Abstract
Background
Traditional T2 weighted MR imaging results are non-specific for the extent of underlying white matter structural abnormalities present in late life depression (LLD). Diffusion tensor imaging provides a unique opportunity to investigate the extent and nature of structural injury, but has been limited by examining only a subset of regions of interest (ROI) and by confounds common to the study of an elderly population, including comorbid vascular pathology. Furthermore, comprehensive correlation of diffusion tensor imaging (DTI) measurements, including axial and radial diffusivity measurements, has not been demonstrated in the late life depression population.
Methods
51 depressed and 16 non-depressed, age- and cerebrovascular risk factor- matched elderly subjects underwent traditional anatomic T1 and T2 weight imaging, as well as DTI. The DTI data were skeletonized using tract based spatial statistics (TBSS), and both regional and global analyses were performed.
Results
Widespread structural abnormalities within white matter were detected in the LLD group, accounting for age, gender and education and matched for cerebrovascular risk factors and global T2 white matter hyperintensities (T2WMH). Regional differences were most prominent in uncinate and cingulate white matter and were generally characterized by an increase in radial diffusivity. Age-related changes particularly in the cingulate bundle were more advanced in individuals with LLD relative to controls. Regression analysis demonstrated significant correlations of regional fractional anisotropy and radial diffusivity with five different neuropsychological factor scores. TBSS analysis demonstrated a greater extent of white matter abnormalities in LLD not responsive to treatment, as compared to controls.
Conclusions
White matter integrity is compromised in late life depression, largely manifested by increased radial diffusivity in specific regions, suggesting underlying myelin injury. A possible mechanism for underlying myelin injury is chronic white matter ischemia related to intrinsic cerebrovascular disease. In some regions such as the cingulate bundle, the white matter injury related to late-life depression appears to be independent of and compounded by age-related changes. The correlations with neuropsychological testing indicate the essential effects of white matter injury on functional status. Lastly, response to treatment may depend on the extent of white matter injury, suggesting a need for intact functional networks.
Keywords: Diffusion tensor imaging, Tract based spatial statistics, Anisotropy, Radial diffusivity
1. INTRODUCTION
Nonspecific deep and subcortical T2WMH (Greenwald et al., 1996; O'Brien et al., 2006; Steffens et al., 2002; Taylor et al., 2005) and DTI variables (Alexopoulos et al., 2002; Alexopoulos et al., 2008; Taylor et al., 2004; Yang et al., 2007; Yuan et al., 2007) are found coincident with LLD. Methods of evaluating white matter injury include visual inspection and automated quantification of T2WMH. Such focal abnormalities underestimate the extent of white matter injury which can be identified by diffusion tensor imaging (Shimony et al., 2009) and which may be a global indicator of widespread white matter disease. Furthermore, T2WMH are found commonly as a function of aging (Guttmann et al., 1998) in individuals without cognitive or mood dysfunction, though regional burden appears to distinguish late onset depression from normal age-matched controls (Sheline et al., 2008). DTI also evaluates structural properties within white matter based upon the directionally constrained diffusion of water. Several studies of LLD have demonstrated abnormal regional white matter by DTI, suggesting disruption of large scale brain networks contributing to mood regulation. Importantly, fiber bundles related to underlying cognitive and emotional function such as the cingulate bundle and uncinate white matter tracts have been implicated by both structural and functional studies in major depression (Greicius et al., 2007; Johansen-Berg et al., 2008; Sheline et al., 2008; Westlye et al., 2009).
Fractional anisotropy (FA) is a measure of directionally dependent restriction of water diffusion, and is well suited to the evaluation of white matter integrity in the brain; high anisotropy is generally characteristic of healthy white matter, reflecting the propensity of water diffusion parallel to the course of the axon fiber bundles. Radial diffusivity (RD) is a measure of diffusion transverse to the orientation of the axonal fiber bundle, while axial diffusivity (AD) is a measure of diffusion along the axis of the axonal fiber bundle. Mean diffusivity (MD) is the average of water diffusion in all directions. Fractional anisotropy is modified by relative changes in RD and AD. For example, an increase in RD or a decrease in AD both result in a lower FA and suggest underlying disruption of myelin insulation or axonal integrity, respectively. Importantly, low FA is also measured in zones of crossing fiber bundles, which are common in many areas of deep white matter. Evaluation of white matter tracts by DTI in the elderly is complicated by age-related atrophy and relatively non-specific T2WMH, which are associated with an increase in mean diffusivity. Traditional ROI and segmentation methods may be confounded by volume averaging, mistaken segmentation due to poor T2 signal contrast, or misalignment given the heterogeneous nature of these brains, particularly when attempting to analyze specific fiber bundles.
To characterize the extent of white matter structural injury in late onset depression independent of T2 white matter hyperintensities, we utilized the tract based spatial statistics (TBSS) module in FSL (Smith et al., 2007), to generate and evaluate skeletonized DTI data. Skeletonization isolates the center of common white matter tracts within the subject group, thereby minimizing effects of volume averaging and avoiding traditional automatic segmentation and selection bias, while allowing for both global and region of interest based analyses.
We posed several questions relating to the DTI data. First, we investigated uncinate and cingulate white matter DTI data, strongly suggested by prior studies to demonstrate structural abnormalities in late life depression (Drevets, 2001; Sheline et al., 2008). We then performed TBSS to study the global extent of white matter abnormalities related to LLD, and to confirm abnormalities seen by ROI based analysis. We investigated age –related changes in the cingulate and uncinate associated white matter in non-depressed controls compared to those with late life depression. We hypothesized that specific, regional white matter structural abnormalities would be detected in those with LLD. We hypothesized that the regional abnormalities would be more widespread than focal lesions described by prior methods, reflecting extensive white matter injury, which may contribute to cognitive dysfunction, altered mood regulation and may be related to response to treatment. In addition, given the large body of work addressing cognitive function in late life depression (Alexopoulos et al., 2005; Boone, 1995; Butters et al., 2004; Hart and Kwentus, 1987; Kramer-Ginsberg et al., 1999; Sheline et al., 2006; 2010), we hypothesized that structural abnormalities identified by TBSS would be correlated with abnormalities in neuropsychological function.
2. EXPERIMENTAL PROCEDURES
2.1 Subjects
Fifty one depressed subjects and 16 nondepressed control subjects ages 59 and older were matched for age, gender, education and total brain volume of T2WMH as determined previously (Sheline et al., 2008; Shimony et al., 2009). These subjects were a subset of the National Alliance for Research on Schizophrenia and Depression-sponsored project, “Decreased White-Matter Connectivity in Late Life Depression” and were recruited from the National Institute of Mental Health study “Treatment Outcome in Vascular Depression”. All subjects were evaluated by board-certified psychiatrists and assigned into the depressed category when meeting the DSM-IV criteria for major depression and assigned into the nondepressed control category when no current or past history of psychiatric illness was ascertained. The depressed subjects were further divided into subtypes as remitters or nonremitters, based upon a Montgomery-Åsberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979) of ≤ 7 following 12 weeks of antidepressant therapy (Sheline et al., 2010). Depressed and control subjects were matched by Framingham criteria on comorbid vascular risk factor scores (Wolf et al., 1991) and none of the subjects had additional severe medical disorders, (e.g., end stage cancer, severe cardiomypothy). All of the subjects had Clinical Dementia Rating (CDR) score of 0, indicating no evidence of either dementia or mild cognitive impairment. All subjects provided written informed consent in accordance with the Washington University Institutional Review Board and the Declaration of Helsinki (Walker, 1991).
