Abstract
Enteric microencapsulation of the potential immunosuppressant TRAM-34 was investigated as a means of enhancing oral drug delivery and minimizing or eliminating hydrolysis of pyrazole substituted triarylmethane to the respective alcohol. Microparticles were successfully formulated with the pH sensitive Eudragit L100 polymer using the coacervation method and water miscible ICH class 3 organic solvents suitable for safe scale up production. The resulting microparticles were spherical and uniform with an average particle size of 460 µm at 15% theoretical loading. The encapsulation efficiency was 90 ± 1.9% and the percentage yield was found to be 91.5 ± 0.3%. Although the oral administration in rhesus macaques of TRAM-34 loaded enteric coated microparticles illustrated 6 times enhancement in its oral bioavailability. However, the TRAM-34 plasma concentration was less than the therapeutic effective level. This could be attributed to the compound’s inherent absorption characteristics and high lipophilicity.
Keywords: Enteric coating, TRAM-34, Microencapsulation, Oral, Bioavailability, Coacervation
1. Introduction
Formulations play a key role in assessing the biological properties of a molecule during drug discovery. Maximizing exposure is the primary objective in early animal experimentation, so that the pharmacokinetics, pharmacodynamics and toxicological signals can be matched to the pharmacodynamic response. However, discovery screening-hits or early lead compounds often exhibit poor physicochemical properties, solubility and pharmacokinetic attributes, making in vivo assessment of the ‘drug-ability’ of the lead compound and enhancement through formulations have become an increasingly important addition to traditional drug efficacy and toxicity evaluations, as pharmaceutical scientists strive to accelerate drug discovery and development processes in a time- and cost-effective manner (Caldwell et al., 2001; Li et al., 2007).
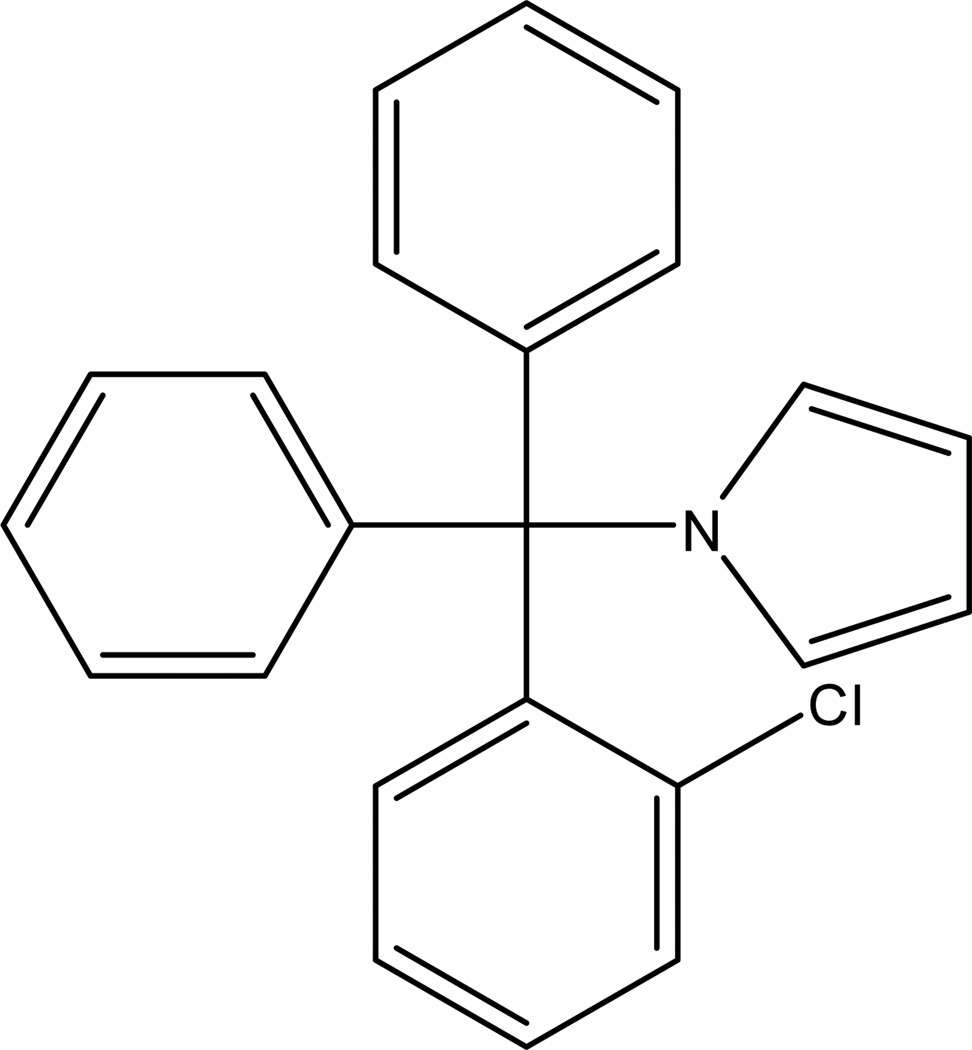
The antimycotic clotrimazole, a potent inhibitor of the intermediate-conductance calcium-activated K+ channel (KCa3.1), was suggested as immunosuppressant (Jensen et al., 2002) based on the fact that KCa3.1 is up-regulated in naïve T cells following mitogenic or antigenic activation (Ghanshani et al., 2000; Wulff et al., 2003). However, clotrimazole’s inhibition of cytochrome P450 enzymes limits its therapeutic value, creating a need for a truly selective KCa3.1 inhibitor. By identifying and exploiting differences in the pharmacophores required for channel block and cytochrome P450 inhibition, (1-[(2-chlorophenyl)-diphenylmethyl]-1H-pyrazole) Wulff et al. designed and synthesized TRAM-34 (Figure 1) (Wulff et al., 2000). TRAM-34 was found to inhibit the cloned and the native KCa3.1 channel in human T lymphocytes with an IC50 of 20 nM, to exhibit an excellent selectivity over other ion channels and to effectively inhibit T cell proliferation (Wulff et al., 2000). Therefore, TRAM-34 was proposed as promising new immunosuppressant. However, the compound’s high lipophilicity (log P = 4) and its susceptibility to acidic hydrolysis hindered the preclinical in vivo testing.
