Abstract
ATP leads to endothelial NO synthase (eNOS)/NO-mediated vasodilation, a process hypothesized to depend on the endothelial caveolar eNOS partitioning and subcellular domain-specific multisite phosphorylation state. We demonstrate herein that, in both the absence and presence of ATP, the uterine artery endothelial caveolae contain specific protein machinery related to subcellular partitioning and act as specific focal “hubs” for NO- and ATP-related proteins. ATP-induced eNOS regulation showed a complex set of multisite posttranslational phosphorylation events that were closely associated with the enzyme’s partitioning between caveolar and noncaveolar endothelial subcellular domains. The comprehensive model that we present demonstrates that ATP repartitioned eNOS between the caveolar and noncaveolar subcellular domains; specifically, the stimulatory PSer635eNOS was substantially higher in the caveolar pool with subcellular domain-independent increased levels on ATP treatment. The stimulatory PSer1179eNOS was not altered by ATP treatment. However, the inhibitory PThr495eNOS was regulated predominantly in the caveolar domain with decreased levels on ATP action. In contrast, the agonist-specific PSer114eNOS was localized in the noncaveolar pool with increased levels on ATP stimulation. Thus, the endothelial caveolar membrane system plays a pivotal role(s) in ATP-associated subcellular partitioning and possesses the relevant protein machinery for ATP-induced NO regulation. Furthermore, these subcellular domain-specific phosphorylation/dephosphorylation events provide evidence relating to eNOS spatio-temporal dynamics.
Keywords: ATP, eNOS, endothelium, vasodilation, phosphorylation
ATP elicits profound vasodilation via its direct action on the endothelial cells.1 Red blood cells,2 platelets,3 and endothelial cells4,5 release ATP into the blood in response to various stimuli, including hypoxia/hypercapnia,6 mechanical deformation,7 shear stress,8 and ATP itself.4 ATP acts subsequently on its receptors in the endothelial membranes9 to produce transient peaks in intracellular calcium,10 leading to complex regulation of endothelial NO synthase (eNOS),11 NO production,12 and a decrease in the vascular tone.13
Under resting unstimulated conditions, eNOS is primarily located in specialized protein-rich plasmalemmal invaginations called caveolae, where its activity is inhibited by direct binding to the scaffolding protein caveolin (cav) 1.14 However, treatment with calcium-mobilizing agonists leads to eNOS translocation from the caveolar membrane into other intracellular sites.15–17 The dynamics of subcellular protein partitioning led us to investigate the entire caveolar proteome using mass spectrometry in the absence and presence of extracellular ATP.
Endothelial cell eNOS activation is further modulated by posttranslational modification via multisite phosphorylation/dephosphorylation.11 However, the sequential modifications of the multisite phosphorylation state of eNOS within the caveolar and noncaveolar subcellular domains are unknown. Interestingly, eNOS protein possesses multiple phosphorylation sites, including Ser1179 and Ser635, which are stimulatory; Thr495, which is inhibitory; and Ser114, which is stimulatory or inhibitory depending on the agonist used.18,19 Although several excellent review articles11,14,17,20,21 have theorized the complex interaction between eNOS partitioning and its phosphorylation/dephosphorylation status, no study has been performed to directly test this hypothesis. Therefore, we hypothesized that posttranslational phosphorylation/dephosphorylation of eNOS is intimately associated with its partitioning in endothelial cells. In addition, subcellular domain-specific multisite phosphorylation/dephosphorylation would occur on ATP stimulation.
We demonstrate herein that the caveolae contain a rich protein machinery relating to a myriad of biological and cellular processes with at least a quarter of the proteome directly relating to subcellular localization or partitioning. We further show that, independent of the ATP effects, the endothelial caveolar subcellular microdomains act as specific focal hubs for NO- and ATP-related proteins. Our findings additionally demonstrate for the first time that ATP stimulates eNOS phosphorylation/dephosphorylation in a site- and subcellular domain-specific manner.
Methods
For complete details on specific methods, please see the online-only Data Supplement.
