Abstract
Natural abundance 15N NMR spectroscopy and ancillary spectroscopic techniques have been employed to study the solution structure of 8-hydroxyadenosine. 8-Hydroxyadenosine is a naturally occurring oxidized nucleic acid adduct that is generally implied to have an 8-hydroxy tautomeric structure. 15N NMR chemical shifts and coupling constants, however, indicate that the modified base exists as an 8-keto tautomer. The pH dependence of 15N NMR and UV spectra showed the presence of two pKa's, at 2.9 and 8.7, corresponding to protonation at N1 and ionization at N7, respectively. The latter results in the formation of an 8-enolate structure. Unusual upfield shifts of the 1H and 15N resonances of the NH2 group, and a reduction in the one-bond coupling constant 1JN6-H6, is indicative of an unfavorable steric or electronic interaction between the NH2 group and the adjacent N7-H proton. This interaction results in a subtle change in the structure of the NH2 group. In addition to being a possible mechanism for alteration of hydrogen bonding in oxidized DNA, this type of interaction gives a better understanding into N7-N9 tautomerism of adenine. Furthermore, the structure of 8-hydroxyadenosine has been related to possible mechanisms for mutations.
Full text
PDF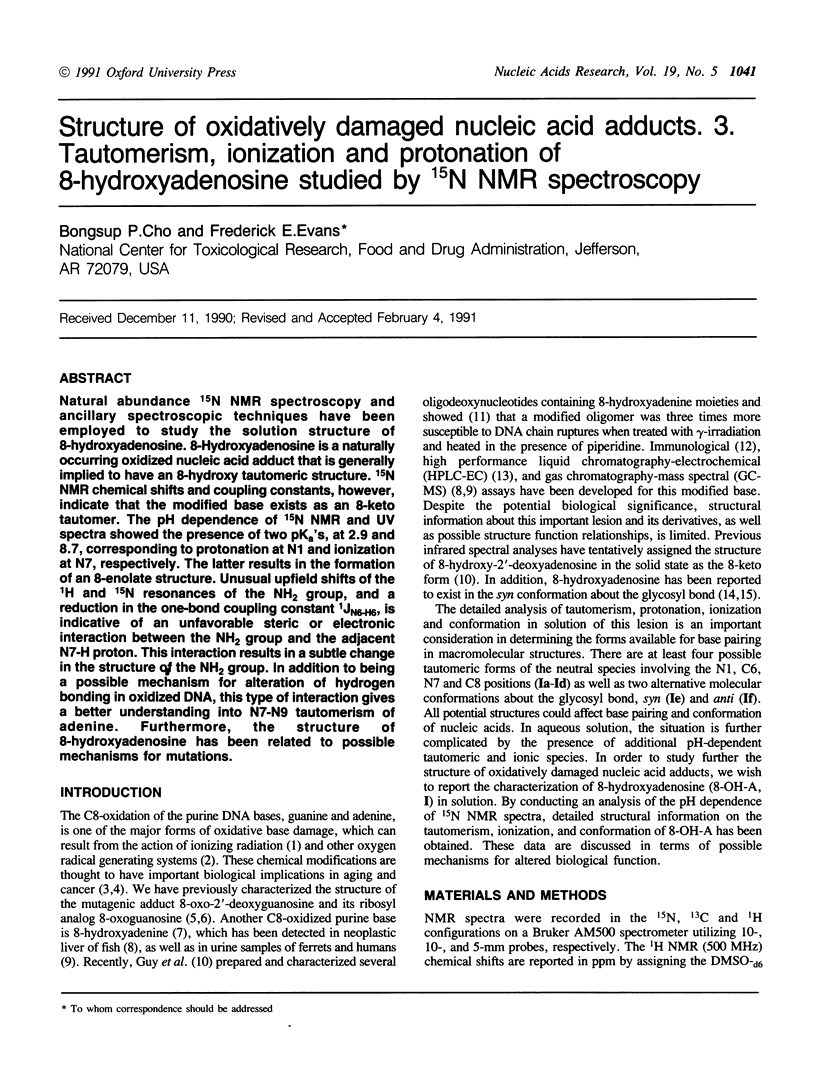



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonicel A., Mariaggi N., Hughes E., Teoule R. In vitro gamma irradiation of DNA: identification of radioinduced chemical modifications of the adenine moiety. Radiat Res. 1980 Jul;83(1):19–26. [PubMed] [Google Scholar]
- Chenon M. T., Pugmire R. J., Grant D. M., Panzica R. P., Townsend L. B. Carbon-13 magnetic resonance. XXVI. A quantitative determination of the tautomeric populations of certain purines. J Am Chem Soc. 1975 Aug 6;97(16):4636–4642. doi: 10.1021/ja00849a028. [DOI] [PubMed] [Google Scholar]
- Cho B. P., Kadlubar F. F., Culp S. J., Evans F. E. 15N nuclear magnetic resonance studies on the tautomerism of 8-hydroxy-2'-deoxyguanosine, 8-hydroxyguanosine, and other C8-substituted guanine nucleosides. Chem Res Toxicol. 1990 Sep-Oct;3(5):445–452. doi: 10.1021/tx00017a010. [DOI] [PubMed] [Google Scholar]
- Cross C. E., Halliwell B., Borish E. T., Pryor W. A., Ames B. N., Saul R. L., McCord J. M., Harman D. Oxygen radicals and human disease. Ann Intern Med. 1987 Oct;107(4):526–545. doi: 10.7326/0003-4819-107-4-526. [DOI] [PubMed] [Google Scholar]
