Abstract
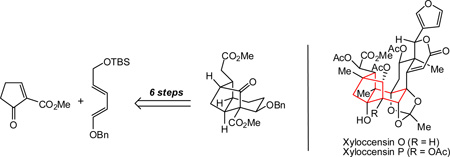
An efficient synthesis of the octahydro-1H-2,4-methanoindene core of phragmalin-type limonoids, such as xyloccensins O and P, is reported. The success of the synthetic route is predicated on the use of network analysis in the retrosynthetic analysis and a Diels-Alder reaction for the synthesis of a key hydrindanone derivative.
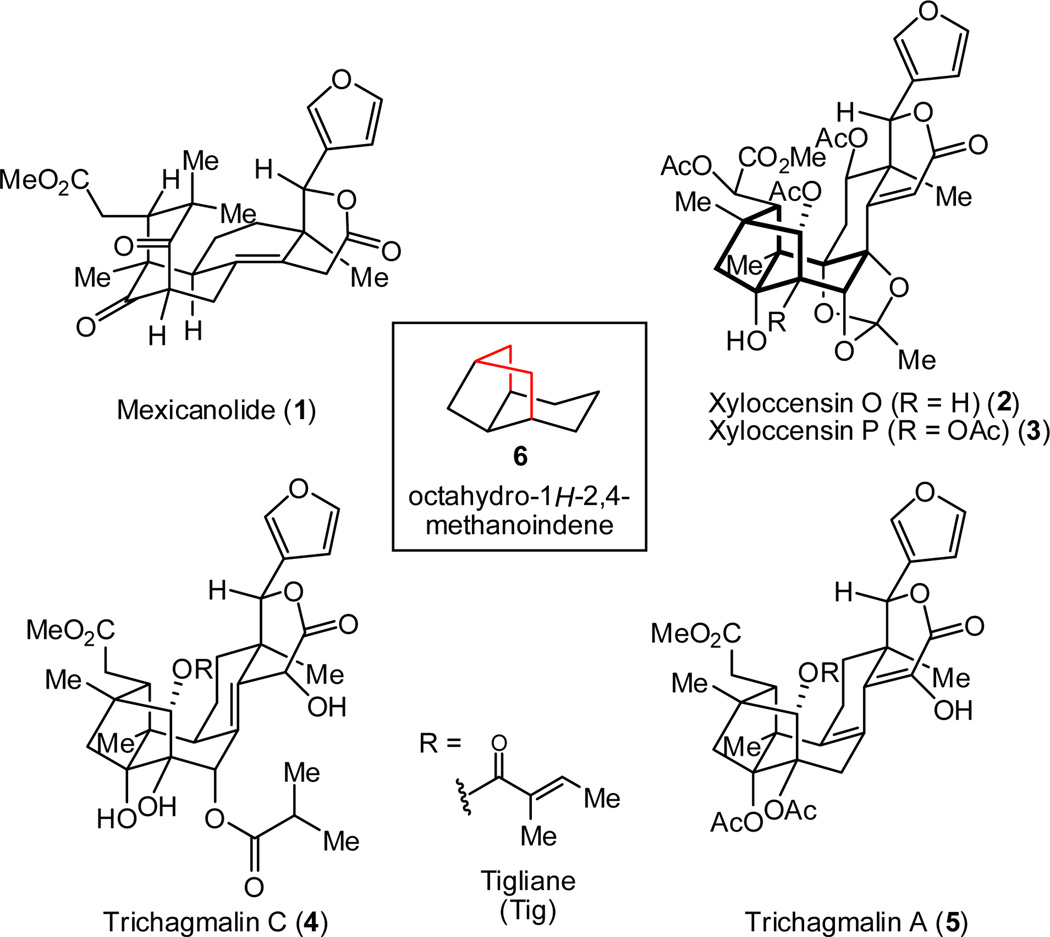
The phragmalin-type limonoids (e.g., xyloccensin O and P, 2 and 3, and trichagmalin A and C, 4 and 5, Figure 1) 1 are highly oxygenated triterpenoid derivatives of daunting molecular complexity that, to date, have not succumbed to total synthesis. The intricate polycyclic and highly oxygenated nature of these compounds constitutes a significant challenge for modern chemical synthesis.2 Central among the challenges associated with the chemical synthesis of this subset of limonoids is identifying effective approaches to the unusual octahydro-1H-2,4-methanoindene framework (highlighted in Figure 1). We reasoned that a synthetic endeavor aimed at this unique carbon skeleton would lead to the discovery of novel strategies and tactics applicable to the synthesis of a variety of the phragmalin-type limonoids. An efficient synthetic route could enable further investigation into the already diverse bioactivities of the limonoid family (e.g., anticancer,3 anti-HIV,4 antibiotic, anti-inflammatory5 and antifeedent6 properties) and potentially, improvement of their pharmacological profiles by derivitization.
