Summary
Parkinson's disease (PD) is a chronic, progressive, as-of-yet incurable, neurodegenerative condition affecting the nigro–striatal dopaminergic system. Emerging evidence suggests the importance of exercise in improving the trajectory of PD. Yet few people with PD are physically active. One challenge that healthcare professionals face in the 21st century is how to deliver physical activity programs to the population of individuals living with PD. A novel approach to delivering physical activity to people with PD is introduced – termed community-based participatory research (CBPR) – which engages people with PD and patient advocates as co-researchers in the development and implementation of community-based exercise programs. The authors describe the CBPR approach and provide several recent examples of community exercise programs that are steps in the direction of developing the CBPR model. This is followed by a discussion of what a more fully realized CBPR model might look like. Finally, the authors describe some obstacles to conducting CBPR and suggest strategies for overcoming them. It is argued that people with PD are an integral component of delivering the exercise intervention.
We believe that one of the most pressing challenges healthcare professionals face in the 21st century is how to deliver physical activity programs to the individuals living with Parkinson's disease (PD). In this article, we introduce a novel approach to delivering physical activity to people with PD – termed community-based participatory research (CBPR) – which engages people with PD and patient-advocates as co-researchers in the development and implementation of community-based exercise programs. In the first section, we present neuroscience evidence supporting the importance of exercise in improving the trajectory of PD and review the literature on physical activity levels of individuals with PD. We will then describe the CBPR approach and provide several recent examples of community exercise programs that are steps in the direction of developing the CBPR model. This is followed by a discussion of what a more fully realized CBPR model might look like. Finally, we describe some obstacles to conducting CBPR and suggest strategies for overcoming them.
Parkinson's disease is a chronic, progressive, as-of-yet incurable, neurodegenerative condition affecting the nigro–striatal dopaminergic system, with effects on motor, cognitive, social and emotional domains [1]. Treatment includes administration of medication (levodopa and other dopaminergic agents) and, in the later stages of the disease, neurosurgical approaches such as deep brain stimulation (DBS). Levodopa and neurosurgery have revolutionized the treatment of PD, but there are no established therapies that can stop or slow the underlying neurodegenerative disease process.
Exercise treatment
Historically, nonpharmacological approaches such as exercise or physiotherapy have been viewed as ‘adjunctive’ (i.e., helpful) in the management of PD. There is ongoing debate as to the efficacy of exercise or physiotherapy [2–5]. A rich vein of evidence now suggests that exercise or physiotherapy have positive effects on PD function including physiologic capacity, gait, balance, range of motion, muscle strength, cognition and quality of life [6–10]. Gait and balance impairment, the cardinal motor features of PD that generally become more prominent with disease severity, are associated with increased mortality [11,12]. Recently, the American Academy of Neurology Quality Standards Subcommittee noted that “exercise may be helpful in improving motor function in people with PD” [13]. While the evidence that exercise or physiotherapy are beneficial for PD is certainly a cause for hope, slowing down disease progression remains a major unmet need in PD therapy [14,15]. Even with optimal pharmacological and surgical intervention, scholars believe that the underlying disease process inevitably progresses, resulting in increased disability in most PD patients over time [16].
Recent, groundbreaking neuroscience studies using animal models of exercise and PD seriously challenges this view of PD, suggesting the physiologic use of exercise may be a curative model (Box 1). Together, the animal studies suggest that forced and/or voluntary exercise interventions confer neuroprotective and neurorestorative effects on nigro–striatal circuitry with or without behavioral recovery [17–19]. Studies using the potent neurotoxins, 6-hydroxydopamine in rats and 1-methyl-4-phenyl-1,2,3,6-tetrahydro-pyridine in mice, report that intense, continuous sensorimotor training before or after diagnosis can alter the neurodegenerative and behavioral effects of these toxic agents, while inactivity may be prodegenerative (i.e., amplify the neurodegenerative disease progression.
Box 1. Plasticity mechanisms in animal models of Parkinson's disease and exercise.
Exercise-induced upregulation of D2 mRNA, downregulation of DAT and increased dopamine release in dorsolateral striatum [62–64]
Exercise-induced sparing of striatal DA neurons and metabolites [65–67]
Exacerbated loss of striatal DA levels, metabolites and DA terminals with forced nonuse [68]
Exercise-induced increase in GDNF mRNA with downregulation of striatal DAT and VMAT2 [69]
Parkinson's disease is characterized by reduced levels of GDNF [70]
Exercise-induced upregulation of striatal GDNF and production of GDNF producing cells (glia) and prevention of downregulation of BDNF signaling pathway in SN and striatum [71–73]
Exercise-induced partial restoration of TH-labeled neurons in SNpc [74]
Lower net DA SNpc neuronal loss with environmental enrichment [75]
Exercise-induced increase in net DA SNpc neurons [76]
Exercise-induced improved mitochondrial function [77]
Exercise-induced striatal angiogenesis [78]
Exercise-induced improved forelimb function without sparing of DA terminals [79]
Exercise-induced loss of TH positive neurons in SNpc [80]
Exercise-induced cardiorespiratory and metabolic adaptations without effects on nigrostriatal DA function [81]
Exercise-induced reduction in DA loss without amelioration of behavioral deficits [82]
BDNF: Brain-derived neurotrophic factor; D2: Dopamine receptor type 2 (DA-D2R); DA: Dopaminergic; DAT: Dopamine transporter; GDNF: Glia cell line-derived neurotrophic factor; SN: Substatia nigra; SNpc: Substantia nigra pars compacta; TH: Tyrosine hydroxylase; VMAT2: Vesicular monoamine transporter.
The mechanisms underlying the effects of exercise-induced neuroplasticity or behavioral recovery in animal models of exercise and PD are yet to be fully understood [19]. Some of the animal studies of exercise and PD suggest neuroprotective or neurorestorative effects of exercise, while other studies suggest behavioral effects without neuroplasticity. Part of the difficultly in generating conclusions from these divergent results may be owing to methodological issues, differences in studies' experimental design, amount or location of neurotoxin used, the timing, mode or amount of exercise, or the animals' age or gender (for overview see [20–23]). While insights from the animal models await replication in human trials, and this may take years, the value of animal models of exercise and PD is that they may lead to new treatment directions. If exercise is as important as the animal studies hint, one of the pressing issues at hand is how to ensure that patients at all stages of PD become more physically active [24,25]. Optimal care that includes development of exercise infrastructure administered by multidisciplinary healthcare teams is one option [24,25]. However, these approaches share a common theme: they place the patient as a more-or-less passive recipient of care and not as an active leader. In this article, we describe a novel approach to multidisciplinary care that aims to empower patients as equal partners in all aspects of their care and also may increase physical activity-seeking behaviors (Figure 1).
Figure 1. Models to plan and deliver community-based exercise interventions for people living with Parkinson's disease.
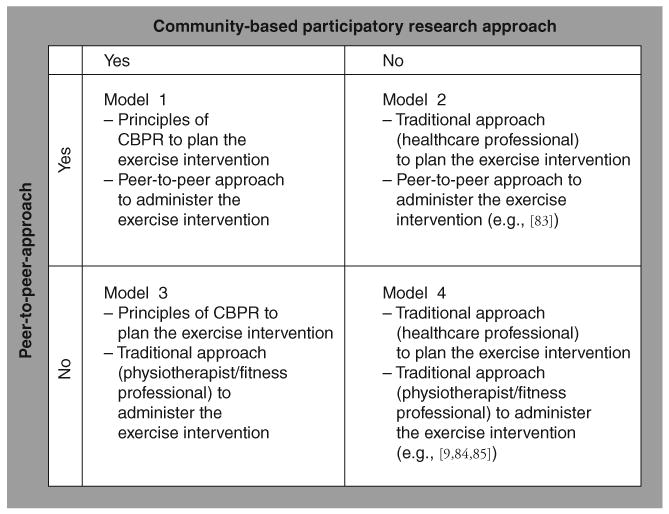
CBPR: Community-based participatory research.