Results
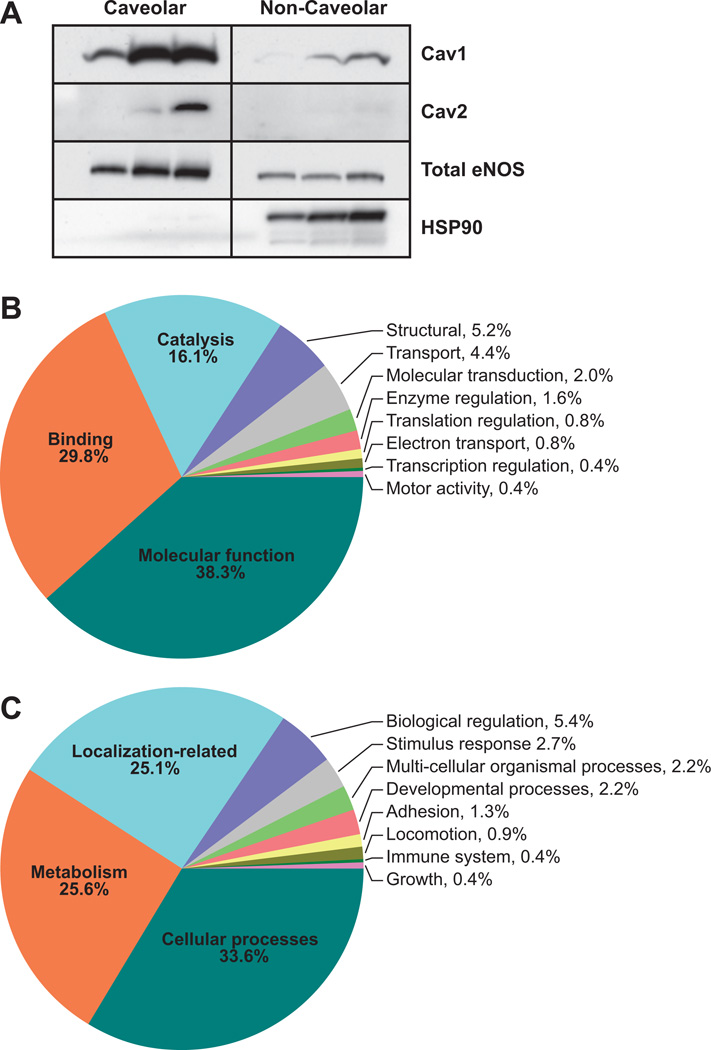
To assess the effect of the calcium-mobilizing agonist ATP on the uterine artery endothelial caveolar protein machinery, we performed liquid chromatography-tandem mass spectroscopy analyses on enriched caveolar fractions obtained from sucrose density gradient centrifugation of endothelial cells treated with or without ATP (100 µmol/L; 5 minutes). We have described previously the endothelial caveolar enrichment process and validated the protocol used in this article with electron microscopy analysis, as well as a caveolae-specific antibody panel.22 In addition, to validate the cell preparations used in the current study, we performed Western blotting using commercially available ovine antibodies for cav-1,23 cav-2,16 eNOS,11 and heat shock protein (HSP) 9014 (Figure 1A). The presence of the classic caveolar proteins cav-1 and eNOS in the proteome (Table S1, available in the online-only Data Supplement) was validated by Western immunoblotting. As expected, Western blotting showed low expression of cav-2, whereas HSP90 was barely detectable in the caveolar domain and was almost exclusively found only in the noncaveolar domain confirming the proteomic observations that cav-2 and HSP90 were not detectable among the 75 most enriched caveolar proteins that were examined in this study.
Figure 1.
A, Immunoblot validation of enriched caveolae from control endothelial cells shows the presence of the caveolar scaffolding protein cav-1 (optical density: caveolar, 0.802±0.295; noncaveolar, 0.0567±0.0383) and endothelial NO synthase (eNOS; optical density: caveolar, 0.725±0.173; noncaveolar, 0.155±0.0274) in the proteome (Table S1). Western blotting showed low expression of cav-2 (optical density: caveolar, 0.387±0.325; noncaveolar, 0.0543±0.00549), whereas heat shock protein (HSP) 90 (optical density: caveolar, 0.00633±0.00203; noncaveolar, 0.595±0.191) was barely detectable in the caveolar domain and was almost exclusively found only in the noncaveolar domain, confirming the proteomic observations that cav-2 and HSP90 were not detected among the 75 most enriched caveolar proteins that were examined in this study. B, Endothelial cells treated with or without ATP (100 µmol/L, 5 minutes) were used for caveolae isolation. The entire caveolar proteome with the Gene Ontology term listed in National Center for Biotechnology Information database was classified according to cellular processes. C, Proteins with Gene Ontology terms were classified according to biological processes.
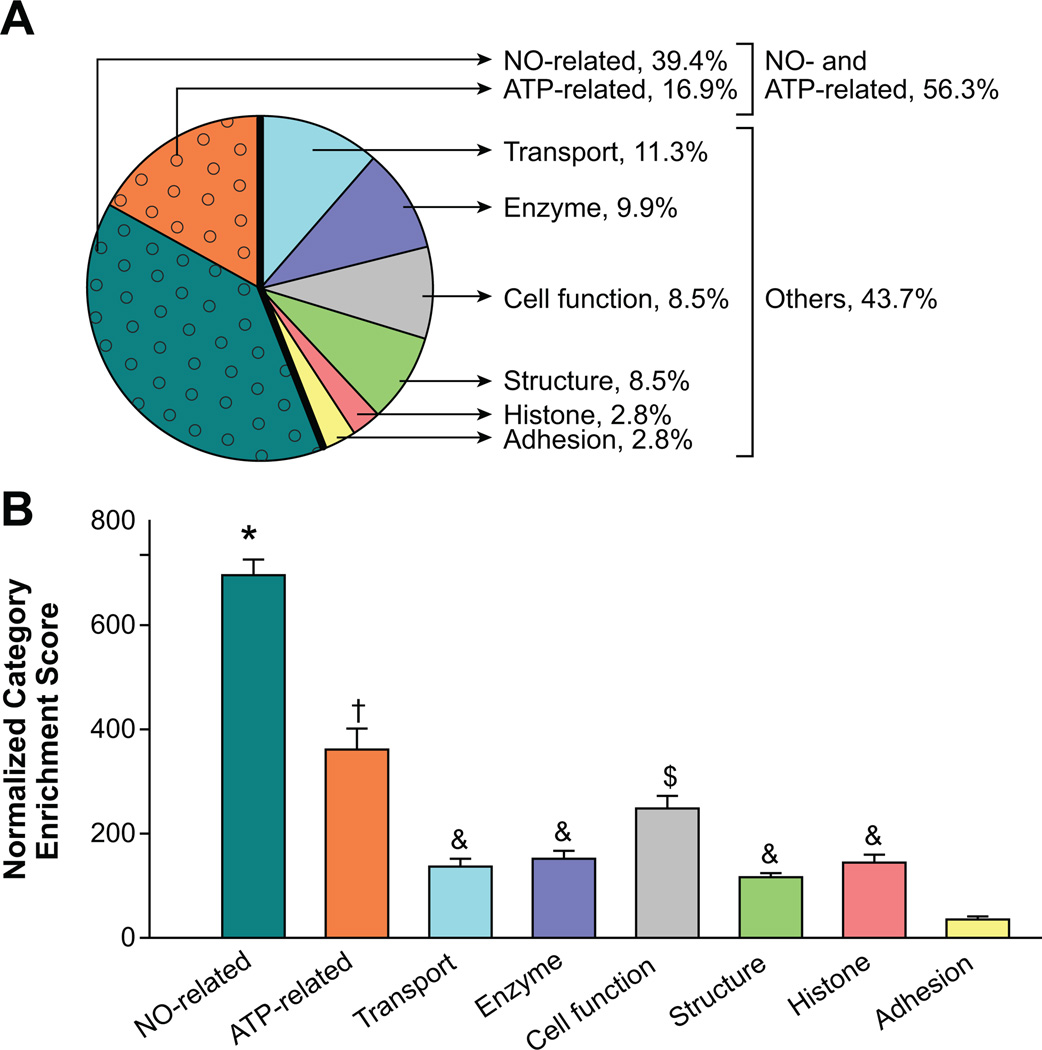
The entire caveolar proteome in the control and ATP treatment groups were populated in Scaffold proteomic software version 2.06.02 and classified based on the cellular and biological processes that they were involved in. First, the caveolar proteins that had a Gene Ontology term listed in the National Center for Biotechnology Information database were classified according to cellular processes (Figure 1B); ≈38% and 30% of the endothelial caveolar proteins were associated with molecular function and protein-protein binding, respectively. The large number of proteins associated with binding probably demonstrates that the caveolae were rich in proteins that may directly regulate subcellular partitioning. The caveolae were also abundant (16.1%) in proteins associated with catalysis. In contrast, proteins associated with all of the other cellular