Figure 1.
Selected limonoid natural products
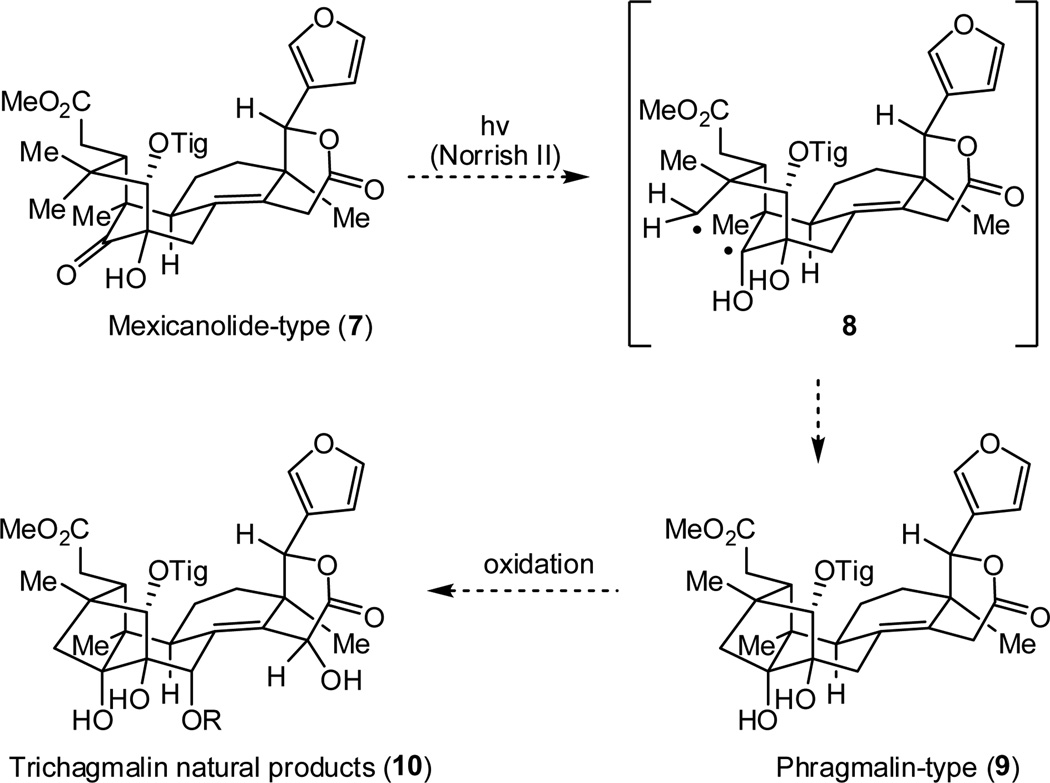
Biosynthetically, the phragmalin-type limonoids likely arise from the related mexicanolide-type natural products (e.g., 7, Scheme 1). A possible biogenetic connection between the mexicanolide-type (7) and corresponding phragmalin derivatives (e.g., 9), proposed by Hao and coworkers,7 is outlined in Scheme 1. This process may begin with a photo-initiated Norrish type II reaction to afford the strained octahydro-1H-2,4-methanoindene framework. Given that the Norrish type II product (9) likely represents the photostationary state, highly strained systems of this type may be accessed.
Scheme 1.
Proposed biogenetic connection between mexicanolide-type and phragmalin-type limonoids
Biomimetic syntheses can often bring an unprecedented level of simplication to a challenging synthetic target;8 however, the unique environs of an enzyme, should one be required, is not easily emulated by synthetic chemists. For the phragmalin-type limonoids in particular, the strict conformational requirements regarding the proximity of reacting partners in photo-reactions, including the Norrish type II process, makes it unclear how high yielding conversion of 7 to 9 would be outside of a biological context. As such, we decided to pursue alternative, more robust, strategies for the formation of the caged phragmalin framework.