Figure 1.
Structure of 1-[(2-chlorophenyl)-diphenylmethyl]-1H-pyrazole (TRAM-34)
Since oral delivery is impossible without passing the acid stomach; we here report the development and the evaluation of drug-loaded enteric microparticles suitable for lipophilic and acid labile compounds such as TRAM-34, as a means of enhancing oral drug delivery.
Among the numerous methods described for microencapsulation (Deasy, 1984), a coacervation process appeared to be most suitable for the enteric coating of TRAM-34, which is practically insoluble in water and soluble in some organic solvents. Eudragit L100 (poly(methacrylic acid-co-methyl methacrylate) 1:1) was utilized as the enteric coating polymer. The formulation of a multiparticulate system is thought to be preferable to a single-unit dosage form because the small particles spread out more uniformly in the gastrointestinal tract. However, multiparticulate system enteric coating could be a challenge sometimes due to the aggregation of microparticles during coacervation. The rapid rise in apparent viscosity of the polymer rich region causes undesirable cohesion and aggregation of microparticles. This could be reduced by using a shock-preventing agent such as Hydroxypropylmethylcellulose (HPMC) or cationic surfactants that are strongly adsorbed at the interface of the coacervate and its surrounding medium, reducing the interfacial tension and reducing the tendency of the particles to aggregate (Deasy, 1984; Dong et al., 2006).
Since toxic organic solvents can interfere with the in vivo testing (Wahlstrom et al., 2007) and also might pose problem for later industrial scale up, we used safe water miscible organic solvents (ICH Class 3 solvents) to dissolve TRAM-34. HPMC and phosphate buffer were then utilized to induce a phase separation of the polymer and subsequent forming of the enteric microparticles by coacervation. To test its bioavailability, TRAM-34 was then administered to Rhesus Macaques intravenously, orally without enteric coating, and orally with enteric coating as microcapsules.
2. Materials and methods
2.1 Materials
TRAM-34 was synthesized as previously described (Wulff et al., 2000). Eudragit L100 was obtained from Degussa AG (Darmstadt, Germany). Hydroxypropyl methylcellulose was obtained from DOW chemical company (Michigan, U.S.A). Acetone, ethanol, monobasic potassium phosphate, sodium hydroxide, and HPLC grade solvents were obtained from Fisher Scientific (New Jersey, U.S.A).