2.2 Neuropsychological testing
Subjects were evaluated with a battery of neuropsychological tests involving several cognitive domains: Executive function, language, processing speed, working memory and episodic memory. The cognitive tasks were grouped and Z-scores were created for the measures of interest as described previously (Sheline et al., 2006). Cross group comparisons of these factor scores were performed with multiple regression/ANCOVA analyses, adjusting for age, gender and whole brain T2 WMH volume.
Executive function included verbal fluency, Trails B (reversed), the color word interference condition of the Stroop (number completed), the Initiation-Perseveration subscales of the Mattis, and the categories completed from the Wisconsin Card Sorting Test.
Processing speed included Symbol-digit modality (number completed), the color naming condition of the Stroop task (number completed), and the Trails A (reversed).
Episodic memory included word list learning (total correct), logical memory (total correct immediate), constructional praxis (memory performance), and the Benton Visual Retention Test (total correct).
Language processing included the Shipley Vocabulary Test (number correct), the Boston Naming Test (number correct) and the Word Reading condition of the Stroop (number completed).
Working memory included digit span forward (number of trials correctly completed), digit span backwards (number of trials correctly completed) and ascending digits (number of trials correctly completed).
2.3 Image Acquisition
All imaging was performed on a 1.5-T Siemens Sonata scanner (Erlangen, Germany). Structural scans included a T1-weighted (T1W) sagittal, magnetization-prepared rapid gradient echo (MPRAGE) (repetition time [TR] = 1900 msec; inversion time [TI] = 1100 msec, echo time [TE] = 3.93 msec, flip angle = 15°, 1 mm × 1 mm × 1.25 mm voxels) and a T2-weighted (T2W) fast spin echo (TR = 4380 msec, TE = 94 msec, 1 mm × 1 mm × 3 mm). The DTI was acquired with a locally modified echo planar imaging (EPI) sequence (TR = 7000 msec, TE = 113 msec, 2.0 mm isotropic voxels, 4.0 mm slice gap), conventional hexahedral (6 direction) encoding, and three levels of diffusion sensitization (b-values = 400, 800, and 1200 sec/mm2). Contiguous coverage was obtained in three spatially interleaved acquisitions. Four complete DTI datasets were acquired in each participant. Total imaging time was approximately 90 min.
2.4 Head Motion Correction of the DTI Data
As previously described (Shimony et al., 2009), each DTI dataset included 19 volumes (18 diffusion sensitized + 1 unsensitized) assembled by collating slices from three interleaved scans. No attempt was made to correct for head motion between interleaved odd/even slices. Each 19-volume dataset was motion-corrected by an iterative procedure that cycled through the following steps: 1) align each volume to the geometric mean volume of each group of images sharing the same degree of sensitization (6 × b = 400 sec/mm2, 6 × b = 800 sec/mm2, 6 × b = 1200 sec/mm2, 1 × b = 0 sec/mm2 (Io image)); 2) re-compute the geometric mean volume; 3) align each group geometric mean to the first acquired I0 image; and 4) algebraically, compose transforms (volume → group geometric mean with group → I0). Three cycles through the preceding steps yielded realignments with errors estimated by internal consistency to be < 0.1 mm. All transforms were 9 parameter affine (rigid body + scanner axis stretch) computed by VGM maximization (Rowland et al., 2005). The I0 volumes of each DTI dataset were aligned with conventional intensity correlation maximization (Snyder 1996). The final, motion-corrected result was obtained by algebraically composing all transforms (saved from the iterative procedure) and averaging all datasets after application of the composed transforms with cubic spline interpolation. The final resampling step output 19 volumes in spatial register with the I0 volume of the first acquired DTI dataset.
2.5 DTI Computations and Skeletonization
Skeletonization was carried out using the TBSS module (Smith et al., 2006) in FSL (Smith et al., 2004). First, the diffusion tensor and three eigenvalues were computed by fitting a tensor model to motion corrected diffusion data using FDT (Oxford). Anisotropy was expressed as fractional anisotropy (FA). Axial diffusivity (AD) was defined as the largest eigenvalue (L1); radial diffusivity (RD) was calculated as the mean of the two small eigenvalues (L2 and L3). The FA image was then brain-extracted using BET (Smith, 2002). All subjects' FA data were then aligned into a common space using the nonlinear registration tool FNIRT (Anderson et al., 2007), which uses a b-spline representation of the registration warp field (Rueckert et al., 1999). Next, the mean FA image was created and thinned to create a mean FA skeleton representing the centers of all tracts common to the group. Each subject's aligned FA data was then projected onto this skeleton. In a similar manner, the RD, AD and MD data were projected onto the skeleton utilizing the FA registration and skeleton projection parameters. Thus, the skeletonized FA, RD, AD and MD data were perfectly coregistered, thereby minimizing volume averaging effects.
2.6 Regional DTI Data Extraction
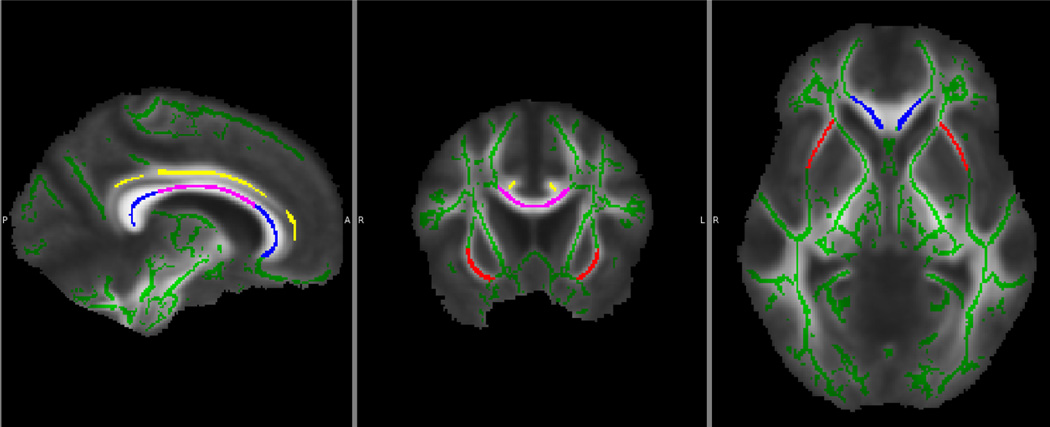
In order to test regional DTI characteristics and correlate regional DTI data with neuropsychological testing, we drew binary masks of the cingulate bundle, corpus callosum (divided into the genu, body and splenium) and uncinate fasciculus on a mean FA image of all subjects, derived from the atlas transformed subjects’ FA images during the TBSS processing described above. The regions of interest were chosen a priori on the basis of prior reports of an inverse correlation of mean diffusivity with processing speed in depressed individuals (Shimony et al., 2009) and the described role of FA in mediating cingulate bundle eletrophysiology (Westlye et al., 2009). In order to minimize the effects of volume averaging on our region of interest analysis, these masks were applied to the skeletonized subject data derived from the TBSS processing, representing the center of white matter bundles. Mean values of each subject’s skeletonized DTI data were extracted for each region, standardized as least square mean z-values, and analyzed for group differences in regional DTI measurements based on depression status, and for regional correlation with cognitive testing scores.