Foremost among our considerations as to how to access the architecturally intricate phragmalin-type tricyclic skeleton was to apply the method of network analysis.9 To forge the caged methanoindene polycycle (see 6 in Figure 1), a disconnection of any of the bonds shown in red would realize the main goal of network analysis—namely, the simplification of the complex caged structure to a fused ring system.8b Despite the powerful reduction in complexity in the retrosynthetic sense that this approach offers, we were cognizant of the significant challenge that the forward C-C bond construction posed due to the accompanying increase in strain.10
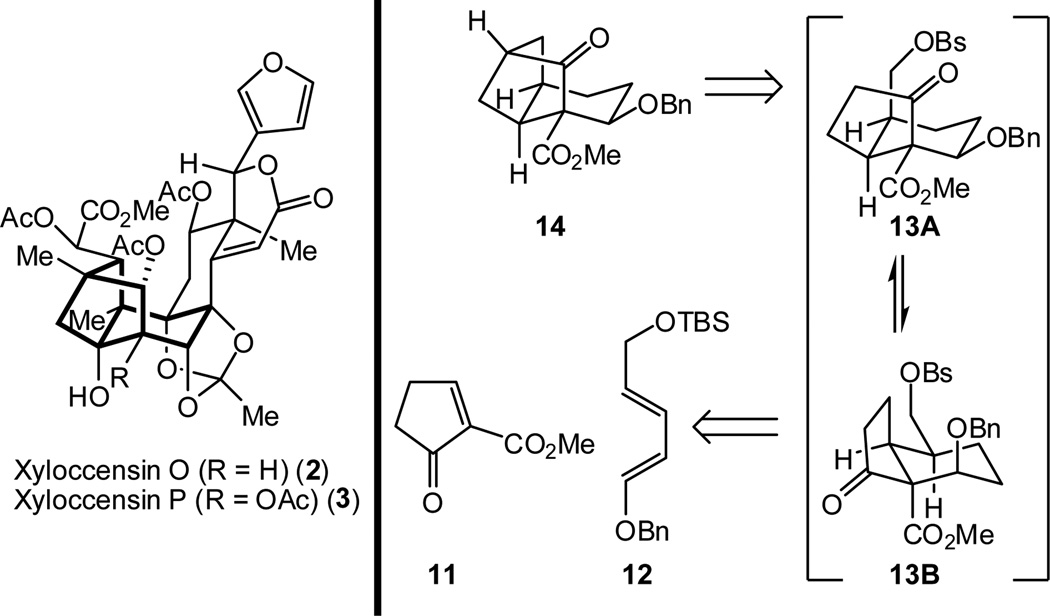
In this communication, we report the successful implementation of network analysis guidelines for forming functionalized octahydro-1H-2,4-methanoindene frameworks (14 and 26, Scheme 2 and 6, respectively), which serve as models for the challenging phragmalin-type caged architecture (e.g., in xyloccensin O, 2).
Scheme 2.
Retrosynthetic analysis of 14
Scheme 6.
Michael addition
Initial studies focused on the construction of 14. This undertaking would demonstrate the feasibility of the intramolecular cylization by using an irreversible alkylation before attempting the bond construction using a potentially reversible Michael addition (vide infra). We envisioned that 14 could arise from conformer 13A of hydrindanone derivative 13 by an alkylative C-C bond formation. Although conformations of hydrindane and hydrindanone systems have been studied,11 there are no reports that describe these dynamics in highly functionalized systems. Despite preliminary computations suggesting conformer 13B to be lower energy, the irreversibility of the alkylation step arising from conformer 13A was expected to lead to productive formation of 14.12 Hydrindanone derivative 13 could in turn arise from a Diels-Alder cycloaddition between cyclopentenone 11 and diene 12.
