Abstract
Recent results using animal models of inflammatory skin conditions have shown that blockers of the voltage-gated potassium channel, Kv1.3 hold great promise for clinical utility. Kv1.3 blockers act as immunosuppressants by modulating the various subsets of inflammatory T and B cells involved in autoimmune disorders. While peptidic inhibitors based on naturally occurring venoms demonstrate potent and selective Kv1.3 blockade, these require parenteral administration and may face potential immunogenicity problems. Small molecule blockers show considerable diversity, however selectivity over other Kv1- family channels has been difficult to achieve. More recent advances have added to the evidence that Kv1.3 channels are a suitable therapeutic target and that the development of novel and selective agents will herald new drugs for inflammatory skin disorders.
Keywords: Psoriasis, Kv1.3 channel, inflammation, drug design, autoimmune disorder
Introduction
Autoimmune disorders can be treated using immunosuppressive agents that also address aspects of the chronic inflammation associated with these illnesses. Quite often these drugs are non-specific in their mode of action resulting in various side effects. In extreme cases, the compounds compromise the immune system making it difficult to fight infections. For example, natalizumab (Tysabri), which is used to treat multiple sclerosis (MS) and Crohn’s disease by preventing T cells from leaving the vasculature and entering tissue, has been associated with patients developing progressive multifocal leukoencephalopathy (PML), a rare and typically fatal viral infection [1,2]. These disproportionate effects of some immunosuppressants indicate that there is a clear need for agents that are more specific for treating autoimmune disorders. Numerous companies are involved in developing immunomodulatory agents with the potential market expected to reach $50 billion in the coming years [3].
Psoriasis is one of the most common autoimmune-mediated skin disorders, affecting about 2.0- 2.5% of the world’s population [4–6]. The most common form of psoriasis is plaque psoriasis which is characterized by red, elevated patches of skin often covered with silvery scales. Other forms of psoriasis include flexural, pustular, guttate, nail and erythrodermic psoriasis as well as psoriatic arthritis [7]. In some cases plaque psoriasis and other variants can coexist or overlap in the same individual. Since most suffers remain affected for the remainder of their lives psoriasis is associated with a significant reduction in the quality of life and an enormous economic burden on the health care system for the “biologics” that are currently increasingly used for treatment [8,9].
The majority of individuals with psoriasis are mildly affected by the disease and are most frequently treated with topical agents including emollients, corticosteroids, retinoids and vitamin D3 analogues [10]. Less frequently used topical compounds include coal tar and dithranol (anthralin), which have low cosmetic appeal and concerns regarding their safety. Of the standard treatments, these have been associated with a range of side effects such as skin irritation and variable efficacy [11]. When topical treatments prove ineffective, phototherapy either in the form of ultraviolet B (UVB) or ultraviolet A following pretreatment with an orally administered photosensitizer such as methoxsalen (i.e. PUVA therapy) can be used [12]. Unfortunately, the risk of non-melanoma skin cancer is marginally increased in these patients [13]. Patients with severe psoriasis that do not respond well to either topical treatment or phototherapy may be treated systemically with retinoids or immunosuppressants [10]. Methotrexate is able to inhibit both DNA synthesis and neutrophil chemotaxis [14] and is the most commonly prescribed systemic immunosuppressive drug used for psoriasis. Long term use of methotrexate can lead to hepatotoxicity and myelosuppression requiring patients to undergo regular monitoring [15]. Due to general advances in the field of immunology many monoclonal antibodies targeting specific immune cells surface receptors or cytokines have been introduced for the treatment of rheumatoid arthritis and psoriasis in the last 5 years. This new class of agents is referred to as “biologics”[16] and includes the TNF-α neutralizing agents etanercept, infliximab and adalimumab which have been approved by the FDA for treatment of psoriasis [17]. While these agents have greatly improved the management of psoriasis and other autoimmune diseases, their long-term therapeutic efficacy in psoriasis is only 60% and more trials are needed to firmly establish them as being beneficial relative to their risk of side effects such as increased rates of serious infections and cancer. In addition, their high cost makes them inaccessible to most individuals. As a consequence, there is a need for improved treatments that are low in cost with acceptable side effect profiles. One rational approach is to seek new targets that affect the underlying immune mechanisms of psoriasis in a more specific manner. This review will focus on one such target, the voltage-gated potassium channel Kv1.3, which plays an important role in the activation and proliferation of so-called effector memory T cells [18,19]. Small molecule inhibitors of Kv1.3 will be outlined together with the more recent patent literature. Finally, the role of serendipity will be explored in the discovery of Kv1.3 blockers and we will introduce our current work in this field.
Pathogenesis of psoriasis
While the precise mechanisms for psoriasis pathogenesis is still unknown, it is clearly associated with an overactive immune system triggered by the activation of T lymphocytes resulting in keratinocyte hyperproliferation [20,21].
T cell mediated immune responses in psoriasis
Psoriasis has a complex pathology with genetic, immunologic, and environmental factors all contributing to its pathogenesis [22,23]. However, activated T cells seem to play a crucial role, which is strongly substantiated by the following observations: (i) immunotherapy targeted against CD4+ T cells clears active plaques of psoriasis [24] and (ii) in SCID mice, transplanted nonlesional psoriatic skin converts to a psoriatic plaque subsequent to intradermal administration of activated T cells [25]. T cell activation during psoriasis is most likely the result of an unknown antigen triggering the immune response through antigen presenting cells (APC, primarily dendritic cells) [16]. This interaction between the APC and T cells activates an immune response resulting in T cells migrating to the dermis and epidermis. Once there, they are reactivated and secrete cytokines, which induce other cells to produce cytokines (e.g. TNF-α, IL-8 and GM-CSF) altering the pathology of the keratinocytes. This cascade of events leads to the excessive proliferation seen in psoriatic plaques [16]. Additionally, in a normal immune response, antigens are eliminated by T cell-stimulated pathways in the skin leading to the termination of the immune response. In psoriasis, this process persists chronically due to an irregularity in the feedback cycle [16,21]. A functional analysis conducted on isolated and cloned T cells obtained from psoriatic skin lesions showed that they were capable of evoking epidermal keratinocyte proliferation by secreting a variety of mediators including IFN-γ, a cytokine implicated in inflammation and T cell proliferation [26].
Central memory and effector memory T cells
Two subsets of human memory T cells; central memory (TCM) and effector memory (TEM) cells have been described based on the expression of the cell surface protein C-C chemokine receptor type 7 (CCR7) [27]. Naïve and memory T cell numbers are roughly maintained in constant numbers to initiate effective immune responses to new antigens whilst keeping high levels of memory cells to deal with previously encountered pathogens [28,29]. CCR7 is used by naïve T cells to gain access into lymph nodes, where they then encounter antigen and are activated to become naïve-effectors that proliferate and secrete inflammatory cytokines. Naïve-effectors then leave the lymph node for the site of antigenic challenge where they are able to carry out their protective role [30]. A number of naïve cells differentiate into long-lived TCM cells, which retain a memory of the specific antigen. These TCM cells also use CCR7 to enter the lymph node where they transform into TCM-effectors after antigen encounter and then migrate to inflamed tissues [30,31]. A repeated antigenic stimulation arising from a chronic infection or autoimmune disease can induce T cells to differentiate into TEM cells which do not need to migrate to lymph nodes for antigen induced activation [31]. TEM cells can become activated in peripheral tissues or typical memory T cell compartments such as the lamina propria or subcutaneous fat pads and then become TEM-effectors that quickly migrate to inflamed sites and secrete large volumes of cytokines to perform immediate effector functions [30].
Kv1.3 Potassium Channels
Role of potassium channels in T cells
Human T cells express two types of K+ channels, the voltage-gated Kv1.3 and the calciumactivated KCa3.1 channel [32–34]. Both Kv1.3 and KCa3.1 play a role in regulating membrane potential and calcium signaling during the activation of T cells [34]. Upon engagement of the T cell receptor there occurs an immediate release of calcium through IP3 receptors from the ER. The resulting depletion of the ER is “sensed” by the single-EF hand protein STIM1 and then initiates a process to assemble calcium-release activated calcium (CRAC) channels in the membrane of the cell [35–37]. Further calcium enters the cell through the CRAC channel ultimately leading to T cell proliferation though activation of calcineurin and the transcription factor NFAT [37]. However, this calcium influx, which is crucial to the process, is only possible if the T cells are able to maintain a negative membrane potential through a counterbalancing potassium efflux via Kv1.3 and/or KCa3.1 [34,38,39]. As has been known for some time, blockade of these potassium channels results in inhibition of T cell activation/proliferation and cytokine secretion [34,39,40].
Kv1.3 and KCa3.1 channels in naïve, TCM and TEM cells
Blockade of Kv1.3 or KCa3.1 channels would appear to be a suitable target for immunomodulatory agents, however we must be careful to avoid a general diminution of immune function. Fortunately, the expression of Kv1.3 and KCa3.1 channels differs across the various immune cell types [31,41]. Both Kv1.3 (250 channels/cell) and KCa3.1 (5–35 channels/cell) channel expression in the resting state of the cells is similar across the naïve, TCM and TEM cell types (Table 1) [18,30]. When activated, naïve and TCM cells up-regulate KCa3.1 to ~500 channels per cell with little or no change in Kv1.3 expression [31,34]. However, in activated TEM cells (both CD4+ and CD8+ subsets), Kv1.3 channels are increased to ~1500–2000 channels per cell with little or no change in KCa3.1 expression [18,30,31]. The dominant channel in TEM cells is therefore the Kv1.3 channel which provides an attractive opportunity for intervention by therapeutic agents.
Table 1.