Exercise levels
There is a small but growing number of studies on physical activity levels in PD. Animal models hinted that “the amount of voluntary physical activity is regulated at least in part by the dopamine system” [26], and now studies in human PD suggest people with PD are prone to exhibiting a more or less sedentary lifestyle [27–31]. In one of the largest studies on daily physical activity patterns, which included 699 patients with PD and 1959 controls, Nimwegen and colleagues [28] report those with PD were 29% less physically active than healthy controls. In an ongoing study by Nimwegen and colleagues (the ParkFit trial [32]), 64% of participants with PD (mean age = 64.1 years, SD = 7.6 years; Hoehn and Yahr stage [33] ≤3), who were screened for physical activity levels at baseline, were classified as being ‘sedentary’. This was defined by the authors using standardized criteria as: less than three times a week of vigorous-intensity physical activity for <60 min; or <three times a week moderate-intensity physical activity for <150 min [34]. Busse and colleagues [29] report reduced 7-day physical activity patterns (measured by step activity monitor) among adults with neurological conditions compared with healthy controls. The ten PD patients in their study had lower step/day counts than healthy adult control subjects. Using accelerometers, Hale and colleagues [30] extended these results demonstrating that people with PD are less physically active than even healthy sedentary adults [30].
Functional factors
With little research having been conducted on physical activity in PD, factors associated with physical activity levels remain elusive. A number of studies [32,35] report high correlations between number of daily steps taken, postural instability and disease severity, with the lowest number of steps taken among patients at mid to higher disease stage (stage 3 and 4). Mak and Pang [35] and Garber [36] report that low scores on standardized measures of PD balance and balance self-efficacy correlate with less distance walked on a standardized test of walking (6-min walk test). Jones and colleagues postulate that cardinal motor characteristics of PD such as step-hesitation, freezing and problems with balance and dyskinesias may interfere with physical activity-seeking behaviors [37]. The authors qualitative study investigated the experiences (e.g., patients' thoughts and feelings) associated with walking among people with PD. Patients experienced walking as an integral component of their self-esteem and independence, and identified walking as integral to their connection with society and feeling like a human being. Results suggest people with PD dislike walking in public, especially negotiating stairs and walking in crowded environments, with the greatest concerns expressed over problems with balance, which could be misconstrued as drunkenness [37]. Prior studies have reported associations between PD and fears of being publicly humiliated [38] and anxiety over interacting socially [39]. These fears are understandable as individuals who might be trying to conceal their symptoms in public settings might be experiencing considerable anxiety and would be less likely to exercise in public. These factors could be exacerbated in community-based public health settings unless strategies are taken to address them.
Several studies report associations between physical fatigue, one of the most disabling symptoms of PD [40], and physical activity levels across disease severity [36,41,42]. Using activity monitors, Rochester and colleagues report patients with PD spent a significant portion of their time sedentary – and these patients displayed the greatest amount of physical fatigue on standardized tests but no statistical relationship was noted between fatigue and physical activity (walking) [42]. Using a cross-sectional design with self-report and standardized measures of physical function, Garber and Friedman report high levels of fatigue correlated with low performance on physical capacity (i.e., maximal oxygen consumption) and associations between high levels of leisure activity and low levels of fatigue [36]. In a recent prospective, longitudinal study on the effect of cueing training in PD [43] in which patients wore activity monitors for 12 weeks, Elbers and colleagues developed a mathematical model of fatigue and physical activity [41]. The authors found greater amounts of fatigue associated with getting less physical activity; however, fatigue explained only 2% of the variability in the amount of physical activity [41]. The authors did not report if the cueing intervention resulted in greater physical activity levels.
One logical avenue to increasing physical activity would be through referrals. Haas and Okun add the following: “most patients with PD are treated by clinicians without specific PD-based training” [25]. According to one study, 25% of PD treating neurologists fails to talk to their PD patients about leading a physically active lifestyle [101]. In addition, “in reality, many patients have limited access to PD services” [25]. Only 34% of PD patients are prescribed medication or physical therapy at diagnosis or within the first year [44], and patients with PD lack awareness for the places in their community to participate in exercise [45,46].
Therefore, people with PD are at special risk toward inactivity and low physical activity levels are an early and ongoing feature of PD. If this situation does not improve, communities with PD could stay disenfranchised from exercise.
Emerging models
A vital component to improving healthcare among people with PD is to educate the community about evidence-based treatment such as exercise, and at the same time, it is important to test the efficacy of exercise intervention for PD, which involves the PD patients themselves as equal partners in the delivery of the interventions (Figures 1 & 2). We question whether it is enough for healthcare professionals to ask patients whether or not they exercise and then to expect them to do it, or to send patients home with a brochure or a booklet with community resources and then to hope for the best. We believe that substantial investments in community-based infrastructure – supporting and empowering places (experiences that nurture greater community-collaboration and self-management of the disease) to exercise – and novel strategies for developing and implementing community-based exercise programs are necessary if we are to expect individuals with PD to exercise for the long-term starting at diagnosis, and, we must all work together in multidisciplinary rehabilitation teams with patients and patient advocates in order to achieve this vision.
Figure 2. Peer trainers with Parkinson's disease are members of the community-based participatory research team.

The photograph shows two individuals with Parkinson's disease who participated in the first author's randomized controlled trial on high-intensity resistance (80% of a four repetition maximum was defined as high-intensity resistance training) and balance training [83]. The study took place at a public fitness facility in Tallahassee, FL, USA. Clinicians might shudder at the thought of two patients serving as peer-trainers. Indeed, at recent conferences in the USA and Europe, physiotherapist-scientists voiced understandable concerns about patient safety and ability of lay-expert trainers to deliver treatment. The photograph shows two individuals; one individual is on the foam, while the other individual is leading the balance intervention. At the time the photograph was taken, both individuals were at Hoehn and Yahr stage 2. The photograph suggests the feasibility of implementing research models that incorporate nontraditional (e.g., peer-to-peer) approaches to deliver the exercise intervention (model 2, Figure 1). The authors wish to emphasize that there are other important models of community-based exercise that are emerging in the literature that are important (for examples see Gruber [84] who uses physioptherapists as personal trainers or King and Horak [85] who employ trained exercise professionals as personal trainers [presumably model 4, Figure 1]). Reproduced with permission from [86].
Why is greater patient involvement in delivery of exercise interventions necessary? In most developed countries, populations of older adults will continue to grow well into the middle of the 21st century, and it is feared that the number of older adults will far outgrow the number of physicians and other allied healthcare professionals needed to provide high-quality care for people with PD [47,48]. With the looming care-giver and professional shortages, we argue that one avenue to solving the problem is to develop collaborative efforts involving the multiple lay stakeholders (e.g., patient, care-partner or fitness professionals) to develop and implement community-placed exercise interventions. We are concerned that, ultimately, our inability to develop collaborative efforts leaves populations with PD with limited resources.