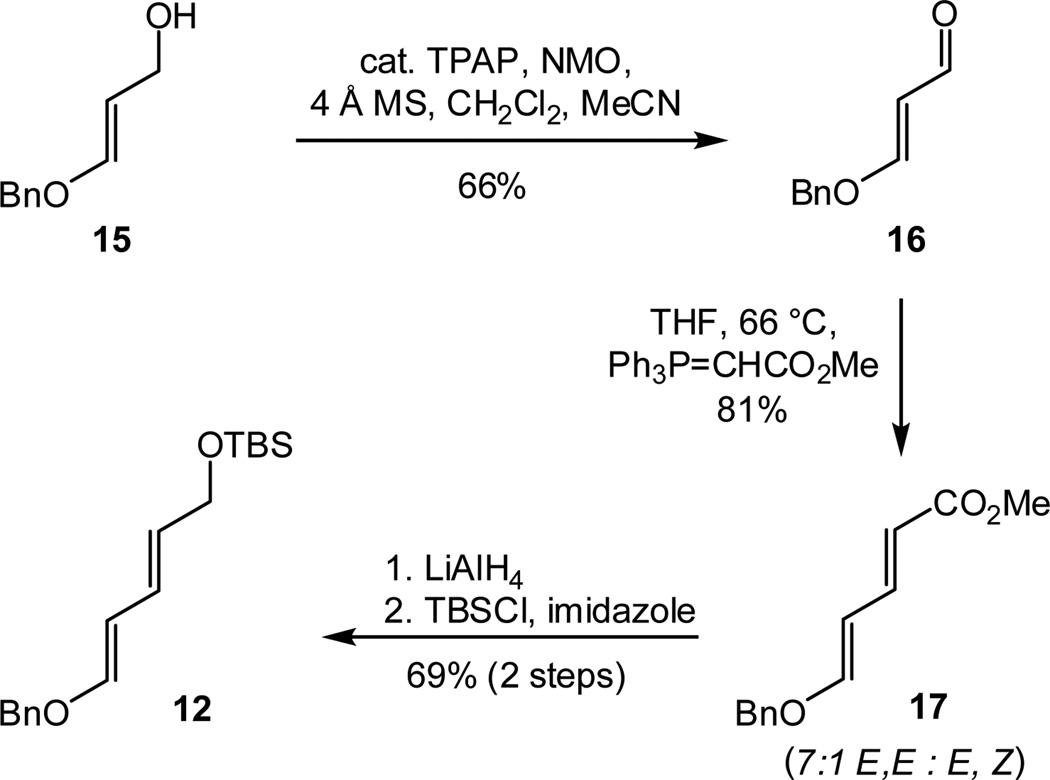
Given that 2-methoxycarbonylcyclopent-2-enone (11) is readily available in multigram quantities,13 our synthesis of 13 commenced with the preparation of diene 12 (Scheme 3). Building on precedent from d’Angelo14 and Grierson,15 we began with Ley oxidation16 of allylic alcohol 15 (prepared from ethyl propiolate),17 affording aldehyde 16 in 66% yield. Wittig olefination using carbomethoxymethylidene phosphorane gave diene ester 17 in 81% yield as a separable 7:1 mixture of E, E and E, Z isomers. Subsequent reduction of the ester group (LiAlH4) and silylation using TBSCl and imidazole provided the desired diene (12) in 69% yield over the two steps.
Scheme 3.
Synthesis of diene 12
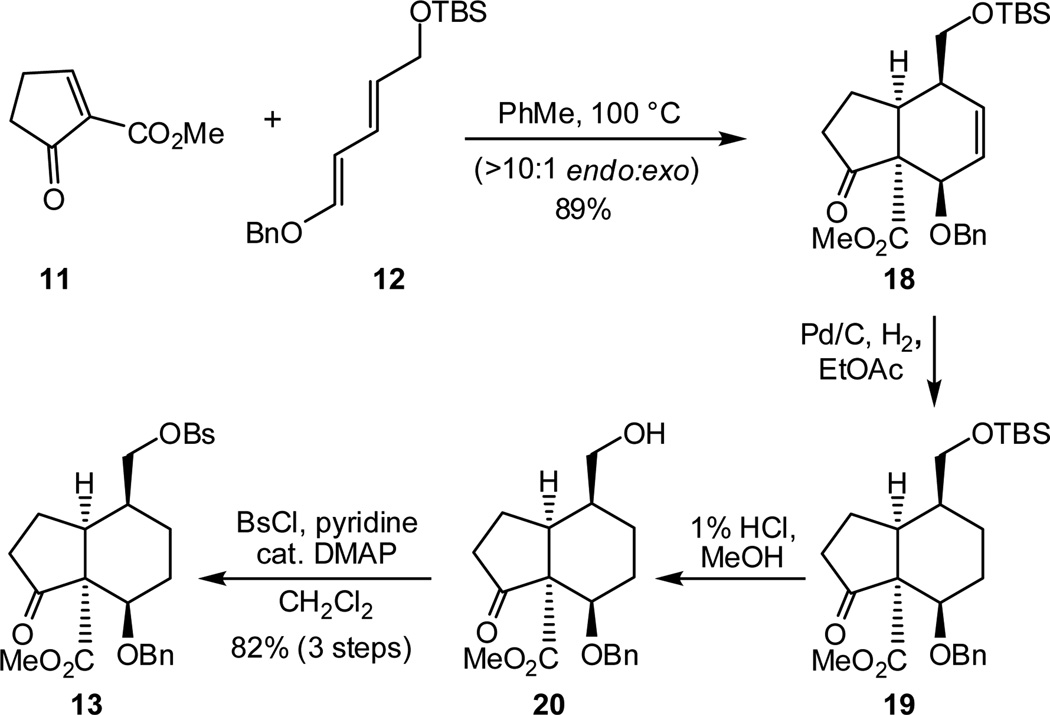
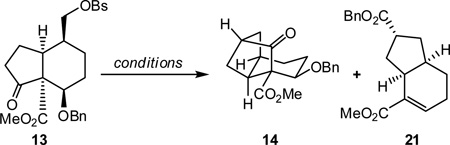
With diene 12 and dienophile 11 in hand, we next pursued the Diels-Alder cycloaddition. Heating a mixture of diene 12 and freshly prepared 11 (1.45 equiv) provided adduct 18 (Scheme 4) in 89% combined yield (>10:1 endo:exo ratio).18,19 Reduction of the double bond in 18 could be accomplished under carefully monitored hydrogenation conditions, which proceeded without hydrogenolytic cleavage of the benzyl ether. Treatment of the crude hydrogenation product (19) with 1% HCl in MeOH furnished alcohol 20, which was sulfonated to produce keto-benzenesulfonate 13 in 82% yield over the three steps.