2.2 Preparation of Drug loaded enteric microparticles
Enteric microparticles were prepared by modified coacervation techniques (Deasy, 1984; Dong et al., 2006). The aqueous polymer phase was prepared by dissolving 1 g HPMC in 1000 mL phosphate buffer (0.1 M, pH 7.2). The organic enteric polymer phase was prepared by dissolving the enteric polymer Eudragit L100 (16.1 % w/w) and TRAM-34 (3.2 %w/w) in acetone. The phase separation of enteric polymer solutions and precipitation of TRAM-34 upon addition of the aqueous phase to the organic polymer/TRAM-34 phase was evaluated by the addition of 1% (w/w) HPMC aqueous solution to 10 g solutions containing 20% (w/w) enteric polymer in either acetone or ethanol (ICH Class 3 solvents) while stirring at 700 rpm (Barnstead International, Iowa, U.S.A). The phase separation and precipitation point was the volume at which the solution became turbid. Acetone was used after trials to optimize the enteric coating formulation process. The enteric microparticles were formed by gradual mixing of 15 g aqueous polymer solution (1% HPMC) into 10 g organic phase containing the enteric polymer and TRAM-34 while stirring at 700 rpm for 8 min. The order of mixing of the organic phase and aqueous polymer solution was investigated. The resulting viscous microparticles suspension was diluted with 50 mL water and stirred for 10 min. The microparticles were then collected by centrifugation (2500 rpm, 10 min), freeze-dried (14 h) and stored in desiccators until use.
2.3. High performance liquid chromatography
The HPLC analysis was performed using Agilent 1100 series chromatographic system (Agilent Technologies, Palo Alto, CA) equipped with a C18 Sunfire ™ column (4.6 mm × 150; 5 µm) (Waters Milford, MA) preceded by a SecurityGuard™ in-line filter frit (Phenomenex®, Torrance, CA). The mobile phase consisted of acetonitrile 60% (v/v), water 25% (v/v), and methanol 15% (v/v) at a flow rate of 1.5 mL/min. The injection volume was 10µl. Chromatographic analysis was performed at 220 nm. The enteric polymer carrier did not interfere at this wavelength and chromatographic conditions. Furthermore, data analysis, including peak recording and integration, was performed utilizing Agilent ChemStation software (version 4.2). The method was validated over the concentration range used and deemed suitable for TRAM-34 analysis.
2.4 In Vitro characterization of Microparticles
The morphology and size distribution of the enteric microparticles were observed using Philips Tecnai 12 Biotwin microscope with an accelerating voltage of 100 kV at the University of Kentucky Medical Center Imaging Facility. A dried microparticle sample was dispersed directly into distilled water, and then a copper grid coated with a carbon film was put into the previous suspension several times and left to incubate for 2.0 min at room temperature. After drying and removal of excess fluid, the samples were negatively stained with 2% uranyl acetate, and the grids were examined and recorded with the transmission electron microscope (TEM) images. The surface morphology of the TRAM-34 loaded microparticles was examined with a scanning electron microscopy (SEM) ((Hitachi SEM-4300) operated at 3 kV. The microparticles were spread and fixed on a holder with double-sided tape and coated with gold-palladium under an argon atmosphere (SCD 040, Balzers Union, Balzers, Lichtenstein).
The TRAM-34 loading in the microencapsules was determined in triplicate by dissolving 100 mg microparticles in 25 mL pH 6.8 phosphate buffer followed by addition of 75 mL of acetonitrile. The drug content of the resulting solution was determined by HPLC as described above. The encapsulation efficiency (EE) of TRAM-34 in the resulting enteric microcapsules was determined as the mass ratio of the actual and theoretical TRAM-34 loading, expressed on a percentage basis. The microparticle yield was the ratio between the amount of microparticles recovered and the weight of drug and polymer used for the preparation of the microparticles, expressed on a percentage basis.
2.5 In vitro TRAM-34 release
In vitro dissolution studies were carried out on the microspheres at 37 C (±0.5 C) at 100 rpm with USP Dissolution Apparatus II using the procedure for enteric-coated products at two successive different pH media (500 mL of 0.1 N HCl or pH 6.8 phosphate buffer; 50 rpm; 37 ± 0.5°C; n = 3) (VanKel 7000, Vankel Industries, NJ, USA). At a predetermined time intervals, 1 mL samples were withdrawn and replaced with fresh dissolution medium.
2.6 Pharmacokinetics of TRAM-34 microcapsules
Four male adult SIV negative rhesus macaques (Macaca mulatta) of comparable age and weight (12–17 kg) were housed in standard steel cages at an ambient temperature of 26 ± 3°C and 20 to 40% humidity, with free access to food and water, at the Yerkes National Primate Research Center (YNPRC) of Emory University. Their housing, care, diet and maintenance was in conformance to the guidelines of the Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council and the Health and Human Services by standard guidelines "Guide for the Care and Use of Laboratory Animals".