2.7 Whole Brain TBSS Analysis
The skeletonized FA, MD, AD and RD data were subsequently analyzed using voxel-wise cross-subject statistics (Randomise, a module of FSL), for comparison of the depressed and control groups, including evaluation of the remitting (n=22) and non-remitting (n=29) subtypes of LLD. Significant clusters were identified using threshold free cluster enhancement (Smith and Nichols, 2009) within the Randomise module, using 5000 random permutations to generate the null distribution and adjusting for age, gender and whole brain T2WMH volume in the general linear model using an FA threshold of 0.25. FSL was then used to back project the skeletonized clusters representing regions of significant difference between groups into the standard space for each subject in order to validate the origin of the DTI data as the center of common white matter tracts.
2.8 Statistical analysis of Group Differences and Correlations of Regional DTI data
All regional statistics were performed with SAS (Cary, North Carolina) version 9.2 or PASW Statistics 17 (SPSS Inc., Chicago, IL). Multiple linear regression/ANCOVA was used for group comparison of depressed and non-depressed subjects, adjusting for age, gender and education. Multiple linear regression analysis adjusted for age, gender and education was performed for evaluation of the regional DTI measurements and cognitive function scores.
3 RESULTS
3.1 Demographics and Neuropsychological Function
As shown in Table 1, depressed and control subjects did not differ in gender, age, education or global T2WMH volume. There were significant differences in the baseline MADRS score between the depressed and control subjects. Episodic memory and executive function were both significantly decreased in depressed elderly subjects compared to non-depressed elderly controls, adjusting for age, gender and T2WMH volume. There was a similar trend in the remaining cognitive domains assessing processing speed, language and working memory, which did not reach significance. Remitting and non-remitting subgroups did not significantly differ by age, gender, education, global T2WMH volume or baseline MADRS score.
| Depressed (n=51) | Control (n=16) | p = | |||
|---|---|---|---|---|---|
| Gender, n (%)a | 1.000 | ||||
| Male | 16 | (31.4 %) | 5 | (31.3 %) | |
| Female | 35 | (68.6 %) | 11 | (68.7 %) | |
| Race, n (%)a | 1.000 | ||||
| White | 45 | (88.2 %) | 15 | (93.8 %) | |
| Black | 5 | (9.8 %) | 1 | (6.2 %) | |
| Asian | 1 | (2.0 %) | 0 | (0 %) | |
| Age, mean (SD)b | 68.3 | (7.5) | 68.1 | (5.7) | 0.912 |
| Education, mean (SD) b | 13.6 | (3.2) | 14.9 | (2.6) | 0.1728 |
| T2 WMH volume (mm3) mean (SD) b | 28070 | (19453) | 29540 | (22700) | 0.8539 |
| Baseline MADRS Score, mean (SD) | 27.37 | (4.8) | 2.17 | (0.98) | <0.0001 |
| Remitters (n=22) | 27.41 | (5.2) | n/a | ||
| Non-remitters (n=29) | 27.34 | (4.6) | n/a | ||
| Neuropsychological Mean Factor Scoresc | |||||
| Episodic memory | −0.4404 | 1.404 | 0.0228 | ||
| Executive function | −0.4117 | 1.527 | 0.0286 | ||
| Processing speed | −0.1235 | 0.8093 | 0.0866 | ||
| Language | −0.1219 | 0.5648 | 0.2597 | ||
| Working memory | −0.2057 | 0.6556 | 0.2061 | ||
Fischer exact test.
Wilcoxon Mann-Whitney Exact Test.
Linear regression/ANCOVA controlling for age, gender and T2 WMH volume.
3.2 Regional Differences in Radial Diffusivity and Fractional Anisotropy
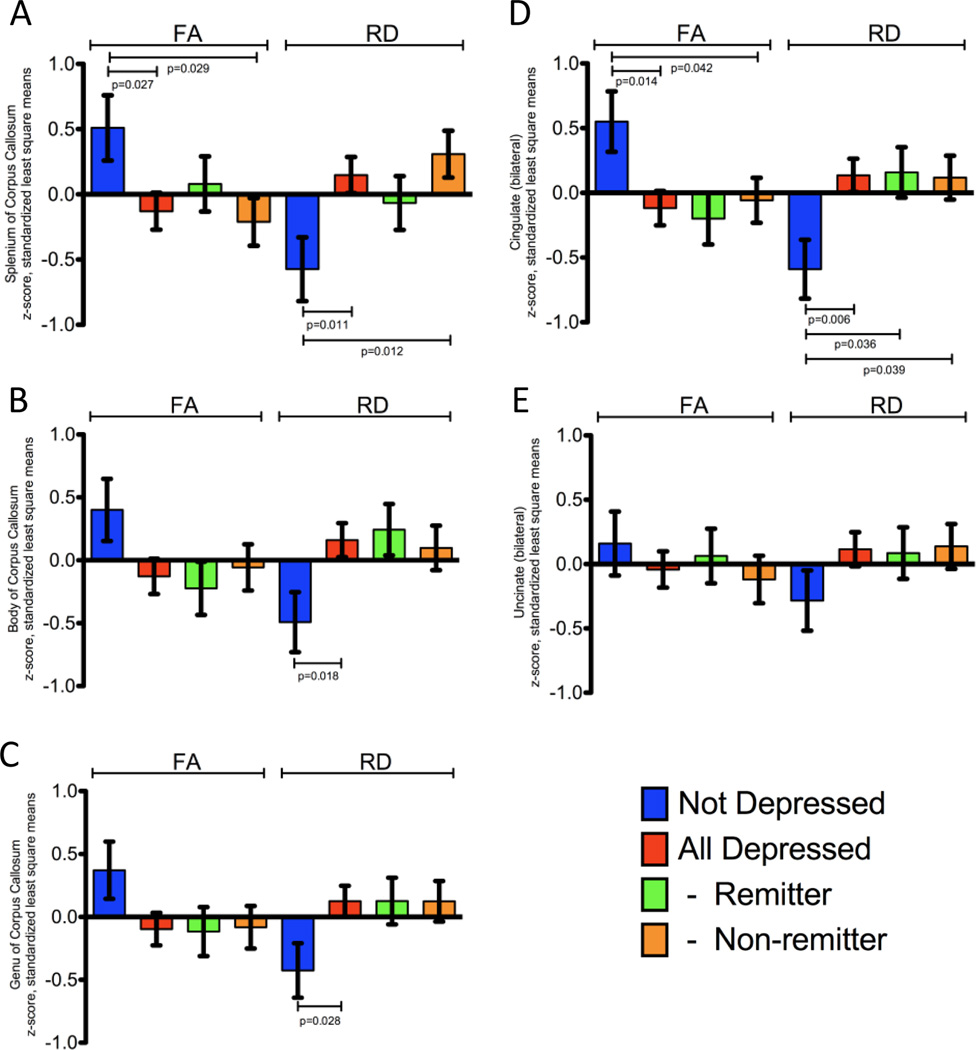
Regional DTI measurements corresponding to the corpus callosum, cingulate bundle and uncinate region (Figure 1) were extracted from the skeletonized data for each individual derived from tract based spatial statistics (TBSS), and standardized as least square mean z-score values. All analyses included adjustment for age, gender and education. In depressed elderly subjects, the splenium, body and genu of the corpus callosum demonstrated significantly increased radial diffusivity in the LLD group (Figure 2A–C); there was a corresponding decrease in FA only in the splenium of the corpus callosum (Figure 2A). The decrease in FA in the body and genu of the corpus callosum trended in a similar manner but did not reach statistical significance (Figure 2B and 2C).
