand the methyl-6-(p-methoxyphenyl)-3,7-dihydroimidazo[1,2-α]pyrazin-3-one (MCLA)-based assay is not sensitive enough for this purpose.44 Nevertheless, we are confident that tempol and mitotempol would scavenge isoflurane-induced ROS as they attenuated the large amount of ROS induced by antimycin A in our measurements. In addition, measurements of ROS production and other mitochondrial parameters were performed in vitro as it is virtually impossible to evaluate subcellular regulation of ROS generation in the intact heart. We also did not assess the interplay between mitochondrial depolarization and ROS generation and this is the aim of ongoing studies in the laboratory.
In summary, our results confirmed the critical role of isoflurane to modulate mitochondrial ROS. The interaction of isoflurane with complex I of the ETC is responsible for generation of signaling ROS at complex I and, indirectly, at complex III. Isoflurane also attenuates reverse electron flow-induced damaging ROS production that occurs during reperfusion.
What we already know about this topic
Anesthetic preconditioning and protection from myocardial ischemic injury involves reactive oxygen species (ROS), but the mechanisms for this are unknown
What this article tells us that is new
In rats, isoflurane preconditioning reduced subsequent myocardial infarction size by 40% and generated ROS primarily through inhibition of Complex I activity in mitochondria
Summary Statement.
Interaction of isoflurane with complex I in mitochondria is responsible not only for reactive oxygen species generation at complex I and III, but also mitigates harmful reverse electron flow-induced reactive oxygen species production.
Acknowledgments
We thank Mary B. Ziebell, Research Technologist, and David Schwabe, B.S., Research Technologist, for technical assistance as well as Terri L. Misorski, A.A.S., Program Coordinator, for editorial assistance (all from Department of Anesthesiology, Medical College of Wisconsin, Milwaukee, Wisconsin).
This work was supported in part by the National Institutes of Health Grants P01GM066730 and R01HL034708 (to Dr. Bosnjak), (to Dr. Bienengraeber) and R01HL054820 (to Dr. Warltier), Bethesda, Maryland
Footnotes
The department and institution: Department of Anesthesiology, Medical College of Wisconsin, Milwaukee, Wisconsin, USA
References
- 1.Kersten JR, Schmeling TJ, Pagel PS, Gross GJ, Warltier DC. Isoflurane mimic preconditioning via activation of K(ATP) channels: Reduction of myocardial infarct size with an acute memory phase. Anesthesiology. 1997;87:361–70. doi: 10.1097/00000542-199708000-00024. [DOI] [PubMed] [Google Scholar]
- 2.Cason BA, Gamperl AK, Slocum RE, Hickey RF. Anesthetic-induced preconditioning: previous administration of isoflurane decreases myocardial infarct size in rabbits. Anesthesiology. 1997;87:1182–90. doi: 10.1097/00000542-199711000-00023. [DOI] [PubMed] [Google Scholar]
- 3.Ljubkovic M, Mio Y, Marinovic J, Stadnicka A, Warltier DC, Bosnjak ZJ, Bienengraeber M. Isoflurane preconditioning uncouples mitochondria and protects against hypoxia-reoxygenation. Am J Physiol Cell Physiol. 2007;292:C1583–90. doi: 10.1152/ajpcell.00221.2006. [DOI] [PubMed] [Google Scholar]