Distribution of potassium channels over T cell subtypes [34]. The values are given as the average number of channels/cell.
| State | Channel | Naïve (CCR7+ CD45RA+) | TCM (CCR7+ CD45RA−) | TEM (CD4+CCR7− CD45RA− and CD8+CCR7− CD45RA−; CD8+CCR7− CD45RA+) |
|---|---|---|---|---|
| Inactive | Kv1.3 | 250 | 250 | 250 |
| KCa3.1 | 5 | 5 | 35 | |
| Active | Kv1.3 | 300 | 300 | 1500 |
| KCa3.1 | 500 | 500 | 50 | |
Potential use of Kv1.3 blockers in psoriasis therapy
Studies on the involvement of the different T cell subsets typically find that TEM cells are present in the target tissue of autoimmune diseases and seem to play an important role in their pathogenesis [42]. For example, in post mortem samples from multiple sclerosis lesions TEM cells are found in abundance [31]. Activated T cells that express CD2 and the IL-2 receptor CD25, and are mainly TEM cells of the CD45RO+ phenotype are also found in psoriasis lesions [43]. Biopsies from psoriasis patients showed that during the early phase of the psoriatic process, CD8+ T cells along with CD45RO+, CD2+ and CD25+ phenotypes are found in the skin [44]. The later phase of psoriasis involves CD94− and CD161-expressing cells [44]. The presence of the cells is also associated with the release of cytokines such as IFN-γ, IL-2 and TNF-α [45,46]. Given the cell types involved in psoriasis and the differential distribution of Kv1.3 channels in the active and inactive states of T cells, there is good reason to believe that Kv1.3 blockers could specifically suppress the disease-causing TEM cells without significantly affecting naïve and TCM cells. This rationale also forms the basis for treatment with the biologic Alefacept (anti-CD2) which reduces the levels of TEM cells while leaving TCM cells and naïve cells mostly unaffected [47]. The biologics used for psoriasis themselves can be classified into two groups; those that neutralize TNF-α (etanercept, infliximab, adalimumab) and agents that modulate pathogenically activated T cells (alefacept, efalizumab). A more extensive discussion on the biologics and psoriasis can be found in Sobell et al. [48,49]. Initial success with the use of biologic agents (particularly alefacept and efalizumab) in psoriasis strengthens the link between T cells and the disorder, and has provided further impetus to explore the Kv1.3 channel as a drug target.
Evidence showing the benefits of blocking the Kv1.3 channel for the treatment of autoimmune disorders has been accumulating for over two decades. The initial work in this area emerged from the use of various peptide toxins particularly following the report of an MS patient who experienced a two month remission from the disorder after being stung by a scorpion [50]. The scorpion toxin in this case probably was charybdotoxin (ChTX) [51] which blocks Kv1.3 channels (IC50 3 nM) and depolarizes human T cells [52–54]. These peptide inhibitors are sourced mainly from venoms derived from sea anemones, scorpions, snakes and marine snails [30]. Particular focus has been placed on ShK (IC50 11 pM) from the sea anemone Stichodactyla helianthus but a lack of selectivity for Kv1.3 led to the synthesis of numerous derivatives [55]. Of these analogues, ShK-186 has proven effective in experimental autoimmune encephalomyelitis [56], delayed type hypersensitivity (DTH) [56] and rheumatoid arthritis [57]. In addition, initial safety tests appear promising [57] and protective immune responses to acute infections like influenza and chlamydia were not compromised [56]. While selectivity has been improved for these peptides as well as their safety profile, the peptide-based inhibitors require parenteral administration and may face potential immunogenicity problems [57]. A detailed overview of peptide inhibitors can be found in Rangaraju et al. [42]. However, despite the undoubted efficacy and selectivity of the peptide Kv1.3 blockers it would be desirable to have orally or topically available small molecule Kv1.3 blockers, which would avoid many of the hurdles facing peptide development.
Small molecule Kv1.3 blockers
The search for drug-like small molecule blockers of Kv1.3 channels is currently being undertaken by a number of groups in academia and industry. The first known inhibitors were standard potassium channel blockers such as 4-aminopyridine [40], tetraethylammonium and the calcium channel blockers verapamil, nifedipine and diltiazem [30]. In addition to these compounds many other small molecule inhibitors have been developed specifically targeting Kv1.3 channels. An occurring problem is the lack of selectivity of these compounds within the Kv1-family of potassium channels; in particular over Kv1.4 and Kv1.5 which are associated with cardiac function [32]. The following discussion provides a brief overview of each of the structural classes of small molecule Kv1.3 blockers highlighting the diversity of chemotypes and providing functional data where available. The reader is also directed to previous reviews on inhibitors of Kv1.3 channels in the following references [18,19,30,58]. This current review will primarily focus on series of compounds where SAR information is available. Other individual compounds will be mentioned where their structure is thought to contribute to the discussion.
Dihydroquinolines
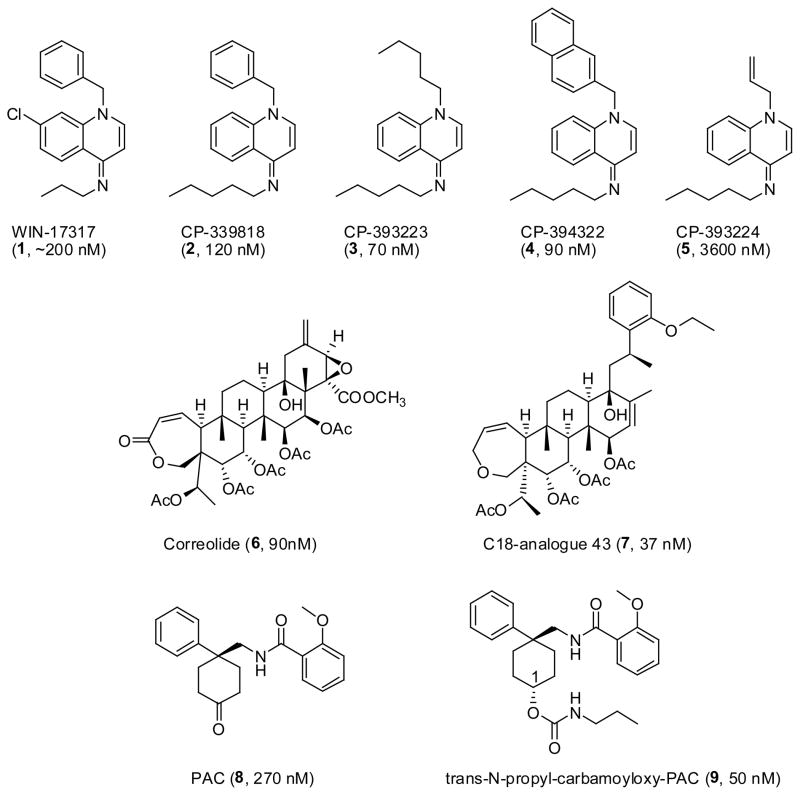
To circumvent the potential drawbacks of peptidic Kv1.3 blockers, several groups have undertaken screening campaigns to find potent small molecule Kv1.3 channel blockers. The first nanomolar compound, WIN-17317 (1) (Figure 1), was found during a high-throughput 125I-ChTXdisplacement screen at Sterling-Winthrop in 1995. While showing only modest affinity (~200 nM) this molecule was functionally active and inhibited T cell proliferation and IL-2 production [59]. Three other analogues were developed, CP-339818 (2), CP-393223 (3) and CP-394322 (4) (Figure 1) showing improved blockade of Kv1.3 channels (120 nM, 70 nM and 90 nM, respectively) [60]. Substitution at the N1 position of the dihydroquinoline ring influenced activity markedly with compound 5 (CP-393224) showing a 10–200 fold reduction in activity across a series of assays [60]. The reduction in activity was attributed to the reduced lipophilicity of the N1 substituent. While CP-339818 (2) was initially promising, this compound showed poor selectivity for Kv1.3 as it also blocked Kv1.4 at comparable concentrations [60]. The initial hit, WIN-17317-3 (1) was also later shown to block neuronal sodium channels with an IC50 of 9 nM, [61] suggesting that all Kv1.3 blockers belonging to this chemotype might have this problem.
Figure 1.
Structures of the dihydroquinoline, correolide and benzamide analogues.
Correolide
Correolide (6) (Figure 1), a natural product from the Costa Rican tree Spachea correa was discovered by scientists at Merck using a high-throughput 86Rb-flux assay and found to block Kv1.3 with an IC50 of 90 nM [62]. Unfortunately, correolide was not specific for Kv1.3 as it bound with equal potency to all Kv1-family channels [63]. Other simplified analogues were synthesized including the C18-correolide analogue 43 (7) (Figure 1) which inhibited Kv1.3 with an IC50 of 37 nM in 86Rb-flux assays [64]. To date, the Kv1.3 specificity of this compound has not been reported as the synthesis of these analogues is hampered by the limited supply of the natural parent compound [64].
Benzamides
Further 86Rb-flux screening by Merck identified PAC (8) (Figure 1), a cyclohexyl-substituted benzamide, which blocked Kv1.3 with an IC50 of 270 nM. A more potent derivative trans-N-propylcarbamoyloxy- PAC (9) (Figure 1), was subsequently synthesized showing improved activity (IC50 50 nM) [65]. Once again only modest selectivity (2–6 fold) over other Kv1 channels was exhibited by these compounds. From an SAR perspective, removal of the phenyl ring, methylation of the amide nitrogen or replacement of the amide carbonyl with a sulfonyl group significantly reduced activity. Modifications to the 2-position of the 2-methoxy phenyl ring were tolerated while substituents in the 3 or 4 position reduced potency. The trans C1 carbamate compounds also showed better selectivity for Kv1.3 over the other Kv1 channels, although at present this appears to be insufficient for clinical utility [65].