In the following section, we highlight recent initiatives that could serve as potential blueprints for developing community-placed exercise interventions. Unlike many pharmacological interventions where the mechanisms are spelled out in detail or explicitly stated, neurorehabilitation studies with physiotherapeutic interventions have not always been detailed enough about the content of the interventions. This makes it challenging, if not impossible, to replicate these studies. The efforts we describe represent an emerging paradigm change in the delivery of health services. This change involves administration of therapy according to evidence-based guidelines of physiotherapy for PD with collaboration by lay-experts. We wish to emphasize that the models are not directly related to the discussion of the PD animal models of exercise but serve as potential models for population-wide programs to empower patients with PD to lead more physically active lifestyles. The amount of detail provided by the models is felt to be a very important reason as to why these initiatives were chosen and not others, as it is felt that the amount of detail provided about the components of the intervention facilitates translation to the community level.
ParkinsonNet model
While there is a growing number of well-designed studies on the efficacy of physiotherapy for PD, the field of physical therapy or exercise for PD is marked by a dearth of studies examining implementation of evidence-based knowledge to the community level. In one of the handful of studies examining whether the physical activity patterns of the PD populations could be affected by an intervention, Rochester and colleagues used objective measures (accelerometers) to examine physical activity levels (defined as the continuous sequence of periods spent sitting/lying, standing and walking at different cadences) [49] before and after DBS of the subthalamic nucleus. A total of 6 months after DBS surgery, there was no change in the patients' amount of physical activity.
Other recent approaches aim to increase physical activity behaviors of patients by making evidence-based physiotherapeutic treatment more accessible by providing services in the patients' home, neighborhood community, instead of the traditional approach of providing services linked to the clinic or hospital. The ParkinsonNet cluster randomized controlled trial is the first and largest study of its kind to bring evidence-based physiotherapy to neighborhoods [50]. Elements of the ParkinsonNet initiative are described in detail in the following section, of which include [51]:
Training of physiotherapists through intensive training workshops according to Dutch evidence-based physiotherapy guidelines
Facilitating communication and collaboration with referring physicians by developing standardized forms to improve the referral into physiotherapy
Web-based and educational materials accessible to patients and healthcare professionals
Results of the ParkinsonNet initiative include reduced healthcare costs among ParkinsonNet clusters compared with patients treated with the usual care [50]; as well as improved PD-specific knowledge of evidence-based care among healthcare professionals involved; enhanced referral process; increased patient volume per treating therapist; and enhanced stakeholder collaboration (cited in [24]). The physiotherapeutic training of the ParkinsonNet professionals, who were licensed physical therapists, was provided by physicians and physical therapists (for more information, see [102] and for practical guidelines to the Dutch evidence-based recommendations for physiotherapy in PD, see [103]).
Using the ParkinsonNet infrastructure [51], the Dutch group followed up with ParkFit [32], an ongoing 2-year multicenter, randomized controlled, single-blind trial with 586 sedentary PD patients enrolled. The primary outcome variable of ParkFit is to increase voluntary physical activity [32]. Secondary outcomes are to monitor the effect of exercise on standardized measures of disease progression (United Parkinson's Disease Rating Scale [UPDRS]), gait, fatigue, anxiety, depression, bone mineral density, aerobic fitness, falls, quality of life and healthcare utilization. Participants are screened for contraindications and randomized into one of two interventions:
Physical therapy focusing on training on how to become more physically active (ParkFit)
Physical therapy with focus on safety and quality of movement (ParkSafe)
In both interventions, patients are treated by ParkinsonNet-experienced physiotherapists in years 1 and 2; and they receive equal number of sessions, brochures with educational materials and a biannual newsletter. In ParkFit, patients interact with therapists schooled in evidence-based behavior modification strategies using the transtheoretical model of health behavior change; they receive educational brochures listing specific exercises, specific strategies to overcome barriers to increasing physical activity in the community such as education about the advantages of leading a physically active life and the dangers of inactivity, encouraging patients to set goals and develop social alliances, and a behavioral health contract, which the patient and the therapist sign before the program begins (for further details on treatment procedure, see [32]).
We believe that community-based exercise provides opportunities for developing social networks that might not develop as readily in clinic or hospital-based settings. Research suggests that social networking may increase physical activity participation in the early stages of the disease [52] and broadening the social network may result in improved compliance with exercise, reduced anxiety or phobic behavior; although studies have not examined this. In ParkFit, patients interact with a physical therapist who is assigned as a lifestyle coach to the participant, and guides the participant in making healthy lifestyle choices and partake in personal training sessions. Patients are trained in exercise goal setting and they receive an ambulatory biofeedback activity monitor with access to a website to track their physical activity patterns to compare results with others. Hopefully, this will provide feedback on the individuals amount of physical activity in comparison with others in the trial and could serve as a further motivator to engage in social networks with a more motivated physical activity-seeking behavior. In ParkSafe, patients receive educational brochures on the benefits of exercise, safety and aims of physical therapy, and are assigned a physical therapist who treats the patient according to agreed upon goals (for further details and rationale of the interventions, see [32]). Results of the ParkFit trial are expected in 2012 [32].
We believe that initiatives such as those previously mentioned should definitely be developed in other healthcare system. However, caution is warranted when translating the ParkinsonNet model directly to other population regions. Future studies should examine how successful ParkinsonNet initiatives are when implemented in more densely populated regions. With 16,485 square miles, The Netherlands is approximately the size of the state of Maryland (MD, USA) and half the size of the state of South Carolina (SC, USA) but has a higher population density (over three times the population of Maryland). So the generalizability of the ParkinsonNet concept might be more applicable to densely populated regions where there is a greater number of physiotherapists per capita, more access to public transportation, and in which patients have shorter travelling time to the therapists, which might increase compliance. Public transportation in the USA, for example, is not as plentiful as in some European countries. In some communities, individuals with PD may live in more rural settings, with fewer resources available (i.e., lack of public transportation). Future studies will need to be conducted to investigate whether the ParkinsonNet concept can be applied in these remote settings.
Community-based participatory models
A hallmark feature of medical care is that lay-communities are often not as directly involved in decisions about their disease management as clinicians would like them to be. In our opinion, this extends to the rehabilitation research world where patients are often involved in research as ‘subjects’ or ‘objects’ of the research, but rarely as ‘colleagues’. Scholars note that rehabilitation researchers have been criticized for doing things to patients rather than with them [53]. Post and colleagues add that: “a challenge is to involve patients more closely in the healthcare process, empowering them to actively participate in the management of their own disease. Effective multidisciplinary care for PD comes with the recognition that patients and their advocates are indispensable members of the healthcare team, with an important role in decision making” [24]. Post and colleagues proceed to state that little progress has been made in translating research to the level of the community, owing to the fact that multidisciplinary team approaches face the formidable challenge of obtaining research funding for partnership building [24].
In the USA, health services research rests on three pillars; bench-to-bedside translational research (T1); clinical trials (T2); and population-based and implementation research (T3; research that aims to translate knowledge from academic centers to community-settings). Researchers that aim to translate the evidence-base to community levels (T3) have historically been under-funded, with the majority of federal support from the NIH in the USA going towards basic science research (including Phase I and II clinical trials) and human clinical research (including controlled observational studies and Phase III clinical trials that aim to translate from the bench to the bedside). The T1 and T2 funding resources have yielded tremendous progress in our understanding of the efficacy of evidence-based physiotherapeutic treatments on PD by increasing the number of meta-analytic studies, systematic reviews and guideline development in PD treatment.