Scheme 4.
Synthesis of hydrindanone derivative 13
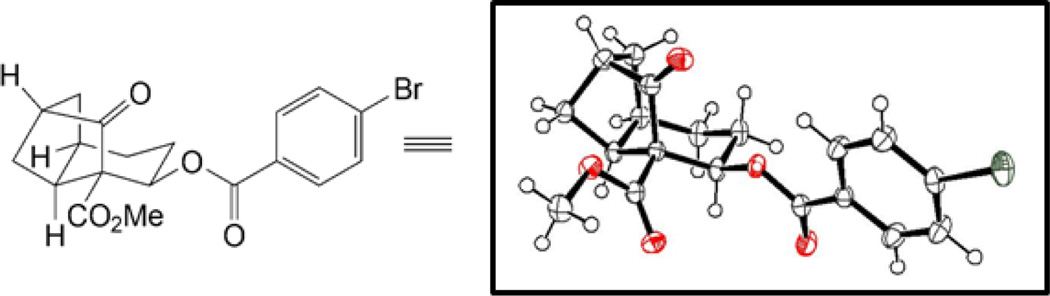
With keto-benzenesulfonate 13 in hand, the stage was set for the key alkylation reaction to build the tricyclic framework of the phragmalin-type limonoids. As shown in Table 1, several conditions were explored with varying levels of success. While excess KHMDS (Table 1, entry 1) led only to decomposition, the use of 1.1 equivalents of KHMDS gave the desired octahydro-1H-2,4-methanoindene framework (entry 2). However, the yields were found to be highly variable, with significant amounts of hydrindene 21 often being formed. Ultimately, a 74% yield of 14 could be consistently obtained under carefully controlled conditions using KHMDS (1.1 equiv) as the base in the presence of triethylamine and tetrabutylammonium iodide (TBAI; 1 equiv) in THF at −78 °C with warming to room temperature over 20 minutes (entry 3). The structure of 14 was unambigously confirmed by X-ray crystallographic analysis of the corresponding p-bromobenzoate derivative 22 (Figure 2).20
Table 1.