Figure 1.
Figure 2.
Likewise, the cingulate bundles demonstrated a significantly lower FA in the LLD group (Figure 2D) when compared to non-depressed elderly controls. This change was largely driven by a significant increase in RD (Figure 2D), while there was no significant difference in AD (not shown).
The central portions of the uncinate regions’ white matter demonstrated no significant differences in FA, MD, RD or AD when considered together (Figure 2E); however, in the LLD group, there was a significant increase in radial diffusivity in the right uncinate region relative to the non-depressed control group, without a corresponding difference in FA (not shown).
In a three-way analysis of the regional DTI measurements, the non-remitting LLD group had both decreased FA and increased RD in the splenium of the corpus callosum compared to the control group (Figure 2A), while the remitting type had a similar trend, but did not reach significance. In the cingulate bundles, there were significant increases in measured RD between both the remitting LLD and non-remitting LLD groups and the control group (Figure 2D). FA was significantly decreased only in the non-remitting group compared to controls (Figure 2D). There were no significant differences between the control, remitting or non-remitting groups in the uncinate region or the body and genu of the corpus callosum (Figures 2B, 2C and 2E).
3.3 Group Differences in White Matter Microstructure by Tract Based Spatial Statistics
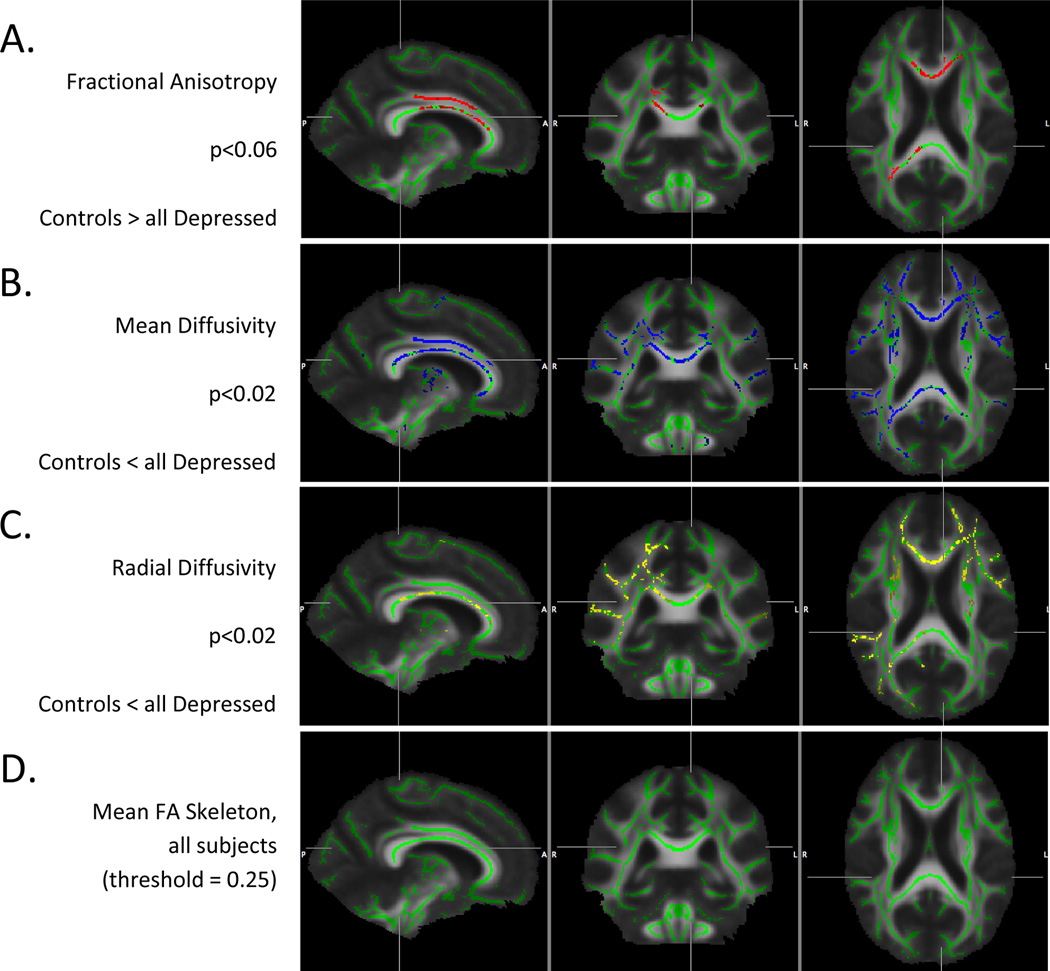
Voxel-wise comparison of depressed and non-depressed elderly subjects demonstrated significant clusters of abnormal white matter microstructure as measured by decreased FA (p < 0.06) and increased MD (p < 0.02) and RD (p < 0.02), following permutation based multiple comparison correction and adjustment for age, gender and education (Figure 3A–C). There were no significant differences of AD between groups. The mean FA image and mean FA skeleton derived from TBSS are displayed in Figure 3D.
Figure 3.
Significant clusters of difference in FA between depressed and non-depressed controls were largely confined to patchy regions within the corpus callosum and more confluent sections of the cingulate bundle. Importantly, there was a greater extent of significant clusters of differences in MD and RD, with much of the change in FA driven primarily by an increase in RD. In depressed elderly subjects, the core white matter RD was increased over many regions of the brain, including the cingulate bundles, segmental regions of the corpus callosum, right precuneus and uncinate regions, and bilateral subcortical parietal/temporal white matter.