Traditionally, scientists make most, if not all, of the decisions in what to research and how to research it, with little or no community input. Since many of these critical decisions are made in isolation, researchers may not be aware of true community needs. At the same time, communities are often not aware of the research resources in their community. To address this issue, in 2004 the NIH, Agency for Healthcare Research and Quality and the CDC inaugurated a new stream of funding known as community participation in research (T3), to encourage healthcare providers and patients to work collaboratively to build healthier communities using an approach to research and interventions such as CBPR. The first steps for encouraging academic and lay-expert involvement in CBPR is not an easy task, as summarized in TABLE 1 and elsewhere [54–57,104–107].
Table 1. Potential obstacles to conducting community-based participatory research.
| Potential obstacle to conducting CBPR | Suggestions for addressing obstacles |
|---|---|
| The true needs of the community are rarely known to researchers | Healthcare professionals could make efforts to get involved politically as patient advocates in Parkinson's policy (such as the Parkinson Action Network, in the USA) or, at the local level, with community organizations (i.e., local or national Parkinson Associations, disability-rights groups), volunteering time to serve on association boards and attending board functions to get to know the issues facing the Parkinson's disease community with translation of programs into policies |
| Healthcare professionals could make efforts to regularly attend Parkinson support group meetings, interact with support group leaders, patients, care-partners and their families. These social interactions can give academicians and clinicians a ground-zero view of the lives of people with PD from the perspectives of the patients and the care-partners own experiences. These are valuable opportunities for developing research partnerships that healthcare professionals and researchers might not otherwise have | |
| Researchers may be unaware of CBPR resources (e.g., funding mechanisms or people in their community currently conducting CBPR) | Contact community-based participatory researchers in your community by signing up to the CCPH blog and information on their website [109] |
| To obtain a list of federally funded North American community-based participatory research projects and principal investigators, the NIH maintains a search engine, the Research Portfolio Online Searching Tool [108]. Using keywords, ‘community-based participatory research’ or CBPR, current and past grant awards can be searched online | |
| Examples of federal and foundation supports for CBPR funding include: | |
| Communities are rarely aware of the research interests of researchers or of the research infrastructure that is available right in their own community | Solicit research ideas by organizing regular town hall meeting or focus groups attended by community leaders. These meetings and agendas should be cofacilitated by researchers and patients. These could aim to identify research topics and research questions of importance to the stakeholders |
| Develop interactive worldwide web sites or blogs and invite consumers and researchers to interact for the purpose of generating ideas for research | |
| CAB | A basic element of CBPR is the formation of a CAB comprised of community leaders, patients, patient advocates and other key stakeholders who provide leadership to the study. CABs could meet monthly throughout the year. For a synthesis of best practices of developing CABs in public health research settings, see the following citation [114] |
| Professionals use technical language (i.e., postural reflex impairment, dyskinesia etc.) or specialized phrasing (e.g., statistical power, external validity and sampling strategy). This may potentially disenfranchise consumers from partnering with researchers in the research process | In planning community-based participatory projects, it is important to provide consumer educational workshops on research methods (data collection, data analysis, data interpretation). While conducting these workshops for consumers (with intact CNSs) might be challenging enough, designing teaching and learning curricula for people with PD – who may have cognitive and communication impairments – adds further layers of complexity to teaching research skills. |
| A key element of a successful CBPR project is to capture the degree of shared decision making during the research project | Team meetings could be cofacilitated/cochaired by healthcare providers/researchers and patients; team meeting agendas can be developed with substantial input from nonresearchers, and at the end of each team meeting, anonymous questionnaires to determine team members' satisfaction with the amount and quality of participation could be administered |
| Meeting minutes should be kept and distributed to the group following the meetings. Team meetings could be audio taped or video taped and transcribed. The transcripts could be examined and analyzed for the level of participation using participatory codes | |
| Ensure that the CAB is comprised of mostly patients and community leader representatives. Ensure that the research team committee has consumer and community leader representation | |
CAB: Community advisory board; CBPR: Community-based participatory research; CCPH: Community-Campus for Health Partnership; PD: Parkinson's disease.
‘Community’ may be defined by geographic location, as the place where individuals live, work, play or study or any group with common interests, including sexual orientation, religious, ethnic or political affiliation, medical specialty, disease or diagnosis [104]. Community-based participatory research initiatives are patient-centered approaches to research that foster collaborative research partnerships between patients, healthcare professionals and communities. During traditional biomedical research, the academically trained researcher develops the idea, writes the grant, collects, analyzes, interprets and disseminates the data. CBPR aims to develop community capacity for research by empowering patients and patient advocates as co-researchers in every step of the research process from generating the idea and writing the grant, to data analysis, data interpretation and dissemination of the results [54–57].
In recent years, funding for CBPR has increased dramatically. In 2004, there were 38 CBPR-funded NIH studies [108]. In 2010, there were 263 studies with a CBPR component and a total of over US$114 million in funding [108]. While CBPR has gained acceptance in North America and internationally as an important approach to improve public health [57], studies have yet to demonstrate benefits of CBPR to health outcomes, and only a few CBPR studies have been conducted with neurological populations [58–61].
As far as we know, there are no published guidelines available for conducting CBPR in populations with neurological or neurodegenerative conditions. As a first step, Table 1 lists the potential obstacles (and potential strategies in overcoming obstacles) in conducting CBPR approaches. Particularly in the early stages of developing CBPR studies, it can be challenging to find funding resources and people with expertise in doing CBPR. A good strategy in getting started with CBPR is to identify CBPR researchers in the community. Websites with information for CBPR are becoming more plentiful. A resource to tap into is Community-Campus Partnerships for Health (CCPH). CCPH is a nonprofit, world-wide network of over 2000 participatory researchers. A webpage and blog are maintained with up-to-date research and tools for conducting CBPR [109]. These resources are invaluable sources of information on CBPR and community partnering. Now that funding for CBPR is more plentiful, it is hoped that healthcare professionals and rehabilitation researchers will begin to collaborate with lay-communities in developing and testing exercise interventions for all patients with PD across disease severity, using CBPR approaches.
Peer-approach
As suggested in Figure 1, there are numerous options for researching the relationship between traditional and CBPR approaches or peer-to-peer and traditional approaches to delivering the exercise interventions. These might include using principles of CBPR in planning the exercise intervention (e.g., using a CBPR approach involving lay experts such as patients, care-partners, and exercise trainers and others in making decisions about which evidence-based training exercises to include in the community-based setting (models 1 and 3, Figure 1); using traditional approaches to planning the exercise intervention (e.g., therapists and clinicians) (models 2 and 4, Figure 1); using peer-to-peer approaches (e.g., patients as trainers) to delivering the intervention (models 1 and 2, Figure 1); or traditional approaches to delivery of the exercise intervention (e.g., physiotherapist or exercise professional; models 3 and 4, Figure 1).
Goodwin and colleagues recently reviewed randomized controlled trials on the effects of exercise interventions on PD [9]. The authors reported that in ten out of 14 studies, the delivery of the exercise intervention was led by medical professionals (e.g., physiotherapists; model 4, Figure 1). In the remainder of the studies, the exercise interventions were delivered by trained exercise leaders (including QiGong teacher and a student nurse) [9]. Presumably, none of the studies reported by Goodwin had substantial input from patients with PD [9]; however, patient involvement in protocol development was not reported. The amount of healthcare professional involvement in delivery of exercise interventions is unlikely to change in the near future, but it does not mean that lay-experts should not or cannot be empowered to work with the healthcare professionals to jointly deliver (and/or develop) the exercise training protocol, or co-lead data analysis/interpretation/dissemination.