Optimization of alkylation conditions
 | ||
|---|---|---|
| entry | conditions | result |
| 1 | KHMDS (2 equiv), THF, −78 °C to rt | decomposition |
| 2 | KHMDS (1.1 equiv), THF, −78 °C to rt | 14 (38–84%) |
| 3 | KHMDS (1.1 equiv), TBAI (1 equiv) THF/NEt3, −78 °C to rt | 14 (74%) |
Figure 2.
ORTEP representation of 22
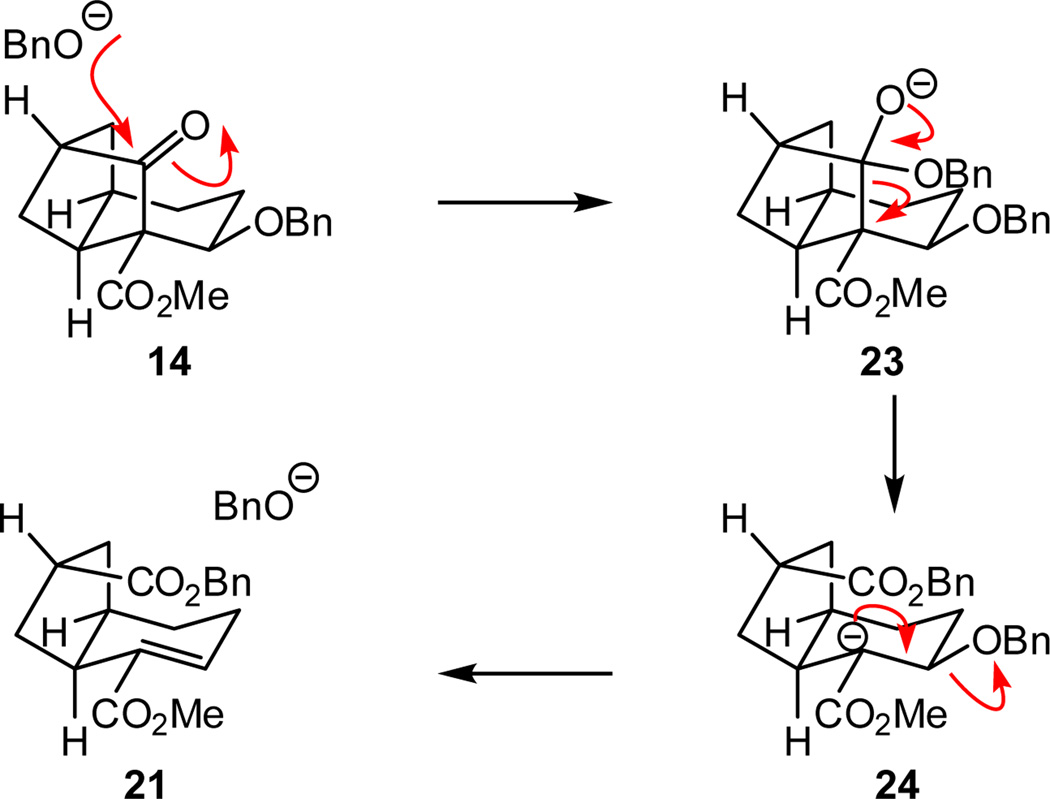
The fragmentation product (i.e., hydrindene 21) likely arises through a retro-Claisen reaction (Scheme 5) where initial nucleophilic attack on the ketone moiety by trace alkoxide generates tetrahedral intermediate 23, which can collapse to form 21 via ester enolate 24 (E1cB type process).21,22
Scheme 5.