The monkeys were randomly divided into two groups to investigate the TRAM-loaded enteric microparticles (25mg/kg) and powdered TRAM-34 (50mg/kg). The animals were fasted but had free access to water overnight. Each monkey received the dose mixed into chocolate treat. The accurate dose was calculated per the ingested amount.
For intravenous (i.v.) administration after two weeks washout period, TRAM-34 was dissolved at 5 mg/mL in a mixture of 25% Cremophor®EL and 75% PBS at 5 mg/kg dose. After dose administration, monkeys were anesthetized with 10 mg/kg of ketamine hydrochloride that was administered intra-muscularly for multiple sampling every 1–2 hr as needed. Blood samples (0.5 mL) were collected into EDTA blood sample collection tubes pre-dose and at 0.5, 1, 2, 4, 6, 8, 12 and 24 hours post-dose. Plasma was then collected by centrifugation at 5000 rpm for 10 min. The obtained plasma samples were stored at −80 °C until analysis by LC-MS.
2.7 LC-MS Assay
Plasma samples were purified using C18 solid phase extraction (SPE) cartridges. Eluted fractions corresponding to TRAM-34 were dried under nitrogen and dissolved in acetonitrile. LC/MS analysis was performed with a Hewlett-Packard 1100 series HPLC stack equipped with a Merck KGaA RT 250-4 LiChrosorb RP-18 column interfaced to a Finnigan LCQ Classic MS. The mobile phase consisted of acetonitrile/water with 0.2% formic acid. The flow rate was 1.0 mL min−1 and the gradient was ramped from 20/80 to 70/30 in 5 min, then to 80/20 over 11 min. With the column temperature maintained at 30°C, TRAM-34 eluted at 14.4 min and was detected by a variable wavelength detector (VWD) set to 190 nm and the MS in series. Using electrospray ionization MS (capillary temperature 270°C, capillary voltage 1V, tube lens offset −15 V, positive ion mode) TRAM-34 was quantified by its base peak of 277 m/z (2-chlorotrityl fragment) and concentrations calculated with a 5-point calibration curve from 25 nM to 2.5 µM. Concentrations above 2.5 µM were quantified by their UV absorption at 190 nM. The related compound TRAM-46 (base peak of 261 m/z, 2-fluorotrityl fragment) was used as an internal standard.
2.8 Pharmacokinetic Analysis
The AUC (area under concentration time curve), Tmax (time at which maximum concentration occurs), Cmax (maximum concentration), Cls (systemic clearance) and Vd (volume of distribution) were calculated when possible for the data using WinNonlin version 5.0 (Pharsight Corp. Mountainview, CA). No statistics were performed since only two RM were used. The bioavailability of the oral microcapsules was calculated using the following formula:
Where AUCpo and Dosepo are the AUC and dose following oral administration of either the powder or the microcapsules, respectively and AUCiv and Doseiv are the AUC and dose for the intravenous dose, respectively.
3. Results and Discussion
TRAM-34 was successfully microencapsulated by a coacervation method. Coacervation was achieved through a separation of the polymeric solution into two immiscible phases; a dense coacervate phase, which is concentrated in polymer and a dilute equilibrium phase (Burgess et al., 2005). In this study, we utilized water miscible organic solvents such as acetone and ethanol, which are considered safe class 3 solvents according to the ICH guideline. We deemed such an approach suitable for safe scale up and for the later application to other compounds of a similar lipholicity. Eudragit L 100, an anionic copolymer based on methacrylic acid, was chosen as enteric polymer.
The amount of HPMC (1% w/w) aqueous solution needed to cause the phase separation when added to 10 g Eudragit L100 (20% w/w) organic solution differed depending on the organic solvent used to dissolve the enteric polymer. A total of 9.2 g of HPMC solution was needed to induce phase separation when ethanol was used, while less HPMC solution (6.1 g) was needed when acetone was the solvent. This can be explained based on the different enteric polymer solubility in the organic solvent. The polymer Eudragit L 100 dissolves better in ethanol than in acetone. Thus, larger amounts of HPMC aqueous solution were needed to induce phase separation when ethanol was the solvent.
Formulation enhancement utilizing different organic solvents revealed that, both Eudragit and TRAM-34 were soluble in acetone. On the other hand, TRAM-34 solubility in ethanol was found to be 4 mg/mL. Thus, we could not obtain a transparent film of polymer and drug solution. Hence, acetone was used as the organic solvent to ensure reproducibility in enteric microencapsulation process. Furthermore, the microparticles obtained with acetone as a solvent were larger in size and uniformly distributed with high reproducibility from batch to batch. We therefore chose acetone for further formulation development.