processes, including caveolar structure, transport, molecular transduction, enzyme regulation, translation regulation, electron transport, transcription regulation, and motor activity together composed only 15.6% of the caveolar proteome. We then classified the endothelial caveolar proteome according to biological processes (Figure 1C). More than half of the proteome was associated with basic cellular processes and metabolism. A quarter of the proteome related to subcellular localization, validating the percentage fraction of proteins associated with binding observed in Figure 1B. Proteins related to all of the other biological processes, including biological regulation, stimulus response, multicellular organismal processes, developmental processes, adhesion, locomotion, immune system, and growth, collectively constituted only 15.5% of the caveolar proteome. Further classification of the 75 most enriched proteins in the caveolae showed that 56.3% of the proteins were related to NO or ATP (ATPases, ATP synthases, and ATP transporters; Figure 2A). Substratification of these proteins revealed that NO-related proteins composed ≈40% of the caveolar proteome, whereas ≈17% were ATP related. Analysis of normalized enrichment score, an estimate of the enrichment of proteins in each category, did not reveal a main effect of ATP treatment. However, independent of ATP effect, the caveolae were significantly more enriched for NO-related (P<0.001), ATP-related (P<0.001), and cell function-related (P<0.005) proteins compared with all of the other categories (Figure 2B). These results demonstrate the substantial importance of ATP-induced NO signaling in the caveolar subcellular domains.
Figure 2.
A, Endothelial cells treated with or without ATP (100 µmol/L, 5 minutes) were used for caveolae isolation. The caveolae are a repository of proteins related to ATP and NO regulation; classification of 75 most enriched proteins in the caveolae showed that 56.3% (shaded with circles) of the total number of proteins were related to NO or ATP. B, Analysis of normalized enrichment score, an estimate of the enrichment of proteins in each category, did not reveal a main effect of ATP treatment. Independent of ATP treatment, NO-related (*), ATP-related (†), and cell function-related ($) protein categories were significantly greater than all of the other categories, including those related to transport, histone, enzymes, structure, and adhesion. & denotes categories that were significantly different from adhesion group alone.
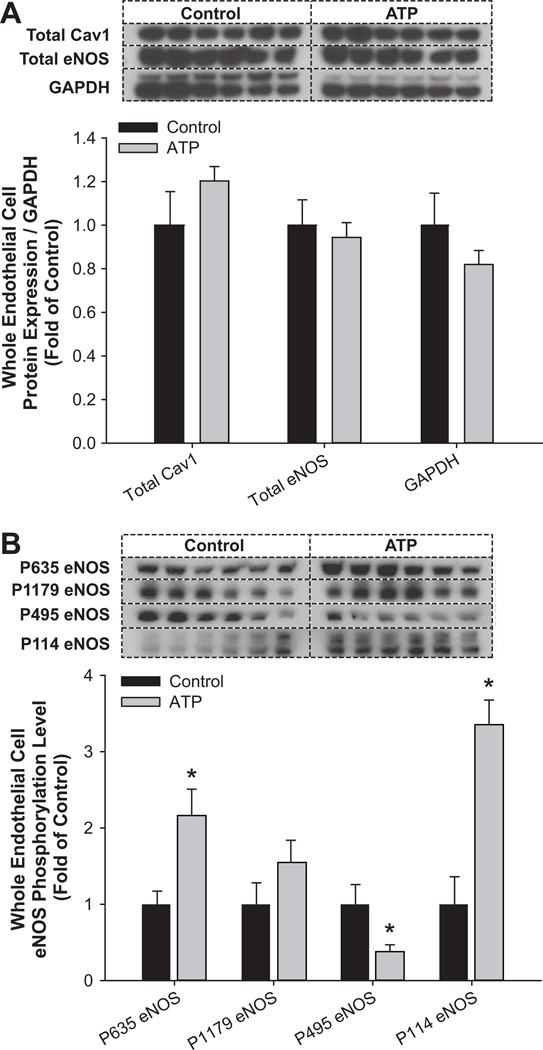
Following our observations on the eNOS- and ATP-associated proteins in these caveolar subcellular domains, we then studied the effect of the calcium mobilizing agonist ATP on eNOS multisite phosphorylation using Western blotting on whole endothelial cell lysates. Total cav-1, total eNOS, and GAPDH were not different between control and ATP treatment groups (Figure 3A). However, ATP treatment differentially altered the eNOS multisite phosphorylation state (Figure 3B). Specifically, stimulatory PSer635eNOS was significantly (P<0.05) increased, whereas the stimulatory PSer1179eNOS was unaltered. In contrast, the inhibitory PThr495eNOS was significantly (P<0.05) decreased in response to ATP compared with the control group. Regulated phosphorylation also occurred at the agonist-specific site Ser114; PSer114eNOS was significantly (P<0.05) increased in response to ATP treatment.