Since research has not examined the effect of CBPR participation on health outcomes in PD, it is conceivable that participating in the planning and implementation of exercise interventions could have a positive or a negative effect on physical activity participation. For example, one hypothesis might be that peer-to-peer approaches to delivery of exercise result in more injuries and generally poorer compliance and poor outcomes. People who are vulnerable to falls and secondary disabling injuries might be especially prone to injuries if they are given too much responsibility for each others safety without taking appropriate steps to protect both the trainer and the trainee from falls. Using some common sense in selecting peer-to-peer trainers (such as selecting peer-to-peer trainers who are in the early stages of the disease before falls and gait problems develop) may be a good strategy before asking people with greater disability and impairment to assist individuals in whom falls are likely to occur during training. It is also possible that peer-to-peer approaches might empower individuals with PD to become physically active. Research suggests that in PD, exercise settings can serve as social networks. By participating in settings with people who have a common interest, community-based exercisers develop social networks that become more important to the individual than, for example, increasing muscle strength. Peer-to-peer approaches to delivering exercise interventions might thus serve to further increase the social network bonds. It is hoped that future research will examine these relationships and the associations of CBPR with peer-to-peer training and health outcomes in greater detail.
Future perspective
It will not be surprising if, in the near future, patients with PD become more involved in the research process as colleagues or co-researchers. Already Parkinson advocacy organizations in the United States such as the Parkinson Disease Foundation have developed specialized training programs that promote greater and direct interaction of patients with the research community (e.g., see PDF Parkinson Action in Research program (PAIR) [115]). Other organizations in Europe, such as the Association of Physiotherapists in Parkinson Disease Europe (APPDE), include patients on their panel of experts. Together with ‘the experts’, the patients are empowered to shape the European clinical care guidelines for physiotherapy in PD [116]. It can be noted that some patient advisors are academically trained medical professionals. Care must be taken to encourage participation of lay-patients and to evaluate the extent of participatory decision making. This is also an opportunity to test whether or not the community-based participatory research process itself could be an intervention which leads to better health outcomes. While these efforts are still in the early stage of their development, the organizations that promote patient co-investigators are to be commended for challenging the traditional paradigm. These efforts will revolutionize research and clinical care implementation. However, it remains to be seen how much actual influence patients will have on specific aspects of the research or clinical care process or if these empowerment efforts turn out to be merely tokenistic.
Practice Points.
Physical therapy and exercise interventions are important adjunctive treatments in Parkinson's disease (PD).
Animal data suggest physical activity is associated with neuroplasticity mechanisms. Some studies of exercise and PD demonstrate a lack of neuroplasticity with or without behavioral recovery. More research is necessary in this area.
At diagnosis and at every office visit, physicians should talk to their patients about exercise interventions and of the potential dangers of inactivity.
At diagnosis, referrals to physiotherapy and community-based exercise programs should be made where patients exercise under appropriate supervision.
Healthcare professionals should partner with community-based wellness facilities and should participate in development and implementation of exercise interventions for patients with PD who do not exhibit contraindications to exercise.
Healthcare professionals, patients and other stakeholders should partner in community-based participatory research interventions.
Acknowledgments
The authors thank Paul D Hirsch for helpful comments and suggestions.
Footnotes
Financial & competing interests disclosure: Partial support by a grant from the Park Foundation, Carolinas Healthcare System Foundation and through National Institute of Child Health and Human Development grant HD055202–01. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Whetten-Goldstein K, Sloan F, Kulad E, et al. The burden of Parkinson's disease on society, family, and the individual. J Am Geriatr Soc. 1997;45:844–849. doi: 10.1111/j.1532-5415.1997.tb01512.x. [DOI] [PubMed] [Google Scholar]
- 2.Deane KH, Jones D, Playford ED, Ben-Shlomo Y, Clarke CE. Physiotherapy for patients with Parkinson's disease: a comparison of techniques. Cochrane Database Syst Rev. 2001;(3) doi: 10.1002/14651858.CD002817. CD002817. [DOI] [PubMed] [Google Scholar]
- 3.Deane KH, Jones D, Ellis-Hill C, Clarke CE, Playford ED, Ben-Shlomo Y. A comparison of physiotherapy techniques for patients with Parkinson's disease. Cochrane Database Syst Rev. 2001;(1) doi: 10.1002/14651858.CD002815. CD002815. [DOI] [PubMed] [Google Scholar]
- 4.Deane KH, Ellis-Hill C, Jones D, et al. Systematic review of paramedical therapies for Parkinson's disease. Mov Disord. 2002;17(5):984–991. doi: 10.1002/mds.10197. [DOI] [PubMed] [Google Scholar]
- 5.Crizzle AM, Newhouse IJ. Is physical exercise beneficial for persons with Parkinson's disease? Clin J Sport Med. 2006;16(5):422–425. doi: 10.1097/01.jsm.0000244612.55550.7d. [DOI] [PubMed] [Google Scholar]
- 6.Kwakkel G, de Goede CJT, van Wegen EEH. Impact of physical therapy for Parkinson's disease: a critical review of the literature. Parkinsonism Relat Disord. 2007;13(Suppl. 3):S478–S487. doi: 10.1016/S1353-8020(08)70053-1. [DOI] [PubMed] [Google Scholar]
- 7.Keus SH, Bloem BR, Hendriks EJ, et al. Evidence-based analysis of physical therapy in Parkinson's disease with recommendations for practice and research. Mov Disord. 2007;22(4):451–460. doi: 10.1002/mds.21244. [DOI] [PubMed] [Google Scholar]
- 8.de Goede CJ, Keus SH, Kwakkel G, Wagenaar RC. The effects of physical therapy in Parkinson's disease: a research synthesis. Arch Phys Med Rehabil. 2001;82(4):509–515. doi: 10.1053/apmr.2001.22352. [DOI] [PubMed] [Google Scholar]
- 9▪.Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL. The effectiveness of exercise interventions for people with Parkinson's disease: a systematic review and meta-analysis. Mov Disord. 2008;23(5):631–640. doi: 10.1002/mds.21922. One of the few meta-analytic review papers on the effectiveness of randomized controlled trials of exercise and physiotherapy on health-related quality of life in Parkinson's disease (PD) [DOI] [PubMed] [Google Scholar]