Piperidines
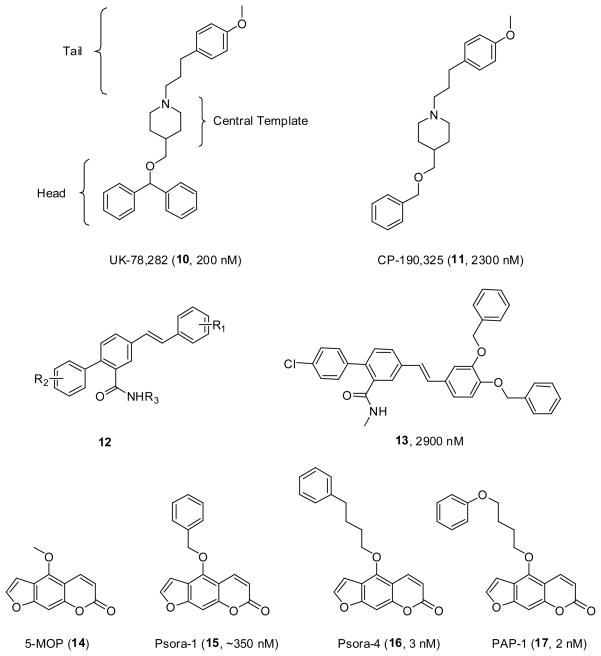
UK-78,282 (10) (Figure 2), a piperidine blocker of Kv1.3 was identified by Pfizer following a large scale screen using a high-throughput human T cell 86Rb efflux assay. This compound blocks Kv1.3 with an IC50 of 200 nM showing 100-fold selectivity for Kv1.3 over most other Kv1-family channels, the only exception being Kv1.4. Albeit a somewhat large molecule (MW 429.6), this compound was chosen as a lead to develop more potent and selective Kv1.3 blockers. The SAR exploration focused on three regions; the head group, central template and tail. The benzhydryl head group was shown to be required for good activity while replacement with a simpler benzyl group (CP-190,325, 11) (Figure 2) reduced activity about 10 fold [66]. The ether oxygen in the head group could be replaced with a carbon atom showing a very marginal increase in activity. Replacement of the central template with piperazine or pyridine lost potency, however a tropane ring maintained activity (400 nM). Deviation away from a para-methoxy phenpropyl group in the tail section of the molecule also reduced potency. While there was a very weak relationship between activity and lipophilicity, the logP of UK-78,282 is estimated to be over 7.0 highlighting the extreme hydrophobicity of these molecules [67]. Interestingly, competition experiments suggest that UK-78,282 can bind to residues at the inner surface of the channel, overlapping with the site of action of verapamil.
Figure 2.
Structures of the piperidine, phenyl stilbene and psoralen series of compounds.
Phenyl Stilbenes
In 1999 Chamberlin and Lew, researchers at the University of California, Irvine, used a de novo ligand based design method to design a new class of micromolar Kv1.3 blockers [68]. A model of the outer vestibule of the Kv1.3 channel was generated and compounds were designed to interact with residues known to be important for toxin binding (Gly380, Asp383 and His404) [69]. Fragments of compounds were joined through suitable spacer groups to form single molecules, which led to the selection of a phenyl stilbene scaffold (12) (Figure 2) for further elucidation. Parallel combinatorial synthesis was undertaken to produce a library of 400 compounds where a number of these compounds showed low micromolar activity in a 125I-ChTX displacement assay. Compound 13 (Figure 2) was the most active compound (IC50 2.9 μM) [68] although it has an estimated logP of over 8.0.
Psoralens
Starting from the natural product 5-methoxypsoralen (5-MOP, 14) (Figure 2) a number of highly potent Kv1.3 blockers were developed by groups at the University of California, Davis and the University of Kiel. The template 5-MOP was initially extracted from the common rue, a plant that had been reported to have beneficial effects in multiple sclerosis, and was found to have modest activity on the neuronal Kv1.2 and the T cell Kv1.3 channel [70]. The development of 5-MOP analogues followed where the methyl group in the 5 position was replaced with a series of substituents of various structure and length. The simplest derivative, Psora-1 (15) (Figure 2), which contains an additional phenyl ring on a single methylene linker, already showed nanomolar activity (IC50 ~ 350 nM). The optimal length for the chain linking the psoralen and aromatic rings was found to be four carbons (Psora-4, 16, 3 nM) [71]. Selectivity of Psora-4 against Kv1.5 was poor however, and this led to the design of PAP-1 (17) (Figure 2) which has comparable Kv1.3 potency (2 nM) but showed greater selectivity (23-fold over Kv1.5) [72]. PAP-1 also demonstrates 33–125 fold selectivity over related Kv1-family channels and 1000-fold selectivity for more distantly related K+ channels like KCa3.1, Kv2.1, Kv3.1 and Kv11.1 (HERG).
PAP-1 has also been considered as a potential human therapeutic agent and has undergone preliminary screens showing that it did not exhibit any cytotoxic or phototoxic effects [72]. This study also demonstrated that PAP-1 was able to suppress the activation of CCR7− human TEM cells (IC50 10 nM) and prevented DTH in Lewis rats dosed either orally or by i.p. injection (at 3 mg/kg). Both effects were ascribed to the Kv1.3 blocking action of PAP-1. The action of PAP-1 in allergic contact dermatitis was further investigated in a rat model using the skin sensitizer oxazolone [73]. Immunohistochemistry showed that PAP-1 was able to prevent CD8+ T cells from infiltrating the skin and reduced the production of the inflammatory cytokines, IFN-γ, IL-2 and IL-17. Topical application also proved effective which is encouraging for a drug that may have clinical utility in dermatology settings [73]. This study further showed that PAP-1 did not act as a skin sensitizer or skin irritant, which is a prerequisite for a topical drug. Taken together this data shows that PAP-1 has great potential for development as a topical drug for the treatment of psoriasis.
Khellinones
In 2004, researchers at the Walter and Eliza Hall Institute (WEHI) of Medical Research in Australia developed two novel classes of Kv1.3 blockers from khellinone (1-(6-hydroxy-4,7- dimethoxy-3a,7a-dihydrobenzofuran-5-yl)ethanone) (18) (Figure 3), itself a weak Kv1.3 blocker (IC50 = 45 μM) [74]. These two classes were khellinone dimers and khellinone chalcones. When khellinone was dimerised at the 6-hydroxy position with a ρ-xylene linker to yield 19 (Figure 3), the potency was greatly increased to an IC50 of 280 nM. Using aliphatic linkers the authors demonstrated that optimal activity was a balance between chain length and the flexibility of the linker. Introduction of an ether into the chain reduced activity suggesting hydrophobicity also influenced biological activity. 19 itself has 10-fold selectivity for Kv1.3 over the related channels, Kv1.1, Kv1.2 and 3-fold selectivity over Kv1.5 [74]. The khellinone chalcones were formed by the reaction of aryl aldehydes with the 5-acetyl group of khellinone. Interestingly, the khellinone chalcone 20 (Figure 3) was found to block Kv1.3 with an IC50 of 400 nM. This compound has 3-fold selectivity over Kv1.1 and has no observable effect on Kv1.2 channels as well as 20-fold selectivity over Kv1.5 [74].
Figure 3.
Structures of compounds based on khellin and khellinone.
Further work by this group on the khellinone scaffold yielded derivatives selectively alkylated on either the 4- or 7-position phenolic groups [75]. Once again submicromolar activity was found with modest selectivity over other potassium channels. Compounds 21 (IC50 480 nM) and 22 (IC50 400 nM) represent the most potent of the derivatives modifying the 4 and 7-positions, respectively (Figure 3). The SAR demonstrated that a range of substituents were tolerated on the benzyl group such as halogen atoms. The correlation between the activities of 19 pairs of derivatives (i.e. comparing the same substituent either in the 4 or 7 position) gave an R value of 0.74 possibly suggesting a common binding mode between these series of compounds.
Recent patents from the WEHI illustrate continued interest in khellinone and related scaffolds [76–78]. In the first of these patents further substitution on positions 5, 6 and 7 of the khellinone ring led to highly potent compounds [76]. 23 was able to block the Kv1.3 channel with an IC50 of 110 nM. Optimisation within this series led to 24 where replacement of the methoxy substituent with a methyl group and the morpholine ring with tetrazol-5-amine gave a 5-fold increase in activity (EC50 = 23 nM) (Figure 3) [76].
In a separate patent filing, synthesis of a series of chromenone derivatives were described using the natural product khellin (25) (Figure 3) as the scaffold [77]. Khellin is found in the plant Ammi visnaga and is structurally related to khellinone (18). Once again a phenoxypropyl side chain was used together with a morpholine group which showed reasonable potency (26, IC50 = 190 nM). In accord with the khellinone series a methyl substituent was more potent than a methoxy group and branching on the phenoxypropyl chain improved activity (27, IC50 = 14 nM) (Figure 3) [77]. The phenoxypropyl side chain is of course reminiscent of the series of compounds based on 5-MOP (e.g. Psora-4, 15). Indeed, it would be interesting to speculate what activity and/or selectivity could be obtained if the same side chain as PAP-1 were adopted within this series of compounds.
One further patent has shown the furan ring of khellinone can be opened with little affect on potency. For example, compound 28 (Figure 3) employed a related branched phenoxypropyl chain to give a potency that was quoted as less than 50 nM. Importantly for this compound it showed 45- fold selectivity over Kv1.1 and 37-fold selectivity against Kv1.5 [78]. Taking together all three patents [76–78] and the previous studies [74,75], it seems that the khellin scaffold can be trimmed back to a single aromatic ring and exploited to derive potent and reasonably selective Kv1.3 blockers. Recent disclosures from the company associated with the patents (Bionomics Ltd.) show that they are pursuing Kv1.3 blockers for a wide range of autoimmune disorders [79]. The compounds appear to have undergone preclinical testing and show efficacy in animal models of both MS and DTH [79].