Figure 3.
Treatment with ATP (100 µmol/L, 5 minutes) altered whole endothelial cell endothelial NO synthase (eNOS) multisite phosphorylation. A, Total cav-1, total eNOS, and GAPDH were not different between control ■ and ATP ▩ groups. B, Stimulatory PSer635eNOS was significantly increased (*) in response to ATP vs the control group. Stimulatory PSer1179eNOS was unaltered. The inhibitory PThr495eNOS was significantly decreased in response to ATP. The agonist-specific PSer114eNOS was significantly increased in response to ATP treatment.
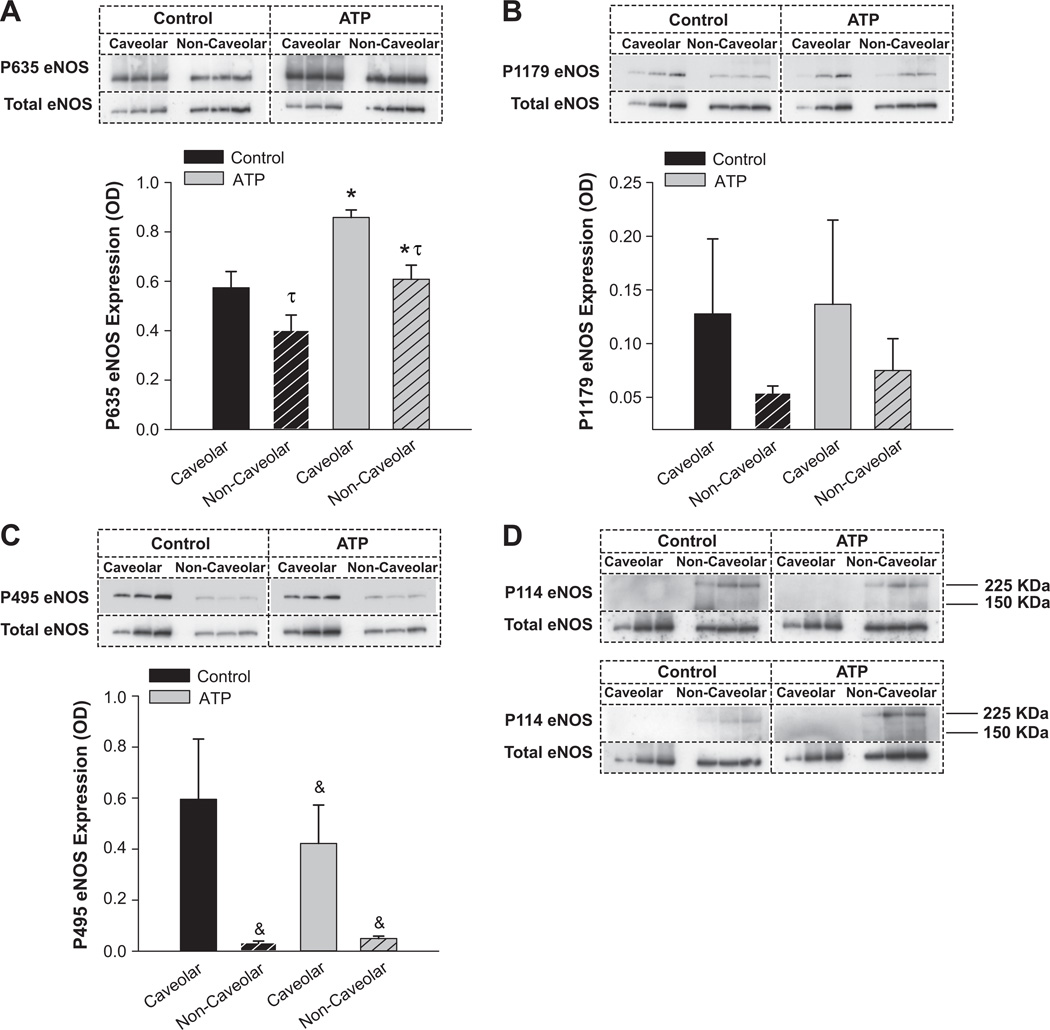
We then examined the subcellular domain-specific multisite phosphorylation/dephosphorylation state of eNOS. Cav-1 immunoisolation showed that, on ATP treatment, eNOS bound to cav-1 significantly (P<0.05) decreased by 46.6%, and the ratio of eNOS to cav-1 significantly (P<0.05) decreased by 55.8% (data not shown). Subsequently, we performed subcellular domain-specific immunoblot analyses on the enriched caveolar and noncaveolar fractions obtained from sucrose density gradient centrifugation (Figure 4). In control unstimulated endothelial cells, stimulatory PSer635eNOS was significantly (P<0.05) more abundant in the caveolar domain compared with the noncaveolar pool. Furthermore, PSer635eNOS was significantly (P<0.05) greater in the ATP group compared with the control group. These data directly validated the whole cell observations. Neither an effect of the subcellular domain nor an effect of ATP was observed with PSer1179eNOS, again confirming the whole endothelial cell data. The inhibitory PThr495eNOS exhibited a strong trend (0.05 < P<0.1) with an interaction between the factors subcellular domain and ATP treatment. However, when the absorbance values were individually divided by their total eNOS protein expression level, a main effect of subcellular domain was detected, with PThr495eNOS being detected predominantly only in the caveolar domain and ATP effect still trending lower toward significance. In contrast, 2 bands (one at the level of whole eNOS and the other at ≈220 kDa) were detected for PSer114eNOS, confirming the bands observed at the whole cell level (Figure 3B). No bands were detected in the caveolar domain, demonstrating that PSer114eNOS was restricted to the noncaveolar pool. However, because of the low expression of the band at 140 to 150 kDa, quantitative analysis could not be accurately performed for PSer114eNOS subcellular domain-specific levels.