Appropriate quantity of water was added afterwards to the viscous suspension of TRAM-34 loaded microparticles and the resulting coacervate was freeze-dried and stored.
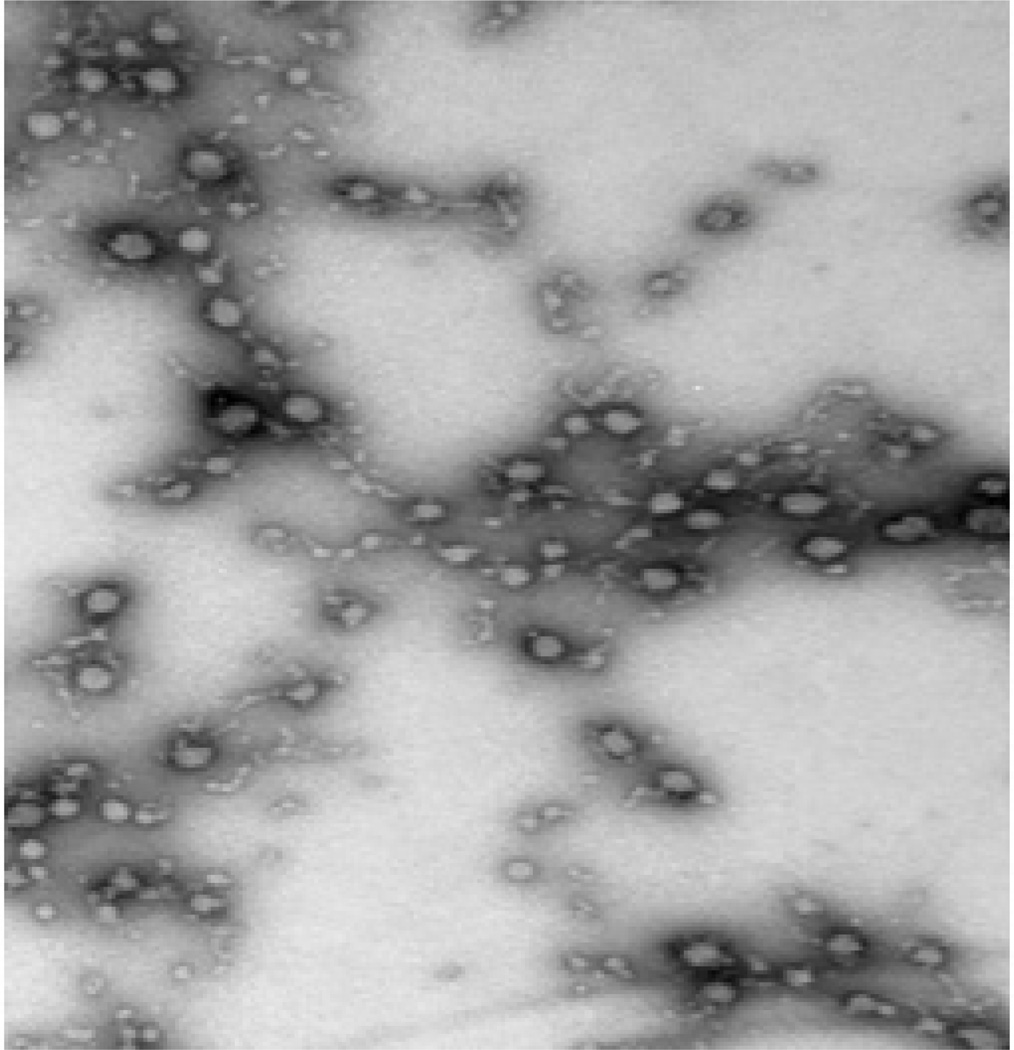
The order of mixing of the organic enteric and the aqueous polymer solution was very important. Adding the organic phase into the aqueous solution resulted in the formation of large polymer precipitates due to the fact that small amounts of the enteric polymer organic solution were in contact with a large excess of the aqueous polymer solution, resulting in a rapid organic solvent diffusion and polymer precipitation because of the complete miscibility of the acetone polymer solvent and the aqueous phase. Such issue could of course be overcome if water immiscible organic solvents were used. However, residual organic solvents will be a limiting factor in a scale up production due to safety concerns. On the other hand, adding the aqueous phase solution drop-wise to the organic phase, while stirring, resulted in the formation of smaller polymer precipitates that were uniform in size (Figure 2). At 15% theoretical TRAM-34 loading, the encapsulation efficiency was 90 ± 1.9% and the percentage yield was found to be 91.5 ± 0.3% (Table 1). Thus, there was no significant difference in encapsulation efficiency or in microparticles percentage yield at the drug loading levels used in this study. Furthermore, SEM imaging (Figure 3) revealed spherical TRAM-34 loaded microparticles with a smooth surface without visible drug crystals, which suggested that TRAM-34 was molecularly entrapped (dissolved) in the polymeric matrix. The average particle size of the microcapsules was 460 ± 120 µm. The particle size was found to increase with increasing drug loading (Table 1). This was most likely due to the formation of hydrogen bond between the p-electron rich pyrazole aromatic ring and carboxylic acid hydrogens of the enteric polymer. Also, the increased lipophilicity of the drug/polymer complex and high drug loadings might lead to a faster drug/polymer precipitation and larger particles. The presence of HPMC in the aqueous phase acted as a shock-preventing agent that reduces the undesirable cohesion and aggregation of the microparticles. The size of the enteric microparticles was also strongly affected by the organic solvent used. Interestingly, the use of acetone resulted in larger particles upon the addition of the aqueous polymer solution. This can be explained based on the moderate hydrogen bond capability and poor solvent property for the enteric polymer when compared to ethanol (Barton, 1983).