Thienopyridines
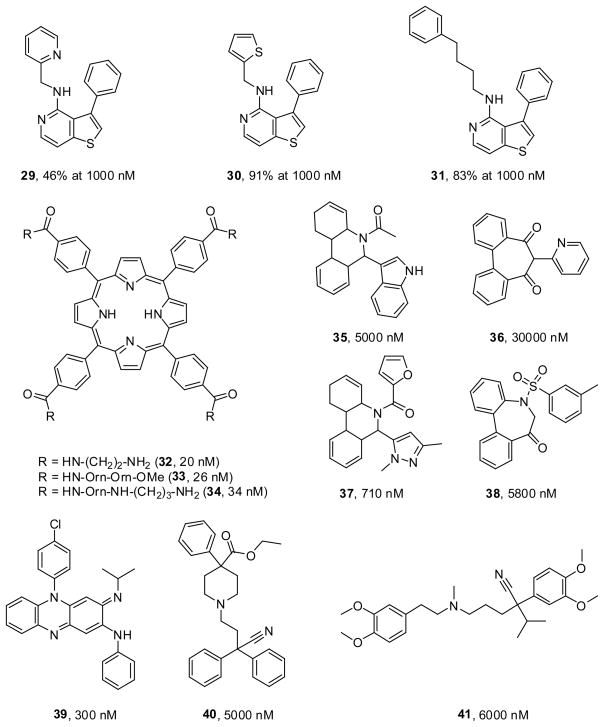
In 2007, a novel class of Kv1.3 blockers was reported by scientists at Xention Ltd. [80] based on thienopyridines, a known class of ADP receptor/P2Y12 inhibitors [81]. Compound 29 showed modest Kv1.3 blockade (46% at 1 μM) while substitution of the pyridine with a thiophene ring (30) increased activity (91% at 1 μM) (Figure 4). Modifications to the phenyl ring were also tolerated and substitution of the thienyl group of 30 with a phenylbutyl chain (31) (Figure 4) maintained activity (83% at 1 μM) [80]. Unfortunately, this set of compounds show no selectivity over Kv1.5. Interestingly, the features of two aromatic systems separated by a chain of 4–6 atoms is found in many compounds as well as this thienopyridine set of molecules.
Figure 4.
Structures of the thienopyridine, tetrahydroporphyrin, dihydrophenanthridine derivatives together with established drugs that inhibit Kv1.3 channels.
Tetraphenylporphyrins
In 2003, researchers at the University of California, Berkley reported the synthesis of a new class of ligands for Kv1.3 using a tetraphenylporphyrin core [82]. The overall design was based on the four-fold symmetry of the homotetrameric structure of the potassium channel. Three of the compounds potently displaced radiolabeled peptide toxins (32, 20 nM; 33, 26 nM; 34, 13 nM) (Figure 4), but inhibited Kv1.3 currents in patch-clamp experiments only at low micromolar concentrations. Although the tetraphenylporphyrins had originally been designed with the idea that their positively charged side chains could interact with four conserved aspartate residues in the outer vestibule in a four-fold symmetry, [82] later solid-phase NMR studies with a KcsA-Kv1.3 chimeric channel demonstrated that one of the four arms of the tetraphenylporphyrin actually penetrated into the selectivity filter [83] and that the compounds were standing upright in the pore like a cross instead of covering it as initially intended [82]. The highly charged nature of these molecules together with their large size will no doubt preclude them from being therapeutic agents, however they represent useful and interesting research tools and could potentially be used for the synthesis of metalloporphyrins for imaging and crystallographic studies.
Dihydrophenanthridines
In 2009, Pegaro et al. reported the discovery of two structurally new classes of Kv1.3 and KCa3.1 inhibitors based on a virtual high throughput screening approach [84]. A homology model of the Kv1.3 channel was created from the crystal structure of the bacterial KcsA channel and used for a virtual screen of around 3.3 million compounds. The search focused on the inner vestibule of the ion channel as this is thought to be the binding site for other Kv1.3 blockers such as verapamil and PAP-1 [71]. From a list of the best 500 docked structures, 37 were selected for patch-clamp testing based on structural diversity and docking scores. Two compounds including the dihydrophenanthridine derivative (35) and dibenzocycloheptanedione derivative (36) were identified giving IC50 values of 5 μM and 30 μM respectively (Figure 4). Variations on the 5–6 dihydrophenanthridine derivative at positions N5 and C6 were performed to yield a variety of compounds showing similar or slightly improved activity with 37 being the most potent (IC50 0.71 μM). Compound 37 was also selective over KCa3.1 showing only 12% inhibition at 20 μM in patchclamp assays. The introduction of an additional methylene group in the main scaffold and derivatisation of the sulfonyl substituents led to other active compounds such as 38 (IC50 5.8 μM) (Figure 4). Compound 38 also showed anti-inflammatory potential in a mouse DTH model, though this may be due to its affect on the IK1 channel (IC50 = 2.1 μM) as Kv1.3 is not upregulated in mouse TEM cells. It has been proposed that these compounds be used as leads for further optimization [84].
Serendipity and new uses for old drugs
Most of the starting points for medicinal chemistry in the Kv1.3 field have come from testing known potassium channel blockers, extracting medicinal plants, screening of actual or virtual libraries or other rational approaches for the design of ligands. In other cases, findings have come from clinical observations. By way of example, in 1976 a 28-year old male with an inflammatory skin condition was treated with clofazimine (39) following the failure of standard therapies. In this case, the leprosy drug clofazimine was able to successfully treat the condition with only minor skin inflammation being observed one year after the treatment was stopped [85]. Testing in 2008 by Ren et al. identified clofazimine as a novel inhibitor of the TCR signaling pathway that worked via the blockade of Kv1.3 channels (IC50 300 nM) and showed that it prevents the rejection of transplanted human foreskin in immunodeficient mice reconstituted with human T cells [86]. The potential link between Kv1.3 blockade and the treatment of an inflammatory skin condition with clofazimine is thus of great interest. It has even been proposed that clofazimine could be used as a new treatment for autoimmune diseases such as psoriasis [86]. When considering this data it is tantalizing to think that this provides consolidating evidence for the utility of Kv1.3 blockers in psoriasis.
In our laboratories we have followed up a similar success story on the use of diphenoxylate for inflammatory skin disorders. In the 1970’s, physician Earl Lanier prescribed a 67-year old woman Lomotil (containing diphenoxylate, 40) to treat her acute diarrhoea. Astonishingly, not only did the drug prove effective in treating her diarrhoea but the patient also experienced a four-month remission of her psoriasis [87]. A further eight patients were treated in open studies with a topical preparation of diphenoxylate. An improvement was observed in all cases after an average of four weeks with many of these patients having no reoccurrence of their lesions while others kept their psoriasis under satisfactory control [87,88]. At the beginning of the 1980’s, Lanier subsequently conducted a larger clinical trial on 35 individuals with an array of inflammatory skin conditions using topical and oral preparations of diphenoxylate, yielding further successful results [89]. Again, many of these patients had no reoccurrence of their symptoms after a short time period while administering the diphenoxylate preparations [89]. However, since Lanier’s work, these promising observations have not been investigated further. Recent work in our laboratory exploring diphenoxylate (40) has found that this compound blocks Kv1.3 with an IC50 of 5 μM in patch-clamp experiments (unpublished results). The possibility exists that the anti-inflammatory action of diphenoxylate observed by Lanier was mediated by the blockade of Kv1.3 channels. Indeed, this is the present subject of our research representing a medicinal chemistry campaign to optimize the activity of diphenoxylate analogues for Kv1.3 activity and selectivity.
Molecular similarity
Our interest in diphenoxylate was sparked by the structure of verapamil (41) (Figure 4), itself a low micromolar Kv1.3 blocker (IC50 6 μM) [90]. From a 2D perspective both molecules share a number of structural similarities such as the two aromatic regions at each end of the molecule, a cyano group and a basic nitrogen atom. In isolation this observation is tenuous, however we noted related groups in UK-78,282 (10) and often observed a separation of two aromatic regions by a chain of around 4–6 atoms in other potent Kv1.3 blockers like PAP-1 (17). Using the Phase software within the Maestro package (Schrodinger Inc. Portland, USA) we were able to generate a simplistic pharmacophore based on compounds 9, 10, 17, 23, 27, 28, 31, 40 and 41. This consists of two aromatic regions separated by 8.8 Å and a hydrogen bond acceptor feature (Figure 5A). While the model can accommodate a range of Kv1.3 chemotypes it only provides three features and is perhaps not specific enough for drug discovery purposes. As a general filter however, it may be useful to reduce the number of compounds selected for screening. Another feature of many Kv1.3 blockers is their high lipophilicity. Table 2 gives the calculated logP and logD7.4 values of the compounds used to derive the pharmacophore. The average value of logD7.4 across these compounds is 4.95 illustrating the high lipophilicity of these molecules. While the compounds listed in Table-2 might not be ideal for oral formulation because of their relatively high logP values, which typically result in large variations in oral availability and so-called “food-effects”, their lipophilicity makes them well suited for topical application. The optimal logP values for transdermal delivery have of course been shown to be in the range of 1–3 [91,92]. However, for the treatment of psoriasis the aim is not to deliver the compounds into the circulation through the skin (which would require a high permeability and mobility), but to deliver them to the thickened and inflamed epidermis and retain them there. As shown by Cross et al. the epidermis and dermis are actually much more lipophilic environments than originally believed and compounds with logP values of 4 and higher very effectively partition into the epidermis and dermis while leaving a considerable reservoir in the stratum corneum [93]. Other properties that are advantageous for dermal drug delivery include; molecular weight <500, high potency, melting point well below 200°C, non-irritant and non-immunogenic [92]. Many of the above mentioned Kv1.3 blockers fulfill many of these criteria and should therefore be suitable for topical use. PAP-1 (17) in fact has already been shown to effectively treat allergic contact dermatitis in rats following topical application [73] at 2% in the lipophilic crème base Eucerin®. (B) Diagram showing the structure of all four subunits of the Kv1.2 channel with the S1–S4 region colored green and the S5-P-S6 region in blue. The potassium ions are shown as yellow spheres while the T1 domain and β-subunit are colored magenta and gold, respectively. The cell membrane is shown in its anticipated position relative to the protein.
Figure 5.
(A) Pharmacophore model developed from compounds 9, 10, 17, 23, 27, 28, 31, 40 and 41 featuring two aromatic groups (indicated with blue circles) and a hydrogen bond acceptor feature (red square).
Table 2.