Figure 4.
A, In control unstimulated endothelial cells, stimulatory PSer635endothelial NO synthase (eNOS) was significantly (τ) more abundant in the caveolar domain vs the noncaveolar pool. Furthermore, PSer635eNOS was significantly (*) greater in the ATP groups ▩ vs the control ■. B, Neither an effect of the subcellular domain nor an effect of ATP was observed with PSer1179eNOS again confirming the whole endothelial cell data. C, The inhibitory PThr495eNOS exhibited a strong trend (&) with an interaction between the factors subcellular domain and ATP treatment. However, when the absorbance values were individually divided by their total protein expression level, a main effect of subcellular domain was detected, with PThr495eNOS being detected predominantly only in the caveolar domain and ATP effect trending lower toward significance. D, Two bands (one at the level of whole eNOS and the other at ≈220 kDa) were detected for PSer114eNOS, confirming the observations at the whole cell level. These findings were confirmed in a replicate blot and are both depicted in the figure. No bands were detected in the caveolar domain, demonstrating that PSer114eNOS was restricted to the noncaveolar pool. However, because of the low expression of the band at 140 to 150 kDa, quantitative analysis was not performed for PSer114eNOS subcellular domain-specific levels. In certain cases, gels were run in replicates and were used for quantification.
Discussion
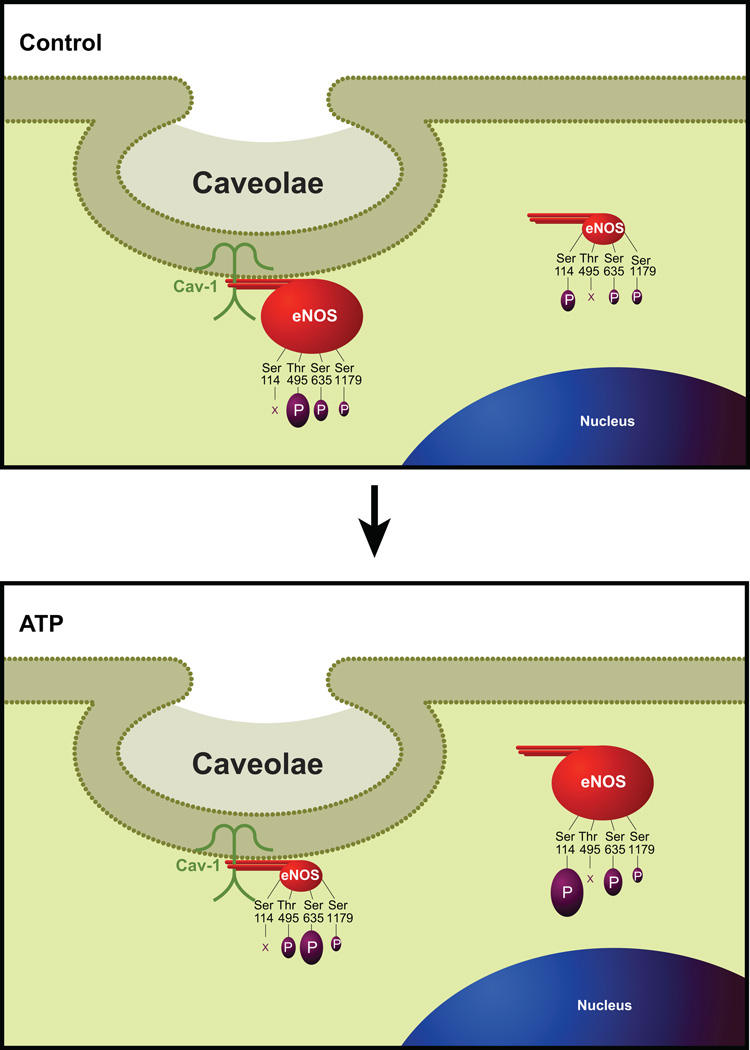
We demonstrate herein that the caveolae contain a rich protein machinery relating to a myriad of biological and cellular processes with at least a quarter of the proteome directly relating to subcellular localization or partitioning. We also show that, independent of the effect of ATP, the endothelial caveolar subcellular microdomains act as specific focal hubs of NO- and ATP-related proteins. The dynamics of ATP-induced eNOS regulation further show, as illustrated in Figure 5, a complex set of multisite posttranslational phosphorylation events that are closely associated with the partitioning of an enzyme between caveolar and noncaveolar endothelial subcellular domains.
Figure 5.
A comprehensive model for ATP-induced partition and subcellular domain-specific multisite phosphorylation (P) state of endothelial NO synthase (eNOS). The level of expression of (P)eNOS is proportional to the depicted size, and X indicates that the specific amino acid is not phosphorylated in the respective subcellular domain. Refer to Discussion for details.