Figure 2.
Transmission electron microscopy (TEM) of enteric coated TRAM-34 loaded microparticles
Table 1.
Characterization of TRAM-34 loaded enteric coated microcapsules
| Theoretical TRAM- 34 loading (%) |
Microparticles Yield (%) |
Encapsulation Efficiency (%) |
Particles size (µm) |
|---|---|---|---|
| 5 | 90.0 ± 1.2 | 89.6 ± 1.4 | 310 ± 90 |
| 15 | 91.5 ± 0.3 | 90.0 ± 1.9 | 460 ± 120 |
| 20 | 90.0 ± 0.8 | 88.2 ± 2.1 | 522 ± 65 |
Figure 3.
Scanning electron micrographs (SEM) of an enteric coated TRAM-34 loaded microparticle
The pH of the gastrointestinal (GI) tract increases progressively from the stomach (pH 2–3), small intestine (pH 6.5–7) to the colon (7.0–8.0) (Ashford et al., 1994). Formulation of acid labile substances for oral use is possible by coating the dosage form with an enteric coating (Amorim et al., 2001). Enteric coating prevents deterioration of the acid labile drugs in the stomach and releases them in the more suitable alkaline region of the intestine. Enteric polymers are insoluble in water and acidic media but soluble beyond pH 5.5 to pH 7.0. They are also good carriers for lipophilic drugs (Dong et al., 2007). The enteric microencapsulation formulation-based approach was utilized in this research to protect TRAM-34 from gastric degradation upon oral ingestion in an effort to increase the compound exposure during animal testing.
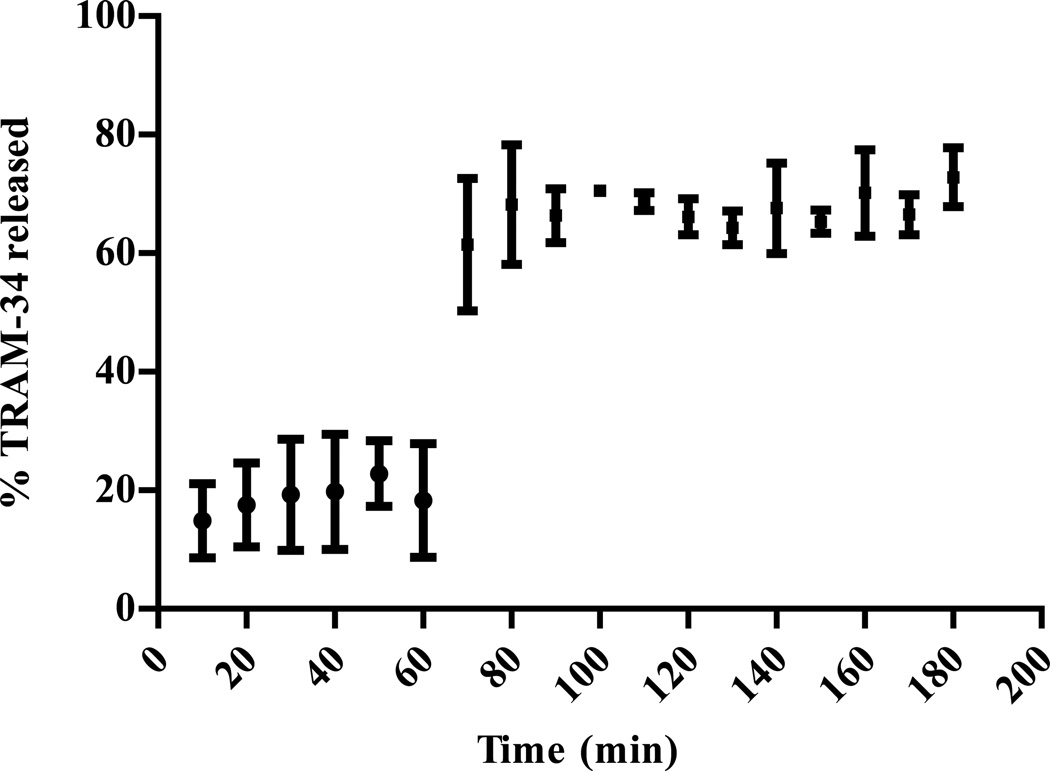
TRAM-34 enteric coated capsules where subjected to dissolution to measure the release of TRAM-34 in both acidic and basic media. Enteric coated particles showed an average release of small amounts around 15% of loaded TRAM-34 in acidic medium (0.1 N HCl) after one hour. Most of the TRAM-34 released during this time in acidic media was due mainly to the initial release and could be removed with an acid wash prior to the dissolution analysis. Since Eudragit L100 polymer is insoluble in this acidic medium, it is expected to have such low levels of TRAM-34 release from the enteric coated microparticles which acts as a protective coat around the compound. The transfer of the microcapsules to pH 6.8 resulted in a total release of around 70% after two hours (Figure 4).
Figure 4.
Effect of pH change (pH 1.2 to pH 6.8 after 60 min) on the TRAM-34 release from Eudragit L100 microcapsules. (Mean ± SD, n = 3)
TRAM-34 was administered to Rhesus Macaques intravenously (5 mg/kg), orally without enteric coating (50 mg/kg) and orally as enteric microcapsules with Eudragit L 100 (25mg/kg). The concentration time profile after TRAM-34 i.v., oral powder, and TRAM-34 enteric microparticles administration are shown in Figures 4 and 6, respectively. The pharmacokinetic parameters are shown in Table 2. The Tmax was delayed two hours for the oral enteric microparticles when compared to TRAM-34 given orally without enteric coating. Cmax