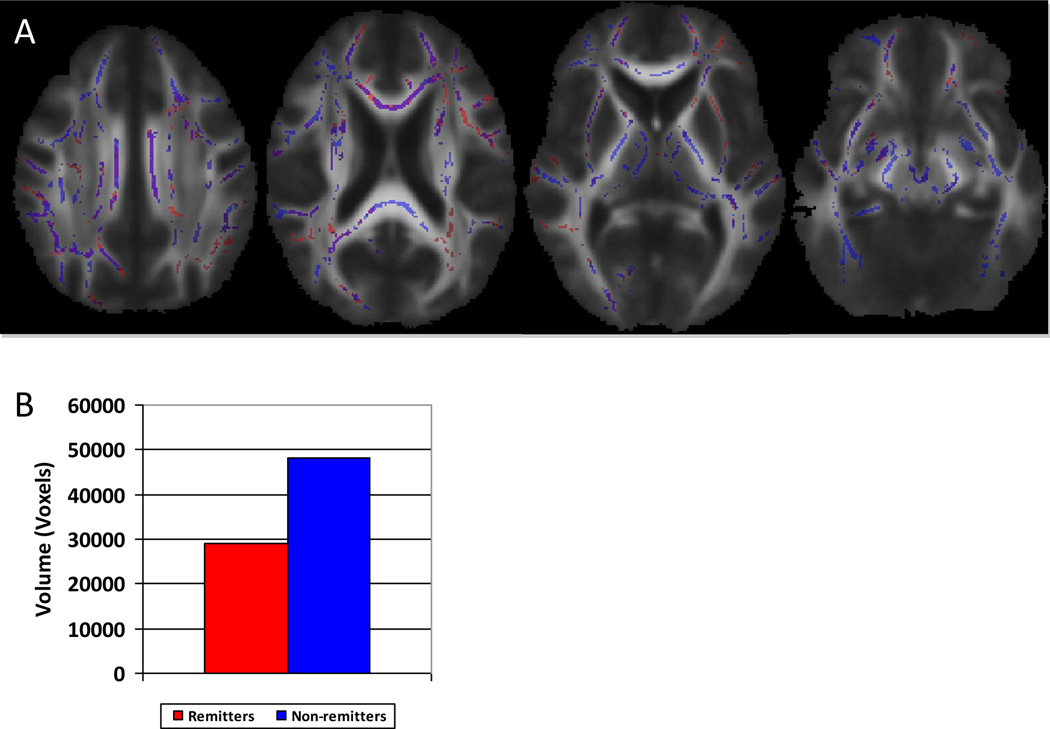
Three-way TBSS analysis comparing non-depressed, remitting type depressed and non-remitting type depressed groups confirmed differences between the non-depressed and depressed sub-types, but failed to show significant clusters of differences between the remitting and non-remitting sub-types (Figure 4A). However, when comparing the statistical p-maps generated from the comparison of the depressed subtypes with the control group, and using identical thresholding of p<0.05, there was a substantial difference in overall volume of affected skeletonized white matter (Figure 4B).
Figure 4.
Following back projection of these data, there was excellent alignment of the skeleton with more central tracts corresponding to the cingulate bundle, corpus callosum, uncinate and retrosplenial white matter (not shown). In contrast, small regions corresponding to the most peripheral, subcortical white matter did not consistently line up well with the native FA defined tracts. Given the high degree of variability in these small subcortical tracts, conclusions drawn from these very peripheral areas are doubtful; many of these questionable areas were excluded from the analysis by using a threshold FA value of 0.25 during the analysis.
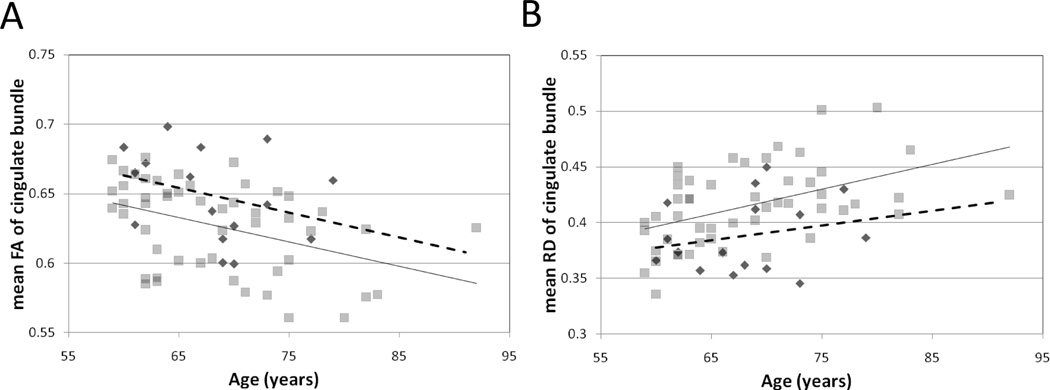
3.4 Age and Depression Status Interactively Affect DTI Measurements
Age related changes in white matter anisotropy and directional diffusivity were pronounced and particularly influential in this elderly cohort. Figure 5 demonstrates the relations of age to measured RD and FA within the cingulate bundles; a similar relationship was present in the uncinate region white matter (not shown). Depression status interacted with age-related changes in FA and RD. As FA declined with age, there was overall greater FA in the control group (Figure 5A). The difference in, and decline of FA, was largely driven by an increase in RD related to age and depression status (Figure 5B).
Figure 5.
3. 5 Correlations of Regional DTI with Neuropsychological Testing
Using the extracted regional DTI data from all subjects’ skeletonized output from TBSS, there were significant correlations of cingulate bundle and uncinate region white matter RD and FA with many of the neuropsychological test factor scores (Table 2). The FA and RD of the corpus callosum were less well correlated with cognitive testing.