Fragmentation pathway of 14
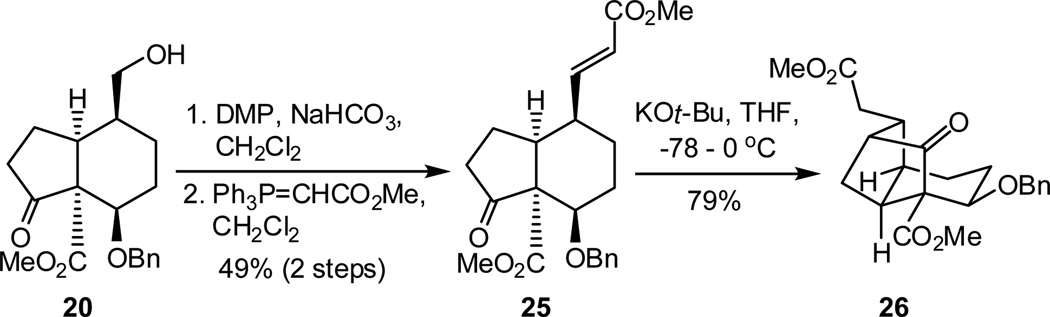
After the success of the intramolecular alkylative cyclization, we next investigated the potentially more challenging Michael addition, which would incorporate the required methylene ester present in the natural products.23 To this end, alcohol 20 was converted to enoate 25 through sequential oxidation and Wittig olefination in 49% yield over two steps. Gratifyingly, treating enone 25 with catalytic amounts of KOt-Bu in THF (−78 – 0 °C) led to the desired 1,4-adduct (26) as a single diastereomer in 79% yield.24 Having accomplished the intramolecular 1,4-conjugate addition, we are currently focused on the preparation of more highly functionalized caged frameworks.
In conclusion, we have developed a rapid and efficient route to the octahydro-1H-2,4-methanoindene core of the phragmalin-type limonoids. Key to the success of this route was the synthesis of the highly substituted hydrindanone system 18, which, in turn, provided access to intramolecular cyclization precursors 13 and 25. This work illustrates the utility of network analysis in defining a rapid path to architecturally complex frameworks such as the octahydro-1H-2,4-methanoindene core of the highly complex phragmalin-type limonoids.
Supplementary Material
Acknowledgement
The authors are grateful to the NIH (NIGMS RO1 084906) and American Cancer Society (RSG-09-017-01 CDD) for support of this work. T.P.L. thanks the NSERC (Canada) for a postdoctoral fellowship. Kathleen A. Durkin (UC Berkeley) is also thanked for computational assistance (NSF CHE-0233882 and CHE-0840505).
Footnotes
Supporting Information Available. Experimental details and characterization for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Wu J, Xiao Q, Huang J, Xiao Z, Qi S, Li Q, Zhang S. Org. Lett. 2004;6:1841–1844. doi: 10.1021/ol049444g. [DOI] [PubMed] [Google Scholar]
- 2.For a recent review of limonoids, see: Heasley BH. Eur. J. Org. Chem. 2011:19–46.
- 3.(a) Brandt GE, Schmidt MD, Prisinzano TE, Blagg BS. J. Med. Chem. 2008;51:6495–6502. doi: 10.1021/jm8007486. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Luo J, Wang J-S, Wang X-B, Luo J-G, Kong L-Y. Chem. Pharm. Bull. 2011;59:225–230. doi: 10.1248/cpb.59.225. [DOI] [PubMed] [Google Scholar]; (c) Cui J, Deng Z, Xu M, Proksch P, Li Q, Lin W. Helv. Chim. Acta. 2009;92:139–150. [Google Scholar]
- 4.Kuo R-Y, Qian K, Morris-Natschke SL, Lee K-H. Nat. Prod. Rep. 2009;26:1321–1344. doi: 10.1039/b810774m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Ravangpai W, Sommit D, Teerawatananond T, Sinpranee N, Palaga T, Pengpreecha S, Muangsin N, Pudhom K. Bioorg. Med. Chem. Lett. 2011;21:4485–4489. doi: 10.1016/j.bmcl.2011.06.010. [DOI] [PubMed] [Google Scholar]; (b) Luo J, Wang J-S, Luo J-G, Wang X-B, Kong L-Y. Tetrahedron. 2011;67:2942–2948. [Google Scholar]
- 6.Wu J, Xiao Q, Zhang S, Li X, Xiao Z, Ding H, Li Q. Tetrahedron. 2005;61:8382–8389. [Google Scholar]
- 7. Zhang Q, Di Y-T, He H-P, Fang X, Chen D-L, Yan X-H, Zhu F, Yang T-Q, Liu L-L, Hao X-J. J. Nat. Prod. 2011;74:152–157. doi: 10.1021/np100428u. For other proposed biosynthetic pathways for more highly oxygenated limonoids see: Taylor DAH. Phytochemistry. 1983;22:1297–1299. Abdelgaleil SAM, Okamura H, Iwagawa T, Sato A, Miyahara I, Doe M, Nakatani M. Tetrahedron. 2001;57:119–126.