Physicochemical properties of the compounds used to derive the pharmacophore model.
| Compound number | ClogP | ClogD7.4 | MW |
|---|---|---|---|
| 9 | 4.55 | 4.55 | 424.5 |
| 10 | 7.24 | 5.27 | 429.6 |
| 17 | 5.05 | 5.05 | 350.4 |
| 23 | 4.23 | 4.19 | 453.5 |
| 27 | 5.14 | 2.98 | 405.5 |
| 28 | 6.75 | 6.75 | 486.0 |
| 31 | 8.04 | 7.99 | 358.5 |
| 40 | 5.88 | 5.45 | 452.6 |
| 41 | 3.90 | 2.33 | 454.6 |
Alongside our computational work, Saini and co-workers [94] have applied QSAR analyses to a series of khellinone derivatives described by Baell et al. [74]. The equation that was generated for a set of 22 compounds showed that lipophilicity was a major factor in understanding their biological activity [94]. In addition, it was found that molar refractivity and polarizability played a lesser role and notably molecules that were too bulky were of lower potency. While this work was able to provide a reasonable equation, the ability to employ this QSAR analysis for other series of compounds remains untested.
Design and future directions
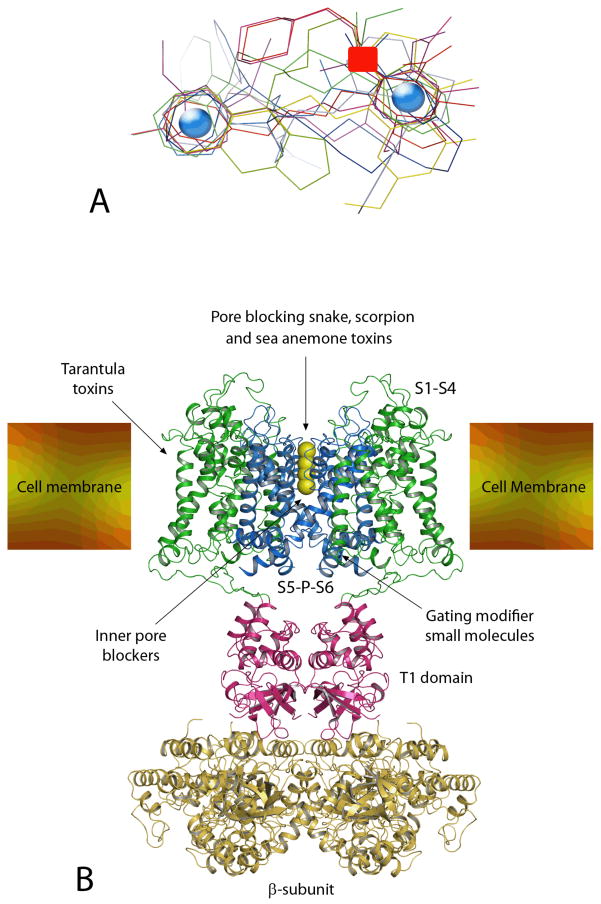
Much of the discussion so far has focused on several series of small molecules with associated SAR but without any information on how they bind to the Kv1.3 channel. To better understand the target macromolecule it is necessary to look at the structure of potassium channels in general. However, with respect to crystal structures one needs to realize that the ion channel field significantly falls behind the kinase field and that we currently only have the structures of a few prokaryotic channels like KcsA [95] and one mammalian voltage-gated K+ channel, the rat Kv1.2 channel [96,97]. Given the high sequence homology between Kv1-family members we should be able to use the 3-D coordinates of Kv1.2, which is assumed to have been crystallized in the open state, as an approximation to gain insights into the structure of Kv1.3. As shown in Figure 5B, Kv1.2 can be broadly “divided” into three parts: transmembrane (TM) domain, tetramerization domain (T1) and β-subunit. The TM domain consists of 24 membrane-spanning helices, 6 from each of the 4 identical α-subunits of the channel. Each 6TM α-subunit consists of a voltage-sensor domain comprising TM segments S1 to S4 and a pore domain composed of segments S5, S6 and a membrane-reentering P-loop. Below the selectivity filter (which in Figure 5B is shown filled with permeating K+ ions) is a hydrophobic cavity (inner vestibule) approximately 9 Å across, which is filled with water [96]. Below the membrane spanning part of the channel is the T1 region, which holds the channel tetramer together and which forms the docking platform for the β subunit for channels that have a β subunit. The β subunit is related to oxido-reductases and the crystal structure of Kv1.2 contains bound molecules of NADP+. This subunit may have a catalytic function and potentially could regulate the activity of the potassium channel [96].
In general, drugs modulating KV channels can interact with these large proteins at multiple sites. Peptide toxins from the venoms of snakes, scorpions, sea anemones and cone snails bind to the outer vestibule of Kv channels and in most cases insert a lysine side chain into the channel pore to occlude it [98–100]. Spider toxins like hanatoxin, which typically contain a cluster of hydrophobic amino acids, partition into the membrane and interact with the voltage-sensor [101,102]. Small molecules like the hydrophobic cations tetrabutylammonium (which was co-crystallized with KcsA [95]) or verapamil (41) occlude the inner pore and have been proposed to project into “niches” between the N-terminal parts of S6 and the P-loop in the case of larger molecules like verapamil [19]. The inner pore of Kv channels can also be targeted by lipophilic molecules like correolide (6), which has been shown through a combination of mutagenesis [103] and molecular modeling to “snuggle” into the hydrophobic surface of the S6 helix with its lipophilic part and to chelate a permeating potassium ion with its polar acetyl groups [104]. Small molecules can further bind to the “gating-hinges” as in the case of the Kv7 channel activator retigabine, which has been found by mutagenesis to bind to a putative hydrophobic pocket formed upon channel opening between the cytoplasmic parts of S5 and S6 [105]. It is further possible for small molecules to bind at the interface between the α- and the β-subunit and disrupt their interaction [106,107]. Another interesting mode of interaction was recently identified for the polycyclic cigutera toxin gambeirol, which is produced by the dinoflagellate Gambierdiscus toxicus. This highly lipophilic molecule (estimated logP 5.41) inserts into the space between S5 and S6 outside of the permeation pathway on the side of the helices facing the lipid and prevents the channel from opening by stabilizing the closed state [108].
Of course it would be ideal to have co-crystals of all the different Kv1.3 blockers with the Kv1.3 channel to ultimately determine where they bind. However, this is currently not feasible because of the great technical difficulties associated with crystallizing membrane proteins of the size of Kv channels and we believe the existing mutagenesis data is sufficient to demonstrate that at least correolide and verapamil bind in the inner vestibule. Because of the structural similarity of many of the compounds described above to verapamil it is reasonable to assume that compounds like PAP-1 (17) and UK-78,282 (10) also bind in the inner vestibule, but their exact orientation is currently impossible to predict without very detailed mapping studies. But it is of course also possible that some of the compounds discussed above bind to other sites, which could include:
The side portals described by Long and co-workers [96] which lie above the T1 domain. These regions have a number of negatively charged amino acids that can attract compounds with positive charges.
The outer pore
The “inner” gating hinges, which form the site of action of retigabine.
The voltage-sensor domain
Interfaces between the TM domains into which lipophilic compounds could insert in a similar manner to gambeirol
The β-subunit would theoretically be a possibility but is unlikely for any of the abovediscussed Kv1.3 blockers since most of the screening assays were performed on expression systems that only contained the α-subunit of the channel.
It may be that the binding sites do not include any of the suggestions above and will need to be identified experimentally for each compound class, ideally through a crystal structure, which then would allow true structure-based drug design. In the interim, classic medicinal chemistry efforts will be needed to optimize activity and selectivity as well as the physicochemical properties of suitable leads.
Conclusions
From the work conducted so far on Kv1.3 channels it is patently clear that this is a good target to pursue for autoimmune disorders such as psoriasis, MS and type-1 diabetes. Animal models have demonstrated in vivo efficacy and if we include unsubstantiated human clinical data, then the case is compelling to further explore Kv1.3 inhibitors as drugs. If we take as an example rat models of MS, chronic administration of ShK-186 was able to significantly reduce a set of key indicators for disease progression [109]. For a fuller discussion on the utility of Kv1.3 blockers in models of inflammation the following reviews are recommended [19,30,42,58]. For all researchers working in this field, a key transition is to successfully progress from animal models to properly conducted human clinical trials.
This review has highlighted a selected set of compounds where SAR data was available, representing a wide range of chemotypes. For many of the potent ligands, a common pharmacophore was found which included two aromatic regions. Use of the pharmacophore may help future design efforts and be of utility in identifying the binding site of these ligands on the K1.3 channel. The lipophilicity of these molecules was also discussed with regard to potential topical use. The range of physicochemical properties that are desirable for topical drugs was underscored which may form the basis of future strategies for Kv1.3 molecular design. Finally, to be able to contribute significantly to this research, structure-based drug design is needed, however this would require gargantuan efforts and is it likely that standard medicinal chemistry practices will dominate in order to optimize a molecule that will ultimately find its way to market.
Acknowledgments
We thank the Cybec Foundation for their generous contribution towards this research. H.W. is supported by National Institute of Health grant RO1 GM076063.
References
- 1.Stuve O, Marra C, Jerome K, Cook L, Cravens P, Cepok S. Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann Neurol. 2006;59:743–747. doi: 10.1002/ana.20858. [DOI] [PubMed] [Google Scholar]
- 2.Van Assche G, Van Ranst M, Sciot R, Dubois B, Vermeire S, Noman M. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med. 2005;353:362–368. doi: 10.1056/NEJMoa051586. [DOI] [PubMed] [Google Scholar]
- 3. [Accessed, August 2009]; http://www.prweb.com/releases/2009/06/prweb2528754.htm.