- 10.Dibble LE, Addison O, Papa E. The effects of exercise on balance in persons with Parkinson's disease: a systematic review across the disability spectrum. J Neurol Phys Ther. 2009;33(1):14–26. doi: 10.1097/NPT.0b013e3181990fcc. [DOI] [PubMed] [Google Scholar]
- 11.Gray WK, Hildreth A, Bilclough JA. Physical assessment as a predictor of mortality in people with Parkinson's disease: a study over 7 years. Mov Disord. 2010;24(13):1934–1940. doi: 10.1002/mds.22610. [DOI] [PubMed] [Google Scholar]
- 12.Kuroda K, Tatara K, Takatorige T, Shinsho F. Effect of physical exercise on mortality in patients with Parkinson's disease. Acta Neurol Scan. 1992;86:55–59. doi: 10.1111/j.1600-0404.1992.tb08054.x. [DOI] [PubMed] [Google Scholar]
- 13.Suchowersky O, Gronseth G, Perlmutter J, Reich S, Zesiewicz, Weiner WJ. Practice parameter: neuroprotective strategies and alternative therapies for Parkinson's disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;66(7):976–982. doi: 10.1212/01.wnl.0000206363.57955.1b. [DOI] [PubMed] [Google Scholar]
- 14.Poewe W. The need for neuroprotective therapies in Parkinson's disease: a clinical perspective. Neurology. 2006;66(10 Suppl. 4):S22S9. doi: 10.1212/wnl.66.10_suppl_4.s2. [DOI] [PubMed] [Google Scholar]
- 15.Koller WC, Cersosimo MG. Neuroprotection in Parkinson's disease: an elusive goal. Curr Neurol Neurosci Rep. 2004;4(4):277–283. doi: 10.1007/s11910-004-0052-2. [DOI] [PubMed] [Google Scholar]
- 16.Shulman LM, Gruber-Baldini AL, Anderson KE, et al. The evolution of disability in Parkinson disease. Mov Disord. 2008;23(6):790–796. doi: 10.1002/mds.21879. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch MA, Farley BG. Exercise, neuroplasticity and Parkinson's disease. Eur J Phys Rehabi l Med. 2009;45(2):215–229. [PubMed] [Google Scholar]
- 18.Speelman AD, van der Warrenburg BP, van Nimwegen M, Petzinger GM, Munneke M, Bloem BR. How might physical activity benefit patients with Parkinson's disease. Nat Rev Neurol. 2011 doi: 10.1038/nrneurol.2011.107. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 19.Archer T, Fredriksson A, Johansson B. Exercise alleviates Parkinsonism: clinical and laboratory evidence. Acta Neurol Scand. 2011;123:73–84. doi: 10.1111/j.1600-0404.2010.01360.x. [DOI] [PubMed] [Google Scholar]
- 20.Gerecke KM, Jiao Y, Pani A, Pagala V, Smeyne RJ. Exercise protects against MPTP-induced neurotoxicity in mice. Brain Res. 2010;1341:72–83. doi: 10.1016/j.brainres.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jucker M. The benefits and limitations of animal models for translational research in neurodegenerative diseases. Nat Med. 2010;16(11):1210–1214. doi: 10.1038/nm.2224. [DOI] [PubMed] [Google Scholar]
- 22.Hartung T. Thoughts on limitations of animal models. Parkinsonism Relat Disord. 2008;14:S81–S82. doi: 10.1016/j.parkreldis.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Antzoulatos E, Jakowec MW, Petzinger GM, Wood RI. Sex differences in motor behavior in the MPTP mouse model of Parkinson's disease. Pharmacol Biochem Behav. 2010;95(4):466–472. doi: 10.1016/j.pbb.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Post B, van der Eijk M, Munneke M, Bloem BR. Multidisciplinary care for Parkinson's disease: not if, but how! Pract Neurol. 2011;11:58–61. doi: 10.1136/jnnp.2011.241604. [DOI] [PubMed] [Google Scholar]
- 25.Haas CJ, Okun MS. Time for comprehensive care networks for Parkinson's disease. Lancet Neurol. 2010;9:20–22. doi: 10.1016/S1474-4422(09)70331-X. [DOI] [PubMed] [Google Scholar]
- 26.Knab AM, Lightfoot JT. Does the difference between physically active and couch potato lie in the dopamine system? Int J Biol Sci. 2010;6(2):133–150. doi: 10.7150/ijbs.6.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chastin S, Rochester L, Jones D, et al. Objective quantification of the pattern of habitual physical activity in advanced Parkinson's disease. Mov Disord. 2009;24(Suppl. 1):S399. [Google Scholar]
- 28.Nimwegen MV, Speelman AD, Hofman EJM, et al. Physical inactivity in Parkinson's disease. J Neurol. 2011 doi: 10.1007/s00415–011–6097–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Busse ME, Pearson OR, Van Dursen R, Wiles CM. Quantified measurement of activity provides insight into motor function and recovery in neurological disease. J Neurol Neurosurg Psychiatry. 2004;75:884–888. doi: 10.1136/jnnp.2003.020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hale LA, Pal J, Becker I. Measuring free-living physical activity in adults with and without neurologic dysfunction with a triaxial accelerometer. Arch Phys Med Rehabil. 2008;89(9):1765–1771. doi: 10.1016/j.apmr.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 31.Skidmore FM, Mackman CA, Breckon P, et al. Daily ambulatory levels in idiopathic Parkinson disease. J Rehabil Res Dev. 2008;45(9):1343–1348. [PubMed] [Google Scholar]
- 32▪▪.Nimwegen MV, Speelman AD, Smulders K, et al. ParkFit Study Group. Design and baseline characteristics of the ParkFit study, a randomized controlled trial evaluating the effectiveness of a multifaceted behavioral program to increase physical activity in Parkinson patients. BMC Neurol. 2010;10:70. doi: 10.1186/1471-2377-10-70. Provides blueprint for conducting a community-based exercise program geared towards increasing physical activity levels in PD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoehn M, Yahr M. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 34.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1435–1445. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- 35.Mak MKY, Pang MYC. Balance self-efficacy determines walking capacity in people with Parkinson's disease. Mov Disord. 2008;23(13):1936–1939. doi: 10.1002/mds.22251. [DOI] [PubMed] [Google Scholar]
- 36.Garber CE, Friedman JH. Effects of fatigue on physical activity and function in patients with Parkinson's disease. Neurology. 2003;60:1119–1124. doi: 10.1212/01.wnl.0000055868.06222.ab. [DOI] [PubMed] [Google Scholar]
- 37.Jones D, Rochester L, Birleson A, et al. Everyday walking with Parkinson's disease: Understanding personal challenges and strategies. Disabil Rehabil. 2008;30(16):1213–1221. doi: 10.1080/09638280701828955. [DOI] [PubMed] [Google Scholar]
- 38.Nijhof G. Parkinson's disease as a problem of shame in public appearance. Social Health Illness. 1995;17(2):193–205. [Google Scholar]
- 39.Krummer A, Cardoso F, Teixeira AL. Frequency of social phobia and psychometric properties of the Liebowitz Social Anxiety Scale in Parkinson's disease. Mov Disord. 2008;23(12):1793–1743. doi: 10.1002/mds.22221. [DOI] [PubMed] [Google Scholar]
- 40.Lou JS. Physical and mental fatigue in Parkinson's disease epidemiology, pathophysiology and treatment. Drugs Aging. 2009;26(3):195–208. doi: 10.2165/00002512-200926030-00002. [DOI] [PubMed] [Google Scholar]
- 41.Elbers R, van Wegen EEH, Rochester L, et al. Is impact of fatigue an independent factor associated with physical activity in patients with idiopathic Parkinson's disease? Mov Disord. 2009;24(10):1512–1518. doi: 10.1002/mds.22664. [DOI] [PubMed] [Google Scholar]