We report for the first time that a quarter of the endothelial caveolar proteome related to subcellular localization when classified based on the biological processes in which the proteins were involved. These numbers were also similar to the fraction of proteins (30%) related to protein-protein binding when classified based on cellular processes. These data demonstrate the pivotal role of the caveolae in subcellular protein partitioning to achieve directed physiological signaling and also support numerous studies that have investigated the caveolae for localization-based protein function regulation.14,24 In addition to these findings, our data also demonstrate that the caveolae contain numerous proteins related to basic cellular processes and metabolism.
Our data demonstrate that, with or without the calcium-mobilizing agonist ATP, the endothelial caveolae contain a rich protein machinery related to the vasodilator NO. These proteins included those related to eNOS trafficking, cell-cell adhesion, and those directly involved in NO metabolism. Prominent eNOS trafficking-associated proteins detected in the endothelial caveolae include filamin and tubulin. This is supportive of earlier reports on a role for filamin and tubulin in facilitating eNOS-HSP90 interaction and subsequent trafficking in and out of the caveolae.25,26 Consistent with other studies, herein we detected numerous adhesion proteins, including platelet endothelial cell adhesion molecule and vascular endothelial cadherin, proteins that play a critical role in NO regulation in the endothelial caveolae.27–29 Finally, we detected a host of proteins directly related to NO metabolism, including eNOS and cav-1.
We observed that the caveolar system is also the “home” for many ATP-related proteins that include ATPases, ATP synthases, and ATP transporters. The current study provides definitive evidence that these mitochondrial and endoplasmic reticulum proteins can actually be enriched from the caveolae, a finding consistent with earlier reports.29–31 In addition, the presence of these proteins in the caveolae has also been attributed to the physical interaction between these organelles with the caveolae at specific contact sites, termed as mitochondria-associated endoplasmic reticulum membranes and plasma membrane-associated endoplasmic reticulum membranes.30
As expected, we observed that ATP treatment resulted in significant dissociation of total eNOS from cav-1, a finding consistent with the idea that calcium-mobilizing agonist stimulation leads to eNOS translocation from the caveolar subcellular compartment into the noncaveolar domain.15,32 In the present study, we also demonstrated that ATP leads to significant phosphorylation and dephosphorylation of specific residues in the eNOS protein. The sequential subcellular domain-specific alteration in the phosphorylation of the enzyme is depicted in Figure 5. ATP significantly increased the phosphorylation of whole endothelial cell PSer635eNOS. Subcellular domain-specific analysis further demonstrated a significant main effect of both subcellular domain and ATP treatment. This site is phosphorylated to a much greater extent in the caveolar pool of the enzyme compared with the noncaveolar domain. Moreover, the PSer635eNOS phosphorylation level is significantly higher on ATP stimulation. Ser635 is a well-documented protein kinase A–dependent excitatory phosphorylation site localized in the flavin mononucleotide-binding domain.18,33 Although information regarding subcellular domain-specific alterations of this site is lacking, it has been reported that phosphorylation on Ser635 peaks between 5 and 10 minutes with sustained levels ≤30 minutes34 and that this site is important for sustained NO production independent of intracellular calcium transients. 18,35 Collectively, these data demonstrate that PSer635eNOS phosphorylation is an important subcellular domain-specific posttranslational modification that appears to play a pivotal role in sustained ATP-induced eNOS activation.
We also observed that neither at the whole endothelial cell level nor at the subcellular level was PSer1179eNOS altered with ATP treatment. Ser1179 is an extensively studied AKT-dependent site.18 However, the lack of subcellular domain-specific alteration of this enzyme is consistent with earlier studies that did not show a role for AKT in ATP signaling.36 Moreover, our data demonstrate that phosphorylation at this site is also not related to the location of the enzyme and that ATP-induced eNOS excitatory phosphorylation exhibits specificity by acting via the Ser635 site.
The current data for inhibitory PThr495eNOS exhibited a strong trend (0.05 < P<0.1) with an interaction between the factors subcellular domain and ATP treatment. However, when the absorbance values were individually divided by their total eNOS protein expression level, a main effect of the subcellular domain was detected, with PThr495eNOS being detected predominantly only in the caveolar domain and ATP effect still trending toward significance. These data are consistent with the previous reports that protein kinase C–dependent Thr495 phosphorylation inhibits eNOS-calmodulin interaction and subsequent repartitioning of the enzyme into the noncaveolar domain and that its dephosphorylation by ATP significantly contributes to elevated eNOS activity.18,34 However, our current study is the first to demonstrate that Thr495 is not phosphorylated in the noncaveolar domain, demonstrating that dephosphorylation on this site is subcellular-domain specific.
At the whole cell level, PSer114eNOS level was significantly greater on ATP treatment compared with the control group. At the subcellular level, however, no bands were detected in the caveolar fractions. However, quantification of the bands observed in the noncaveolar fractions could not be performed, because 2 bands were detected, one at 140 to 150 kDa and the other at ≈220 kDa, and the lower band was too low for quantification. Taking together the absence of bands in the caveolar domain and the significant increase in their levels in whole endothelial cell preparations, we conclude that, unlike PThr495eNOS, PSer114eNOS regulation is restricted only to the noncaveolar domain and is phosphorylated on ATP treatment. Ser114 is the only known phosphorylation site in the oxygenase domain of the eNOS enzyme, and the function of this site is agonist specific, with VEGF promoting Ser114 dephosphorylation and shear stress phosphorylating this site.18 Because myristoylation and palmitoylation all occur in the amino acid residues that are localized in the eNOS oxygenase domain,14,37 we reason that phosphorylation of noncaveolar Ser114 must prevent eNOS trafficking back to the caveolae on ATP stimulation.