- Culp S. J., Cho B. P., Kadlubar F. F., Evans F. E. Structural and conformational analyses of 8-hydroxy-2'-deoxyguanosine. Chem Res Toxicol. 1989 Nov-Dec;2(6):416–422. doi: 10.1021/tx00012a010. [DOI] [PubMed] [Google Scholar]
- Evans F. E., Levine R. A. Conformation and configuration at the central amine nitrogen of a nucleotide adduct of the carcinogen 2-(acetylamino)fluorene as studied by 13C and 15N NMR spectroscopy. J Biomol Struct Dyn. 1986 Apr;3(5):923–934. doi: 10.1080/07391102.1986.10508474. [DOI] [PubMed] [Google Scholar]
- FOX J. J., SHUGAR D. Spectrophotometric studies of nucleic acid derivatives and related compounds as a function of pH. II. Natural and synthetic pyrimidine nucleosides. Biochim Biophys Acta. 1952 Oct;9(4):369–384. doi: 10.1016/0006-3002(52)90181-9. [DOI] [PubMed] [Google Scholar]
- Floyd R. A. The role of 8-hydroxyguanine in carcinogenesis. Carcinogenesis. 1990 Sep;11(9):1447–1450. doi: 10.1093/carcin/11.9.1447. [DOI] [PubMed] [Google Scholar]
- Giessner-Prettre C., Pullman B. On the conformational dependence of the proton chemical shifts in nucleosides and nucleotides. II. Proton shifts in the ribose ring of purine nucleosides as a function of the torsion angle about the glycosyl bond. J Theor Biol. 1977 Mar 7;65(1):189–201. doi: 10.1016/0022-5193(77)90083-2. [DOI] [PubMed] [Google Scholar]
- Ikehara M., Tada H., Kaneko M. Studies of nucleosides and nucleotides. XXXV. Purine cyclonucleosides. 5. Synthesis of purine cyclonucleoside having 8,2'-O-anhydro linkage and its cleavage reactions. Tetrahedron. 1968 Apr;24(8):3489–3498. doi: 10.1016/s0040-4020(01)92646-8. [DOI] [PubMed] [Google Scholar]
- Lappi D. A., Evans F. E., Kaplan N. O. Reduced nicotinamide 8-(alkylamino)adenine dinucleotides: enzyme-coenzyme interactions with different adenyl glycosyl bond conformations. Biochemistry. 1980 Aug 5;19(16):3841–3845. doi: 10.1021/bi00557a030. [DOI] [PubMed] [Google Scholar]
- Lin T. S., Cheng J. C., Ishiguro K., Sartorelli A. C. 8-Substituted guanosine and 2'-deoxyguanosine derivatives as potential inducers of the differentiation of Friend erythroleukemia cells. J Med Chem. 1985 Sep;28(9):1194–1198. doi: 10.1021/jm00147a012. [DOI] [PubMed] [Google Scholar]
- Malins D. C., Haimanot R. 4,6-Diamino-5-formamidopyrimidine, 8-hydroxyguanine and 8-hydroxyadenine in DNA from neoplastic liver of English sole exposed to carcinogens. Biochem Biophys Res Commun. 1990 Dec 14;173(2):614–619. doi: 10.1016/s0006-291x(05)80079-8. [DOI] [PubMed] [Google Scholar]