- 4.Fitzpatrick T, Johnson R, Wolff K. Color atlas and synopsis of clinical dermatology. 4. McGraw-Hill; New York: 2001. [Google Scholar]
- 5.Marks R, Plunkett A, Merlin K, Jenner N. Atlas of common skin diseases in Australia. Department of Dermatology, St Vincents Hospital; Melbourne: 1999. [Google Scholar]
- 6.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 7.Menter A, Gottlieb A, Feldman SR, Van Voorhees AS, Leonardi CL, Gordon KB, Lebwohl M, Koo JY, Elmets CA, Korman NJ, Beutner KR, Bhushan R. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826–850. doi: 10.1016/j.jaad.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 8.Gelfand JM, Feldman SR, Stern RS, Thomas J, Rolstad T, Margolis DJ. Determinants of quality of life in patients with psoriasis: a study from the US population. J Am Acad Dermatol. 2004;51:704–708. doi: 10.1016/j.jaad.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Horn EJ, Fox KM, Patel V, Chiou CF, Dann F, Lebwohl M. Association of patientreported psoriasis severity with income and employment. J Am Acad Dermatol. 2007;57:963–971. doi: 10.1016/j.jaad.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 10.Pariser DM, Bagel J, Gelfand JM, Korman NJ, Ritchlin CT, Strober BE, Van Voorhees AS, Young M, Rittenberg S, Lebwohl MG, Horn EJ. National Psoriasis Foundation clinical consensus on disease severity. Arch Dermatol. 2007;143:239–242. doi: 10.1001/archderm.143.2.239. [DOI] [PubMed] [Google Scholar]
- 11.Lebwohl M, Freeman A, Chapman M, Feldman S, Hartle J, Henning A. Tacrolimus ointment is effective for facial and intertriginous psoriasis. J Am Acad Dermatol. 2004;51:723–730. doi: 10.1016/j.jaad.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Zanolli M. Phototherapy arsenal in the treatment of psoriasis. Dermatol Clin. 2004;22:397–406. doi: 10.1016/j.det.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Luba KM, Stulberg DL. Chronic plaque psoriasis. Am Fam Physician. 2006;73:636–644. [PubMed] [Google Scholar]
- 14.Weinstein GD, Jeffes E, McCullough JL. Cytotoxic and immunologic effects of methotrexate in psoriasis. J Invest Dermatol. 1990;95:49S–52S. doi: 10.1111/1523-1747.ep12505777. [DOI] [PubMed] [Google Scholar]
- 15.Lee M, Kalb RE. Systemic therapy for psoriasis. Dermatol Nurs. 2008;2:105–111. [PubMed] [Google Scholar]
- 16.Sinclair R. A new approach to managing moderate to severe chronic plaque psoriasis. Aust J Pharm. 2006;87:66–69. [Google Scholar]
- 17.Kircik LH, Del Rosso JQ. Anti-TNF agents for the treatment of psoriasis. J Drugs Dermatol. 2009;8:546–559. [PubMed] [Google Scholar]
- 18.Wulff H, Pennington M. Targeting effector memory T-cells with Kv1.3 blockers. Curr Opin Drug Discov Dev. 2007;10:438–445. [PubMed] [Google Scholar]
- 19.Wulff H, Zhorov BS. K+ channel modulators for the treatment of neurological disorders and autoimmune diseases. Chem Rev. 2008;108:1744–1773. doi: 10.1021/cr078234p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vena GA, Cassano N. Emerging drugs for psoriasis. Expert Opin Emerg Drugs. 2006;11:567–596. doi: 10.1517/14728214.11.4.567. [DOI] [PubMed] [Google Scholar]
- 21.Prinz JC. The role of T cells in psoriasis. J Eur Acad Derm Vener. 2003;17:257–270. doi: 10.1046/j.1468-3083.2003.00720.x. [DOI] [PubMed] [Google Scholar]
- 22.Sagoo GS, Tazi-Ahnini R, Barker JW, Elder JT, Nair RP, Samuelsson L, Traupe H, Trembath RC, Robinson DA, Iles MM. Meta-analysis of genome-wide studies of psoriasis susceptibility reveals linkage to chromosomes 6p21 and 4q28-q31 in Caucasian and Chinese Hans population. J Invest Dermatol. 2004;122:1401–1405. doi: 10.1111/j.0022-202X.2004.22607.x. [DOI] [PubMed] [Google Scholar]
- 23.Bowcock AM, Krueger JG. Getting under the skin: the immunogenetics of psoriasis. Nat Rev Immunol. 2005;5:699–711. doi: 10.1038/nri1689. [DOI] [PubMed] [Google Scholar]
- 24.Gottlieb AB, Lebwohl M, Shirin S, Sherr A, Gilleaudeau P, Singer G, Solodkina G, Grossman R, Gisoldi E, Phillips S, Neisler HM, Krueger JG. Anti-CD4 monoclonal antibody treatment of moderate to severe psoriasis vulgaris: results of a pilot, multicenter, multiple-dose, placebo-controlled study. J Am Acad Dermatol. 2000;43:595–604. doi: 10.1067/mjd.2000.107945. [DOI] [PubMed] [Google Scholar]
- 25.Wrone-Smith T, Nickoloff BJ. Dermal injection of immunocytes induces psoriasis. J Clin Invest. 1996;98:1878–1887. doi: 10.1172/JCI118989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prinz JC, Groß B, Vollmer BS, Trommler P, Strobel I, Meurer M, Plewig G. T cell clones from psoriasis skin lesions can promote keratinocyte proliferation in vitro via secreted products. Eur J Immunol. 1994;24:593–598. doi: 10.1002/eji.1830240315. [DOI] [PubMed] [Google Scholar]
- 27.Sallusto F, Lenig D, Förster RLM, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 28.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naïve, central memory and effector memory CD4+ T cells. Pathol Biol. 2003;51:64–66. doi: 10.1016/s0369-8114(03)00098-1. [DOI] [PubMed] [Google Scholar]
- 29.Baccala R, Theofilopoulos AN. The new paradigm of T-cell homeostatic proliferationinduced autoimmunity. Trends Immunol. 2005;26:5–8. doi: 10.1016/j.it.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Chandy GK, Wulff H, Beeton C, Calabresi PA, Gutman GA, Pennington M. In: Voltage-Gated Ion Channels as Drug Targets. Triggle D, editor. Wiley-VCH Verlag GmbH & Co; New York: 2006. pp. 214–274. [Google Scholar]
- 31.Wulff H, Calabresi PA, Allie R, Yun S, Pennington M, Chandy GK. The voltage-gated Kv1.3 K+ channel in effector memory T cells as new target for MS. J Clin Invest. 2003;111:1703–1713. doi: 10.1172/JCI16921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wulff H, Beeton C, Chandy KG. Potassium channels as therapeutic targets for autoimmune disorders. Curr Opin Drug Discov Dev. 2003;6:640–647. [PubMed] [Google Scholar]
- 33.Chandy KG, Cahalan M, Pennington M, Norton RS, Wulff H, Gutman GA. Potassium channels in T lymphocytes: toxins to therapeutic immunosuppressants. Toxicon. 2001;39:1269–1276. doi: 10.1016/s0041-0101(01)00120-9. [DOI] [PubMed] [Google Scholar]
- 34.Chandy KG, Wulff H, Beeton C, Pennington M, Gutman GA, Cahalan MD. K+ channels as targets for specific immunomodulation. Trends Pharmacol Sci. 2004;25:280–289. doi: 10.1016/j.tips.2004.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 37.Cahalan MD, Chandy KG. The functional network of ion channels in T lymphocytes. Immunol Rev. 2009;231:59–87. doi: 10.1111/j.1600-065X.2009.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cahalan MD, Wulff H, Chandy KG. Molecular properties and physiological roles of ion channels in the immune system. J Clin Immunol. 2001;21:235–252. doi: 10.1023/a:1010958907271. [DOI] [PubMed] [Google Scholar]
- 39.Lin CS, Boltz RC, Blake JT, Nguyen M, Talento A, Fischer PA, Springer MS, Sigal NH, Slaughter RS, Garcia ML. Voltage-gated potassium channels regulate calciumdependent pathways involved in human T lymphocyte activation. J Exp Med. 1993;177:637–645. doi: 10.1084/jem.177.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeCoursey TE, Chandy KG, Gupta S, Cahalan MD. Voltage-gated K+ channels in human T lymphocytes: a role in mitogenesis? Nature. 1984;307:465–468. doi: 10.1038/307465a0. [DOI] [PubMed] [Google Scholar]
- 41.Wulff H, Knaus HG, Pennington M, Chandy KG. K+ channel expression during B cell differentiation: implications for immunomodulation and autoimmunity. J Immunol. 2004;173:776–786. doi: 10.4049/jimmunol.173.2.776. [DOI] [PubMed] [Google Scholar]
- 42.Rangaraju S, Chi V, Pennington MW, Chandy KG. Kv1.3 potassium channels as a therapeutic target in multiple sclerosis. Expert Opin Ther Targets. 2009;13:909–924. doi: 10.1517/14728220903018957. [DOI] [PubMed] [Google Scholar]
- 43.Krueger JG. The immunologic basis for the treatment of psoriasis with new biologic agents. J Am Acad Dermatol. 2002;46:1–23. doi: 10.1067/mjd.2002.120568. [DOI] [PubMed] [Google Scholar]
- 44.Vissers WH, Arndtz CH, Muys L, Van Erp PE, de Jong EM, van de Kerkhof PC. Memory effector (CD45RO+) and cytotoxic (CD8+) T cells appear early in the margin zone of spreading psoriatic lesions in contrast to cells expressing natural killer receptors, which appear late. Br J Dermatol. 2004;150:852–859. doi: 10.1111/j.1365-2133.2004.05863.x. [DOI] [PubMed] [Google Scholar]
- 45.Ghoreschi K, Thomas P, Breit S, Dugas M, Mailhammer R, van Eden W, van der Zee R, Biedermann T, Prinz J, Mack M, Mrowietz U, Christophers E, Schlondorff D, Plewig G, Sander CA, Rocken M. Interleukin-4 therapy of psoriasis induces Th2 responses and improves human autoimmune disease. Nat Med. 2003;9:40–46. doi: 10.1038/nm804. [DOI] [PubMed] [Google Scholar]
- 46.Austin LM, Ozawa M, Kikuchi T, Walters IB, Krueger JG. The majority of epidermal T cells in Psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999;113:752–759. doi: 10.1046/j.1523-1747.1999.00749.x. [DOI] [PubMed] [Google Scholar]