Perspectives
In summary, the endothelial actions of the physiological vasodilator ATP are mediated through caveolar microdomains that possess the relevant protein machinery for eNOS-NO regulation. The current study directly infers the cause-and-effect relationship between eNOS subcellular partition and multisite phosphorylation; thus, these data underpin the intimate relationship between these 2 factors in NO regulation. Furthermore, the subcellular domain-specific multisite phosphorylation state of eNOS illustrates a unique role for each of the regulatory amino acid residues in eNOS regulation that is intimately associated with the intracellular location and/or trafficking of the enzyme, thus providing for the first time direct spatio-temporal indications to eNOS activation states.
Supplementary Material
Acknowledgments
We acknowledge Robert J. Gordon and Kreg M. Grindle for helping in figure preparations. We also thank Sheikh O. Jobe, Mayra B. Pastore, and Mary Y. Sun for their suggestions during article development.
Sources of Funding
This work was supported by National Institutes of Health grants AA19446, HL49210, HD38843, HL87144, and HL70562.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Gordon JL. Extracellular ATP: Effects, sources and fate. Biochem J. 1986;233:309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sprague RS, Hanson MS, Achilleus D, Bowles EA, Stephenson AH, Sridharan M, Adderley S, Procknow J, Ellsworth ML. Rabbit erythrocytes release ATP and dilate skeletal muscle arterioles in the presence of reduced oxygen tension. Pharmacol Rep. 2009;61:183–190. doi: 10.1016/s1734-1140(09)70020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beigi R, Kobatake E, Aizawa M, Dubyak GR. Detection of local atp release from activated platelets using cell surface-attached firefly luciferase. Am J Physiol. 1999;276:C267–C278. doi: 10.1152/ajpcell.1999.276.1.C267. [DOI] [PubMed] [Google Scholar]
- 4.Bodin P, Burnstock G. ATP-stimulated release of atp by human endothelial cells. J Cardiovasc Pharmacol. 1996;27:872–875. doi: 10.1097/00005344-199606000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Yang S, Cheek DJ, Westfall DP, Buxton IL. Purinergic axis in cardiac blood vessels: Agonist-mediated release of ATP from cardiac endothelial cells. Circulation research. 1994;74:401–407. doi: 10.1161/01.res.74.3.401. [DOI] [PubMed] [Google Scholar]
- 6.Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res. 1992;26:40–47. doi: 10.1093/cvr/26.1.40. [DOI] [PubMed] [Google Scholar]
- 7.Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. ATP: The red blood cell link to no and local control of the pulmonary circulation. Am J Physiol. 1996;271:H2717–H2722. doi: 10.1152/ajpheart.1996.271.6.H2717. [DOI] [PubMed] [Google Scholar]
- 8.Milner P, Bodin P, Loesch A, Burnstock G. Rapid release of endothelin and atp from isolated aortic endothelial cells exposed to increased flow. Biochem Biophys Res Commun. 1990;170:649–656. doi: 10.1016/0006-291x(90)92141-l. [DOI] [PubMed] [Google Scholar]
- 9.Mathie RT, Ralevic V, Alexander B, Burnstock G. Nitric oxide is the mediator of ATP-induced dilatation of the rabbit hepatic arterial vascular bed. Br J Pharmacol. 1991;103:1602–1606. doi: 10.1111/j.1476-5381.1991.tb09834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gifford SM, Yi FX, Bird IM. Pregnancy-enhanced ca2+ responses to ATP in uterine artery endothelial cells is due to greater capacitative ca2+ entry rather than altered receptor coupling. J Endocrinol. 2006;190:373–384. doi: 10.1677/joe.1.06635. [DOI] [PubMed] [Google Scholar]
- 11.Dudzinski DM, Michel T. Life history of eNOS: Partners and pathways. Cardiovasc Res. 2007;75:247–260. doi: 10.1016/j.cardiores.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yi FX, Magness RR, Bird IM. Simultaneous imaging of [ca2+]i and intracellular no production in freshly isolated uterine artery endothelial cells: Effects of ovarian cycle and pregnancy. Am J Physiol Regul Integr Comp Physiol. 2005;288:R140–R148. doi: 10.1152/ajpregu.00302.2004. [DOI] [PubMed] [Google Scholar]
- 13.Bird IM, Zhang L, Magness RR. Possible mechanisms underlying pregnancy-induced changes in uterine artery endothelial function. Am J Physiol Regul Integr Comp Physiol. 2003;284:R245–R258. doi: 10.1152/ajpregu.00108.2002. [DOI] [PubMed] [Google Scholar]
- 14.Shaul PW. Regulation of endothelial nitric oxide synthase: location, location, location. Annu Rev Physiol. 2002;64:749–774. doi: 10.1146/annurev.physiol.64.081501.155952. [DOI] [PubMed] [Google Scholar]
- 15.Goetz RM, Thatte HS, Prabhakar P, Cho MR, Michel T, Golan DE. Estradiol induces the calcium-dependent translocation of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1999;96:2788–2793. doi: 10.1073/pnas.96.6.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gratton JP, Bernatchez P, Sessa WC. Caveolae and caveolins in the cardiovascular system. Circ Res. 2004;94:1408–1417. doi: 10.1161/01.RES.0000129178.56294.17. [DOI] [PubMed] [Google Scholar]