was higher for TRAM-34 microcapsules as well (mean of 38 nM for enteric microparticles compared to 22.5 nM for TRAM-34 powder). The volume of distribution following i.v. administration was 11.1 L/kg. This high volume of distribution is consistent with the lipophilicity of TRAM-34. The systemic clearance was calculated to be 3.2 L/hr/kg. When calculating the bioavailability for TRAM-34 oral powder and TRAM-34 oral microcapsules, the average bioavailability values were 0.003 and 0.017, respectively. Although the bioavailability for the TRAM-34 enteric microparticles exceeded the TRAM-34 neat powder form 6 times, it was still relatively low (1.7%). The microcapsules enhanced the bioavailability due to bypassing the gastric acidity. However, there are other factors that need to be considered here. The low oral bioavailability might be due to the inherent absorption characteristics of TRAM-34. The compound’s high lipophilicity is probably the greatest obstacle to its absorption (Wils et al., 1994). It was reported that drugs with Log P greater than 3.5 exhibit decrease in the in vitro permeability through intestinal epithelial cell models (Wils et al., 1994). Furthermore, first-pass metabolism and efflux transport effects reducing bioavailability cannot be ruled out at this stage. Currently, formulations and structural modifications are investigated to improve the compound’s oral delivery.
Figure 6.
Concentration-time profile of TRAM-34 powder at 50mg/kg oral dose (♦) and TRAM-34 enteric microparticles at 25 mg/kg oral dose (■) to Rhesus Macaques primates.
Table 2.
Pharmacokinetic parameters average calculated for TRAM-34 in Rhesus Macaques after i.v. and oral administration of TRAM-34 powder and TRAM 34 enteric microcapsules
| Pharmacokinetic parameter |
Dose (mg/kg) |
AUC (0–24) (hr*nM) |
Cmax (nM) |
Tmax (hr) |
Cls (L/hr/kg) |
Vd (L/kg) |
Bioavailability (F) |
|---|---|---|---|---|---|---|---|
| Intravenous | 5 mg/kg | 3643 | - | - | 3.2 | 11.1 | 1 |
| Oral powder | 50 mg/kg | 102.5 | 22.5 | 2 | - | 6.5 | 0.003 |
| Oral enteric microparticles | 25 mg/kg | 311 | 38 | 4 | - | 6.6 | 0.017 |
Conclusion
TRAM-34 was successfully microencapsulated by a coacervation method utilizing water miscible organic solvents such as acetone and ethanol, which are considered safe class 3 solvents per the ICH guideline. This enteric microencapsulation method could also be used for other acid labile lipophilic compounds. The bioavailability of TRAM-34 was improved 6 times using enteric coated microparticles compared to TRAM-34 in powder form. However, the oral bioavailability of TRAM-34 from the enteric microcapsules was still poor (1.7%) and the compound plasma concentration is below the therapeutic effective dose. This could be attributed to the compound’s inherent absorption characteristics and high lipophilicity.