- 4.Pravdic D, Sedlic F, Mio Y, Vladic N, Bienengraeber M, Bosnjak ZJ. Anesthetic-induced preconditioning delays opening of mitochondrial permeability transition pore via protein kinase C-ε-mediated pathway. Anesthesiology. 2009;111:267–74. doi: 10.1097/ALN.0b013e3181a91957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sedlic F, Pravdic D, Ljubkovic M, Marinovic J, Stadnicha A, Bosnjak ZJ. Differences in production of reactive oxygen species and mitochondrial uncoupling as events in the preconditioning signaling cascade between desflurane and sevoflurane. Anesth Analg. 2009;109:405–11. doi: 10.1213/ane.0b013e3181a93ad9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka K, Weihrauch D, Kehl F, Ludwig LM, LaDisa JF, Jr, Kersten JR, Pagel PS, Warltier DC. Mechanism of preconditioning by isoflurane in rabbits: A direct role for reactive oxygen species. Anesthesiology. 2002;97:1485–90. doi: 10.1097/00000542-200212000-00021. [DOI] [PubMed] [Google Scholar]
- 7.Kevin LG, Novalija E, Riess ML, Camara AK, Rhodes SS, Stowe DF. Sevoflurane exposure generates superoxide but leads to decreased superoxide during ischemia and reperfusion in isolated hearts. Anesth Analg. 2003;96:949–55. doi: 10.1213/01.ANE.0000052515.25465.35. [DOI] [PubMed] [Google Scholar]
- 8.Novalija E, Kevin LG, Eells JT, Henry MM, Stowe DF. Anesthetic preconditioning improves adenosine triphosphate synthesis and reduces reactive oxygen species formation in mitochondria after ischemia by a redox dependent mechanism. Anesthesiology. 2003;98:1155–63. doi: 10.1097/00000542-200305000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Riess ML, Novalija E, Camara AK, Eells JT, Chen Q, Stowe DF. Preconditioning with sevoflurane reduces changes in nicotinamide adenine dinucleotide during ischemia-reperfusion in isolated hearts: Reversal by 5-hydroxydecanoic acid. Anesthesiology. 2003;98:387–95. doi: 10.1097/00000542-200302000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Redal A, Lange M, Jazbutyte V, Lots C, Smul TM, Roewer N, Kehl F. Activation of mitochondrial large-conductance calcium-activated K+ channels via protein kinase A mediates desflurane-induced preconditioning. Anesth Analg. 2008;106:384–91. doi: 10.1213/ane.0b013e318160650f. [DOI] [PubMed] [Google Scholar]
- 11.Sedlic F, Sepac A, Pravdic D, Camara AK, Bienengraeber M, Brzezinska AK, Wakatsuki T, Bosnjak ZJ. Mitochondrial depolarization underlies delay in permeability transition by preconditioning with isoflurane: Roles of ROS and Ca2+ Am J Physiol Physiol. 2010;299:C506–15. doi: 10.1152/ajpcell.00006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanley PJ, Ray J, Brandt U, Daut J. Halothane, isoflurane and sevoflurane inhibit NADH: Ubiquinone oxidoreductase (complex I) of cardiac mitochondria. J Physiol. 2002;544:687–93. doi: 10.1113/jphysiol.2002.025015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sedlic F, Pravdic D, Hirata N, Mio Y, Sepac A, Camara AK, Wakatsuki T, Bosnjak ZJ, Bienengraeber M. Monitoring mitochondrial electron fluxes using NAD(P)H-flavoprotein fluorometry reveals complex action of isoflurane on cardiomyocytes. Biochim Biophys Acta. 2010;1797:1749–58. doi: 10.1016/j.bbabio.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pravdic D, Mio Y, Sedlic F, Pratt PF, Wartier DC, Bosnjak ZJ, Bienengraeber M. Isoflurane protects cardiomyocytes and mitochondria by immediate and cytosol-independent at reperfusion. Br J Pharmacol. 2010;160:220–32. doi: 10.1111/j.1476-5381.2010.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: Central role of complex III. J Biol Chem. 2003;278:36027–31. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 16.Ludwig LM, Tanaka K, Eells JT, Weihrauch D, Pagel PS, Kersten JR, Warltier DC. Preconditioning by isoflurane is mediated by reactive oxygen species generated from mitochondrial electron transport chain complex III. Anesth Analg. 2004;99:1308–15. doi: 10.1213/01.ANE.0000134804.09484.5D. [DOI] [PubMed] [Google Scholar]