- 42.Rochester L, Jones D, Hetherington V, et al. Gait and gait-related activities and fatigue in Parkinson's disease: what is the relationship? Disabil Rehabil. 2006;28(22):1365–1371. doi: 10.1080/09638280600638034. [DOI] [PubMed] [Google Scholar]
- 43.Nieuwboer A, Kwakkel G, Rochester L, et al. Cueing training in the home improves gait-related mobility in Parkinson's disease: the Rescue trial. J Neurol Neurosurg Psychiatry. 2007;78:134–140. doi: 10.1136/jnnp.200X.097923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dahodwala N, Xie M, Noll E, Siderowf A, Mandell DS. Treatment disparities in Parkinson's disease. Ann Neurol. 2009;66:142–145. doi: 10.1002/ana.21774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leritz E, Loftis C, Crucian G, Friedman W, Bowers D. Self-awareness of deficits in Parkinson disease. Clin Neuropsychol. 2004;18(3):352–361. doi: 10.1080/1385404049052412. [DOI] [PubMed] [Google Scholar]
- 46.Aas RW, Grotle M. Clients using community occupational therapy services: Sociodemographic factors and occurrence of diseases and disabilities. Scand J Occup Ther. 2007;14(3):150–9. doi: 10.1080/11038120600968811. [DOI] [PubMed] [Google Scholar]
- 47.Reuben DB, Zwanziger J, Bradley TB, et al. How many physicians will be needed to provide medical care for older persons Physician manpower needs for the twenty-frst century. J Am Geriat Soc. 1993;41:444–453. doi: 10.1111/j.1532-5415.1993.tb06955.x. [DOI] [PubMed] [Google Scholar]
- 48.Flemming KC, Evans JM, Chutka DS. Caregiver and clinician shortages in an aging nation. Mayo Clin Proc. 2003;78(8):1026–1040. doi: 10.4065/78.8.1026. [DOI] [PubMed] [Google Scholar]
- 49.Rochester L, Chastin SFM, Baker K, Jones D, Burn DJ. Effect of deep brain stimulation of the STN on novel metrics of habitual physical activity patterns in advanced Parkinson's disease. Mov Disord. 2010;25(Suppl. 3):S672. [Google Scholar]
- 50▪▪.Munneke M, Nijkrake MJ, Keus SHJ, et al. Efficacy of community-based physiotherapy networks for patients with Parkinson's disease: a cluster randomized trial. Lancet Neurol. 2009;9(1):46–54. doi: 10.1016/S1474-4422(09)70327-8. Results of the ParkinsonNet intervention provides useful information on developing a community-based program for people with PD. [DOI] [PubMed] [Google Scholar]
- 51.Nijkrake MJ, Keus SHJ, Overeem S, et al. The ParkinsonNet concept: development, implementation and initial experience. Mov Disord. 2010;25(7):823–829. doi: 10.1002/mds.22813. [DOI] [PubMed] [Google Scholar]
- 52.Ravenek MJ, Schneider MA. Social support for physical activity and perceptions of control in early Parkinson's disease. Disabil Rehabil. 2009;31(23):1925–1936. doi: 10.1080/09638280902850261. [DOI] [PubMed] [Google Scholar]
- 53.Stone E, Priestly M. Parasites, pawns and partners: disability researchers and the role of non-disabled researchers. Br J Sociol. 1996;47(4):699–716. [PubMed] [Google Scholar]
- 54.Jones L, Wells K. Strategies for academic and clinician engagement in community-participatory partnered research. JAMA. 2007;31(297):407–410. doi: 10.1001/jama.297.4.407. [DOI] [PubMed] [Google Scholar]
- 55.Macaulay AC, Commanda LE, Freeman WL, et al. Participatory research maximizes community and lay involvement North American Primary Care Research Group. BMJ. 1999;319:774–778. doi: 10.1136/bmj.319.7212.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.White MA, Verhoef MJ. Toward a patient-centered approach Incorporating principles of participatory action research into clinical studies. Integr Cancer Ther. 2005;4(1):21–24. doi: 10.1177/1534735404273727. [DOI] [PubMed] [Google Scholar]
- 57.Macaulay AC, Jagosh J, Seller R, et al. Assessing the benefits of participatory research: a rationale for a realist review. Glob Health Promot. 2011;18(2):45–48. doi: 10.1177/1757975910383936. [DOI] [PubMed] [Google Scholar]
- 58.Hammel J, Jones R, Gossett A, Morgan E. Examining barriers and supports to community living and participation after a stroke from a participatory action research approach. Top Stroke Rehabil. 2006;13(3):43–58. doi: 10.1310/5X2G-V1Y1-TBK7-Q27E. [DOI] [PubMed] [Google Scholar]
- 59.Twillert S, Postema K, Geertzen JHB, Hemminga T, Lettinga AT. Improving rehabilitation treatment in a local setting a case study of prosthetic rehabilitation. Clin Rehabil. 2009;23:938–947. doi: 10.1177/0269215509338125. [DOI] [PubMed] [Google Scholar]
- 60.Kneightley ML, Ratnayake R, Minore B, et al. Rehabilitation challenges for aboriginal clients recovering from brain injury: qualitative study engaging health care practitioners. Brain InJ. 2009;23(3):250–261. doi: 10.1080/02699050902748331. [DOI] [PubMed] [Google Scholar]
- 61.Sohlberg MM, Ehlhardt LA, Fickas S, Sutcliffe A. A pilot study exploring electronic (or e-mail) mail in users with acquired cognitive-linguistioc impairments. Brain Inj. 2003;17(7):609–629. doi: 10.1080/0269905031000070189. [DOI] [PubMed] [Google Scholar]
- 62.Fisher BE, Petzinger GM, Nixon K, et al. Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse basal ganglia. J Neurosci Res. 2004;77(3):378–90. doi: 10.1002/jnr.20162. [DOI] [PubMed] [Google Scholar]
- 63.Petzinger GM, Walsh JP, Akopian G, et al. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci. 2007;27(20):5291–300. doi: 10.1523/JNEUROSCI.1069-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vuckovic MG, Li QZ, Fisher B, et al. Exercise elevates dopamine D2 receptor in a mouse model of Parkinson's disease: In vivo imaging with [F-18] fallypride. Mov Disord. 2010;25(16):2777–2784. doi: 10.1002/mds.23407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65▪▪.Tillerson JL, Cohen AD, Philhower J, Miller GW, Zigmond MJ, Schallert T. Forced limb-use effects on the behavioral and neurochemical effects of 6-hydroxydopamine. J Neurosci. 2001;21(12):4427–4435. doi: 10.1523/JNEUROSCI.21-12-04427.2001. Model for neuroprotection. Early use promotes functional recovery. Delayed use leads to partial or no recovery of motor function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66▪.Tillerson JL, Caudle WM, Reveron ME, Miller GW. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson's disease. Neuroscience. 2003;119(3):899–911. doi: 10.1016/s0306-4522(03)00096-4. Provides possible mechanisms explaining how brief interruptions in training or inactivity impact severity of PD signs and symptoms and neurochemical deficits using animal model of exercise and PD. [DOI] [PubMed] [Google Scholar]
- 67.Mabandla M, Kellaway L, St Clair Gibson A, Russell VA. Voluntary running provides neuroprotection in rats after 6-hydroxydopamine injection into the medial forebrain bundle. Metab Brain Dis. 2004;19(1–2):43–50. doi: 10.1023/b:mebr.0000027416.13070.c3. [DOI] [PubMed] [Google Scholar]