- 17.Feron O, Saldana F, Michel JB, Michel T. The endothelial nitric-oxide synthase-caveolin regulatory cycle. J Biol Chem. 1998;273:3125–3128. doi: 10.1074/jbc.273.6.3125. [DOI] [PubMed] [Google Scholar]
- 18.Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial no synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol. 2007;42:271–279. doi: 10.1016/j.yjmcc.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 19.Boo YC, Jo H. Flow-dependent regulation of endothelial nitric oxide synthase: Role of protein kinases. Am J Physiol Cell Physiol. 2003;285:C499–C508. doi: 10.1152/ajpcell.00122.2003. [DOI] [PubMed] [Google Scholar]
- 20.Fulton D, Gratton JP, Sessa WC. Post-translational control of endothelial nitric oxide synthase: Why isn’t calcium/calmodulin enough? J Pharmacol Exp Ther. 2001;299:818–824. [PubMed] [Google Scholar]
- 21.Sessa WC. eNOS at a glance. J Cell Sci. 2004;117:2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- 22.Liao WX, Feng L, Zhang H, Zheng J, Moore TR, Chen DB. Compartmentalizing vegf-induced ERK2/1 signaling in placental artery endothelial cell caveolae: A paradoxical role of caveolin-1 in placental angiogenesis in vitro. Mol Endocrinol. 2009;23:1428–1444. doi: 10.1210/me.2008-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez FA, Savalia NB, Duran RG, Lal BK, Boric MP, Duran WN. Functional significance of differential enos translocation. Am J Physiol Heart Circ Physiol. 2006;291:H1058–H1064. doi: 10.1152/ajpheart.00370.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Q, Patton WF, Hechtman HB, Shepro D. A novel anti-inflammatory peptide inhibits endothelial cell cytoskeletal rearrangement, nitric oxide synthase translocation, and paracellular permeability increases. J Cell Physiol. 1997;172:171–182. doi: 10.1002/(SICI)1097-4652(199708)172:2<171::AID-JCP4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 26.Su Y, Kondrikov D, Block ER. Cytoskeletal regulation of nitric oxide synthase. Cell Biochem Biophys. 2005;43:439–449. doi: 10.1385/CBB:43:3:439. [DOI] [PubMed] [Google Scholar]
- 27.Dusserre N, L’Heureux N, Bell KS, Stevens HY, Yeh J, Otte LA, Loufrani L, Frangos JA. Pecam-1 interacts with nitric oxide synthase in human endothelial cells: implication for flow-induced nitric oxide synthase activation. Arterioscler Thromb Vasc Biol. 2004;24:1796–1802. doi: 10.1161/01.ATV.0000141133.32496.41. [DOI] [PubMed] [Google Scholar]
- 28.Govers R, Bevers L, de Bree P, Rabelink TJ. Endothelial nitric oxide synthase activity is linked to its presence at cell-cell contacts. Biochem J. 2002;361:193–201. doi: 10.1042/0264-6021:3610193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramadoss J, Liao WX, Chen DB, Magness RR. High-throughput caveolar proteomic signature profile for maternal binge alcohol consumption. Alcohol. 2010;44:691–697. doi: 10.1016/j.alcohol.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMahon KA, Zhu M, Kwon SW, Liu P, Zhao Y, Anderson RG. Detergent-free caveolae proteome suggests an interaction with er and mitochondria. Proteomics. 2006;6:143–152. doi: 10.1002/pmic.200500208. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto K, Shimizu N, Obi S, Kumagaya S, Taketani Y, Kamiya A, Ando J. Involvement of cell surface atp synthase in flow-induced atp release by vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2007;293:H1646–H1653. doi: 10.1152/ajpheart.01385.2006. [DOI] [PubMed] [Google Scholar]
- 32.Oess S, Icking A, Fulton D, Govers R, Muller-Esterl W. Subcellular targeting and trafficking of nitric oxide synthases. Biochem J. 2006;396:401–409. doi: 10.1042/BJ20060321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michell BJ, Harris MB, Chen ZP, Ju H, Venema VJ, Blackstone MA, Huang W, Venema RC, Kemp BE. Identification of regulatory sites of phosphorylation of the bovine endothelial nitric-oxide synthase at serine 617 and serine 635. J Biol Chem. 2002;277:42344–42351. doi: 10.1074/jbc.M205144200. [DOI] [PubMed] [Google Scholar]
- 34.Cale JM, Bird IM. Dissociation of endothelial nitric oxide synthase phosphorylation and activity in uterine artery endothelial cells. Am J Physiol Heart Circ Physiol. 2006;290:H1433–H1445. doi: 10.1152/ajpheart.00942.2005. [DOI] [PubMed] [Google Scholar]
- 35.Boo YC, Sorescu GP, Bauer PM, Fulton D, Kemp BE, Harrison DG, Sessa WC, Jo H. Endothelial no synthase phosphorylated at ser635 produces no without requiring intracellular calcium increase. Free Radic Biol Med. 2003;35:729–741. doi: 10.1016/s0891-5849(03)00397-6. [DOI] [PubMed] [Google Scholar]
- 36.Sullivan JA, Grummer MA, Yi FX, Bird IM. Pregnancy-enhanced endothelial nitric oxide synthase (eNOS) activation in uterine artery endothelial cells shows altered sensitivity to ca2+, u0126, and wortmannin but not ly294002: evidence that pregnancy adaptation of eNOS activation occurs at multiple levels of cell signaling. Endocrinology. 2006;147:2442–2457. doi: 10.1210/en.2005-0399. [DOI] [PubMed] [Google Scholar]
- 37.Shaul PW, Smart EJ, Robinson LJ, German Z, Yuhanna IS, Ying Y, Anderson RG, Michel T. Acylation targets emdothelial nitric-oxide synthase to plasmalemmal caveolae. J Biol Chem. 1996;271:6518–6522. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.