- 17.Wang C, Weihrauch D, Schwabe DA, Bienengraeber M, Warltier DC, Kersten JR, Pratt PF, Jr, Pagel PS. Extracellular signal-regulated kinases trigger isoflurane preconditioning concomitant with upregulation of hypoxia-inducible factor-1alpha and vascular endothelial growth factor expression in rats. Anesth Analg. 2006;103:281–8. doi: 10.1213/01.ane.0000226094.94877.98. [DOI] [PubMed] [Google Scholar]
- 18.Warltier DC, Zyvoloski MG, Gross GJ, Hardman HF, Brooks HL. Determination of experimental myocardial infarct size. J Pharmacol Methods. 1981;6:199–210. doi: 10.1016/0160-5402(81)90109-1. [DOI] [PubMed] [Google Scholar]
- 19.Dröse S, Brandt U. The mechanism of mitochondrial superoxide production by the cytochrome bc1 complex. J Biol Chem. 2008;283:21649–54. doi: 10.1074/jbc.M803236200. [DOI] [PubMed] [Google Scholar]
- 20.Votyakova TV, Reynolds IJ. Detection of hydrogen peroxide with amplex red: Interference by NADH and reduced glutathione auto-oxidation. Arch Biochem Biophys. 2004;431:138–44. doi: 10.1016/j.abb.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 21.Krähenbühl S, Talos C, Wiesmann U, Hoppel CL. Development and evaluation of spectrophotometric assay for complex III in isolated mitochondria, tissues and fibroblasts from rats and humans. Clin Chim Acta. 1994;230:177–87. doi: 10.1016/0009-8981(94)90270-4. [DOI] [PubMed] [Google Scholar]
- 22.O'Toole JF, Patel HV, Naples CJ, Fujioka H, Hoppel CL. Decreased cytochrome c mediates an age-related decline of oxidative phosphorylation in rat kidney mitochondria. Biochem J. 2010;427:105–12. doi: 10.1042/BJ20091373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trumpower BL, Edwards CA. Purification of a reconstitutively active iron-sulfur protein (oxidation factor) from succinate cytochrome c reductase complex of bovine heart mitochondria. J Biol Chem. 1979;254:8697–706. [PubMed] [Google Scholar]
- 24.Dröse W. Free redicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 25.Tritto I, D'Andrea D, Eramo N, Scognamiglio A, De Simone C, Violante A, Esposito A, Chiariello M, Ambrosio G. Oxygen radicals can induce preconditioning in rabbit hearts. Circ Res. 1997;80:743–8. doi: 10.1161/01.res.80.5.743. [DOI] [PubMed] [Google Scholar]
- 26.Riess ML, Kevin LG, McCormick J, Jiang MT, Rhodes SS, Stowe DF. Anesthetic preconditioning: The role of free radical in sevoflurane-induced attenuation of mitochondrial electron transport in guinea pig isolated hearts. Anesth Analg. 2005;100:46–53. doi: 10.1213/01.ANE.0000139346.76784.72. [DOI] [PubMed] [Google Scholar]
- 27.Zaugg M, Lucchinetti E, Uecker M, Pasch T, Schaub MC. Anaesthetics and cardiac preconditioning. Part I. Signaling and cytoprotective mechanisms. Br J Anaesth. 2007;99:603–6. doi: 10.1093/bja/aeg205. [DOI] [PubMed] [Google Scholar]
- 28.Wilcox CS, Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol Rev. 2008;60:418–69. doi: 10.1124/pr.108.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trnka J, Blaikie FH, Smith RA, Murphy MP. A mitochondria-targeted nitroxide is reduced to its hydroxylamine by ubiquinol in mitochondria. Free Radic Biol Med. 2008;44:1406–19. doi: 10.1016/j.freeradbiomed.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 30.Dröse S, Hanley PJ, Brandt U. Ambivalent effects of diazoxide on mitochondrial ROS production at respiratory chain complexes I and III. Biochim Biophys Acta. 2009;1790:558–65. doi: 10.1016/j.bbagen.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Garlid KD, Paucek P. Mitochondrial potassium transport: the K(+) cycle. Biochim Biophys Acta. 2003;1606:23–41. doi: 10.1016/s0005-2728(03)00108-7. [DOI] [PubMed] [Google Scholar]