| A. | Significance of positive correlation of Factor scores with Fractional Anisotropy | |||||
|---|---|---|---|---|---|---|
| Region | Episodic Memory |
Executive Function |
Processing Speed |
Language | Working Memory |
|
| Genu | 0.1762 | 0.2529 | 0.2883 | 0.050 | 0.4876 | |
| CC (body) | 0.0671 | 0.1037 | 0.2804 | 0.0835 | 0.2258 | |
| Splenium | 0.1941 | 0.1378 | 0.1317 | 0.0301 | 0.3337 | |
| Cingulate | ||||||
| Left | 0.0409 | 0.0360 | 0.0295 | 0.0101 | 0.1399 | |
| Right | 0.0283 | 0.0276 | 0.0220 | 0.0026 | 0.1021 | |
| Uncinate | ||||||
| Left | 0.0409 | 0.0019 | 0.1450 | 0.0066 | 0.0446 | |
| Right | 0.0011 | 0.0160 | 0.1064 | 0.0124 | 0.2560 | |
| B. | Significance of negative correlation of Factor Scores with Radial Diffusivity | |||||
|---|---|---|---|---|---|---|
| Region | Episodic Memory |
Executive Function |
Processing Speed |
Language | Working Memory |
|
| Genu | 0.3854 | 0.1209 | 0.2345 | 0.0561 | 0.2958 | |
| CC (body) | 0.0810 | 0.0245 | 0.1130 | 0.0775 | 0.0988 | |
| Splenium | 0.2203 | 0.0368 | 0.0423 | 0.0431 | 0.1981 | |
| Cingulate | ||||||
| Left | 0.1057 | 0.0046 | 0.0131 | 0.0162 | 0.0468 | |
| Right | 0.1211 | 0.0053 | 0.0080 | 0.0246 | 0.0531 | |
| Uncinate | ||||||
| Left | 0.0066 | 0.0004 | 0.0180 | 0.0065 | 0.0060 | |
| Right | 0.0273 | 0.0025 | 0.0158 | 0.0214 | 0.1024 | |
The highest correlations with regional FA, adjusting for age, gender and education, were the following: Left cingulate with language; right cingulate with episodic memory and language; left uncinate with executive function and language; right uncinate with episodic memory. The highest correlations with regional RD were: left cingulate with executive function; right cingulate with executive function and processing speed; left uncinate with executive function, language and working memory; right uncinate with executive function.
4 DISCUSSION
4.1 Conclusions
The key finding of this study was that individuals with LLD have widespread significant differences in white matter diffusion characteristics, compared to elderly controls, even when matched for global T2WMH burden and vascular risk factors and adjusting for age, gender and education. Further, these abnormalities were significantly correlated with performance on measures of neuropsychological function, and were more extensive in the non-remitting LLD subgroup. Although age- and vascular risk factor- related T2WMH are seen with greater frequency in certain brain regions in depressed individuals (Sheline et al., 2008), white matter diffusion abnormalities appear to be more extensive than predicted by traditional T2 imaging (Shimony et al., 2009) suggesting that T2WMH measures underestimate the extent of overall white matter abnormalities.
By utilizing TBSS, a measure of whole brain diffusion characteristics with automatic correction for multiple comparisons, we were able to examine white matter integrity in the entire brain in a more comprehensive way than with a region-of-interest approach. TBSS provides robust voxel-wise analysis, and thus we were able to define white matter abnormality independent of segmentation schemes or a priori selection. Furthermore, TBSS utilizes a strategy which narrows the comparison to the center of white matter tracts, thereby diminishing volume averaging effects which compromise traditional ROI based approaches, particularly in elderly individuals with varying degrees of brain atrophy. The diffusion abnormalities identified in this study support the presence of accelerated axonal injury or dysfunction within white matter critical for connectivity between cortical regions related to mood regulation, specifically within the uncinate fasciculus and cingulate bundles.
4.2 Discussion of greater abnormality in RD and MD than AD and implications
Mean diffusivity differences were extensive in portions of the corpus callosum, medial frontal, parietal and cingulate white matter. Differences in fractional anisotropy and mean diffusivity were largely driven by increases in RD, whereas there were no significant differences between the groups with regard to AD. In order to further characterize these white matter abnormalities, we compared MD, RD and AD between depressed and control elderly individuals in specific regions of the brain (Figure 2), since RD and AD both contribute to the determination of FA. RD is considered to be a measure of myelin integrity, attributable to restriction of free diffusion of water perpendicular to the axon (Song et al., 2002); in contradistinction, AD represents diffusion along the course of the axon, such as may be seen consequent to intrinsic axonal injury (Budde et al., 2009). In the current study, the pattern of differences in RD was more extensive than the differences seen with FA, while there were no significant differences in any of the regions tested with regard to AD. This pattern suggests an underlying white matter abnormality, which may be detected with greater sensitivity by evaluation of RD.
4.3 Discussion of regional differences in white matter integrity
The uncinate fasciculus and cingulate bundle are important mediators of anterior-posterior limbic connectivity (Schmahmann et al., 2007), while the corpus callosum is an important mediator of inter-hemispheric connectivity. A region of interest approach utilizing skeletonized diffusion data derived with TBSS demonstrated significant differences in FA and RD within the splenium of the corpus callosum (Figure 2A) and the cingulate bundles (Figure 2D) in depressed individuals. This difference was more pronounced in non-remitting depression, not responding to standardized treatment, suggesting the possibility that sustained anterior-posterior network connectivity and interhemispheric connectivity is required for pharmacological efficacy in the treatment of late life depression. While there was a trend towards a lower fractional anisotropy within the remaining corpus callosum of depressed elderly individuals, these differences did not reach significance. Finally, utilizing the ROI based approach, no significant differences were seen between control and depressed groups within the uncinate region. These relatively modest differences in fractional anisotropy were similar to prior studies (Kieseppa et al., 2010).
Fractional anisotropy within the cingulate bundle decreased with age and was lower over the LLD age spectrum measured in this study, as demonstrated in Figure 5. A similar relationship was found in the uncinate region white matter (not shown). However, the rate of change with age (slope) was not different between depressed and non-depressed individuals. This result suggests that FA depends on two independent factors, one being age, the other manifesting as depression. The available evidence suggests that this second factor is cerebrovascular disease, which leads both to depression and to impaired cognition (see below). Alternatively, an additional factor such as inflammation could mediate both of these effects.
4.4 Discussion of global differences in white matter integrity in LLD, including differences between remitting- and non-remitting subgroups of LLD
With TBSS, compromised white matter integrity in LLD was observed in the corpus callosum, particularly the body and genu, as well as white matter bundles within the medial frontal lobes, parietal lobes and cingulate gyri (Figure 3). The differences were more widespread when analyzing the RD data, suggesting a greater degree of sensitivity for differences than FA. The cingulate gyri and cingulate bundles are components of the limbic system, and likely interact with subgenual cortical structures, thalamus and precuneus. Importantly, abnormalities of diffusion within the cingulate gyrus are correlated with electrophysiologic abnormalities, confirming the ability of diffusion imaging to detect microstructural abnormalities associated with physiological defects (Westlye et al., 2009).
There were no significant differences in FA, MD, RD or AD between remitting and non-remitting sub-types of individuals with LLD. Both sub-types demonstrated clusters of significant differences when compared to the control group in a 3-way analysis. Importantly, despite a trend toward greater T2WMH volume in the non-remitting group, there was no significant difference between the remitting and non-remitting subgroups. However, the volume of affected skeletonized white matter when compared to normal controls was much greater in the non-remitting LLD group than in the remitting LLD group (Figure 4B), suggesting an overall trend toward greater white matter disease in individuals who are refractory to treatment, and supporting the findings from the region of interest approach. This difference in volume of affected white matter may explain, in part, the lack of clinical response to mood altering drugs which may require intact functional networks in order to be effective (Diaconescu et al., 2011) or may predict treatment response, as suggested by longitudinal functional studies of anteroposterior networks (Walsh et al., 2007).