- 47.Chamian F, Lin SL, Lee E, Kikuchi T, Gilleaudeau P, Sullivan-Whalen M, Cardinale I, Khatcherian A, Novitskaya I, Wittkowski KM, Krueger JG, Lowes MA. Alefacept (anti- CD2) causes a selective reduction in circulating effector memory T cells (Tem) and relative preservation of central memory T cells (Tcm) in psoriasis. J Transl Med. 2007;5:27. doi: 10.1186/1479-5876-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sobell JM, Kalb RE, Weinberg JM. Management of moderate to severe plaque psoriasis (part 2): clinical update on T-cell modulators and investigational agents. J Drugs Dermatol. 2009;8:230–238. [PubMed] [Google Scholar]
- 49.Sobell JM, Kalb RE, Weinberg JM. Management of moderate to severe plaque psoriasis (part I): clinical update on antitumor necrosis factor agents. J Drugs Dermatol. 2009;8:147–154. [PubMed] [Google Scholar]
- 50.Breland AE, Currier RD. Scorpion venom and multiple sclerosis. Lancet. 1983;2:1021. doi: 10.1016/s0140-6736(83)90996-0. [DOI] [PubMed] [Google Scholar]
- 51.Miller C, Moczydlowski E, Latorre R, Phillips M. Charybdotoxin, a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature. 1985;313:316–318. doi: 10.1038/313316a0. [DOI] [PubMed] [Google Scholar]
- 52.Sands S, Lewis R, Cahalan M. Charybdotoxin blocks voltage-gated K+ channels in human and murine T lymphocytes. J Gen Physiol. 1989;93:1061–1074. doi: 10.1085/jgp.93.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ying-Duo Gao MLG. Interaction of agitoxin2, charybdotoxin, and iberiotoxin with potassium channels: Selectivity between voltage-gated and Maxi-K channels. Proteins: Structure, Function, and Genetics. 2003;52:146–154. doi: 10.1002/prot.10341. [DOI] [PubMed] [Google Scholar]
- 54.Grissmer S, Nguyen AN, Aiyar J, Hanson DC, Mather RJ, Gutman GA, Karmilowicz MJ, Auperin DD, Chandy KG. Pharmacological characterization of five cloned voltagegated K+ channels, types Kv1.1, 1.2, 1.3, 1.5, and 3.1, stably expressed in mammalian cell lines. Mol Pharmacol. 1994;45:1227–1234. [PubMed] [Google Scholar]
- 55.Beeton C, Pennington MW, Wulff H, Singh S, Nugent D, Crossley G, Khaytin I, Calabresi PA, Chen CY, Gutman GA, Chandy KG. Targeting effector memory T cells with a selective peptide inhibitor of Kv1.3 channels for therapy of autoimmune diseases. Mol Pharmacol. 2005;67:1369–1381. doi: 10.1124/mol.104.008193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matheu MP, Beeton C, Garcia A, Chi V, Rangaraju S, Safrina O, Monaghan K, Uemura MI, Li D, Pal S, de la Maza LM, Monuki E, Flugel A, Pennington MW, Parker I, Chandy KG, Cahalan MD. Imaging of effector memory T cells during a delayedtype hypersensitivity reaction and suppression by Kv1. 3 channel block. Immunity. 2008;29:602–614. doi: 10.1016/j.immuni.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beeton C, Wulff H, Standifer NE, Azam P, Mullen KM, Pennington MW, Kolski- Andreaco A, Wei E, Grino A, Counts DR, Wang PH, LeeHealey CJ, BSA, Sankaranarayanan A, Homerick D, Roeck WW, Tehranzadeh J, Stanhope KL, Zimin P, Havel PJ, Griffey S, Knaus HG, Nepom GT, Gutman GA, Calabresi PA, Chandy KG. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc Natl Acad Sci U S A. 2006;103:17414–17419. doi: 10.1073/pnas.0605136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baell JB. Potassium channel blockers as immunosuppressants. Expert Opin Ther Patents. 2005;15:1209–1220. [Google Scholar]
- 59.Hill RJ, Grant AM, Volberg W, Rapp L, Faltynek C, Miller D, Pagani K, Baizman E, Wang S, Guiles JW, Krafte DS. WIN 17317–3: novel nonpeptide antagonist of voltageactivated K+ channels in human T lymphocytes. Mol Pharmacol. 1995;48:98–104. [PubMed] [Google Scholar]
- 60.Nguyen A, Kath JC, Hanson DC, Biggers MS, Canniff PC, BDC, JMR, JBM, Rauer H, Aiyar J, Lepple-Wienhues A, Gutman GA, Grissmer S, Cahalan MD, Chandy KG. Novel nonpeptide agents potently block the C-type inactivated conformation of Kv1.3 and suppress T cell activation. Mol Pharmacol. 1996;50:1672–1679. [PubMed] [Google Scholar]
- 61.Wanner SG, Glossmann H, Knaus HG, Baker R, Parsons W, Rupprecht KM, Brochu R, Cohen CJ, Schmalhofer W, Smith M, Warren V, Garcia ML, Kaczorowski GJ. WIN 17317–3, a new high-affinity probe for voltage-gated sodium channels. Biochemistry. 1999;38:11137–11146. doi: 10.1021/bi990336p. [DOI] [PubMed] [Google Scholar]
- 62.Felix JP, Bugianesi RM, Schmalhofer WA, Borris R, Goetz MA, Hensens OD, Bao JM, Kayser F, Parsons WH, Rupprecht K, Garcia ML, Kaczorowski GJ, Slaughter RS. Identification and biochemical characterization of a novel nortriterpene inhibitor of the human lymphocyte voltage-gated potassium channel, Kv1. 3. Biochemistry. 1999;38:4922–4930. doi: 10.1021/bi982954w. [DOI] [PubMed] [Google Scholar]
- 63.Hanner M, Schmalhofer WA, Green B, Bordallo C, Liu J, Slaughter RS, Kaczorowski GJ, Garcia ML. Binding of correolide to K(v)1 family potassium channels. Mapping the domains of high affinity interaction. J Biol Chem. 1999;274:25237–25244. doi: 10.1074/jbc.274.36.25237. [DOI] [PubMed] [Google Scholar]
- 64.Bao J, Miao S, Kayser F, Kotliar AJ, Baker RK, Doss GA, Felix JP, Bugianesi RM, Slaughter RS, Kaczorowski GJ, Garcia ML, Ha SN, Castonguay L, Koo GC, Shah K, Springer MS, Staruch MJ, Parsons WH, Rupprecht KM. Potent Kv1.3 inhibitors from correolide-modification of the C18 position. Bioorg Med Chem Lett. 2005;15:447–451. doi: 10.1016/j.bmcl.2004.10.058. [DOI] [PubMed] [Google Scholar]
- 65.Miao S, Bao J, Garcia ML, Goulet JL, Hong XJ, Kaczorowski GJ, Kayser F, Koo GC, Kotliar A, Schmalhofer WA, Shah K, Sinclair PJ, Slaughter RS, Springer MS, Staruch MJ, Tsou NN, Wong F, Parsons WH, Rupprecht KM. Benzamide derivatives as blockers of Kv1.3 ion channel. Bioorg Med Chem Lett. 2003;13:1161–1164. doi: 10.1016/s0960-894x(03)00014-3. [DOI] [PubMed] [Google Scholar]
- 66.Hanson DC, Nguyen A, Mather RJ, Rauer H, Koch K, Burgess LE, Rizzi JP, Donovan CB, Bruns MJ, Canniff PC, Cunningham AC, Verdries KA, Mena E, Kath JC, Gutman GA, Cahalan MD, Grissmer S, Chandy KG. UK-78,282, a novel piperidine compound that potently blocks the Kv1.3 voltage-gated potassium channel and inhibits human T cell activation. Br J Pharmacol. 1999;126:1707–1716. doi: 10.1038/sj.bjp.0702480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burgess LE, Koch K, Cooper K, Biggers MS, Ramchandani M, Smitrovich JH, Gilbert EJ, Bruns MJ, Mather RJ, Donovan CB, Hanson DC. The SAR of UK-78,282: a novel blocker of human T cell Kv1.3 potassium channels. Bioorg Med Chem Lett. 1997;7:1047–1052. [Google Scholar]
- 68.Lew A, Chamberlin AR. Blockers of human T cell Kv1.3 potassium channels using de novo ligand design and solid-phase parallel combinatorial chemistry. Bioorg Med Chem Lett. 1999;9:3267–3272. doi: 10.1016/s0960-894x(99)00601-0. [DOI] [PubMed] [Google Scholar]
- 69.Hidalgo P, MacKinnon R. Revealing the architecture of a K+ channel pore through mutant cycles with a peptide inhibitor. Science. 1995;268:307–310. doi: 10.1126/science.7716527. [DOI] [PubMed] [Google Scholar]
- 70.Bohuslavizki KH, Hänsel W, Kneip A, Koppenhöfer E, Niemöller E, Sanmann K. Mode of action of psoralens, benzofurans, acridinons, and coumarins on the ionic currents in intact myelinated nerve fibres and its significance in demyelinating diseases. Gen Physiol Biophys. 1994;13:309–328. [PubMed] [Google Scholar]
- 71.Vennekamp J, Wulff H, Beeton C, Calabresi PA, Grissmer S, Hänsel W, Chandy KG. Kv1.3-Blocking 5-Phenylalkoxypsoralens: A New Class of Immunomodulators. Mol Pharmacol. 2004;65:1364–1374. doi: 10.1124/mol.65.6.1364. [DOI] [PubMed] [Google Scholar]
- 72.Schmitz A, Sankaranarayanan A, Azam P, Schmidt-Lassen K, Homerik D, Hansel W, Wulff H. Design of PAP-1, a selective small molecule Kv1.3 blocker, for the suppression of effector memory T cells in autoimmune diseases. Mol Pharmacol. 2005;68:1254–1270. doi: 10.1124/mol.105.015669. [DOI] [PubMed] [Google Scholar]
- 73.Azam P, Sankaranarayanan A, Homerick D, Griffey S, Wulff H. Targeting effector memory T cells with the small molecule Kv1.3 blocker PAP-1 suppresses allergic contact dermatitis. J Invest Dermatol. 2007;127:1419–1429. doi: 10.1038/sj.jid.5700717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baell JB, Gable RW, Harvey AJ, Nathan T, Herzog T, Hansel W, Wulff H. Khellinone derivatives as blockers of the voltage-gated potassium channel Kv1.3: Synthesis and immunosuppressive activity. J Med Chem. 2004;47:2326–2336. doi: 10.1021/jm030523s. [DOI] [PubMed] [Google Scholar]
- 75.Harvey AJ, Baell JB, Toovey N, Homerick D, Wulff H. A new class of blockers of the voltage-gated potassium channel Kv1.3 via modification of the 4- or 7-position of khellinone. J Med Chem. 2006;49:1433–1441. doi: 10.1021/jm050839v. [DOI] [PubMed] [Google Scholar]
- 76.Flynn B, Baell JB, Harvey AJ, Chaplin JH, Paul D, Mould JA. WO/2008/040057. Novel benzofuran potassium channel blockers and uses thereof. 2008
- 77.Flynn B, Baell JB, Harvey AJ, Chaplin JH, Paul D, Mould JA. WO/2008/040058. Novel chromenone potassium channel blockers and uses thereof. 2008
- 78.Flynn BL, Baell JB, Chaplin JH, Gill GS, Grobelny DW, Harvey AJ, Mould JA, Paul D. WO/2009/043117. Novel aryl potassium channel blockers and uses thereof. 2009
- 79. [Accessed October 2009]; http://www.bionomics.com.au/page.php?section=105.