- 32.Costa AD, Quinlan CL, Andrukhiv A, West IC, Jaburek M, Garlid KD. The direct physiological effects of mitoK(ATP) opening on heart mitochondria. Am J Physiol Heart Circ Physiol. 2006;290:H406–15. doi: 10.1152/ajpheart.00794.2005. [DOI] [PubMed] [Google Scholar]
- 33.Carroll R, Grant VA, Yellon DM. Mitochondrial K(ATP) channel opening protects a human atrial-derived cell line by a mechanism involving free radical generation. Cardiovasc Res. 2001;51:691–700. doi: 10.1016/s0008-6363(01)00330-3. [DOI] [PubMed] [Google Scholar]
- 34.Minners J, Lacerda L, Yellon DM, Opie LH, McLeod CJ, Sack MN. Diazoxide-induced respiratory inhibition – a putative mitochondrial K (ATP) channel independent mechanism of pharmacological preconditioning. Mol Cell Biochem. 2007;294:11–8. doi: 10.1007/s11010-005-9066-6. [DOI] [PubMed] [Google Scholar]
- 35.Dröse S, Brandt U, Hanley PJ. K+-independent actions of diazoxide question the role of inner membrane KATP channels in mitochondrial cytoprotective signaling. J Biol Chem. 2006;281:23733–9. doi: 10.1074/jbc.M602570200. [DOI] [PubMed] [Google Scholar]
- 36.Kohro S, Hogan QH, Nakae Y, Yamakage M, Bosnjak ZJ. Anesthetic effects on mitochondrial ATP-sensitive K channel. Anesthesiology. 2001;95:1435–40. doi: 10.1097/00000542-200112000-00024. [DOI] [PubMed] [Google Scholar]
- 37.McLeod CJ, Aziz A, Hoyt RF, McCoy JP, Jr, Sack MN. Uncoupling proteins 2 and 3 function in concert to augment tolerance to cardiac ischemia. J Biol Chem. 2005;280:33470–6. doi: 10.1074/jbc.M505258200. [DOI] [PubMed] [Google Scholar]
- 38.Nadtochiy SM, Tompkins AJ, Brookes PS. Different mechanisms of mitochondrial proton leak in ischaemia/reperfusion injury and preconditioning: implications for pathology and cardioprotection. Biochem J. 2006;395:611–8. doi: 10.1042/BJ20051927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ackrell BA, Kearney EB, Mayr M. Role of oxalacetate in the regulation of mammalian succinate dehydrogenase. J Biol Chem. 1974;249:2021–7. [PubMed] [Google Scholar]
- 40.Esteitie N, Hinttala R, Wibom R, Nilsson H, Hance N, Naess K, Tear-Fahnehjelm K, von Dobeln U, Majamaa K, Larsson NG. Secondary metabolic effects in complex I deficiency. Ann Neurol. 2005;58:544–52. doi: 10.1002/ana.20570. [DOI] [PubMed] [Google Scholar]
- 41.Grivennikova VG, Vinogradov AD. Generation of superoxide by the mitochondrial complex I. Biochim Biophys Acta. 2006;1757:553–61. doi: 10.1016/j.bbabio.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 42.Bittl JA, Shine KI. Protection of ischemic rabbit myocardium by glutamic acid. Am J Physiol. 1983;245:H406–12. doi: 10.1152/ajpheart.1983.245.3.H406. [DOI] [PubMed] [Google Scholar]
- 43.Hoyer S, Krier C. Ischemia and aging brain. Studies on glucose and energy metabolism in rat cerebral cortex. Neurobiol Aging. 1986;7:23–9. doi: 10.1016/0197-4580(86)90022-9. [DOI] [PubMed] [Google Scholar]
- 44.Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: Progress, pitfall, and proscpects. Free Radic Biol Med. 2007;43:995–1022. doi: 10.1016/j.freeradbiomed.2007.06.026. [DOI] [PubMed] [Google Scholar]