4.5 Implications regarding the association of white matter disease with neuropsychological abnormalities
Late life depression is associated with global deficits in neuropsychological functioning, including slowing of cognitive processing speed (Sheline et al., 2010), and pervasive executive dysfunction (Herrmann et al., 2008). Impaired cognition may persist despite effective treatment of depression (Alexopoulos et al., 2008), although significant improvements in episodic memory and executive control have also been demonstrated following treatment (Barch et al., 2011).
Furthermore, abnormal diffusion in fronto-striatal-limbic white matter is correlated with poor performance on neuropsychological testing (Murphy et al., 2007). The widespread distribution of white matter abnormalities in frontal, parietal and cingulate areas may explain, in part, the association of depression and cognitive dysfunction in the elderly population. In particular, the cingulate bundle represents a major link within the connectivity core of the brain (Greicius et al., 2009; Hagmann et al., 2008). As well, the cingulate bundle constitutes a major connection of the subgenual cingulate bundle (SGACC). Deep brain stimulation of the SGACC has been used to treat refractory depression (Johansen-Berg et al., 2008). Hence, pathology within the cingulum bundle may be expected to affect multiple cognitive domains as well as impair mood regulation.
Observed correlations of regional FA and RD with neuropsychological function (factor scores) highlight the role of the cingulate bundle in mediating cognitive tasks impacted in depression, such as executive function and processing speed (Butters et al., 2004; Sheline et al., 2006; Murphy et al., 2007; Shimony et al., 2009). Measures of episodic and working memory were less well correlated with cingulate white matter microstructural integrity. Regional uncinate FA and RD, shown not to be significantly different between depressed and non-depressed groups, were correlated with a broad array of factor scores suggesting a relation to more global impairment not specific to depression. Lastly, better correlation of factor scores with FA and RD was observed in the left uncinate region, which may be related to functional lateralization of the cortex (Steffens et al., 2011) or may be related to lateralizing differences previously described in bipolar and unipolar depression (Versace et al., 2010).
The current study provides further evidence for the widespread nature of white matter changes. The radial diffusivity abnormalities suggest disruptions in myelination and, as would be expected from a diffuse disruption of white matter tracts, are associated with multiple domains of cognitive dysfunction and poor response to clinical intervention.
Highlights.
Diffuse white matter abnormalities are present in late life depression, largely manifested as increased radial diffusivity.
Age related change of fractional anisotropy and radial diffusivity in the cingulate bundle is independent of and compounded by late life depression.
Neuropsychological testing correlates with regional white matter abnormalities.
White matter abnormalities are more extensive within depressed elderly who are refractory to treatment.
Acknowledgements
This work was supported by a collaborative RO1 for Clinical Studies of Mental Disorders Grant Number MH60697 (YIS). Dr. Sheline also receives support from NIMH K24 65421. Additionally this work was supported by grant to the WUSM General Clinical Research Center RR00036, and grants to the Alzheimer’s Disease Research Center P50AG05681 and Healthy Aging and Senile Dementia Project P01AG03991.
This publication was made possible by Grant Numbers 1 UL1 RR024992-01, 1 TL1 RR024995-01 and 1 KL2 RR 024994-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
Abbreviations
- LLD
Late life depression
- ROI
Region of interest
- DTI
Diffusion tensor imaging
- T2WMH
T2 white matter hyperintensities
- FA
Fractional anisotropy
- MD
Mean diffusivity
- RD
Radial diffusivity
- AD
Axial diffusivity
- TBSS
Tract based spatial statistics
- CDR
Clinical Dementia Rating
- MADRS
Montgomery-Asberg Depression Rating Scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Joseph M Mettenburg, Email: mettenburgj@wustl.edu.
Tammie L.S. Benzinger, Email: benzingert@wustl.edu.
Joshua S Shimony, Email: shimonyj@wustl.edu.
Abraham Z. Snyder, Email: avi@wustl.edu.
Yvette I Sheline, Email: yvette@npg.wustl.edu.
References
- Alexopoulos GS, Kiosses DN, Choi SJ, Murphy CF, Lim KO. Frontal white matter microstructure and treatment response of late-life depression: a preliminary study. Am J Psychiatry. 2002;159:1929–1932. doi: 10.1176/appi.ajp.159.11.1929. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Kiosses DN, Heo M, Murphy CF, Shanmugham B, Gunning-Dixon F. Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58:204–210. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Latoussakis V, Kanellopoulos D, Klimstra S, Lim KO, Hoptman MJ. Microstructural white matter abnormalities and remission of geriatric depression. Am J Psychiatry. 2008;165:238–244. doi: 10.1176/appi.ajp.2007.07050744. [DOI] [PubMed] [Google Scholar]
- Anderson VM, Fernando KT, Davies GR, Rashid W, Frost C, Fox NC, Miller DH. Cerebral atrophy measurement in clinically isolated syndromes and relapsing remitting multiple sclerosis: a comparison of registration-based methods. J Neuroimaging. 2007;17:61–68. doi: 10.1111/j.1552-6569.2006.00081.x. [DOI] [PubMed] [Google Scholar]
- Barch D, D'Angelo G, Pieper C, Wilkins C, Welsh-Bohmer K, Taylor W, Garcia K, Gersing K, Doraiswamy PM, Sheline YI. Cognitive Improvement Following Treatment in Late Life Depression: Relationship to Vascular Risk and Age of Onset. 2011 doi: 10.1097/JGP.0b013e318246b6cb. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone DE. A cross-sectional analysis of WAIS-R aging patterns with psychiatric inpatients: support for Horn's hypothesis that fluid cognitive abilities decline. Percept Mot Skills. 1995;81:371–379. doi: 10.1177/003151259508100204. [DOI] [PubMed] [Google Scholar]