- 8.(a) Nicolaou KC, Montagnon T, Snyder SA. Chem. Commun. 2003:551–564. doi: 10.1039/b209440c. [DOI] [PubMed] [Google Scholar]; (b) Heathcock CH. Angew. Chem. Int. Ed. 1992;31:665–681. [Google Scholar]
- 9.(a) Corey EJ, Ohno M, Vatakencherry PA, Mitra RB. J. Am. Chem. Soc. 1961;83:1251–1253. [Google Scholar]; (b) Corey EJ, Ohno M, Mitra RB, Vatakencherry PA. J. Am. Chem. Soc. 1964;86:478–485. [Google Scholar]; (c) Corey EJ, Howe WJ, Orf HW, Pensak DA, Petersson PG. J. Am. Chem. Soc. 1975;97:6116–6124. [Google Scholar]; (d) Corey EJ. Angew. Chem. Int. Ed. 1991;30:455–465. [Google Scholar]
-
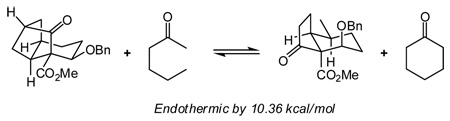
10.For example, the following homodesmotic calculation (Gaussian DFT; basis set B3LYP/6-31G(d,p); see the Supporting Information for details) indicates that formation of the tricycle from the bicycle is uphill by 10.36 kcal/mol. For discussion on homodesmotic calculations see:
Wheeler SE, Houk KN, Schleyer PvR, Allen WD. J. Am. Chem. Soc. . 2009;131:2547–2560. doi: 10.1021/ja805843n.
- 11.(a) Sokolova IM, Petrov AA. Neftekhimiya. 1977;17:498. [Google Scholar]; Chem. Abstr. 87 184059b. [Google Scholar]; (b) Lo cicero B, Weisbuch F, Dana G. J. Org. Chem. 1981;46:914–919. [Google Scholar]; (c) Schneider H-J, Nguyen-Ba N. Org. Magn. Reson. 1982;18:38. [Google Scholar]; (d) Moniz WB, Dixon JA. J. Am. Chem. Soc. 1961;83:1671–1675. [Google Scholar]; (e) Lack RE, Roberts JD. J. Am. Chem. Soc. 1968;90:6997–7001. [Google Scholar]
- 12.Our preliminary conformational search of 13 using Macromodel minimization/Monte Carlo search parameters has identified compound 13B as more energetically stable than compound 13A.
- 13.Wang C, Gu X, Yu MS, Curran DP. Tetrahedron. 1998;54:8355–8370. [Google Scholar]
- 14.Maddaluno J, d’Angelo J. Tetrahedron Lett. 1983;24:895–898. [Google Scholar]
- 15.Prabhakaran J, Lhermitte H, Das J, Sasi-Kumar TK, Grierson DS. Synlett. 2000;5:658–662. [Google Scholar]
- 16.(a) Griffith WP, Ley SV, Whitcombe GP, White AD. J. Chem. Soc. Chem. Commun. 1987:1625–1627. [Google Scholar]; (b) Hinzen B, Ley SV. J. Chem. Soc. Perkin Trans. 1997;1:1907–1908. [Google Scholar]
- 17.(a) Fujiwara K, Goto A, Sato D, Kawai H, Suzuki T. Tetrahedron Letters. 2005;46:3465–3468. [Google Scholar]; (b) Tellam JP, Kociok-Köhn G, Carbery DR. Org. Lett. 2008;10:5199–5202. doi: 10.1021/ol802169j. [DOI] [PubMed] [Google Scholar]
- 18.The endoand exo diastereomers could be easily separated by column chromatography.
- 19.Of note, the double bond in 18 could provide a functional handle for the annulation of the remainder of the phragmalin framework.
- 20.Ortep-3 for Windows: Farrugia LJ. J. Appl. Cryst. 1997;30:565.
- 21.Support for this hypothesis is found by treatment of 14 with KOBn which results in clean conversion to 21.
- 22.Interestingly, fragmentation of the polycyclic framework is not observed upon reduction of the carbonyl group of 14 with NaBH4. For more information, see the Supporting Information.
- 23.The success of this cyclization illustrates the potential for further elaboration of this core towards the phragmalin-type limonoids.
- 24.Stereochemistry of 26 predicated on A1,3 considerations.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.