- Markowski V., Sullivan G. R., Roberts J. D. Nitrogen-15 nuclear magnetic resonance spectroscopy of some nucleosides and nucleotides. J Am Chem Soc. 1977 Feb 2;99(3):714–718. doi: 10.1021/ja00445a009. [DOI] [PubMed] [Google Scholar]
- Neidle S., Kuroda R., Broyde S., Hingerty B. E., Levine R. A., Miller D. W., Evans F. E. Studies on the conformation and dynamics of the C8-substituted guanine adduct of the carcinogen acetylaminofluorene; model for a possible Z-DNA modified structure. Nucleic Acids Res. 1984 Nov 12;12(21):8219–8233. doi: 10.1093/nar/12.21.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizkalla B. H., Robins R. K., Broom A. D. Purine nucleosides. XXVII. The synthesis of 1- and 7-methyl-8-oxoguanosine and related nucleosides. The use of the N-amino group as a selective blocking agent in nucleoside synthesis. Biochim Biophys Acta. 1969 Dec 16;195(2):285–293. [PubMed] [Google Scholar]
- Simic M. G., Bergtold D. S., Karam L. R. Generation of oxy radicals in biosystems. Mutat Res. 1989 Sep;214(1):3–12. doi: 10.1016/0027-5107(89)90192-9. [DOI] [PubMed] [Google Scholar]
- Stillwell W. G., Xu H. X., Adkins J. A., Wishnok J. S., Tannenbaum S. R. Analysis of methylated and oxidized purines in urine by capillary gas chromatography-mass spectrometry. Chem Res Toxicol. 1989 Mar-Apr;2(2):94–99. doi: 10.1021/tx00008a004. [DOI] [PubMed] [Google Scholar]
- Téoule R., Duplaa A. M. Free radical and radiation-induced DNA damage to oligonucleotides. Free Radic Res Commun. 1989;6(2-3):121–122. doi: 10.3109/10715768909073446. [DOI] [PubMed] [Google Scholar]
- Uesugi S., Ikehara M. Carbon-13 magnetic resonance spectra of 8-substituted purine nucleosides. Characteristic shifts for the syn conformation. J Am Chem Soc. 1977 May 11;99(10):3250–3253. doi: 10.1021/ja00452a008. [DOI] [PubMed] [Google Scholar]
- Ward J. F. DNA damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation, and reparability. Prog Nucleic Acid Res Mol Biol. 1988;35:95–125. doi: 10.1016/s0079-6603(08)60611-x. [DOI] [PubMed] [Google Scholar]
- West G. J., West I. W., Ward J. F. Radioimmunoassay of 7,8-dihydro-8-oxoadenine (8-hydroxyadenine). Int J Radiat Biol Relat Stud Phys Chem Med. 1982 Nov;42(5):481–490. doi: 10.1080/09553008214551421. [DOI] [PubMed] [Google Scholar]