- Budde MD, Xie M, Cross AH, Song SK. Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. J Neurosci. 2009;29:2805–2813. doi: 10.1523/JNEUROSCI.4605-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH, Zmuda MD, Bhalla R, Meltzer CC, Pollock BG, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61:587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- Diaconescu AO, Kramer E, Hermann C, Ma Y, Dhawan V, Chaly T, Eidelberg D, McIntosh AR, Smith GS. Distinct functional networks associated with improvement of affective symptoms and cognitive function during citalopram treatment in geriatric depression. Hum Brain Mapp. 2011;32:1677–1691. doi: 10.1002/hbm.21135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Greenwald BS, Kramer-Ginsberg E, Krishnan RR, Ashtari M, Aupperle PM, Patel M. MRI signal hyperintensities in geriatric depression. Am J Psychiatry. 1996;153:1212–1215. doi: 10.1176/ajp.153.9.1212. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttmann CR, Jolesz FA, Kikinis R, Killiany RJ, Moss MB, Sandor T, Albert MS. White matter changes with normal aging. Neurology. 1998;50:972–978. doi: 10.1212/wnl.50.4.972. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart RP, Kwentus JA. Psychomotor slowing and subcortical-type dysfunction in depression. J Neurol Neurosurg Psychiatry. 1987;50:1263–1266. doi: 10.1136/jnnp.50.10.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann LL, Le Masurier M, Ebmeier KP. White matter hyperintensities in late life depression: a systematic review. J Neurol Neurosurg Psychiatry. 2008;79:619–624. doi: 10.1136/jnnp.2007.124651. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Gutman DA, Behrens TE, Matthews PM, Rushworth MF, Katz E, Lozano AM, Mayberg HS. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;18:1374–1383. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieseppa T, Eerola M, Mantyla R, Neuvonen T, Poutanen VP, Luoma K, Tuulio-Henriksson A, Jylha P, Mantere O, Melartin T, et al. Major depressive disorder and white matter abnormalities: a diffusion tensor imaging study with tract-based spatial statistics. J Affect Disord. 2010;120:240–244. doi: 10.1016/j.jad.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Kramer-Ginsberg E, Greenwald BS, Krishnan KR, Christiansen B, Hu J, Ashtari M, Patel M, Pollack S. Neuropsychological functioning and MRI signal hyperintensities in geriatric depression. Am J Psychiatry. 1999;156:438–444. doi: 10.1176/ajp.156.3.438. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Murphy CF, Gunning-Dixon FM, Hoptman MJ, Lim KO, Ardekani B, Shields JK, Hrabe J, Kanellopoulos D, Shanmugham BR, Alexopoulos GS. White-matter integrity predicts stroop performance in patients with geriatric depression. Biol Psychiatry. 2007;61:1007–1010. doi: 10.1016/j.biopsych.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JT, Firbank MJ, Krishnan MS, van Straaten EC, van der Flier WM, Petrovic K, Pantoni L, Simoni M, Erkinjuntti T, Wallin A, et al. White matter hyperintensities rather than lacunar infarcts are associated with depressive symptoms in older people: the LADIS study. Am J Geriatr Psychiatry. 2006;14:834–841. doi: 10.1097/01.JGP.0000214558.63358.94. [DOI] [PubMed] [Google Scholar]
- Rowland DJ, Garbow JR, Laforest R, Snyder AZ. Registration of [18F]FDG microPET and small-animal MRI. Nucl Med Biol. 2005;32:567–572. doi: 10.1016/j.nucmedbio.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18:712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D'Arceuil HE, de Crespigny AJ, Wedeen VJ. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Pieper CF, Barch DM, Welsh-Bohmer K, McKinstry RC, MacFall JR, D'Angelo G, Garcia KS, Gersing K, Wilkins C, et al. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010;67:277–285. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Vaishnavi SN, Mintun MA, Barch DM, Epstein AA, Wilkins CH, Snyder AZ, Couture L, Schechtman K, et al. Regional white matter hyperintensity burden in automated segmentation distinguishes late-life depressed subjects from comparison subjects matched for vascular risk factors. Am J Psychiatry. 2008;165:524–532. doi: 10.1176/appi.ajp.2007.07010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Garcia K, Gersing K, Pieper C, Welsh-Bohmer K, Steffens DC, Doraiswamy PM. Cognitive function in late life depression: relationships to depression severity, cerebrovascular risk factors and processing speed. Biol Psychiatry. 2006;60:58–65. doi: 10.1016/j.biopsych.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Shimony JS, Sheline YI, D'Angelo G, Epstein AA, Benzinger TL, Mintun MA, McKinstry RC, Snyder AZ. Diffuse microstructural abnormalities of normal-appearing white matter in late life depression: a diffusion tensor imaging study. Biol Psychiatry. 2009;66:245–252. doi: 10.1016/j.biopsych.2009.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols TE, Miller KL, Robson MD, Jones DK, Klein JC, Bartsch AJ, et al. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat Protoc. 2007;2:499–503. doi: 10.1038/nprot.2007.45. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Bosworth HB, Provenzale JM, MacFall JR. Subcortical white matter lesions and functional impairment in geriatric depression. Depress Anxiety. 2002;15:23–28. doi: 10.1002/da.1081. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Taylor WD, Denny KL, Bergman SR, Wang L. Structural integrity of the uncinate fasciculus and resting state functional connectivity of the ventral prefrontal cortex in late life depression. PLoS One. 2011;6:e22697. doi: 10.1371/journal.pone.0022697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, MacFall JR, Payne ME, McQuoid DR, Provenzale JM, Steffens DC, Krishnan KR. Late-life depression and microstructural abnormalities in dorsolateral prefrontal cortex white matter. Am J Psychiatry. 2004;161:1293–1296. doi: 10.1176/appi.ajp.161.7.1293. [DOI] [PubMed] [Google Scholar]
- Taylor WD, MacFall JR, Payne ME, McQuoid DR, Steffens DC, Provenzale JM, Krishnan RR. Greater MRI lesion volumes in elderly depressed subjects than in control subjects. Psychiatry Res. 2005;139:1–7. doi: 10.1016/j.pscychresns.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Versace A, Almeida JR, Quevedo K, Thompson WK, Terwilliger RA, Hassel S, Kupfer DJ, Phillips ML. Right orbitofrontal corticolimbic and left corticocortical white matter connectivity differentiate bipolar and unipolar depression. Biol Psychiatry. 2010;68:560–567. doi: 10.1016/j.biopsych.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A. Health and the environment. Population: more than a numbers game. BMJ. 1991;303:1194–1197. doi: 10.1136/bmj.303.6811.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh ND, Williams SC, Brammer MJ, Bullmore ET, Kim J, Suckling J, Mitterschiffthaler MT, Cleare AJ, Pich EM, Mehta MA, et al. A longitudinal functional magnetic resonance imaging study of verbal working memory in depression after antidepressant therapy. Biol Psychiatry. 2007;62:1236–1243. doi: 10.1016/j.biopsych.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Bjornerud A, Due-Tonnessen P, Fjell AM. Error-related negativity is mediated by fractional anisotropy in the posterior cingulate gyrus--a study combining diffusion tensor imaging and electrophysiology in healthy adults. Cereb Cortex. 2009;19:293–304. doi: 10.1093/cercor/bhn084. [DOI] [PubMed] [Google Scholar]
- Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- Yang Q, Huang X, Hong N, Yu X. White matter microstructural abnormalities in latelife depression. Int Psychogeriatr. 2007;19:757–766. doi: 10.1017/S1041610207004875. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Zhang Z, Bai F, Yu H, Shi Y, Qian Y, Zang Y, Zhu C, Liu W, You J. White matter integrity of the whole brain is disrupted in first-episode remitted geriatric depression. Neuroreport. 2007;18:1845–1849. doi: 10.1097/WNR.0b013e3282f1939f. [DOI] [PubMed] [Google Scholar]