- 68▪.Tillerson JL, Cohen AD, Caudle WM, Zigmond MJ, Schallert T, Miller GW. Forced nonuse in unilateral parkinsonian rats exacerbates injury. J Neurosci. 2002;22(15):6790–6799. doi: 10.1523/JNEUROSCI.22-15-06790.2002. Inactivity unmasks behavioral and neurochemical defcits. Physically active animals were protected from the effects of a mild neurotoxin. Life-long exercise matters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Faherty CJ, Raviie-Shepherd K, Herasimtschuk A, Smeyne RJ. Environmental enrichment in adulthood eliminates neuronal death in experimental Parkinsonism. Brain Res Mol Brain Res. 2005;134(1):170–179. doi: 10.1016/j.molbrainres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 70.Chauhan NB, Siegel GJ, Lee LM. Depletion of glial cell line-derived neurotrophic factor in substantia nigra neurons of Parkinson's disease brain. J Chem Neuroanat. 2001;21:277–288. doi: 10.1016/s0891-0618(01)00115-6. [DOI] [PubMed] [Google Scholar]
- 71▪▪.Cohen AD, Tillerson JL, Smith AD, Schallert T, Zigmond MJ. Neuroprotective effects of prior limb use in 6-hydroxydopamine-treated rats: possible role of GDNF. J Neurochem. 2003;85(2):299–305. doi: 10.1046/j.1471-4159.2003.01657.x. Glial cell line-derived neurotrophic factor was increased on the side of the exercised limb. [DOI] [PubMed] [Google Scholar]
- 72.Steiner B, Winter C, Hosman K, et al. Enriched environment induces cellular plasticity in the adult substantia nigra and improves motor behavior function in the 6-OHDA rat model of Parkinson's disease. Exp Neurol. 2006;199(2):291–300. doi: 10.1016/j.expneurol.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 73.Wu SY, Wang TF, Yu L, et al. Running exercise protects the substantia nigra dopaminergic neurons against infammation-induced degeneration via the activation of BDNF signaling pathway. Brain Behav Immun. 2011;25(1):135–146. doi: 10.1016/j.bbi.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 74.Goldberg NRS, Haack AK, Meshul CK. Enriched environment promotes similar neuronal and behavioral recovery in a young and aged mouse model of Parkinson's disease. Neuroscience. 2011;172:443–452. doi: 10.1016/j.neuroscience.2010.09.062. [DOI] [PubMed] [Google Scholar]
- 75.Anastasia A, Torre L, de Erausquin GA, et al. Enriched environment protects the nigrostriatal dopaminergic system and induces astroglial reaction in the 6-OHDA rat model of Parkinson's disease. J Neurochem. 2009;109:755–765. doi: 10.1111/j.1471-4159.2009.06001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith BA, Goldberg NRS, Meshul CK. Effects of treadmill exercise on behavioral recovery and neural changes in the substantia nigra and striatum of the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse. Brain Res. 2011;1386:70–80. doi: 10.1016/j.brainres.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lau YS, Patki G, Das-Panja K, Le WD, Ahmad SO. Neuroprotective effects and mechanisms of exercise in a chronic mouse model of Parkinson's disease with moderate neurodegeneration. Eur J Neurosci. 2011;33(7):1264–1274. doi: 10.1111/j.1460-9568.2011.07626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Al-Jarrah M, Jamous M, Al Zailaey K, Bweir SO. Endurance exercise training promotes angiogenesis in the brain of chronic/progressive mouse model of Parkinson's disease. Neurorehabilitation. 2010;26(4):369–373. doi: 10.3233/NRE-2010-0574. [DOI] [PubMed] [Google Scholar]
- 79.O'Dell SJ, Gross NB, Fricks AN, Casiano BD, Nguyen TB, Marshall JF. Running wheel exercise enhances recovery from nigrostriatal dopamine injury without inducing neuroprotection. Neuroscience. 2007;144(3):1141–1151. doi: 10.1016/j.neuroscience.2006.10.042. [DOI] [PubMed] [Google Scholar]
- 80.Pothakos K, Kurz MJ, Lau YS. Restorative effect of endurance exercise on behavioral deficits in the chronic mouse model of Parkinson's disease with severe neurodegeneration. BMC Neurosci. 2009;10:6. doi: 10.1186/1471-2202-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Al-Jarrah M, Pothakos K, Novikova L, et al. Endurance exercise promotes cardiorespiratory rehabilitation without neurorestoration in the chronic mouse model of Parkinsonism with severe neurodegeneration. Neurosci. 2007;149:28–37. doi: 10.1016/j.neuroscience.2007.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Poulton NP, Muir GD. Treadmill training ameliorates dopamine loss but not behavioral deficits in hemi-Parkinsonian rats. Exp Neurol. 2005;193:181–197. doi: 10.1016/j.expneurol.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 83.Hirsch MA, Toole T, Maitland CG, Rider RA. The Effects of balance training and high-intensity resistance training on persons with idiopathic Parkinson's disease. Arch Phys Med Rehabil. 2003;84(8):1109–1117. doi: 10.1016/s0003-9993(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 84.Gruber RA, Elman JG, Huijbregts MPJ. Self-management programs for people with Parkinson's disease. Top Geriatr Rehabil. 2008;24(2):141–150. [Google Scholar]
- 85▪.King LA, Horak F. Delaying mobility disability in people with Parkinson disease using a sensorimotor agility exercise program. Physical Therapy. 2009;89:384–393. doi: 10.2522/ptj.20080214. Provides specific exercises to be used in a fitness or wellness setting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86▪.Hirsch MA, Rider RA, Toole T, et al. Falls prevention in individuals with Parkinson's disease: part I: the importance of balance and resistance training. Palaestra. 1997;13(3):15–20. [Google Scholar]
Websites
- 101.National Parkinson's Disease Foundation; 2008. Parkinson's Knowledge and Needs Exchange Survey. www.pdf.org/en/media_news/release/pr_1226015239. [Google Scholar]
- 102.ParkinsonNet homepage. www.parkinsonnet.nl/welcome.aspx.
- 103.APPDE homepage. www.appde.eu.
- 104.NIH: Successful Models of Community-Based Participatory Research. Final Report http://ehp.niehs.nih.gov/members/2002/suppl-2/155–159ofallon/ofallon-full.html.
- 105.The Welleseley Institute: Peer research in action I. Models of practice www.wellesleyinstitute.com/uncategorized/peer-research-in-action.
- 106.IDS: So what difference does it make? Mapping the outcomes of citizen engagement. www.ids.ac.uk/go/idspublication/so-what-difference-does-it-make-mapping-the-outcomes-of-citizen-engagement.
- 107.Involve: exploring impact: public involvement in NHS, public health and social care research. www.invo.org.uk/viewnews.asp?valueid=457.
- 108.NIH RePORT (Research Portfolio Online Reporting Tool) http://projectreporter.nih.gov/reporter.cfm.
- 109.CCPH homepage. www.ccph.info.
- 110.National Institute for Health Research (UK) website promoting public involvement in public health and social care research www.invo.org.uk.
- 111.Agency for Healthcare Research and Quality (USA) doi: 10.1080/15360280802537332. www.ahrq.gov. [DOI] [PubMed]
- 112.WK Kellogg Foundation (USA) www.wkkf.org/what-we-support/civic-engagement.aspx.
- 113.Centers for Disease Control and Prevention (USA) www.cdc.gov/prc/research-projects/community-partnership.htm.
- 114.CDC Community advisory boards in community-based participatory research: a synthesis of best processes. www.cdc.gov/pcd/issues/2011/may/10_0045.htm. [PMC free article] [PubMed]
- 115.Parkinson's Advocates in Research (PAIR) www.pdf.org/en/pair.
- 116.European guideline for physiotherapy in Parkinson's disease. www.appde.eu/EN/pdfs/newsletters/EU_Guideline_Parkinson_Newsletter2__ July2011.pdf.