- 80.Ford J, Madge DJ, Payne HJ, Knight JD. WO/2007/066127. Thieno(3,2-c)pyridine compounds. 2007
- 81.Horiuchi H. Recent advance in antiplatelet therapy: The mechanisms, evidence and approach to the problems. Ann Med. 2006;38:162–172. doi: 10.1080/07853890600640657. [DOI] [PubMed] [Google Scholar]
- 82.Gradl SN, Felix JP, Isacoff EY, Garcia ML, Trauner D. Protein surface recognition by rational design: nanomolar ligands for potassium channels. J Am Chem Soc. 2003;125:12668–12669. doi: 10.1021/ja036155z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ader C, Schneider R, Hornig S, Velisetty P, Wilson EM, Lange A, Giller K, Ohmert I, Martin-Eauclaire MF, Trauner D, Becker S, Pongs O, Baldus M. A structural link between inactivation and block of a K+ channel. Nat Struct Mol Biol. 2008;15:605–612. doi: 10.1038/nsmb.1430. [DOI] [PubMed] [Google Scholar]
- 84.Pegoraro S, Lang M, Dreker T, Kraus J, Hamm S, Meere C, Feurle J, Tasler S, Prütting S, Kuras Z, Visan V, Grissmer S. Inhibitors of potassium channels Kv1.3 and IK-1 as immunosuppressants. Bioorg Med Chem Lett. 2009;19:2299–2304. doi: 10.1016/j.bmcl.2009.02.077. [DOI] [PubMed] [Google Scholar]
- 85.Piamphongsant T, Chuaprapaisilp T. Treatment of pustular psoriasis with clofazimine. Br J Dermatol. 1978;99:303–305. doi: 10.1111/j.1365-2133.1978.tb02001.x. [DOI] [PubMed] [Google Scholar]
- 86.Ren YR, Pan F, Parvez S, Fleig A, Chong CR, Xu J, Dang Y, Zhang J, Jiang H, Penner R, Liu JO. Clofazimine inhibits human Kv1. 3 potassium channel by perturbing calcium oscillation in T lymphocytes. PLoS ONE. 2008;3:e4009. doi: 10.1371/journal.pone.0004009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lanier EW. Diphenoxylate therapy for psoriasis. Arch Dermatol. 1985;121:1486. [PubMed] [Google Scholar]
- 88.Lanier EW. Basic diphenoxylate therapy. Arch Dermatol. 1986;122:382. [PubMed] [Google Scholar]
- 89.Lanier EW. US 4,383,000. Method for treatment of various dermatological conditions. 1983
- 90.Grissmer S, Dethlefs B, Wasmuth JJ, Goldin AL, Gutman GA, Cahalan MD, Chandy KG. Expression and chromosomal localization of a lymphocyte K+ channel gene. Proc Natl Acad Sci U S A. 1990;87:9411–9415. doi: 10.1073/pnas.87.23.9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lu GW, Flynn GL. In: Modern Pharmaceutics, Applications and Advances. Florence AT, Siepmann J, editors. Informa Healthcare; New York: 2009. pp. 43–100. [Google Scholar]
- 92.Finnin BC, Morgan TM. Transdermal penetration enhancers: applications, limitations, and potential. J Pharm Sci. 1999;88:955–958. doi: 10.1021/js990154g. [DOI] [PubMed] [Google Scholar]
- 93.Cross SE, Magnusson BM, Winckle G, Anissimov Y, Roberts MS. Determination of the effect of lipophilicity on the in vitro permeability and tissue reservoir characteristics of topically applied solutes in human skin layers. J Invest Dermatol. 2003;120:759–764. doi: 10.1046/j.1523-1747.2003.12131.x. [DOI] [PubMed] [Google Scholar]
- 94.Saini L, Gupta SP, Kumar Satluri VS. A QSAR study on some series of sodium and potassium channel blockers. Med Chem. 2009;5:570–576. doi: 10.2174/157340609790170524. [DOI] [PubMed] [Google Scholar]
- 95.Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2. 0 A resolution. Nature. 2001;414:43–48. doi: 10.1038/35102009. [DOI] [PubMed] [Google Scholar]
- 96.Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltagedependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 97.Long SB, Tao X, Campbell EB, MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450:376–382. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- 98.MacKinnon R, Miller C. Mutant potassium channels with altered binding of charybdotoxin, a pore-blocking peptide inhibitor. Science. 1989;245:1382–1385. doi: 10.1126/science.2476850. [DOI] [PubMed] [Google Scholar]
- 99.MacKinnon R, Heginbotham L, Abramson T. Mapping the receptor site for charybdotoxin, a pore-blocking potassium channel inhibitor. Neuron. 1990;5:767–771. doi: 10.1016/0896-6273(90)90335-d. [DOI] [PubMed] [Google Scholar]
- 100.Chandy KG, Cahalan M, Pennington M, Norton RS, Wulff H, Gutman GA. Potassium channels in T lymphocytes: toxins to therapeutic immunosuppressants. Toxicon. 2001;39:1269–1276. doi: 10.1016/s0041-0101(01)00120-9. [DOI] [PubMed] [Google Scholar]
- 101.Phillips LR, Milescu M, Li-Smerin Y, Mindell JA, Kim JI, Swartz KJ. Voltage-sensor activation with a tarantula toxin as cargo. Nature. 2005;436:857–860. doi: 10.1038/nature03873. [DOI] [PubMed] [Google Scholar]
- 102.Lee SY, MacKinnon R. A membrane-access mechanism of ion channel inhibition by voltage sensor toxins from spider venom. Nature. 2004;430:232–235. doi: 10.1038/nature02632. [DOI] [PubMed] [Google Scholar]
- 103.Hanner M, Green B, Gao YD, Schmalhofer WA, Matyskiela M, Durand DJ, Felix JP, Linde AR, Bordallo C, Kaczorowski GJ, Kohler M, Garcia ML. Binding of correolide to the K(v)1. 3 potassium channel: characterization of the binding domain by site-directed mutagenesis. Biochemistry. 2001;40:11687–11697. doi: 10.1021/bi0111698. [DOI] [PubMed] [Google Scholar]
- 104.Bruhova I, Zhorov BS. Monte Carlo-energy minimization of correolide in the Kv1.3 channel: possible role of potassium ion in ligand-receptor interactions. BMC Struct Biol. 2007;7:5. doi: 10.1186/1472-6807-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wuttke TV, Seebohm G, Bail S, Maljevic S, Lerche H. The new anticonvulsant retigabine favors voltage-dependent opening of the Kv7.2 (KCNQ2) channel by binding to its activation gate. Mol Pharmacol. 2005;67:1009–1017. doi: 10.1124/mol.104.010793. [DOI] [PubMed] [Google Scholar]
- 106.Zhang ZH, Rhodes KJ, Childers WE, Argentieri TM, Wang Q. Disinactivation of N-type inactivation of voltage-gated K channels by an erbstatin analogue. J Biol Chem. 2004;279:29226–29230. doi: 10.1074/jbc.M403290200. [DOI] [PubMed] [Google Scholar]
- 107.Lu Q, Peevey J, Jow F, Monaghan MM, Mendoza G, Zhang H, Wu J, Kim CY, Bicksler J, Greenblatt L, Lin SS, Childers W, Bowlby MR. Disruption of Kv1.1 N-type inactivation by novel small molecule inhibitors (disinactivators) Bioorg Med Chem. 2008;16:3067–3075. doi: 10.1016/j.bmc.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 108.Kopljar I, Labro AJ, Cuypers E, Johnson HW, Rainier JD, Tytgat J, Snyders DJ. A polyether biotoxin binding site on the lipid-exposed face of the pore domain of Kv channels revealed by the marine toxin gambierol. Proc Natl Acad Sci U S A. 2009;106:9896–9901. doi: 10.1073/pnas.0812471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Matheu MP, Beeton C, Garcia A, Chi V, Rangaraju S, Safrina O, Monaghan K, Uemura MI, Li D, Pal S, de la Maza LM, Monuki E, Flugel A, Pennington MW, Parker I, Chandy KG, Cahalan MD. Imaging of effector memory T cells during a delayedtype hypersensitivity reaction and suppression by Kv1. 3 channel block. Immunity. 2008;29:602–614. doi: 10.1016/j.immuni.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]