Figure 5.
Concentration-time profile of intravenous dosing of TRAM-34 to Rhesus Macaques at single dose of 5 mg/kg.
Acknowledgement
This work was supported by RO1-GM076063 to HW with a subcontract to AMA.
References
- Amorim MJ, Ferreira JP. Microparticles for delivering therapeutic peptides and proteins to the lumen of the small intestine. Eur. J. Pharm. Biopharm. 2001;52:39–44. doi: 10.1016/s0939-6411(01)00148-5. [DOI] [PubMed] [Google Scholar]
- Ashford M, Fell JT. Targeting drugs to the colon: delivery systems for oral administration. J. Drug. Target. 1994;2:241–257. doi: 10.3109/10611869408996806. [DOI] [PubMed] [Google Scholar]
- Barton AFM. CRC Handbook of Solubility Parameters and Other Cohesion Parameters. Florida, USA: CRC Press Inc.; 1983. [Google Scholar]
- Burgess DJ. Injectable dispersed systems: formulation, processing, and performance. Boca Raton, USA: Taylor & Francis; 2005. [Google Scholar]
- Caldwell GW, Ritchie DM, Masucci JA, Hageman W, Yan Z. The new pre- preclinical paradigm: compound optimization in early and late phase drug discovery. Curr. Top. Med. Chem. 2001;1:353–366. doi: 10.2174/1568026013394949. [DOI] [PubMed] [Google Scholar]
- Deasy PB. Drugs and the Pharmaceutical Sciences: Microencapsulation and Related Drug Processes. New York, USA: Marcel Dekker; 1984. [Google Scholar]
- Dong W, Bodmeier R. Encapsulation of lipophilic drugs within enteric microparticles by a novel coacervation method. Int. J. Pharm. 2006;326:128–138. doi: 10.1016/j.ijpharm.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Dong WY, Maincent P, Bodmeier R. In vitro and in vivo evaluation of carbamazepine-loaded enteric microparticles. Int. J. Pharm. 2007;331:84–92. doi: 10.1016/j.ijpharm.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Ghanshani S, Wulff H, Miller MJ, Rohm H, Neben A, Gutman GA, Cahalan MD, Chandy KG. Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J. Biol. Chem. 2000;275:37137–37149. doi: 10.1074/jbc.M003941200. [DOI] [PubMed] [Google Scholar]
- Jensen BS, Hertz M, Christophersen P, Madsen LS. The Ca2+-activated K+ channel of intermediate conductance: A possible target for immune suppression. Expert. Opin. Ther. Targets. 2002;6:623–636. doi: 10.1517/14728222.6.6.623. [DOI] [PubMed] [Google Scholar]
- Li P, Zhao L. Developing early formulations: Practice and perspective. Int. J. Pharm. 2007;341:1–19. doi: 10.1016/j.ijpharm.2007.05.049. [DOI] [PubMed] [Google Scholar]
- Wahlstrom JL, Chiang P, Ghosh S, Warren CJ, Wene SP, Albin LA, Smith ME, Roberds SL. Pharmacokinetic evaluation of a 1,3-dicyclohexylurea nanosuspension formulation to support early efficacy assessment. Nanoscal. Res. Lett. 2007;2:291–296. [Google Scholar]
- Wils P, Warnery A, Phung-Ba V, Legrain S, Scherman D. High lipophilicity decreases drug transport across intestinal epithelial cells. J. Pharmacol. Exp. Ther. 1994;269:654–658. [PubMed] [Google Scholar]
- Wulff H, Miller MJ, Hansel W, Grissmer S, Cahalan MD, Chandy KG. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc. Natl. Acad. Sci. 2000;97:8151–8156. doi: 10.1073/pnas.97.14.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff H, Calabresi PA, Allie R, Yun S, Pennington M, Beeton C, Chandy KG. The voltage-gated Kv1.3 K+ channel in effector memory T cells as new target for MS. J. Clin. Invest. 2003;111:1703–1713. doi: 10.1172/JCI16921. [DOI] [PMC free article] [PubMed] [Google Scholar]